Introduction
In recent decades, the search for active compounds
from natural sources, mainly for pharmacological applications, has
represented an important challenge (1). The increasing incidence of severe
diseases, such as cancer, demand an urgent need to discover new
drugs. Standard drugs have notable toxicity and their use is often
associated with tumor resistance, thus the development of more
effective therapies is required. In addition, natural anticancer
compounds, unlike synthetic drugs, are able to inhibit tumor growth
with minimal side effects (2).
Particularly intriguing is the identification of molecules from the
marine environment to be used in pharmaceuticals. Notably, oceans
cover >70% of the earth surface and display higher biodiversity
than the terrestrial environment; therefore, they have become an
interesting source for the discovery of novel drugs. To date,
oceans are still a largely unexplored environment, suggesting them
as promising candidates for sources of biologically active natural
compounds (3,4). Notably, soft-bodied sessile marine
invertebrates, such as sponges, are able to produce several
secondary metabolites for their survival in different habitats,
which are used to counterattack predators, and in competition for
space and nutrients. These bioactive compounds could be useful in
pharmacological, nutraceutical and cosmeceutical applications
(5,6).
Sponges represent the most studied marine organisms
as sources of bioactive compounds (1,3,4).
Previous studies have reported the bioactivity of different marine
sponge extracts in several diseases and some extracts have been
used to produce commercial anticancer drugs (7). Among sponges, the bioactivity of the
Mediterranean sponge Geodia cydonium has been poorly
characterized. Our previous study reported the anti-inflammatory
and anticancer effects of G. cydonium organic extracts on
breast cancer cells (8,9).
To the best of our knowledge, the present study was
the first to evaluate the antiproliferative potential of G.
cydonium extract (GEOCYDO) in mesothelioma, which is a rare and
aggressive type of cancer associated with exposure to asbestos
fibers that exhibits high chemoresistance (10). The present study used three human
mesothelioma cell lines, MSTO-211H (MSTO), NCI-H2452 (NCI) and
Ist-Mes2 (Mes2), which differ in their sensitivity (MSTO and NCI)
and resistance (Mes2) to standard combined treatment with cisplatin
and piroxicam (11). The aim of
the present study was to analyze the effect of GEOCYDO on
mesothelioma, a type of cancer characterized by high
chemoresistance. The present study indicated that the extract
affects mesothelioma cell viability and that the fraction C could
be the one responsible for its antiproliferative effects, being the
most active against the three mesothelioma cell lines. Furthermore,
preliminary chemical analysis of fraction C (12) revealed a complex metabolic profile,
which requires further fractionation for identification of the
active metabolite. To the best of our knowledge, the present study
provided novel findings, as despite the large number of marine
compounds assessed as drug candidates in various types of cancer
(13), no previous study has
referred to their use in mesothelioma.
Materials and methods
Collection of biological material
The present study did not involve protected species.
G. cydonium (order, Tetractinellida; family, Geodiidae)
samples were collected at 20 m in depth by scuba diving at the
‘Parco Sommerso di Baia’ (Naples, Italy). As soon as the samples
were collected, they were stored at −20°C until further
analysis.
G. cydonium extraction
Lyophilized sponge tissue (200 g wet weight) was
extracted with methanol at room temperature. After sonication (5
min, 59 KHz, 26°C), the organic extract was dried under nitrogen
flow and maintained at −20°C until further use. The extraction step
was repeated three times. The extract was filtered through Whatman
filter paper to recover solvent residues, and was then evaporated
at low pressure in a rotavapor at 28°C and dissolved in methanol.
The final extract was dried and stored at −20°C until use. For the
NMR analysis (Pulprog: zg), the dry extract was dissolved in
deuterated methanol (CD3OD) and transferred to a NMR
tube. NMR spectra were recorded on Bruker DRX 600 spectrometer
equipped with an inverse TCI CryoProbe (Bruker Corporation).
Chemical shift values are reported in ppm (δ) and referenced to
internal signals of residual protons (CD3OD,
1H 3.34).
Cell culture and chemicals
Human mesothelioma cell lines MSTO and NCI, and the
human mesothelial cell line MeT-5A were grown in RPMI supplemented
with 10% FBS (both from Euroclone SpA), glutamine (2 mM), sodium
pyruvate and antibiotics (0.02 IU/ml penicillin and 0.02 mg/ml
streptomycin). Human mesothelioma Mes2 cells were cultured in DMEM
(Euroclone SpA), supplemented with 10% FBS, glutamine (2 mM), 1%
nonessential amino acids and antibiotics (0.02 IU/ml penicillin and
0.02 mg/ml streptomycin). MSTO, NCI and MeT-5A cells were obtained
from the American Type Culture Collection, and Mes2 cells were
obtained from the Istituto Nazionale per la Ricercasul
Cancro-Genova.
