Ovarian cancer is the most deadly gynecologic
malignancy in women worldwide due to its diagnosis at advanced
stages of disease (1). There were an
estimated 22,240 new cases with 14,070 deaths in the United States
in 2018 (2). To date, most patients
with ovarian cancer undergo tumor reduction surgery followed by
chemotherapy with platinum/paclitaxel-based regimens; however,
chemoresistance often develops, resulting in treatment failure and
a mortality rate of >90% (3).
Thus, an improved understanding of ovarian cancer pathogenesis and
the development of novel therapeutic strategies may help medical
oncologists to clinically control ovarian cancer. Previous studies
have shown that the mRNA expression levels of interleukin (IL)-33
and the suppressor of tumorigenicity 2 (ST2) receptor are
significantly upregulated in ovarian cancer tissues and tumor
metastatic lesions compared with those of normal ovarian tissues
(3,4). In addition, IL-33 exhibits
significantly higher expression in both serous and mucinous ovarian
malignancies compared with in benign ones, which is associated with
an increase in tumor grade (5).
Other studies have reported that increased IL-33 and ST2 expression
is associated with a shortened survival time of patients with
epithelial ovarian cancer (4,6). The
IL-33 receptor ST2 is expressed as both a membrane-anchored
receptor (ST2L) activated by IL-33, and as a soluble variant (sST2)
that exhibits anti-inflammatory properties (7). In the present review, the importance of
the IL-33/ST2L axis in the progression of ovarian cancer was
discussed, as well as the therapeutic approaches to control the
IL-33/ST2L axis for patients with ovarian cancer.
The worldwide incidence and mortality rates of
ovarian cancer have increased to 41.2% during the past decades from
1990 to 2010 (8). The high-risk
groups for ovarian cancer include women who have not given birth to
children (9), women with early first
menstruation or late menopause (10), obesity, patients treated with hormone
replacement therapy (11) and
carriers of germline BRCA1 or BRCA2 mutations (12). The high mortality rate is mainly due
to its diagnosis at advanced stages of the disease, with <20% of
patients with ovarian cancer diagnosed at early stages (1).
Resection surgery, chemotherapy and radiotherapy are
the primary options to treat ovarian cancer clinically; however,
frequent recurrence of advanced ovarian cancer after chemoradiation
therapy remains a clinical challenge (13). Altered tumor cell metabolism has
drawn much attention as one of the causes of cancer
chemoresistance; for example, cancer stem cells are known to be
highly chemo-resistant and to maintain survival by altering key
metabolic pathways (14). Therefore,
the combination of chemotherapeutic agents with immune-targeting
methods is a promising approach for overcoming drug resistance
(15). For example, patients with
advanced ovarian cancer in remission treated with
cyclophosphamide-modulated vaccination had a trend towards
improvement in survival compared with those treated with
vaccination alone (16). In
addition, accumulated evidence revealed that cancer stem cells
serve a role in disease relapse after chemotherapy (1). Moreover, patients with ovarian cancer
with BRCA1 or BRCA2 mutations exhibit an improved response rate to
platinum/paclitaxel chemotherapy compared with non-carriers
(17,18). Furthermore, hormonal therapy and
immunotherapy (19,20), as well as palliative care, are also
utilized to treat ovarian cancer (21).
Epithelial ovarian cancer is considered as an
immunogenic tumor that can be recognized by the immune system of
patients (22). Previous studies
have reported that tumor-activated T lymphocytes and antibodies can
be detected in the blood, tumor and ascite samples of patients with
advanced-stage ovarian cancer (23,24). The
recruitment of regulatory T lymphocytes (Tregs) into the ovarian
cancer microenvironment confers immunity privilege and is
associated with a poor prognosis and a decreased survival (25). Additionally, high mRNA expression
levels of programmed death-ligand 1 are associated with a poor
prognosis in epithelial ovarian cancer (26). These altered immune responses serve
an important role in ovarian cancer pathogenesis and disease
progression. Thus, new and more effective immunotherapies may
provide a novel option to treat ovarian cancer, although this
treatment remains to be assessed clinically (27).
