Introduction
Glioblastoma multiforme (GBM) is a heterogeneous
brain tumor with a high degree of malignancy, which can be divided
into two subtypes, primary and secondary, both of which have potent
invasive and proliferative abilities. GBM accounts for 70% of brain
tumors diagnosed (1). GBM stem
cells (GSCs) were recently discovered in GBM and are considered to
be pivotal to the disease, driving tumor initiation due to their
regenerative capacity, which is capable of reproducing all the
biological functions of GBM tumors (2). GSCs show stronger resistance to
conventional therapies than normal neural stem cells (3), resulting in a poor prognosis in
patients with GBM, with a median survival rate of only 12–15 months
(4). Therefore, novel effective
and successful treatment methods are urgently required to eliminate
the entire tumor mass.
ETS variant transcription factor 4 (ETV4) is
involved in the progression of a variety of types of cancer. For
example, in colorectal cancer, it was discovered to promote the
epithelial-mesenchymal transition via the ERK/EGFR signaling
pathway (5). In clear cell renal
cell carcinoma (ccRCC), ETV4 was found to activate the
metastasis-promoting gene FOS like 1, AP-1 transcription factor
subunit in a PI3K/AKT-dependent manner to promote the migration and
invasion of ccRCC cells (6). In
addition, in non-small cell lung cancer, ETV4 promoted cancer
progression by upregulating paxillin and MMP1 expression (7). However, to the best of our knowledge,
the expression pattern of ETV4 in GBM has yet to be reported. Our
previous study found that silencing EMP1 could inhibit the
proliferation and migration of GBM cells by inhibiting the
activation of the PI3K/AKT signaling pathway, which reduced the
expression of the GSC stemness marker, CD44 (8). Furthermore, analysis from the Gene
Expression Profiling Interactive Analysis (GEPIA) database showed
that the expression of epithelial membrane protein 1 (EMP1) was
highly positively associated with ETV4, and subsequent analysis
from the JASPAR CORE database identified a binding site for ETV4 on
the promoter of EMP1. Miao et al (9) also reported that EMP1 could promote
the proliferation and invasion of glioma cells by activating the
PI3K/AKT/mTOR signaling pathway. This previous study also
successfully knocked down the expression of EMP1 in athymic nude
mice, which was discovered to successfully inhibit the growth of
tumors in vivo, subsequently improving the overall survival
rate of tumor-bearing animals. Furthermore, another study
demonstrated that inhibiting the expression of the autophagy
inhibitor, mTOR, could effectively activate and promote lethal
autophagy in GBM (10). In fact,
autophagy-dependent apoptosis has been suggested to be able to
synergistically enhance the cytotoxicity of chemotherapy drugs
towards GBM, alleviate the resistance of GBM cells to chemotherapy
drugs and promote tumor mass ablation (11).
The present study aimed to determine whether ETV4
could influence the activation of the PI3K/AKT/mTOR signaling
pathway to affect the autophagy and apoptosis of GBM cells by
regulating the transcriptional activity of EMP1. The expression
levels of ETV4 were analyzed in several GBM cell lines, and the
relationship between EMP1 and EMP1 was determined. In addition,
ETV4 expression was knocked down to determine its effect on the
EMP1/PI3K/AKT/mTOR signaling axis and GBM cell autophagy-dependent
apoptosis.
Materials and methods
Bioinformatics analysis
The expression of ETV4 in GBM was analyzed using the
GEPIA database (http://gepia.cancer-pku.cn). Survival outcomes
according to ETV4 status in primary glioma were analyzed using the
Chinese Glioma Genome Atlas (CGGA) database (http://www.cgga.org.cn/analyse/RNA-data.jsp;
dataset no. mRNAseq_325). The binding sites of ETV4 on the EMP1
promoter were predicted using the JASPAR CORE database (http://jaspar.genereg.net).
Cell lines and culture
A172 (cat. no. CRL-1620; American Type Culture
Collection), LN-229 (cat. no. CRL-2611; American Type Culture
Collection), BS149 (provided by the Clinical and Experimental
Pathology Laboratory at the Xuzhou Medical University, Xuzhou,
China) and U251 (serial no. TCHu 58; The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences) were cultured in
DMEM (Beijing Solarbio Science & Technology Co., Ltd.)
supplemented with 10% FBS (Beijing Solarbio Science &
Technology Co., Ltd.) and 1% penicillin-streptomycin, and
maintained at 37°C in a humidified incubator with 5%
CO2. All cell lines were used within 10 passages of
their initial culture. The normal human astrocytes (NHAs) cells
(cat. no. CC-2565; Lonza Group, Ltd.) were cultured according to
the manufacturer's recommendations. Rapamycin (RAP; 100 nM; Beijing
Solarbio Science & Technology Co., Ltd.), as a specific
inhibitor of mTOR protein, was incubated with cells at 37°C for 24
h.
Cell transfection
Small interfering RNA (siRNA/si) sequences against
ETV4 (si-ETV4-1/si-ETV4-2) and a non-targeting si-negative control
(NC) were synthesized and purified by Guangzhou RiboBio Co., Ltd.
The sequences were as follows: si-ETV4-1,
5′-TTGGATGTTGGAGAAAATGGA-3′; si-ETV4-2,
5′-TCCAGATCATTCCTTTAGTTT-3′; and si-NC, 5′-GGCTCTAGAAAAGCCTATGC-3′.
The siRNAs (10 nM) were transfected into LN-229 cells
(3×105 cells/well in 6-well plates) for 48 h at 37°C
using Lipofectamine® RNAiMAX transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols.
