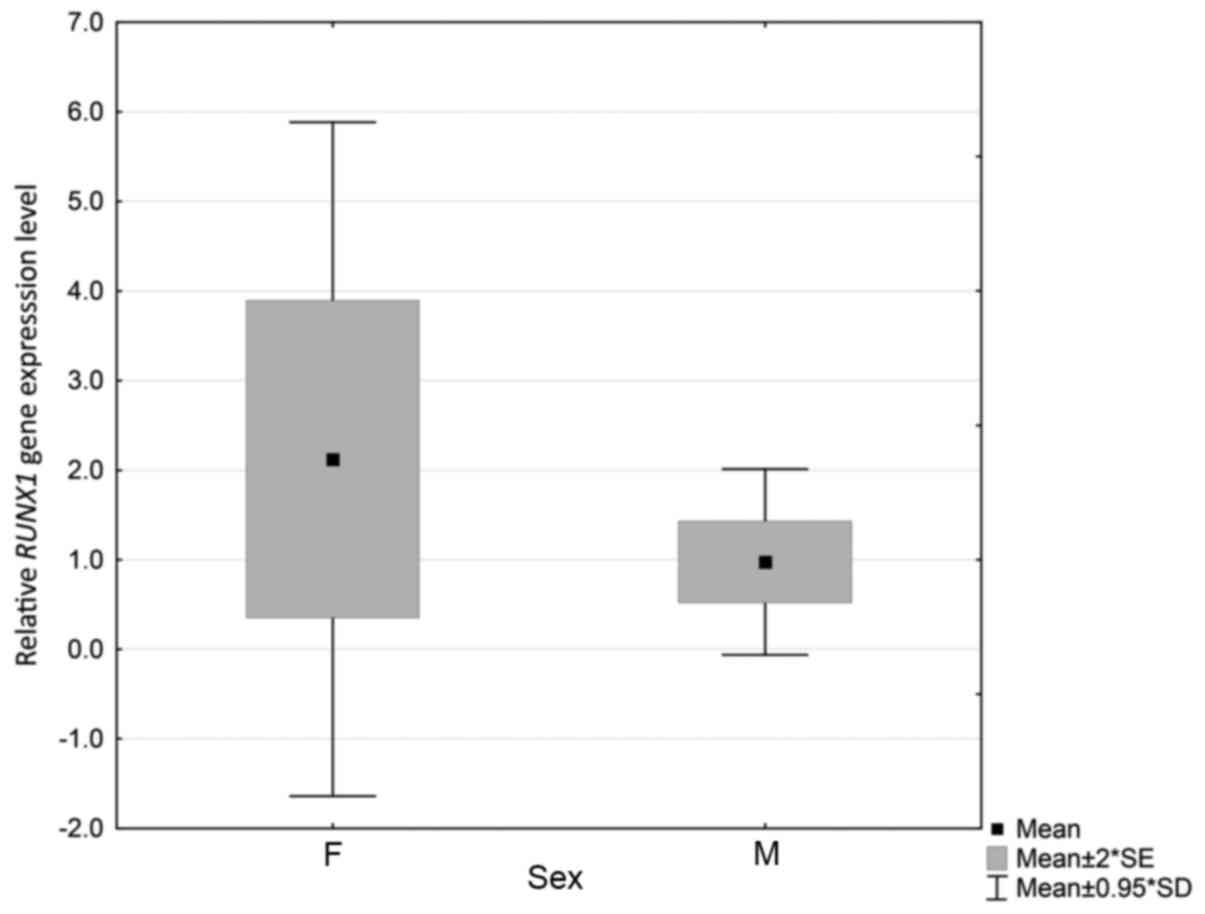
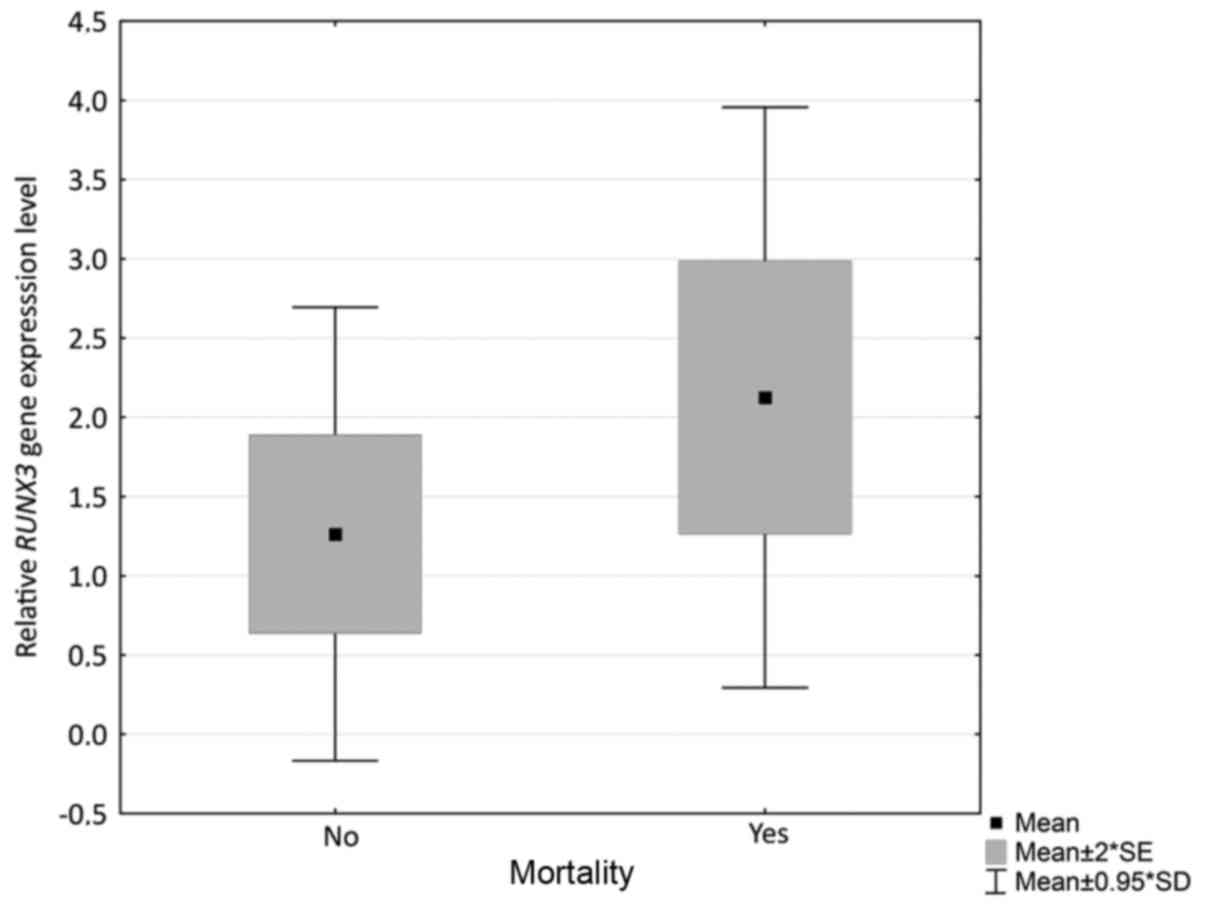
|
1
|
Saultz JN and Garzon R: Acute myeloid
leukemia: A concise review. J Clin Med. 5:pii: E33. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fey MF and Buske C: ESMO Guidelines
Working Group: Acute myeloblastic leukaemias in adult patients:
ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24 Suppl 6:vi138–vi143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thein MS, Ershler WB, Jemal A, Yates JW
and Baer MR: Outcome of older patients with acute myeloid leukemia:
An analysis of SEER data over three decades. Cancer. 119:2720–2727.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 375:1136–1152. 2015.
View Article : Google Scholar
|
|
5
|
Döhner H, Estey E, Grimwade D, Amadori S,
Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA,
et al: Diagnosis and management of AML in adults: 2017 ELN
recommendations from an international expert panel. Blood.
129:424–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ito Y: Oncogenic potential of the RUNX
gene family: ‘Overview’. Oncogene. 23:4198–4208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng CK, Li L, Cheng SH, Lau KM, Chan NP,
Wong RS, Shing MM, Li CK and Ng MH: Transcriptional repression of
the RUNX3/AML2 gene by the t(8;21) and inv(16) fusion proteins in