IL-33 was originally identified in canine
vasospastic cerebral arteries following subarachnoid hemorrhage
(28) and subsequently in human
tissues in 2003 (29). In 2005,
IL-33 was identified as a member of the IL-1 superfamily of
cytokines and as a ligand of ST2 (30). The IL33 gene is localized at
human chromosome 9p24.1, and IL-33 cDNA encodes polypeptides of 270
amino acid in humans, with a protein mass of 30 kDa (31). IL-33 protein is composed of two
evolutionary conserved domains: The amino (N)-terminal nuclear
domain and the carboxyl (C)-terminal IL-1-like cytokine domain
(32). It has been demonstrated that
the N-terminal part of IL-33 is necessary for the nuclear targeting
of epitope tagged (GFP-fused) IL-33 and for translocating IL-33
(tagged with N-term-discosoma recombinant red fluorescent protein)
to the nucleus in a mouse model (33,34).
Regarding the C-terminal IL-1-like cytokine domain of IL-33, this
has a β-trefoil fold that interacts with the extracellular domain
of ST2, thereby exerting the cytokine function of IL-33 (35).
IL-33 is a dual function protein that acts
intracellularly as a nuclear factor regulating transcription and
extracellularly as a potent cytokine (42). Full-length IL-33 can target the
nucleus to bind to histones H2A and H2B as a chromatin-related
nuclear factor (32,43). In addition, IL-33 can repress the
expression levels of NF-κB-regulated genes that are necessary for
pro-inflammatory signaling by interacting with the N-terminal
domain of the p65 subunit of NF-κB (44). A study in multiple sclerosis revealed
that IL-33 can activate histone deacetylase 3 activities, thereby
affecting gene expression through remodeling chromatin structure
and epigenetic mechanisms (45).
Furthermore, IL-33 has been demonstrated to be a
tissue-derived nuclear cytokine and is expressed in epithelial,
endothelial and fibroblastlike cells under both homeostatic and
inflammatory conditions (12).
Additionally, IL-33 functions as a stress-response protein, since
it is highly expressed in the nucleus of endothelial and epithelial
cells after damage or infection with a pathogen, resulting in its
release from the nucleus to the extracellular space as an
endogenous ‘danger’ signal to alert the immune system (46–48).
IL-33 is widely expressed in various organs, including the brain,
heart, liver, kidney, spleen and lung (49). IL-33 possesses pleiotropic activities
during Th1, Th2 and regulatory immune responses, and serves an
important role in fibrotic, infectious and chronic inflammatory
diseases (36). IL-33 functions to
promote or inhibit disease progression depending on the disease
type (31). For example, IL-33 mRNA
expression is significantly increased in the inflammatory mucosa of
patients with inflammatory bowel disease and in mice with dextran
sulfate sodium-induced colitis (50). In addition, previous studies have
revealed that the IL-33/ST2 axis demonstrates an adverse effect on
the pathogenesis of systemic lupus erythematosus (51), as well as promoting tubular cell
injury and interstitial fibrosis in obstructive kidney disease
(52). Moreover, the serum levels of
IL-33 and sST2 are elevated in patients with sepsis, suggesting the
involvement of the IL-33/ST2 axis in the pathogenesis and
progression of sepsis (53–55). Another study has demonstrated that
IL-33 expression is upregulated in the serum and synovial fluid
samples of patients with rheumatoid arthritis and is associated
with disease progression (56).
However, IL-33 and ST2 expression is beneficial in a non-alcoholic
fatty liver disease mouse model primarily by decreasing high-flow
dialysis-induced hepatic steatosis and serum alanine
aminotransferase levels, while improving insulin resistance and
glucose tolerance (31).
In human cancer, the IL-33/ST2 axis promotes lung
cancer cell migration and invasion through the protein kinase B
pathway (57). In addition, IL-33
expression is upregulated in the serum of patients with gastric
(58), non-small cell lung (59) and breast cancer (60). By contrast, IL-33 possesses a
tumor-suppressor role in sporadic colon cancer; the IL-33/ST2 axis
regulates the tumor microenvironment by recruiting immune cells
that support malignant hyperplasia or alter antitumor immunity
(61). In addition, IL-33/ST2L
signals trigger the transcription of downstream inflammatory genes
and anti-inflammatory genes by activating various intracellular
kinases and factors, thereby activating the inflammatory immune
response (62). The latest data
indicate that the IL33/ST2 axis in Tregs is an important pathway
that allows Tregs to accumulate in the tumor microenvironment,
suggesting that the IL33/ST2 axis may be a potential therapeutic
target for cancer immunotherapy (63). Overall, the aforementioned studies
demonstrate the pleiotropy of IL-33 in regulating antitumor
immunity or tumor growth.