The plenti6/V5-DEST vector was purchased from
Shaanxi Youbio Technology Co., Ltd., and used to carry ETV4 or EMP1
cDNA. Briefly, the sequences of ETV4 or EMP1 cDNA were cloned into
the BamHI and AscI sites of the plenti6/V5-DEST
vector; a non-targeting cDNA sequence [overexpression (Oe)-NC] was
used as the control. After amplification and DNA sequencing, the
vectors (50 nM) were transduced into LN-229 cells (3×105
cells/well in 6-well plates) using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. Transfection efficiency was detected via
reverse transcription-quantitative PCR (RT-qPCR) at 48 h after
transfection.
RT-qPCR
Total RNA was extracted from the cultured cell lines
using TRIzol® reagent (Thermo Fisher Scientific, Inc.).
Total RNA was reverse transcribed into cDNA using a PrimeScript™ RT
reagent kit (Perfect Real Time) (Takara Bio, Inc.) according to the
manufacturer's instructions. qPCR was subsequently performed on a
StepOnePlus™ Real-Time PCR system (Thermo Fisher Scientific, Inc.)
using a One Step TB Green® PrimeScript™ plus RT-PCR kit
(Perfect Real Time) (Takara Bio, Inc.), according to the
manufacturer's protocol. The following thermocycling conditions
were used for qPCR: Initial denaturation at 42°C for 5 min and 95°C
for 10 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30
sec. The following primers pairs for ETV4, EMP1 and β-actin were
used for qPCR: ETV4 forward, 5′-GAAAAACAAGTCGGTGCGCT-3′ and
reverse, 5′-GGGGGCCGAGACCTGA-3′; EMP1 forward,
5′-ATGCCAGTGAAGATGCCCTC-3′ and reverse, 5′-TGTAGATGGACACCCCCACA-3′;
and β-actin forward, 5′-GCCGCCAGCTCACCAT-3′ and reverse,
5′-TCGTCGCCCACATAGGAATC-3′. β-actin was used for normalization. The
relative expression levels of target genes were calculated using
the 2−ΔΔCq method (12).
Western blotting
For western blotting, the protein samples were
prepared as previously described (8). Proteins (20 µg/lane) were separated
via 10% SDS-PAGE (Beyotime Institute of Biotechnology) and
subsequently transferred onto PVDF membranes (MilliporeSigma).
After incubating the membranes in 5% skimmed milk powder dissolved
in TBS with 0.1% Tween-20 (TBST) for 2 h at room temperature, they
were incubated with a primary antibody at 4°C overnight. Following
the primary antibody incubation, the membranes were washed three
times with TBST and incubated with a HRP-conjugated secondary
antibody for 1 h at room temperature. Protein bands were visualized
using SuperSignal West Pico Plus (Thermo Fisher Scientific, Inc.)
and a GS800 densitometer scanner (Bio-Rad Laboratories, Inc.).
Densitometric analysis was performed using ImageJ v1.46 software
(National Institutes of Health). The following primary antibodies
were used: Mouse anti-ETV4 (1:1,500; cat. no. sc-113; Santa Cruz
Biotechnology, Inc.), rabbit anti-EMP1 (1:1,000; cat. no. ab230445;
Abcam), mouse anti-GAPDH (1:5,000; cat. no. sc-47724; Santa Cruz
Biotechnology, Inc.), mouse anti-Beclin-1 (1:2,000; cat. no.
ab118148; Abcam), rabbit anti-LC3B I and II (1:2,000; cat. no.
ab192890; Abcam), rabbit anti-p62 (1:40,000; cat. no. ab109012;
Abcam), rabbit anti-cleaved-caspase 9 (1:500; cat. no. ab2324;
Abcam), rabbit anti-caspase 9 (1:10,000; cat. no. ab32539; Abcam),
rabbit anti-Bcl-2 (1:1,000; cat. no. ab32124; Abcam), rabbit
anti-Bax (1:2,000; cat. no. ab182733; Abcam), rabbit
anti-phosphorylated (p)-AKT (1:2,000; cat. no. 4060S; Cell
Signaling Technology, Inc.), rabbit anti-AKT (1:1,000; cat. no.
9272S; Cell Signaling Technology, Inc.), rabbit anti-p-mTOR
(1:1,000; cat. no. ab177734; Abcam) and rabbit anti-mTOR (1:3,000;
cat. no. ab32028; Abcam). The following HRP-conjugated secondary
antibodies were used: Goat anti-rabbit IgG H&L (HRP; 1:20,000;
cat. no. ab6721; Abcam) and goat anti-mouse IgG H&L (HRP;
1:10,000; cat. no. ab6789; Abcam).
TUNEL assay
Apoptosis was analyzed using a TUNEL apoptosis
detection kit (Beyotime Institute of Biotechnology). Briefly, the
transfected LN-229 cells were seeded into a 24-well plate at a
density of 5×104 cells/well, and fixed with 4%
paraformaldehyde for 30 min at room temperature. The cells were
then washed once with PBS and incubated in 0.3% Triton-X 100 in PBS
for 5 min at room temperature, before being washed again with PBS.
TUNEL detection solution was prepared according to the
manufacturer's protocol and 50 µl was added to each sample and
incubated at 37°C for 60 min in the dark. DAPI staining solution
(10 µg/ml) was used to visualize nuclei for 5 min at 37°C.
Following the incubation, the cells were washed three times with
PBS, mounted with anti-fluorescence quenching mounting solution and
three random fields were visualized using a fluorescence microscope
(Olympus Corporation). The apoptosis levels were estimated as the
ratio of the number of TUNEL-positive cells to the number of
Hoechst-positive cells, and each experiment was repeated in
triplicate.
Dual luciferase gene reporter
assay
A dual luciferase reporter gene assay kit (Beyotime
Institute of Biotechnology) was used to detect the regulation of
ETV4 on EMP1 promoter elements, according to the manufacturer's
protocol. Briefly, the promoter region of EMP1 (amplified by
Shanghai GenePharma Co., Ltd.) containing wild-type (WT,
GGAGGAAGAC) and mutant (MUT, GAGAATTCCC) sequences, located from
positions −405 to −396 upstream of the EMP1 transcription start
site, were inserted as XhoI/Bg1II fragments upstream
of the luciferase gene into pGL4-luc (Promega Corporation). The
luciferase reporter plasmids and regulatory factors were
co-transfected into LN-229 cells using Lipofectamine 3000 reagent.
