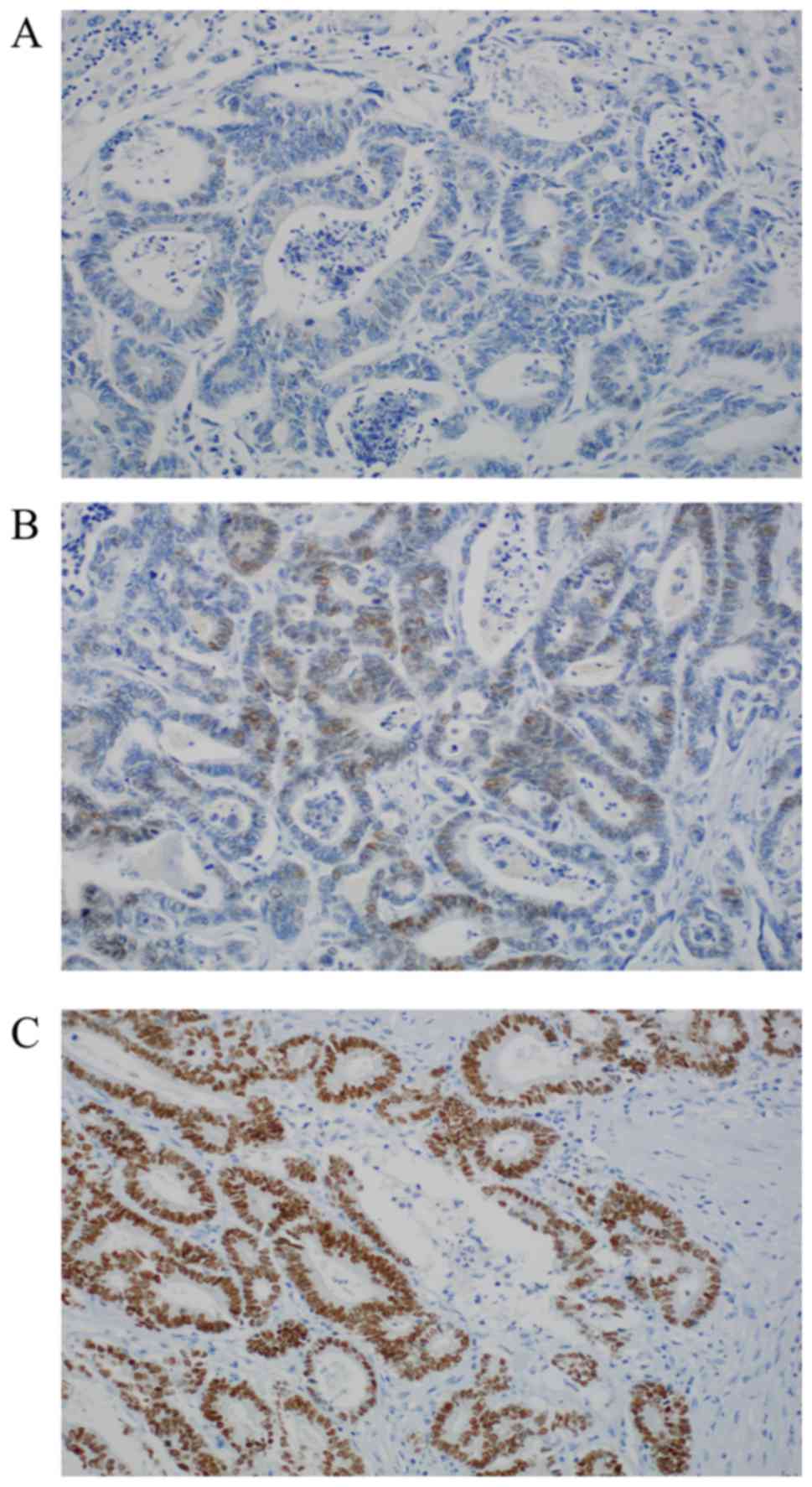
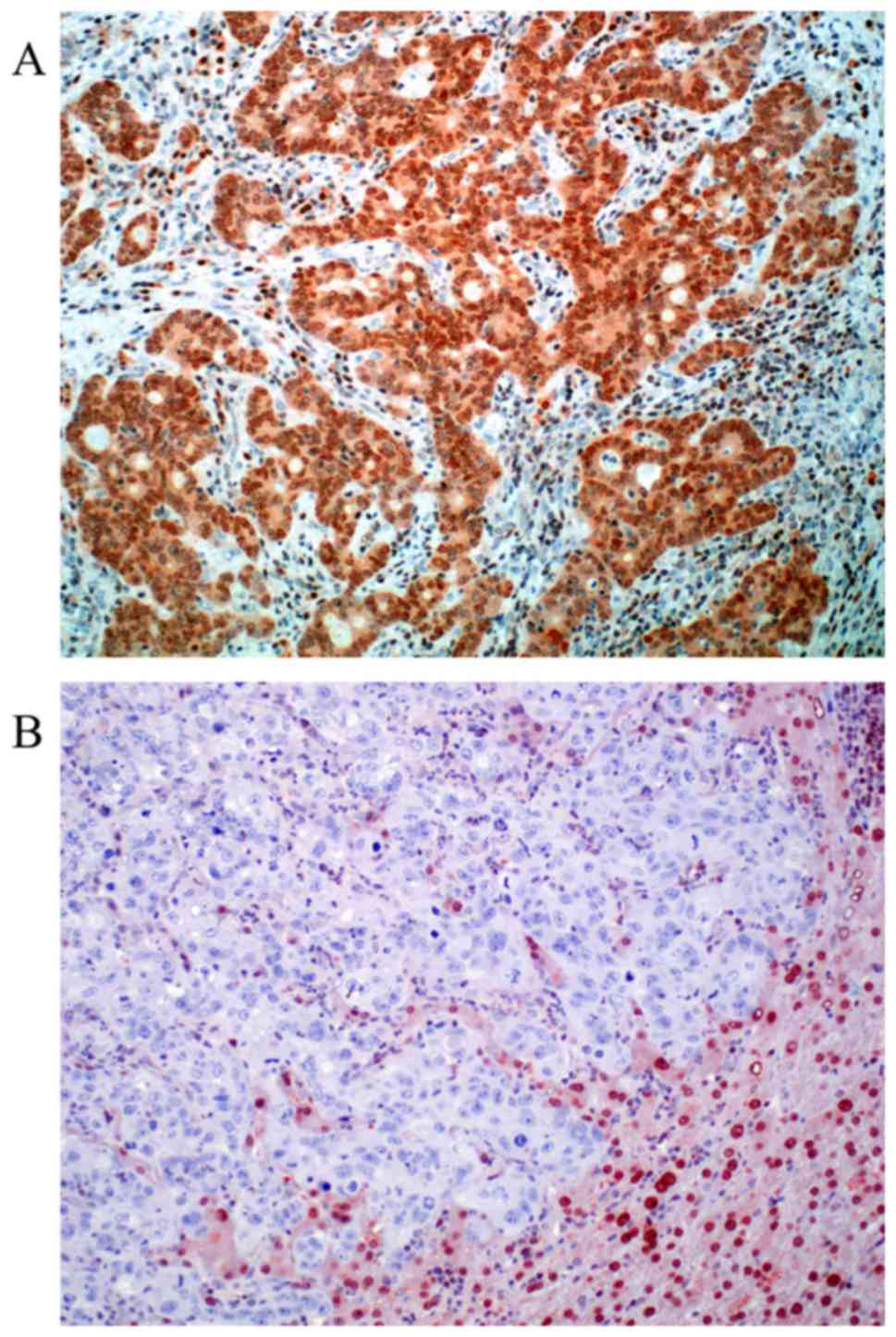
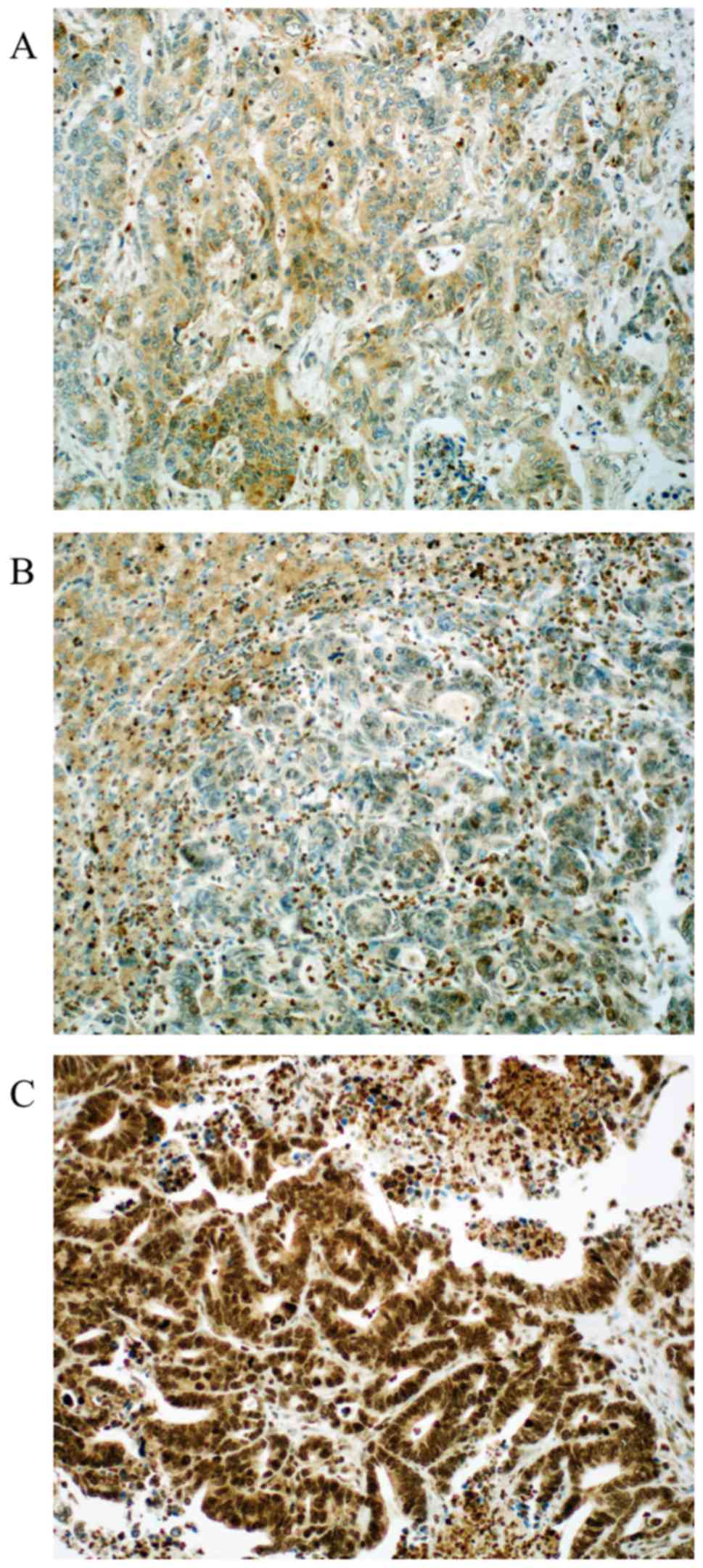
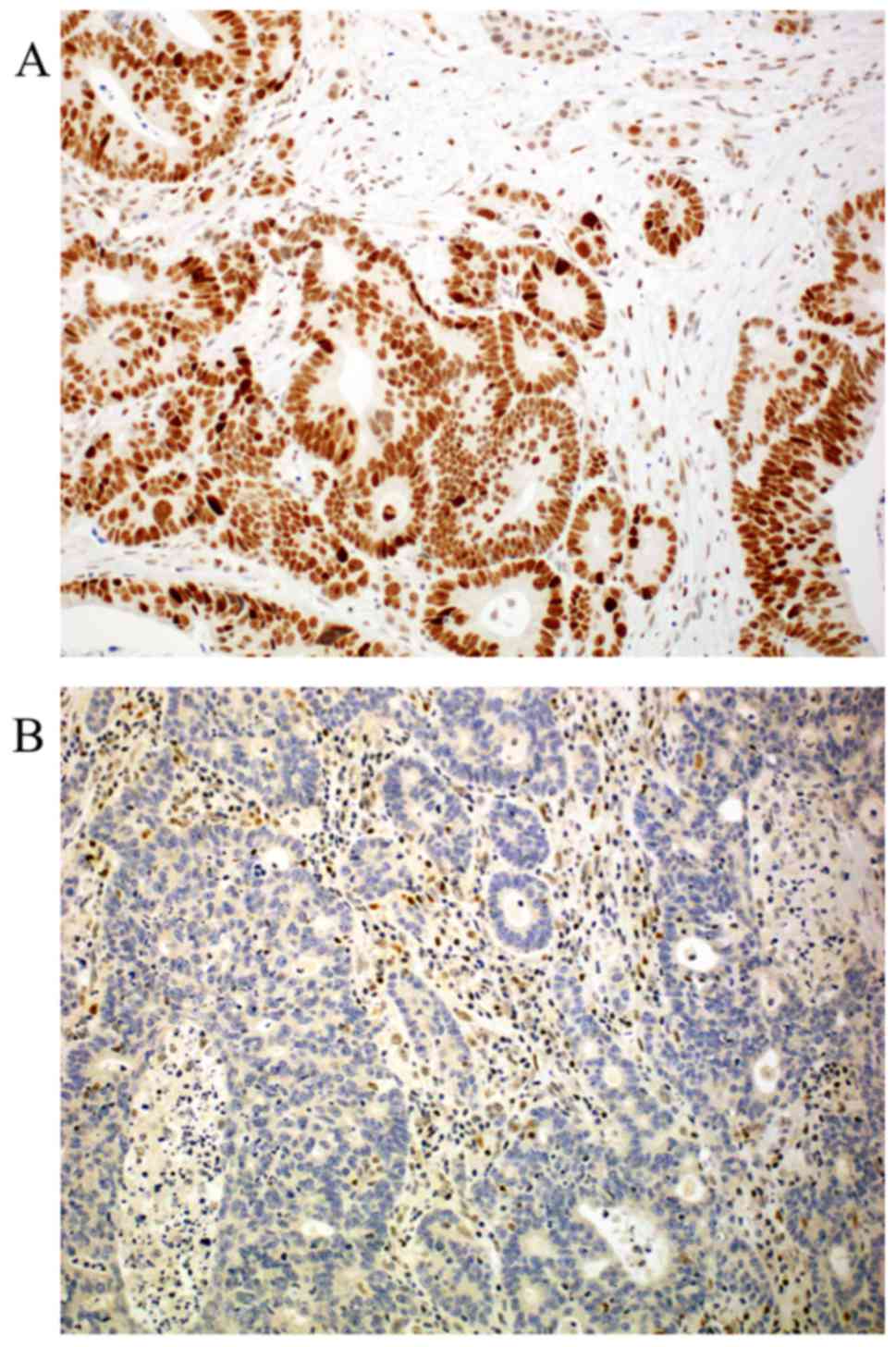
|
1
|
Hryniuk A, Grainger S, Savory JG and
Lohnes D: Cdx1 and Cdx2 function as tumor suppressors. J Biol Chem.
289:33343–33354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Misiakos EP, Karidis NP and Kouraklis G:
Current treatment for colorectal liver metastases. World J