IL-33 expression fluctuates with specific ovarian
functions, such as ovulation and estrus cycles (64). Studies have revealed that IL-33 is
the most significantly upregulated immune molecule during ovulation
(64,65). Numerous randomly sampled ovaries
expressed a higher level of IL-33 compared with other organs,
except with ovaries sampled at IL-33 expression peak in the estrous
cycle and during ovulation (64).
This suggests that some additional unknown factors may affect IL-33
expression. It has been revealed that IL-33 expression is
significantly upregulated in ovarian cancer tissues and metastatic
tumor lesions compared with in benign ones (5). In addition, compared with in normal
human ovarian tissue samples, upregulation of ST2 was detected in
66% primary ovarian tumors and 87% metastatic ovarian tumors, which
was more marked than the upregulation of IL-33 expression in 59% of
primary sites and 76% of metastatic ovarian tumors (6). Another study has demonstrated that high
IL-33 and ST2 expression is closely associated with a poor overall
survival of patients with epithelial ovarian cancer (6), indicating that the altered expression
of the IL-33/ST2 axis serves an important role in the progression
of ovarian cancer and that the detection of their expression may be
utilized as a biomarker for a poor prognosis in patients with
ovarian cancer.
Since 70% of ovarian cancers are diagnosed at
advanced stages of disease with metastasized tumors, it is crucial
to assess and investigate the molecular mechanisms involved in the
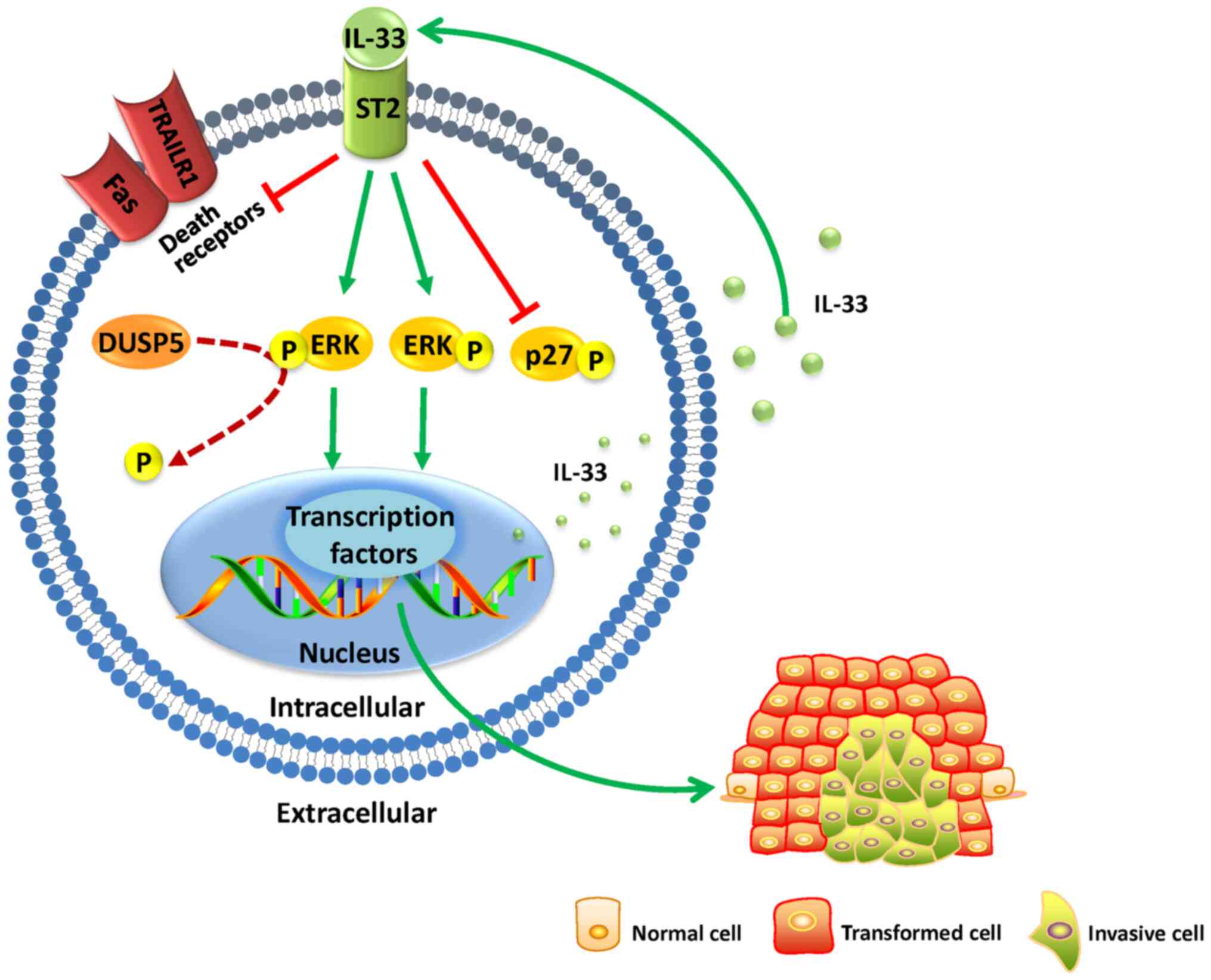
invasiveness and metastasis of ovarian cancer (66,67). A
previous study has analyzed the microarray data from the Gene
Expression Omnibus comprehensive database and found that patients
with low dual-specificity phosphatase 5 (DUSP5) expression have
significantly shorter overall survival than those with high
expression (68). DUSP5 is a nuclear
ERK1/2-selective phosphatase induced by ERK signaling in mammalian
cells, which selectively binds and inactivates ERK1 and ERK2 in
vivo, and is a direct transcriptional target of the tumor
suppressor p53 (69–71). The latest data indicate that
silencing of DUSP5 transcription is able to increase the expression
and secretion of IL-33, thereby promoting the proliferation,
migration and invasion of ovarian cancer cells (68). Furthermore, a previous study has
revealed changes in signal transduction pathways after treatment of
epithelial ovarian cancer cells with full-length human IL-33, with