After 48 h, cells were subsequently lysed with lysis buffer and
incubated with firefly luciferase detection reagent. The relative
light unit (RLU) was measured after mixing, and the reporter gene
cell lysate was obtained as a blank control. Following this step,
Renilla luciferase detection working solution was added to
the cells and the RLU was measured after mixing. The activation
degree of the reporter gene was defined as the value obtained by
dividing the firefly RLU by the Renilla RLU, with
Renilla luciferase acting as the internal reference. Each
experiment was repeated at least three times.
Chromatin immunoprecipitation
assay
The SimpleChIP® Enzymatic Chromatin IP
kit (Cell Signaling Technology, Inc.) was used to detect the
binding between ETV4 and EMP1 promoters, according to the
manufacturer's protocol. Briefly, the LN-229 cells were plated into
a 15-cm plate and cross-linked at 37°C for 10 min with a final
concentration of 1% formaldehyde. Glycine (0.125 M) was
subsequently added to terminate the cross-linking, following which
the cells were washed with PBS and centrifuged at 1,000 × g for 5
min at 4°C. The supernatant was removed and SDS lysis buffer
containing a protease inhibitor complex (Beijing Solarbio Science
& Technology Co., Ltd.) was added to obtain an
immunoprecipitated (IP) preparation. Then, 0.5 µl micrococcal
nuclease was added to each IP preparation, which was mixed by
inverting the centrifuge tube several times, and then incubated at
37°C for 20 min to digest the DNA to a length of ~150–900 bp. EDTA
was subsequently added to terminate the digestion, and the cells
were centrifuged at 16,000 × g for 1 min at 4°C to pellet the
nuclei and remove the supernatant. A VirTis Virsonic 100 Ultrasonic
Homogenizer/Sonicator (set to 6 and equipped with a 1/8-inch probe
for 4 sets of 15-sec pulses) was used to lyse LN-229 cell nuclei.
The lysate was centrifuged in a microcentrifuge at 9,400 × g for 10
min at 4°C to clarify the lysate and collect the supernatant.
Subsequently, RNase A and Proteinase K were used for chromatin
digestion and analysis of product concentration. Part of the
product was set aside to act as the input group, while 1 µg mouse
anti-ETV4 (1:50; cat. no. sc-113; Santa Cruz Biotechnology, Inc.)
or 1 µg mouse anti-IgG (1:50; cat. no. sc-2025; Santa Cruz
Biotechnology, Inc.) antibodies and 40 µl protein A/G magnetic
beads (MilliporeSigma) were added to the remaining product and
incubated overnight at 4°C. Following the incubation, the immune
complexes were precipitated and washed. After decrosslinking at
65°C overnight, DNA was recovered and subjected to RT-qPCR analysis
as described above.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8 software (GraphPad Software, Inc.). Data are presented as
the mean ± SD of at least three independent experiments.
Statistical differences between two groups were determined using an
unpaired Student's t-test, while those between multiple groups were
determined using a one-way ANOVA followed by Tukey's post hoc test.
In addition, survival rates were statistically analyzed using the
Kaplan-Meier analysis followed by a log-rank test and the
correlation between ETV4 and EMP1 in GBM was determined using
Pearson's analysis. P<0.05 was considered to indicate a
statistically significant difference, and |R|>0.3 (for Pearson's
analysis) was considered to indicate a significant correlation.
Results
ETV4 is upregulated in GBM and
associated with poor prognosis
The expression levels of ETV4 in GBM tissues were
discovered to be significantly upregulated compared with those in
normal tissues (Fig. 1A),
according to analysis by the GEPIA database. Similar results were
also observed in GBM cell lines (A172, BS149, U251 and LN-229)
compared with NHAs via western blotting (Fig. 1C and D) and RT-qPCR (Fig. 1E). The expression of ETV4 was
upregulated to the greatest extent in LN-229 cells; therefore, this
cell line was selected for use in subsequent experiments. Analysis
from the CGGA database also revealed a poor survival prognosis for
patients with high ETV4 expression (Fig. 1B), which suggested that ETV4 may
play an important role in promoting GBM progression.
si-ETV4 promotes autophagy and
apoptosis in LN-229 cells
siRNA was used to silence ETV4 expression in LN-229
cells. Following the analysis of the transfection efficiency
(Fig. 2A), si-ETV4-1 was found to
exert the most effective interference effect. In order to reduce
the possibility of an off-target effect, si-ETV4-1 and si-ETV4-2
were both selected for use in follow-up experiments to explore the
effect of ETV4 on autophagy and apoptosis.
 | Figure 2.Low expression of ETV4 in LN-229 cells
promotes autophagy and apoptosis. (A) Knockdown efficiency of
si-ETV4-1/2 was analyzed via reverse transcription-quantitative PCR
(n=3). (B) Western blotting was used to analyze Beclin-1, LC3B II/I
and p62 expression levels in different groups (control, si-NC,
si-ETV4-1 and si-ETV4-2; n=3). GAPDH served as the loading control.