Gastroenterol. 17:4067–4075. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olsen J, Espersen ML, Jess P, Kirkeby LT
and Troelsen JT: The clinical perspectives of CDX2 expression in
colorectal cancer: A qualitative systematic review. Surg Oncol.
23:167–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olsen AK, Coskun M, Bzorek M, Kristensen
MH, Danielsen ET, Jørgensen S, Olsen J, Engel U, Holck S and
Troelsen JT: Regulation of APC and AXIN2 expression by intestinal
tumor suppressor CDX2 in colon cancer cells. Carcinogenesis.
34:1361–1369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zlobec I, Bihl M, Foerster A, Rufle A and
Lugli A: Comprehensive analysis of CpG island methylator phenotype
(CIMP)-high, -low and -negative colorectal cancers based on protein
marker expression and molecular features. J Pathol. 225:336–343.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Renouf B, Soret C, Saandi T, Delalande F,
Martin E, Vanier M, Duluc I, Gross I, Freund JN and Domon-Dell C:
Cdx2 homeoprotein inhibits non-homologous end joining in colon
cancer but not in leukemia cells. Nucleic Acids Res. 40:3456–3469.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Hu F, Wang Y, Yao X, Zhang Z, Wang
F, Sun G, Cui BB, Dong X and Zhao Y: CpG island methylator
phenotype and prognosis of colorectal cancer in Northeast China.