increased phosphorylation of JNK and ERK proteins; however, after
using sST2 to neutralize IL-33, the phosphorylation of ERK and JNK
was completely blocked (6). By
contrast, treatment of human ovarian cancer CAOV3 and HO8910 cells
with the selective ERK inhibitor U0126 significantly suppressed ERK
phosphorylation, blocking the effects of IL-33-mediated increase in
tumor cell migration and invasion, as well as tumor cell viability
and proliferation (6). Additionally,
the JNK pathway inhibitor SP600125 inhibited JNK phosphorylation in
CAOV3 and HO8910 cells, but had no effect on IL-33-induced cell
migration and invasion, even though they were able to block tumor
cell viability and proliferation in IL-33-induced CAOV3 cells
(6). Furthermore, a recent study
reported that IL-33 promoted the proliferation and inhibited the
apoptosis of ovarian cancer cells by downregulating p27, Fas cell
surface death receptor and tumor necrosis factor-related
apoptosis-inducing ligand receptor 1 (TRAILR1) in vitro
(4). The aforementioned studies
indicated that low DUSP5 expression decreases the dephosphorylation
of ERK, thereby increasing IL-33 expression, which promotes tumor
cell invasion and metastasis through the phosphorylation of ERK and
the other aforementioned pathways. In summary, low DUSP5 expression
may decrease the dephosphorylation of ERK, thereby increasing IL-33
expression; IL-33 may then promote the proliferation of ovarian
cancer cells and inhibit their apoptosis by downregulating p27, Fas
and TRAILR1 expression. This process may lead to the survival of
patients with low DUSP5 expression to be shorter than those with
high expression (Fig. 1). However,
the precise underlying mechanism of IL-33/ST2-promoting tumor
growth and metastasis remains to be determined.
In addition, further investigations regarding the
conflicting functions of IL-33 in different types of cancer are
required. For example, one study has revealed that IL-33 is a key
mediator in the development of inflammation-associated pancreatic
cancer by upregulating the secretion of pro-inflammatory IL-6 and
IL-8 (72), whereas another study
has reported that transgenic IL-33 was able to activate natural
killer and CD8+ T cells to inhibit the growth and
metastasis of melanoma and lung cancer in animal models (73). However, in ovarian cancer, studies
have demonstrated that a local intraperitoneal injection of IL-33
is able to delay ovarian cancer peritoneal metastases, indicating
the efficacy of IL-33 for the treatment of abdominal metastatic
cancer (74,75).