Semi-quantification of (C) Beclin-1, (D) LC3B II/I and (E) p62
protein expression levels. (F) TUNEL assay was used to determine
the effect of ETV4 on LN-229 cell apoptosis (n=3; TUNEL, green;
Hoechst, blue). Scale bar, 50 µm. (G) Percentage of TUNEL-positive
cells. (H) Representative western blots demonstrating Bcl-2, Bax,
cleaved-caspase 9 and caspase 9 expression levels in different
groups (control, si-NC, si-ETV4-1 and si-ETV4-2; n=3). GAPDH served
as the loading control. Semi-quantification of (I) Bcl-2, (J) Bax,
(K) cleaved-caspase 9 protein expression levels. Untransfected
cells were used as the control. **P<0.01, ***P<0.001 vs.
si-NC. ETV4, ETS variant transcription factor 4; LC3B II/I, LC3B
II/LC3B I; si, small interfering RNA; NC, negative control. |
The protein expression levels of the autophagy
marker, Beclin-1, were first analyzed, and it was found that,
compared with those observed in the si-NC group, the expression
levels of Beclin-1 in the si-ETV4 group were significantly
upregulated (Fig. 2B and C). In
addition, following the knockdown of ETV4 expression, the
expression levels of LC3B II/I (the ratio of LC3B II/LC3B I) were
discovered to be upregulated, while p62 expression levels were
downregulated (Fig. 2B, D and E),
indicating that silencing ETV4 expression may activate autophagic
flux in LN-229 cells.
Silencing ETV4 expression also increased the number
of TUNEL-positive cells (green fluorescence) (Fig. 2F). The cell apoptosis rate was
significantly higher in the si-ETV4-1 group compared with the si-NC
group (Fig. 2G). These results
were validated via western blotting. In more detail, compared with
the si-NC group, the expression levels of cleaved-caspase 9 were
found to be upregulated in the si-ETV4 groups (Fig. 2H and K). Notably, the activation of
caspase was not caused by the increase in the concentration of
zymogen (Fig. 2H). Furthermore,
the expression levels of the anti-apoptotic protein, Bcl-2, were
found to be downregulated (Fig. 2H and
I), while the expression levels of the proapoptotic protein,
Bax, were upregulated (Fig. 2H and
J). Since, similar results were obtained using si-ETV4-1 and
si-ETV4-2 specific knockdown of ETV4 in LN-229 cells, it was
concluded that the knockdown of ETV4 may activate the endogenous
apoptotic pathway of LN-299 cells, which is regulated by the
caspase 9 cascade. si-ETV4-1 was selected for subsequent
experiments due to its more effective interference effect.
ETV4 activates EMP1 transcription in
LN-229 cells
A previous study revealed that the upregulated
expression of EMP1 promoted the proliferation and invasion of
glioma cells by activating the PI3K/AKT signaling pathway (7). The JASPAR database was used to
predict the binding site of ETV4 in the EMP1 promoter region
(Fig. 3B). Therefore, it was
hypothesized that ETV4 may participate in the regulation of GBM
disease progression by activating the transcription of EMP1. To
test this hypothesis, the effect of silencing ETV4 expression in
LN-229 cells on the expression of EMP1 was first analyzed.
Although, using GEPIA database analysis, the correlation
coefficients (R=0.26) of EMP1 and ETV4 indicated that they were
negligibly correlated in GBM (Fig.
3A). The results revealed that the mRNA expression levels of
EMP1 were significantly downregulated following the knockdown of
ETV4 (Fig. 3C). Next, an ETV4
overexpression vector was successfully constructed in vitro
(Fig. 3D), and the results of the
dual luciferase reporter gene assay, which was used to detect the
promoter activity of EMP1, found that the overexpression of ETV4
increased luciferase expression driven by an EMP1 promoter fragment
containing a putative ETV4 binding site (Fig. 3E). The results of the chromatin
immunoprecipitation assay in LN-229 cells also demonstrated that
ETV4 bound to the promoter region of EMP1 (Fig. 3F). These results indicated that
ETV4 may target the promoter region of EMP1 to positively regulate
the expression of EMP1 in LN-229 cells.
Overexpression of EMP1 reverses the
promoting effect of si-ETV4 on autophagy and apoptosis
To verify whether the pro-autophagic and
proapoptotic effects of si-ETV4 in LN-229 cells were achieved by
downregulating the expression of EMP1, an EMP1 overexpression
vector was successfully established in vitro (Fig. 4A-C). The vector was transfected
into LN-299 cells and western blotting was used to determine the
expression levels of autophagy- and apoptosis-related proteins. The
expression levels of Beclin-1 and LC3B II/I were significantly
downregulated and p62 was upregulated in LN-229 cells
co-transfected with si-ETV4 + Oe-EMP1 compared with in cells
co-transfected with si-ETV4 + Oe-NC (Fig. 4D-G). Moreover, it was found that
si-ETV4-induced apoptosis was partially reversed in the si-ETV4 +
Oe-EMP1 group compared with the si-ETV4 + Oe-NC group, with a
decreased number of TUNEL-positive cells along with the
downregulation of Bax and cleaved-caspase 9 expression levels and
the upregulation of Bcl-2 expression (Fig. 4H-M). These findings suggested that
the effect of si-ETV4 on promoting autophagy and apoptosis may be
achieved by reducing the transcriptional activation of EMP1 by
ETV4.