acute myeloid leukemia. Blood. 112:3391–3402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito Y, Bae SC and Chuang LS: The RUNX
family: Developtal regulators in cancer. Nature Reviews Cancer.
15:81–95. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Medinger M, Lengerke C and Passweg J:
Novel prognostic and therapeutic mutations in acute myeloid
leukemia. Cancer Genomics Proteomics. 13:317–329. 2016.PubMed/NCBI
|
|
10
|
Tracey WD and Speck NA: Potential roles
for RUNX1 and its orthologs in determining hematopoietic cell fate.
Semin Cell Dev Biol. 11:337–342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
GeneCards® Human Gene Database:
RUNX3 gene. http://www.genecards.org/cgi-bin/carddisp.pl?gene=RUNX3April
24–2017
|
|
12
|
Asou N: The role of a Runt domain
transcription factor AML1/RUNX1 in leukemogenesis and its clinical
implications. Crit Rev Oncol Hematol. 45:129–150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bangsow C, Rubins N, Glusman G, Bernstein
Y, Negreanu V, Goldenberg D, Lotem J, Ben-Asher E, Lancet D,
Levanon D and Groner Y: The RUNX3 gene-sequence, structure and
regulated expression. Gene. 279:221–232. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Otto F, Stock M, Fliegauf M, Fenaux P,
Preudhomme C and Lübbert M: Absence of somatic mutations within the
Runt domain of AML2/RUNX3 in acute myeloid leukaemia. Leukemia.