Nearly 30 years have passed since the discovery of
IL-33, and numerous studies have been conducted to determine the
molecular structure, distribution, receptor binding and signaling
pathways of IL-33. The knowledge regarding the molecular basis of
IL-33 signaling is relatively comprehensive. To date, the
literature presents great progress in understanding the function of
IL-33 in ovarian cancer. However, future studies are required to
fully understand the role of IL-33 in the regulation of signaling
pathways and regulatory networks in ovarian cancer. Targeting IL-33
and its signaling pathways may function as a novel strategy to
control ovarian cancer progression.
Not applicable.
No funding was received.
Not applicable.
NL, YY and SW conceived and designed the study. NL,
JC, YZ, MZ and LP performed the literature search and drafted the
manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Li SS, Ma J and Wong AST: Chemoresistance
in ovarian cancer: Exploiting cancer stem cell metabolism. J
Gynecol Oncol. 29:e322018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Hansen DM, Timko NJ, Zhu Z, Ames A,
Qin C, Nicholl MB, Bai Q, Chen X, Wakefield MR, et al: Association
between interleukin-33 and ovarian cancer. Oncol Rep. 41:1045–1050.
2019.PubMed/NCBI
|
|
5
|
Saied EM and El-Etreby NM: The role and
prognostic value of inducible nitric oxide synthase (iNOS) and
interleukin-33 (IL-33) in serous and mucinous epithelial ovarian
tumours. Ann Diagn Pathol. 27:62–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tong X, Barbour M, Hou K, Gao C, Cao S,
Zheng J, Zhao Y, Mu R and Jiang HR: Interleukin-33 predicts poor
prognosis and promotes ovarian cancer cell growth and metastasis
through regulating ERK and JNK signaling pathways. Mol Oncol.
10:113–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De la Fuente M, MacDonald TT and Hermoso
MA: The IL-33/ST2 axis: Role in health and disease. Cytokine Growth
Factor Rev. 26:615–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoffman Bl SJ, Schaffer JI, Halvorson LM,
Bradshaw KD and Cunningham FG: Epithelian ovarian cancer. Williams
Gynecology. 2nd edition. McGraw-Hill; New York, NY: pp. 853–878.
2012
|
|
10
|
Gong TT, Wu QJ, Vogtmann E, Lin B and Wang
YL: Age at menarche and risk of ovarian cancer: A meta-analysis of
epidemiological studies. Int J Cancer. 132:2894–2900. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Longo DL, Fauci A, Kasper D, Hauser S,
Jameson JL and Loscalzo J: Harrison's Principles of Internal
Medicine. 18th edition. McGraw-Hill; New York, NY: 2012
|
|
12
|
Kanchi KL, Johnson KJ, Lu C, McLellan MD,
Leiserson MD, Wendl MC, Zhang Q, Koboldt DC, Xie M, Kandoth C, et
al: Integrated analysis of germline and somatic variants in ovarian
cancer. Nat Commun. 5:31562014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deshmukh A, Deshpande K, Arfuso F,
Newsholme P and Dharmarajan A: Cancer stem cell metabolism: A
potential target for cancer therapy. Mol Cancer. 15:692016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen G and Emens LA: Chemoimmunotherapy:
Reengineering tumor immunity. Cancer Immunol Immunother.
62:203–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu CS, Boyer J, Schullery DS, Gimotty PA,
Gamerman V, Bender J, Levine BL, Coukos G, Rubin SC, Morgan MA, et
al: Phase I/II randomized trial of dendritic cell vaccination with
or without cyclophosphamide for consolidation therapy of advanced
ovarian cancer in first or second remission. Cancer Immunol
Immunother. 61:629–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hyman DM, Zhou Q, Iasonos A, Grisham RN,
Arnold AG, Phillips MF, Bhatia J, Levine DA, Aghajanian C, Offit K,
et al: Improved survival for BRCA2-associated serous ovarian cancer
compared with both BRCA-negative and BRCA1-associated serous
ovarian cancer. Cancer. 118:3703–3709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang D, Khan S, Sun Y, Hess K, Shmulevich
I, Sood AK and Zhang W: Association of BRCA1 and BRCA2 mutations
with survival, chemotherapy sensitivity, and gene mutator phenotype
in patients with ovarian cancer. JAMA. 306:1557–1565. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanduc D: Oligopeptides for immunotherapy
approaches in ovarian cancer treatment. Curr Drug Discov Technol.
16:285–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Argento M, Hoffman P and Gauchez AS:
Ovarian cancer detection and treatment: Current situation and
future prospects. Anticancer Res. 28((5B)): 3135–3138.