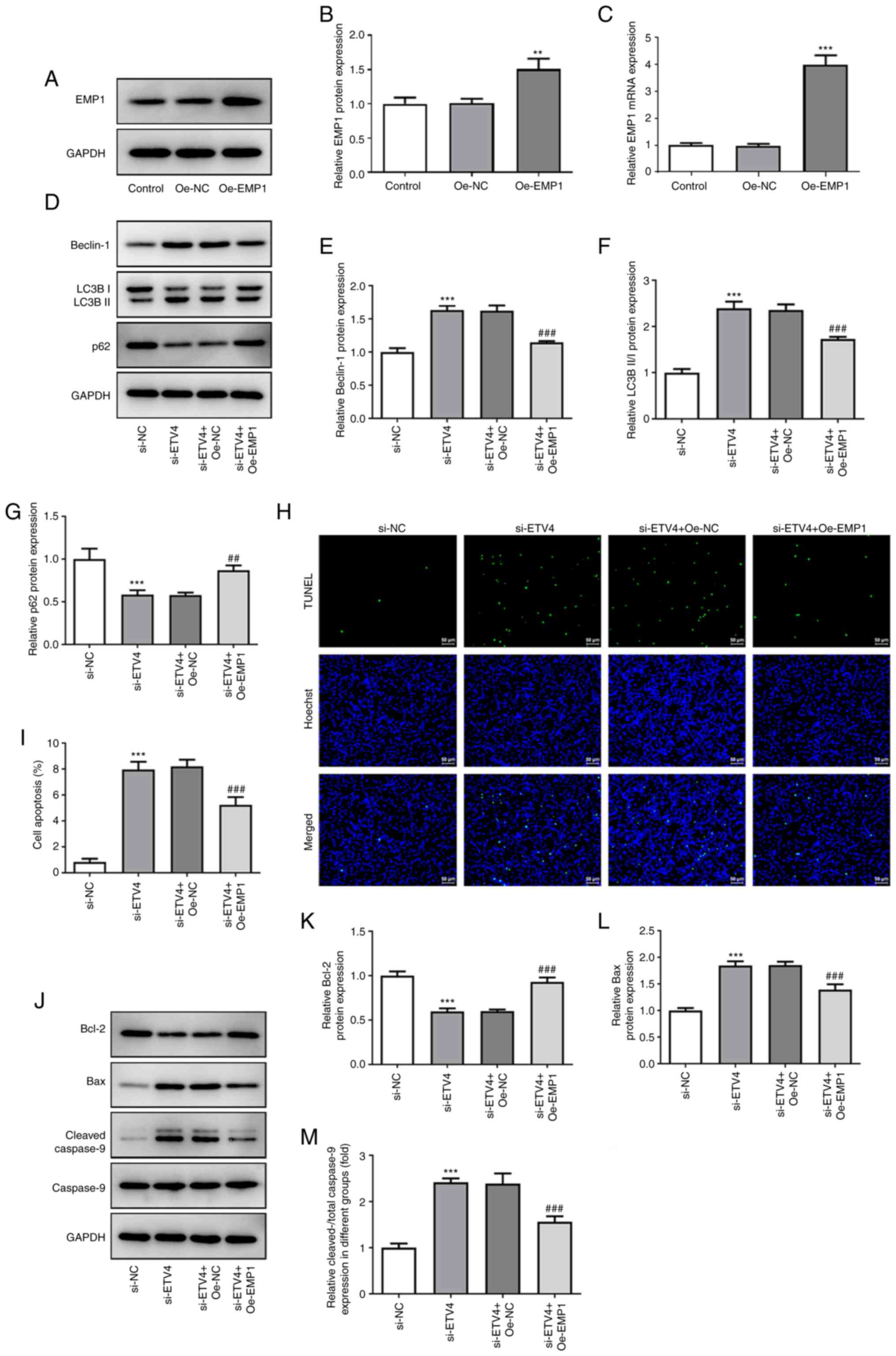 | Figure 4.Overexpression of EMP1 in LN-229 cells
reverses the promoting effects of si-ETV4 on autophagy and
apoptosis. (A) Representative western blots showing EMP1 expression
after transfection with Oe-EMP1. Untransfected cells were used as
the control. GAPDH served as the loading control. (B) Histograms
showing the statistical results from part (A). (C) Reverse
transcription-quantitative PCR was used to determine the
transfection efficiency of Oe-EMP1. n=4. **P<0.01, ***P<0.001
vs. Oe-NC. (D) Representative western blots demonstrating Beclin-1,
LC3B II/I and p62 expression levels in different groups (si-NC,
si-ETV4, si-ETV4 + Oe-NC and si-ETV4 + Oe-EMP1). GAPDH served as
the loading control. Semi-quantification of (E) Beclin-1, (F) LC3B
II/I and (G) p62 protein expression. (H) TUNEL assay was used to
determine the effect of EMP1 on LN-229 cell apoptosis (TUNEL,
green; Hoechst, blue). Scale bar, 50 µm. (I) Percentage of
TUNEL-positive cells. (J) Representative western blots
demonstrating Bcl-2, Bax, cleaved-caspase 9 and caspase 9
expression levels in different groups (si-NC, si-ETV4, si-ETV4 +
Oe-NC and si-ETV4 + Oe-EMP1). GAPDH served as the loading control.
Semi-quantification of (K) Bcl-2, (L) Bax and (M) cleaved-caspase 9
protein expression. n=3. ***P<0.001 vs. si-NC;
##P<0.01, ###P<0.001 vs. si-ETV4 +
Oe-NC. EMP1, epithelial membrane protein 1; ETV4, ETS variant
transcription factor 4; si, small interfering RNA; Oe,
overexpression; NC, negative control; LC3B II/I, LC3B II/LC3B
I. |
Interfering with the ETV4/EMP1 axis
promotes autophagy-dependent apoptosis and inhibits the mTOR
signaling pathway
Rapamycin (RAP) is a specific inhibitor of the mTOR
protein; it can bind to the intracellular receptor, FK506 binding
protein (FKBP)-12, to form a complex and directly act on the
FKBP-12-RAP binding domain of mTOR to inhibit protein activity
(13). In the present study,
LN-229 cells were co-incubated with RAP and si-ETV4, and the
results of the TUNEL staining assay showed that the co-treatment
could upregulate apoptosis compared with the RAP + si-NC group,
while the concurrent overexpression of EMP1 could downregulate
apoptosis compared with the RAP + si-ETV4 + Oe-NC group (Fig. 5A and B). At the protein level, the
co-incubation of RAP with si-ETV4 was discovered to upregulate the
expression levels of cleaved-caspase 9 and Bax, and downregulate
the expression levels of Bcl-2 compared with the RAP + si-NC group.
Following the concurrent overexpression of EMP1, these effects were
partially reversed (Fig. 5C-G).