Biomed Res Int. 2014:2363612014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inno A, Fanetti G, Di Bartolomeo M, Gori
S, Maggi C, Cirillo M, Iacovelli R, Nichetti F, Martinetti A and de
Braud F: Role of MGMT as biomarker in colorectal cancer. World J
Clin Cases. 2:835–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hassen S, Ali N and Chowdhury P: Molecular
signaling mechanisms of apoptosis in hereditary non-polyposis
colorectal cancer. World J Gastrointest Pathophysiol. 3:71–79.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sayar I, Akbas EM, Isik A, Gokce A, Peker
K, Demirtas L and Gürbüzel M: Relationship among mismatch repair
deficiency, CDX2 loss, p53 and E-cadherin in colon carcinoma and
suitability of using a double panel of mismatch repair proteins by
immunohistochemistry. Pol J Pathol. 66:246–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Z, Qing Y, Guan W, Li M, Peng Y, Zhang
S, Xiong Y and Wang D: Predictive value of APE1, BRCA1, ERCC1 and
TUBB3 expression in patients with advanced non-small cell lung
cancer (NSCLC) receiving first-line platinum-paclitaxel
chemotherapy. Cancer Chemother Pharmacol. 74:777–786. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ruzzo A, Graziano F, Loupakis F, Rulli E,
Canestrari E, Santini D, Catalano V, Ficarelli R, Maltese P,
Bisonni R, et al: Pharmacogenetic profiling in patients with
advanced colorectal cancer treated with first-line FOLFOX-4
chemotherapy. J Clin Oncol. 25:1247–1254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Persad S, Troussard AA, McPhee TR,
Mulholland DJ and Dedhar S: Tumor suppressor PTEN inhibits nuclear
accumulation of beta-catenin and T cell/lymphoid enhancer factor
1-mediated transcriptional activation. J Cell Biol. 153:1161–1174.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henderson BR and Fagotto F: The ins and
outs of APC and beta-catenin nuclear transport. EMBO Rep.
3:834–839. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
16
|
Camp RL, Charette LA and Rimm DL:
Validation of tissue microarray technology in breast carcinoma. Lab
Invest. 80:1943–1949. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torhorst J, Bucher C, Kononen J, Haas P,
Zuber M, Köchli OR, Mross F, Dieterich H, Moch H, Mihatsch M, et
al: Tissue microarrays for rapid linking of molecular changes to
clinical endpoints. Am J Pathol. 159:2249–2256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Umar A, Boland CR, Terdiman JP, Syngal S,
de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ,
Hamelin R, et al: Revised bethesda guidelines for hereditary
nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite
instability. J Natl Cancer Inst. 96:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toth C, Meinrath J, Herpel E, Derix J,
Fries J, Buettner R, Schirmacher P and Heikaus S: Expression of the
apoptosis repressor with caspase recruitment domain (ARC) in liver
metastasis of colorectal cancer and its correlation with DNA
mismatch repair proteins and p53. J Cancer Res Clin Oncol.
142:927–935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gerhard R, Carvalho A, Carneiro V, Bento
RS, Uemura G, Gomes M, Albergaria A and Schmitt F:
Clinicopathological significance of ERCC1 expression in breast
cancer. Pathol Res Pract. 209:331–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dawson H, Galvan JA, Helbling M, Muller
DE, Karamitopoulou E, Koelzer VH, Economou M, Hammer C, Lugli A and
Zlobec I: Possible role of Cdx2 in the serrated pathway of
colorectal cancer characterized by BRAF mutation, high-level CpG
Island methylator phenotype and mismatch repair-deficiency. Int J
Cancer. 134:2342–2351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dawson H, Koelzer VH, Lukesch AC, Mallaev
M, Inderbitzin D, Lugli A and Zlobec I: Loss of Cdx2 expression in
primary tumors and lymph node metastases is specific for mismatch
repair-deficiency in colorectal cancer. Front Oncol. 3:2652013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halford S, Rowan A, Sawyer E, Talbot I and
Tomlinson I: O(6)-methylguanine methyltransferase in colorectal
cancers: Detection of mutations, loss of expression and weak
association with G:C>A:T transitions. Gut. 54:797–802. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakagawachi T, Soejima H, Urano T, Zhao W,
Higashimoto K, Satoh Y, Matsukura S, Kudo S, Kitajima Y, Harada H,
et al: Silencing effect of CpG island hypermethylation and histone
modifications on O6-methylguanine-DNA methyltransferase (MGMT) gene
expression in human cancer. Oncogene. 22:8835–8844. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cabrini G, Fabbri E, Lo Nigro C, Dechecchi
MC and Gambari R: Regulation of expression of O6-methylguanine-DNA
methyltransferase and the treatment of glioblastoma (Review). Int J
Oncol. 47:417–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Molenaar RJ, Verbaan D, Lamba S, Zanon C,
Jeuken JW, Boots-Sprenger SH, Wesseling P, Hulsebos TJ, Troost D,
van Tilborg AA, et al: The combination of IDH1 mutations and MGMT
methylation status predicts survival in glioblastoma better than
either IDH1 or MGMT alone. Neuro Oncol. 16:1263–1273. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen L, Kondo Y, Rosner GL, Xiao L,
Hernandez NS, Vilaythong J, Houlihan PS, Krouse RS, Prasad AR,
Einspahr JG, et al: MGMT promoter methylation and field defect in
sporadic colorectal cancer. J Natl Cancer Inst. 97:1330–1338. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalogeraki A, Karvela-Kalogeraki I,
Tamiolakis D, Petraki P, Saridaki Z and Tzardi M: ERCC1 expression
correlated with EGFR and clinicopathological variables in patients
with non-small cell lung cancer. An immunocytochemical study on
fine-needle aspiration biopsies samples. Rev Port Pneumol.