2008.PubMed/NCBI
|
|
21
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schlienger K, Chu C S, Woo E Y, Rivers P
M, Toll A J, Hudson B, Maus MV, Riley JL, Choi Y and Coucos G:
TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class
I-restricted T cells specific for autologous tumor in late-stage
ovarian cancer patients. Clin Cancer Res. 9:1517–1527.
2003.PubMed/NCBI
|
|
24
|
Goodell V, Salazar LG, Urban N, Drescher
CW, Gray H, Swensen RE, McIntosh MW and Disis ML: Antibody immunity
to the p53 oncogenic protein is a prognostic indicator in ovarian
cancer. J Clin Oncol. 24:762–768. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating
CD8+ T lymphocytes are prognostic factors of human
ovarian cancer. Proc Natl Acad Sci USA. 104:3360–3365. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hardwick N, Frankel PH and Cristea M: New
approaches for immune directed treatment for ovarian cancer. Curr
Treat Options Oncol. 17:142016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Onda H, Kasuya H, Takakura K, Hori T,
Imaizumi T, Takeuchi T, Inoue I and Takeda J: Identification of
genes differentially expressed in canine vasospastic cerebral
arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab.
19:1279–1288. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baekkevold ES, Roussigné M, Yamanaka T,
Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M,
Haraldsen G and Girard JP: Molecular characterization of NF-HEV, a
nuclear factor preferentially expressed in human high endothelial
venules. Am J Pathol. 163:69–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Z, Chang B, Gao M, Zhang J and Zou Z:
IL-33-ST2 axis in liver disease: Progression and challenge.
Mediators Inflamm. 2017:53142132017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roussel L, Erard M, Cayrol C and Girard
JP: Molecular mimicry between IL-33 and KSHV for attachment to
chromatin through the H2A-H2B acidic pocket. EMBO Rep. 9:1006–1012.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bessa J, Meyer CA, de Vera Mudry MC,
Schlicht S, Smith SH, Iglesias A and Cote-Sierra J: Altered
subcellular localization of IL-33 leads to non-resolving lethal
inflammation. J Autoimmun. 55:33–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xi H, Katschke KJ Jr, Li Y, Truong T, Lee
WP, Diehl L, Rangell L, Tao J, Arceo R, Eastham-Anderson J, et al:
IL-33 amplifies an innate immune response in the degenerating
retina. J Exp Med. 213:189–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Hammel M, He Y, Tainer JA, Jeng US,
Zhang L, Wang S and Wang X: Structural insights into the
interaction of IL-33 with its receptors. Proc Natl Acad Sci USA.
110:14918–14923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cayrol C and Girard JP: Interleukin-33
(IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev.
281:154–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cayrol C and Girard JP: IL-33: An alarmin
cytokine with crucial roles in innate immunity, inflammation and
allergy. Curr Opin Immunol. 31:31–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mirchandani AS, Salmond RJ and Liew FY:
Interleukin-33 and the function of innate lymphoid cells. Trends
Immunol. 33:389–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lingel A, Weiss TM, Niebuhr M, Pan B,
Appleton BA, Wiesmann C, Bazan JF and Fairbrother WJ: Structure of
IL-33 and its interaction with the ST2 and IL-1RAcP
receptors-insight into heterotrimeric IL-1 signaling complexes.
Structure. 17:1398–1410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tago K, Noda T, Hayakawa M, Iwahana H,
Yanagisawa K, Yashiro T and Tominaga S: Tissue distribution and
subcellular localization of a variant form of the human ST2 gene
product, ST2V. Biochem Biophys Res Commun. 285:1377–1383. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Iwahana H, Yanagisawa K, Ito-Kosaka A,
Kuroiwa K, Tago K, Komatsu N, Katashima R, Itakura M and Tominaga
S: Different promoter usage and multiple transcription initiation
sites of the interleukin-1 receptor-related human ST2 gene in UT-7
and TM12 cells. Eur J Biochem. 264:397–406. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Larsen KM, Minaya MK, Vaish V and Peña
MMO: The role of IL-33/ST2 pathway in tumorigenesis. Int J Mol Sci.