These findings suggested that the effect of the ETV4/EMP1 axis on
the autophagy and apoptosis of LN-299 cells may be associated with
the mTOR signaling pathway. Next, western blotting was used to
measure the expression levels of PI3K/AKT/mTOR signaling
pathway-related proteins. The results demonstrated that the
activation of AKT and mTOR was inhibited after the incubation of
RAP and/or si-ETV4, while the concurrent overexpression of EMP1
could partially reduce the degree of inhibition (Fig. 5H-J). These results indicated that
interference with the ETV4/EMP1 signaling axis may promote
autophagy-induced apoptosis and inhibit the activation of the mTOR
signaling pathway in LN-229 cells.
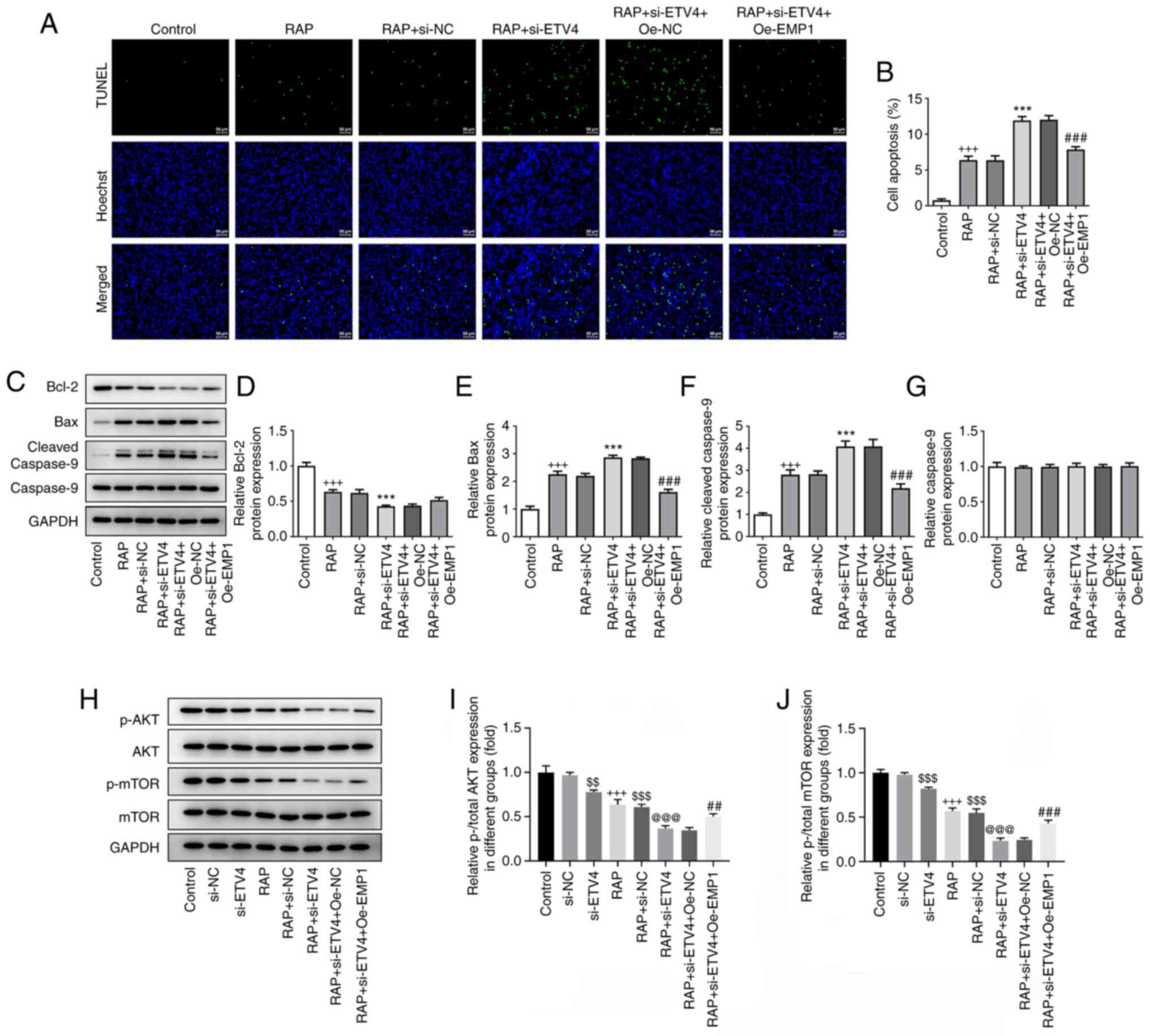 | Figure 5.Interference of the ETV4/EMP1 axis in
LN-229 cells promotes autophagy-dependent apoptosis and inhibits
the mTOR signaling pathway. (A) TUNEL assay was used to detect
apoptosis in LN-299 cells transfected with si-NC, si-ETV4, si-ETV4
+ Oe-NC or si-ETV4 + Oe-EMP1 after 3 h of incubation with 100 nM
RAP or treatment with RAP only (TUNEL, green; Hoechst, blue).
Untreated/untransfected cells were used as the control. Scale bar,
50 µm. (B) Percentage of TUNEL-positive cells. (C) Representative
western blots demonstrating Bcl-2, Bax, cleaved-caspase 9 and
caspase 9 expression levels in different groups (control, RAP, RAP
+ si-NC, RAP + si-ETV4, RAP + si-ETV4 + Oe-NC and RAP + si-ETV4 +
Oe-EMP1). GAPDH served as the loading control. Semi-quantification
of (D) Bcl-2, (E) Bax, (F) cleaved-caspase 9 and (G) caspase 9
protein expression. Untransfected cells were used as the control.
(H) Representative western blots demonstrating p-AKT, AKT, p-mTOR
and mTOR expression levels in different groups (control, si-NC,
si-ETV4, RAP, RAP + si-NC, RAP + si-ETV4, RAP + si-ETV4 + Oe-NC and
RAP + si-ETV4 + Oe-EMP1). GAPDH served as the loading control.