20:200–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Facista A, Nguyen H, Lewis C, Prasad AR,
Ramsey L, Zaitlin B, Nfonsam V, Krouse RS, Bernstein H, Payne CM,
et al: Deficient expression of DNA repair enzymes in early
progression to sporadic colon cancer. Genome Integr. 3:32012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smith DH, Fiehn AM, Fogh L, Christensen
IJ, Hansen TP, Stenvang J, Nielsen HJ, Nielsen KV, Hasselby JP,
Brünner N and Jensen SS: Measuring ERCC1 protein expression in
cancer specimens: Validation of a novel antibody. Sci Rep.
4:43132014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen HY, Shao CJ, Chen FR, Kwan AL and
Chen ZP: Role of ERCC1 promoter hypermethylation in drug resistance
to cisplatin in human gliomas. Int J Cancer. 126:1944–1954.
2010.PubMed/NCBI
|
|
33
|
Borrmann L, Schwanbeck R, Heyduk T,
Seebeck B, Rogalla P, Bullerdiek J and Wisniewski JR: High mobility
group A2 protein and its derivatives bind a specific region of the
promoter of DNA repair gene ERCC1 and modulate its activity.
Nucleic Acids Res. 31:6841–6851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garofalo M and Croce CM: MicroRNAs as
therapeutic targets in chemoresistance. Drug Resist Updat.
16:47–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hawkins NJ, Lee JH, Wong JJ, Kwok CT, Ward
RL and Hitchins MP: MGMT methylation is associated primarily with
the germline C>T SNP (rs16906252) in colorectal cancer and
normal colonic mucosa. Mod Pathol. 22:1588–1599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang KJ, Min BH, Ryu KJ, Kim KM, Chang DK,
Kim JJ, Rhee JC and Kim YH: The role of the CpG island methylator
phenotype on survival outcome in colon cancer. Gut Liver.
9:202–207. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pai SG, Carneiro BA, Mota JM, Costa R,
Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK and Giles FJ:
Wnt/beta-catenin pathway: Modulating anticancer immune response. J
Hematol Oncol. 10:1012017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Liu X, Gusev E, Wang C and Fagotto
F: Regulation of the phosphorylation and nuclear import and export
of β-catenin by APC and its cancer-related truncated form. J Cell
Sci. 127:1647–1659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Efstathiou JA, Noda M, Rowan A, Dixon C,
Chinery R, Jawhari A, Hattori T, Wright NA, Bodmer WF and
Pignatelli M: Intestinal trefoil factor controls the expression of
the adenomatous polyposis coli-catenin and the E-cadherin-catenin
complexes in human colon carcinoma cells. Proc Natl Acad Sci USA.
95:pp. 3122–3127. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Neufeld KL: Nuclear APC. Adv Exp Med Biol.
656:13–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fagman H, Larsson F, Arvidsson Y, Meuller
J, Nordling M, Martinsson T, Helmbrecht K, Brabant G and Nilsson M:
Nuclear accumulation of full-length and truncated adenomatous
polyposis coli protein in tumor cells depends on proliferation.
Oncogene. 22:6013–6022. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fodde R, Smits R and Clevers H: APC,
signal transduction and genetic instability in colorectal cancer.
Nat Rev Cancer. 1:55–67. 2001. View Article : Google Scholar : PubMed/NCBI
|