19:E26762018. View Article : Google Scholar
|
|
43
|
Carriere V, Roussel L, Ortega N, Lacorre
DA, Americh L, Aguilar L, Bouche G and Girard JP: IL-33, the
IL-1-like cytokine ligand for ST2 receptor, is a
chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci
(USA). 104:282–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ali S, Mohs A, Thomas M, Klare J, Ross R,
Schmitz ML and Martin MU: The dual function cytokine IL-33
interacts with the transcription factor NF-κB to dampen
NF-κB-stimulated gene transcription. J Immunol. 187:1609–1616.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang F, Tossberg JT, Spurlock CF, Yao SY,
Aune TM and Sriram S: Expression of IL-33 and its epigenetic
regulation in Multiple Sclerosis. Ann Clin Transl Neurol.
1:307–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Monticelli LA, Sonnenberg GF, Abt MC,
Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ,
Yang CY, Sathaliyawala T, et al: Innate lymphoid cells promote
lung-tissue homeostasis after infection with influenza virus. Nat
Immunol. 12:1045–1054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chang YJ, Kim HY, Albacker LA, Baumgarth
N, McKenzie AN, Smith DE, Dekruyff RH and Umetsu DT: Innate
lymphoid cells mediate influenza-induced airway hyper-reactivity
independently of adaptive immunity. Nat Immunol. 12:631–638. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yasuda K, Muto T, Kawagoe T, Matsumoto M,
Sasaki Y, Matsushita K, Taki Y, Futatsugi-Yumikura S, Tsutsui H,
Ishii KJ, et al: Contribution of IL-33-activated type II innate
lymphoid cells to pulmonary eosinophilia in intestinal
nematode-infected mice. Proc Natl Acad Sci (USA). 109:3451–3456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen WY, Li LC and Yang JL: Emerging roles
of IL-33/ST2 axis in renal diseases. Int J Mol Sci. 18:E7832017.
View Article : Google Scholar
|
|
50
|
Sun M, He C, Wu W, Zhou G, Liu F, Cong Y
and Liu Z: Hypoxia inducible factor-1α-induced interleukin-33
expression in intestinal epithelia contributes to mucosal
homeostasis in inflammatory bowel disease. Clin Exp Immunol.
187:428–440. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu SL, Wong CK and Tam LS: The alarmin
functions of high-mobility group box-1 and IL-33 in the
pathogenesis of systemic lupus erythematosus. Expert Rev Clin
Immunol. 9:739–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen WY, Chang YJ, Su CH, Tsai TH, Chen
SD, Hsing CH and Yang JL: Upregulation of interleukin-33 in
obstructive renal injury. Biochem Biophys Res Commun.
473:1026–1032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Çekmez F, Fidanci MK, Ayar G, Saldir M,
Karaoglu A, Gündüz RC, Tunc T and Kalkan G: Diagnostic value of
upar, IL-33, and ST2 Levels in childhood sepsis. Clin Lab.
62:751–755. 2016. View Article : Google Scholar
|
|
54
|
Parenica J, Malaska J, Jarkovsky J,
Lipkova J, Dastych M, Helanova K, Litzman J, Tomandl J, Littnerova
S, Sevcikova J, et al: Soluble ST2 levels in patients with
cardiogenic and septic shock are not predictors of mortality. Exp
Clin Cardiol. 17:205–209. 2012.PubMed/NCBI
|
|
55
|
Xu H, Turnquist HR, Hoffman R and Billiar
TR: Role of the IL-33-ST2 axis in sepsis. Mil Med Res.
4:32017.PubMed/NCBI
|
|
56
|
Matsuyama Y, Okazaki H, Tamemoto H, Kimura
H, Kamata Y, Nagatani K, Nagashima T, Hayakawa M, Iwamoto M, Yoshio
T, et al: Increased levels of interleukin-33 in sera and synovial
fluid from patients with active rheumatoid arthritis. J Rheumatol.