17:1677–1678. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lau QC, Raja E, Salto-Tellez M, Liu Q, Ito
K, Inoue M, Putti TC, Loh M, Ko TK, Huang C, et al: RUNX3 is
frequently inactivated by dual mechanisms of protein
mislocalization and promoter hypermethylation in breast cancer.
Cancer Res. 66:6512–6520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae SC and Choi JK: Tumor suppressor
activity of RUNX3. Oncogene. 23:4336–4340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo C, Ding J, Yao L, Sun L, Lin T, Song
Y, Sun L and Fan D: Tumor suppressor gene Runx3 sensitizes gastric
cancer cells to chemotherapeutic drugs by downregulating Bcl-2,
MDR-1 and MRP-1. Int J Cancer. 116:155–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kabel A, Zamzami F, Al-Talhi M, Al-Dwila K
and Hamza R: Acute myeloid leukemia: A focus on risk factors,
clinical presentation, diagnosis and possible lines of management.
Cancer Res Treat. 5:62–67. 2017.
|
|
19
|
Silver N, Best S, Jiang J and Thein SL:
Selection of housekeeping genes for gene expression studies in
human reticulocytes using real-time PCR. BMC Mol Biol. 7:332006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu L, Fu H, Tian L, Xu K, Hu K, Wang J,
Wang J, Jing H, Shi J and Ke X: High expression of RUNX1 is
associated with poorer outcomes in cytogenetically normal acute
myeloid leukemia. Oncotarget. 7:15828–15839. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lam K and Zhang DE: RUNX1 and RUNX1-ETO:
Roles in hematopoiesis and leukemogenesis. Front Biosci (Landmark
Ed). 17:1120–1139. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ge T, Yin M, Yang M, Liu T and Lou G:
MicroRNA-302b suppresses human epithelial ovarian cancer cell
growth by targeting RUNX1. Cell Physiol Biochem. 34:2209–2220.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrari N, Mohammed ZM, Nixon C, Mason SM,
Mallon E, McMillan DC, Morris JS, Cameron ER, Edwards J and Blyth
K: Expression of RUNX1 correlates with poor patient prognosis in
triple negative breast cancer. PLoS One. 9:e1007592014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silva FP, Morolli B, Storlazzi CT, Anelli
L, Wessels H, Bezrookove V, Kluin-Nelemans HC and Giphart-Gassler
M: Identification of RUNX1/AML1 as a classical tumor suppressor
gene. Oncogene. 22:538–547. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osato M: Point mutations in the RUNX1/AML1
gene: Another actor in RUNX leukemia. Oncogene. 23:4284–4296. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ben-Ami O, Friedman D, Leshkowitz D,
Goldenberg D, Orlovsky K, Pencovich N, Lotem J, Tanay A and Groner
Y: Addiction of t(8;21) and inv(16) acute myeloid leukemia to
native RUNX1. Cell Rep. 4:1131–1143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goyama S, Schibler J, Cunningham L, Zhang
Y, Rao Y, Nishimoto N, Nakagawa M, Olsson A, Wunderlich M, Link KA,
et al: Transcription factor RUNX1 promotes survival of acute
myeloid leukemia cells. J Clin Invest. 123:3876–3888. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goyama S, Huang G, Kurokawa M and Mulloy
JC: Posttranslational modifications of RUNX1 as potential
anticancer targets. Oncogene. 34:3483–3492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilkinson AC, Ballabio E, Geng H, North P,
Tapia M, Kerry J, Biswas D, Roeder RG, Allis CD, Melnick A, et al:
RUNX1 is a key target in t(4;11) leukemias that contributes to gene
activation through an AF4-MLL complex interaction. Cell Rep.
3:116–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inoue K, Ozaki S, Shiga T, Ito K, Masuda
T, Okado N, Iseda T, Kawaguchi S, Ogawa M, Bae SC, et al: RUNX3
controls the axonal projection of proprioceptive dorsal root
ganglion neurons. Nat Neurosci. 5:946–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taniuchi I, Osato M, Egawa T, Sunshine MJ,
Bae SC, Komori T, Ito Y and Littman DR: Differential requirements
for Runx proteins in CD4 repression and epigenetic silencing during
T lymphocyte development. Cell. 111:621–633. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li QL, Ito K, Sakakura C, Fukamachi H,
Inoue Ki, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, et al: Causal
relationship between the loss of RUNX3 expression and gastric
cancer. Cell. 109:113–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang Y, Tong D, Lou G, Zhang Y and Geng
J: Expression of RUNX3 gene, methylation status and
clinicopathological significance in breast cancer and breast cancer
cell lines. Pathobiology. 75:244–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lacayo NJ, Meshinchi S, Kinnunen P, Yu R,
Wang Y, Stuber CM, Douglas L, Wahab R, Becton DL, Weinstein H, et
al: Gene expression profiles at diagnosis in de novo child-hood AML
patients identify FLT3 mutations with good clinical outcomes.
Blood. 104:2646–2654. 2004. View Article : Google Scholar : PubMed/NCBI
|