Semi-quantification of (I) p-/total AKT and (J) p-/total mTOR
protein expression levels. Untransfected cells were used as the
control. n=3. +++P<0.001 vs. control;
$$P<0.01, $$$P<0.001 vs. si-NC;
@@@P<0.001 vs. si-ETV4; ***P<0.001 vs. RAP +
si-NC; ##P<0.01, ###P<0.001 vs. RAP +
si-ETV4 + Oe-NC. EMP1, epithelial membrane protein 1; ETV4, ETS
variant transcription factor 4; si, small interfering RNA; NC,
negative control; Oe, overexpression; p-, phosphorylated; RAP,
rapamycin. |
Discussion
GBM is a heterogenous malignant brain tumor that
accounts for ~80% of primary brain tumors and is extremely
aggressive and lethal (14),
seriously affecting the survival and cognitive function of
patients. The treatment regimen for GBM comprises standard surgery,
radiotherapy and chemotherapy; however, once the disease begins to
deteriorate, most patients succumb to the disease within 2 years of
treatment (15). The results of
the present study demonstrated that si-ETV4 promoted the autophagy
and apoptosis of LN-229 GBM cells. Thus, this study further
explored the potential underlying molecular mechanism of ETV4 in
GBM.
As a common cancer-promoting transcription factor,
ETV4 has been shown to promote the occurrence and development of a
variety of types of cancer (6,7,16–18),
including non-small cell lung, prostate and endometrial cancer. In
the current study, the analysis of the CGGA database revealed that
patients with primary glioma and high expression levels of ETV4 had
a poor survival rate. Current research into the role of ETV4 in
glioma has focused on its ability to promote tumor cell
proliferation and invasion. Padul et al (19) found that in oligodendroglioma, ETV4
promoted tumor cell aggressiveness by regulating the Notch
signaling pathway. Jiang et al (20) also suggested that the pro-invasive
effect of ETV4 in glioma may also be regulated by the
co-transcriptional complex formed between itself and the
transcription factor, Sp1. However, to the best of our knowledge,
there are currently no studies reporting the role of ETV4 in GBM.
In the present study, the expression of ETV4 was found to be
upregulated in 163 patients with GBM from the GEPIA database, which
was validated in several GBM cell lines in vitro.
Autophagy is a common method used by tumors to
generate metabolic precursors and ATP to survive in extreme
environments. If the homeostatic autophagic balance is disrupted,
inadequate repair or major cellular stress will trigger autophagic
death (21). Autophagy-regulated
tumor cell death has been a research hotspot in recent decades, and
there have been numerous studies on GBM that have found that this
may occur via the involvement of the mTOR, Src and ERK signaling
pathways (11,13,22).
In the present study, the knockdown of ETV4, using siRNA, was
discovered to promote autophagy and apoptosis in LN-229 cells.
Through analysis of the GEPIA database, it was discovered that ETV4
was weakly positively correlated with EMP1. Meanwhile, si-ETV4 was
found to significantly decrease the expression of EMP1 in LN-229
cells. Thus, it was hypothesized that the transcription of ETV4 may
positively regulate the expression of EMP1. The results of the dual
luciferase reporter and chromatin immunoprecipitation assays
further verified this hypothesis, as ETV4 was identified to bind to
the EMP1 promoter (−405-396) region. In GBM, EMP1 has been found to
upregulate CD44 by activating the PI3K/AKT/mTOR signaling pathway
to maintain stemness and promote cell proliferation and invasion
(8). Subsequently, tumor
suppression and a longer lifespan were found in tumor-bearing mice
that had knocked out EMP1 expression (8,9). In
the current study, an EMP1 overexpression vector was constructed to
explore whether the effect of ETV4 on the biological behavior of
LN-299 cell autophagy and apoptosis occurred via EMP1. The results
of the western blotting and TUNEL staining analyses revealed that
the overexpression of EMP1 may partially reverse autophagy and
apoptosis induced by ETV4 knockdown. These findings indicated that
inhibiting the ETV4/EMP1 signaling axis may play an important role
in promoting autophagy and apoptosis in LN-229 GBM cells.
mTOR has been reported to be upregulated in GBM, and
the PI3K/AKT/mTOR signaling pathway was discovered to play a
crucial role in regulating cell proliferation, apoptosis and
autophagy (22). In the present
study, LN-229 cells were co-incubated with RAP, si-ETV4 and Oe-EMP1
alone or in combination, and the expression levels of p-AKT and
p-mTOR, which indicate that the PI3K/AKT/mTOR signaling pathway has
been activated, were measured. Furthermore, the effect of the
ETV4/EMP1 axis on the PI3K/AKT/mTOR signaling pathway was explored.
The results found that the inhibition of the ETV4/EMP1 axis induced
autophagy and apoptosis in LN-229 cells, which was discovered to
occur via the inhibition of the PI3K/AKT/mTOR signaling
pathway.
In conclusion, the findings of the present study
suggested that ETV4 may be upregulated in GBM tissues and several
GBM cell lines (A172, BS149, U251 and LN-229), which was found to
reduce the survival probability of patients with primary GBM.
Mechanistically, silencing ETV4 gene expression in LN-229 cells was
discovered to promote autophagy-induced apoptosis. This effect was
found to be associated with EMP1. ETV4 was identified to target the
promoter region of EMP1 to positively regulate its expression, and
interference of the ETV4/EMP1 axis promoted autophagy-dependent
apoptosis and inhibited the mTOR signaling pathway. These findings
are of great significance to the current understanding of the
progression of GBM, and provide a novel insight and theoretical
foundations for the treatment of GBM via regulation of
autophagy-dependent apoptosis. However, most of the experimental
data obtained in this study are based on the LN-229 cell line.