37:18–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang Z, Gao X, Wang J, Xu L, Zheng Y and
Xu Y: Interleukin-33 enhanced the migration and invasiveness of
human lung cancer cells. OncoTargets Ther. 11:843–849. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun P, Ben Q, Tu S, Dong W, Qi X and Wu Y:
Serum interleukin-33 levels in patients with gastric cancer. Dig
Dis Sci. 56:3596–3601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu LA, Fu Y, Zhang DN and Zhang J: Serum
IL-33 as a diagnostic and prognostic marker in non- small cell lung
cancer. Asian Pac J Cancer Prev. 14:2563–2566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu J, Shen JX, Hu JL, Huang WH and Zhang
GJ: Significance of interleukin-33 and its related cytokines in
patients with breast cancers. Front Immunol. 5:1412014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Eissmann MF, Dijkstra C, Wouters MA,
Baloyan D, Mouradov D, Nguyen PM, Davalos-Salas M, Putoczki TL,
Sieber OM, Mariadason JM, et al: Interleukin-33 signaling restrains
sporadic colon cancer in an interferon-γ-dependent manner. Cancer
Immunol Res. 6:409–421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Casciaro M, Cardia R, Di Salvo E, Tuccari
G, Ieni A and Gangemi S: Interleukin-33 involvement in nonsmall
cell lung carcinomas: An update. Biomolecules. 9:E2032019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Son J, Cho JW, Park HJ, Moon J, Park S,
Lee H, Lee J, Kim G, Park SM, Lira SA, et al: Tumor-infiltrating
regulatory T-cell accumulation in the tumor microenvironment is
mediated by IL33/ST2 signaling. Cancer Immunol Res. 8:1393–1406.
2020.PubMed/NCBI
|
|
64
|
Carlock CI, Wu J, Zhou C, Tatum K, Adams
HP, Tan F and Lou Y: Unique temporal and spatial expression
patterns of IL-33 in ovaries during ovulation and estrous cycle are
associated with ovarian tissue homeostasis. J Immunol. 193:161–169.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu J, Carlock C, Zhou C, Nakae S, Hicks J,
Adams HP and Lou Y: IL-33 is required for disposal of unnecessary
cells during ovarian atresia through regulation of autophagy and
macrophage migration. J Immunol. 194:2140–2147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Goff BA, Mandel L, Muntz HG and Melancon
CH: Ovarian carcinoma diagnosis. Cancer. 89:2068–2075. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Heintz AP, Odicino F, Maisonneuve P, Quinn
MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S and Beller U:
Carcinoma of the ovary. FIGO 26th annual report on the results of
treatment in gynecological cancer. Int J Gynaecol Obstet. 95 (Suppl
1):S161–S192. 2006. View Article : Google Scholar
|
|
68
|
Wang L, Hu J, Qiu D, Gao H, Zhao W, Huang
Y, Jiang T, Zhou J and Chen Y: Dual-specificity phosphatase 5
suppresses ovarian cancer progression by inhibiting IL-33
signaling. Am J Transl Res. 11:844–854. 2019.PubMed/NCBI
|
|
69
|
Rushworth LK, Kidger AM, Delavaine L,
Stewart G, van Schelven S, Davidson J, Bryant CJ, Caddye E, East P,
Caunt CJ, et al: Dual-specificity phosphatase 5 regulates nuclear
ERK activity and suppresses skin cancer by inhibiting mutant
Harvey-Ras (HRasQ61L)-driven SerpinB2 expression. Proc Natl Acad
Sci (USA). 111:18267–18272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kutty RG, Talipov MR, Bongard RD, Lipinski
RAJ, Sweeney NL, Sem DS, Rathore R and Ramchandran R: Dual
specificity phosphatase 5-substrate interaction: A mechanistic
perspective. Compr Physiol. 7:1449–1461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang H, Zheng H, Mu W, He Z, Yang B, Ji Y
and Hui L: DUSP16 ablation arrests the cell cycle and induces
cellular senescence. FEBS J. 282:4580–4594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Schmieder A, Multhoff G and Radons J:
Interleukin-33 acts as a pro-inflammatory cytokine and modulates
its receptor gene expression in highly metastatic human pancreatic
carcinoma cells. Cytokine. 60:514–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gao X, Wang X, Yang Q, Zhao X, Wen W, Li
G, Lu J, Qin W, Qi Y, Xie F, et al: Tumoral expression of IL-33
inhibits tumor growth and modifies the tumor microenvironment
through CD8+ T and NK cells. J Immunol. 194:438–445.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Perales-Puchalt A, Svoronos N, Villarreal
DO, Zankharia U, Reuschel E, Wojtak K, Payne KK, Duperret EK,
Muthumani K, Conejo-Garcia JR, et al: IL-33 delays metastatic
peritoneal cancer progression inducing an allergic
microenvironment. OncoImmunology. 8:e15150582018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Melichar B and Freedman R S; Immunology of
the peritoneal cavity, : Relevance for host-tumor relation. Int J
Gynecol Canc. 12:3–17. 2012. View Article : Google Scholar : PubMed/NCBI
|