Although it is a commonly used cell line for studying gliomas, the
usage of only one cellular model may weaken the data because they
could result from just the genotypic/phenotypic features of this
specific cell line. Therefore, in future studies we will verify the
results of this experiment in other glioma cell lines and explore
the significance of silencing ETV4 for the treatment of GBM in
in vivo animal models.
Acknowledgements
Not applicable.
Funding
This work was supported by Youth Science and Technology Project
of ‘Invigorating Health Through Science and Education’ in Suzhou
(grant no. KJXW2020067).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, CS, JL and MG conceived and designed the study.
JW, CS, JL, HJ and YQ collected and analyzed the data. All authors
were involved in the writing of the manuscript and revised the
manuscript. JW and MG confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gimple RC, Bhargava S, Dixit D and Rich
JN: Glioblastoma stem cells: Lessons from the tumor hierarchy in a
lethal cancer. Genes Dev. 33:591–609. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hambardzumyan D, Squatrito M, Carbajal E
and Holland EC: Glioma formation, cancer stem cells, and akt
signaling. Stem cell Rev. 4:203–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jawhari S, Ratinaud MH and Verdier M:
Glioblastoma, hypoxia and autophagy: A survival-prone
‘ménage-à-trois’. Cell Death Dis. 7:e24342016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leng Y, Chen Z, Ding H, Zhao X, Qin L and
Pan Y: Overexpression of microRNA-29b inhibits
epithelial-mesenchymal transition and angiogenesis of colorectal
cancer through the ETV4/ERK/EGFR axis. Cancer Cell Int. 21:172021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu L, Hu H, Zheng LS, Wang MY, Mei Y, Peng
LX, Qiang YY, Li CZ, Meng DF, Wang MD, et al: ETV4 is a theranostic
target in clear cell renal cell carcinoma that promotes metastasis
by activating the pro-metastatic gene FOSL1 in a PI3K-AKT dependent
manner. Cancer Lett. 482:74–89. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Ding X, Liu B, Li M, Chang Y, Shen
H, Xie SM, Xing L and Li Y: ETV4 overexpression promotes
progression of non-small cell lung cancer by upregulating PXN and
MMP1 transcriptionally. Mol Carcinog. 59:73–86. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Li X, Wu H, Wang H, Yao L, Deng Z
and Zhou Y: EMP1 regulates cell proliferation, migration, and
stemness in gliomas through PI3K-AKT signaling and CD44. J Cell
Biochem. 120:17142–17150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miao L, Jiang Z, Wang J, Yang N, Qi Q,
Zhou W, Feng Z, Li W, Zhang Q, Huang B, et al: Epithelial membrane
protein 1 promotes glioblastoma progression through the
PI3K/AKT/mTOR signaling pathway. Oncol Rep. 42:605–614.
2019.PubMed/NCBI
|
|
10
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiapaer S, Furuta T, Tanaka S, Kitabayashi
T and Nakada M: Potential strategies overcoming the temozolomide
resistance for glioblastoma. Neurol Med Chir (Tokyo). 58:405–421.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JE, Yoon SS and Moon EY:
Curcumin-induced autophagy augments its antitumor effect against
A172 human glioblastoma cells. Biomol Ther (Seoul). 27:484–491.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dolecek TA, Propp JM, Stroup NE and
Kruchko C: CBTRUS statistical report: Primary brain and central
nervous system tumors diagnosed in the United States in 2005–2009.
Neuro Oncol. 14 (Suppl 5):v1–v49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grossman SA, Ye X, Piantadosi S, Desideri
S, Nabors LB, Rosenfeld M, Fisher J and NABTT CNS Consortium:
Survival of patients with newly diagnosed glioblastoma treated with
radiation and temozolomide in research studies in the United
States. Clin Cancer Res. 16:2443–2449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cosi I, Pellecchia A, De Lorenzo E, Torre
E, Sica M, Nesi G, Notaro R and De Angioletti M: ETV4 promotes late
development of prostatic intraepithelial neoplasia and cell
proliferation through direct and p53-mediated downregulation of
p21. J Hematol Oncol. 13:1122020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodriguez AC, Vahrenkamp JM, Berrett KC,
Clark KA, Guillen KP, Scherer SD, Yang CH, Welm BE, Janát-Amsbury
MM, Graves BJ and Gertz J: ETV4 is necessary for estrogen signaling
and growth in endometrial cancer cells. Cancer Res. 80:1234–1245.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu ST, Sun BH, Ge JN, Shi JL, Zhu MS, Wei
ZG, Li TT, Zhang ZC, Chen WS and Lei ST: CRLF1-MYH9 interaction
regulates proliferation and metastasis of papillary thyroid
carcinoma through the ERK/ETV4 axis. Front Endocrinol (Lausanne).
11:5352020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Padul V, Epari S, Moiyadi A, Shetty P and
Shirsat NV: ETV/Pea3 family transcription factor-encoding genes are
overexpressed in CIC-mutant oligodendrogliomas. Genes Chromosomes
Cancer. 54:725–733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang J, Wei Y, Shen J, Liu D, Chen X,
Zhou J, Zong H, Yun X, Kong X, Zhang S, et al: Functional
interaction of E1AF and Sp1 in glioma invasion. Mol Cell Biol.
27:8770–8782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Azad MB, Chen Y, Henson ES, Cizeau J,
McMillan-Ward E, Israels SJ and Gibson SB: Hypoxia induces
autophagic cell death in apoptosis-competent cells through a
mechanism involving BNIP3. Autophagy. 4:195–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang Y, Liu J, Hong W, Fei X and Liu R:
Arctigenin inhibits glioblastoma proliferation through the AKT/mTOR
pathway and induces autophagy. Biomed Res Int. 2020:35426132020.
View Article : Google Scholar : PubMed/NCBI
|



