Fractionation of the methanolic
extract of G. cydonium
A small amount of the active methanol extract (~40
mg) was subject to SPE using CHROMABOND® HRX cartridges
(6 ml/500 mg; Macherey-Nagel) on a GX-271 ASPEC (Gilson, Inc.)
(12). The extract was suspended
in 1 ml distilled water and sonicated (59 KHz, 26°C), for a few
seconds in an ultrasonic bath before loading onto the column, which
was previously conditioned with 3 ml methanol and equilibrated with
6 ml distilled water. This fractionation yielded five fractions (A,
B, C, D and E) obtained by stepwise elution with H2O (6
ml), CH3OH/H2O 7:3 (9 ml),
CH3CN/H2O 7:3 (9 ml), CH3CN (9 ml)
and CH2Cl2/CH3OH 9:1 (9 ml),
respectively. The total extract (TE) and SPE fractions B-E were
tested for cytotoxicity. The organic extract and fractions were
then analyzed by thin layer chromatography (TLC) stained with
Ce(SO4)2 and a preliminary chemical
characterization was carried out by 1H-NMR spectrum in
CD3OD. Each SPE fraction was dissolved in deuterated
solvent (CD3OD for GCYD-2B and 2C; CDCl3 for
GCYD-2D and 2E) and transferred to an NMR tube to acquire
1H-NMR spectra (Pulprog: zg), as already reported for
G. cydonium extract. Bidimensional NMR experiments
heteronuclear single quantum coherence edited (HSQCed) (Fig. S1) and heteronuclear multiple bond
correlation (HMBC) (Fig. S2) in
CD3OD were also acquired on the active SPE fraction C.
NMR spectra were recorded on a Bruker DRX 600 spectrometer equipped
with an inverse TCI CryoProbe. Chemical shift values are reported
in ppm (δ) and referenced to internal signals of residual protons
(CD3OD, 1H 3.34; CDCl3,
1H 7.26).
The extract and fractions were dissolved in 100 mM
DMSO and dilutions were made to obtain the different concentrations
to be tested, with a final concentration of 0.05% DMSO. Details on
fractionation and analysis are reported in Figs. S1 and S2.
To evaluate the bioactivity of SPE fractions, MSTO,
NCI and Mes2 cells were treated with 200 µg/ml of the four enriched
samples (B-E) for 24 h at 37°C; this concentration was selected as
this concentration of the total extract did not affect cell
viability.
Cell viability assay
For each cell line, ~1×104 cells/well
were plated in 48-well plates and were treated with GEOCYDO at
different concentrations (50, 150, 300 and 500 µg/ml) for 16, 24 or
48 h at 37°C in humified atmosphere containing 5% CO2.
For fraction bioactivity analysis, cells were treated with 200
µg/ml SPE fractions B, C, D and E for 24 h at 37°C. Subsequent
analysis with 50, 100, 150 and 200 µg/ml fraction C for 24 h at
37°C was performed done to establish the half maximal inhibitory
concentration (IC50) concentration. For all experiments,
cells treated with the same amount of vehicle (0.1% DMSO;
MilliporeSigma) present in the extract were used as a control.
Cell viability was evaluated counting live cells
using MTS assay (CellTiter 96; Promega Corporation) according to
the manufacturers' instructions. For the MTS assay, treated cells
were incubated with 20 µl MTS reagent for 2 h at 37°C. The
absorbance was recorded on a microplate reader at a wavelength of
490 nm (VICTOR Multilabel Plate Reader; PerkinElmer, Inc.). All
experiments were performed in triplicate and data are expressed as
the mean ± SD.
Colony formation assay
Colony formation was assessed as previously reported
by our group (14). For each cell
line, 500 cells/well were plated in six-well plates, incubated for
7 days and then treated with 500 µg/ml GEOCYDO for 24 or 48 h under
culture conditions before replacing the media. The growth was
assessed for a further 7 days and then crystal violet was used to
stain the colonies, which were successively counted. Briefly, cells
were fixed with formaldehyde (3.7%) for 10 min at room temperature,
then washed with PBS and stained for 10 min with crystal violet
(0.5%) at room temperature. The absorbance was measured at 595 nm
using a microplate reader (Cytation3 ASHI; BioTek Corporation). A
scanner (Epson Stylus Photo, PX 650; Epson) was used to capture
images of representative plates. All experiments were performed in
triplicate and data are expressed as the mean ± SD.
Wound-healing assay
The wound-healing assay was performed as previously
reported (14,15). For each cell line,
~3×105 cells/well were seeded in six-well plates. After
overnight incubation at 37°C, cells were at 90% confluence and
wounds were created using a 200-µl pipette tip. After wound
generation, cells were treated using the aforementioned culture
medium, with 500 µg/ml GEOCYDO or 0.1% DMSO for 24 or 48 h. To
analyze cell migration, at least six representative images for each
scratch were taken in different areas at different time points. A
phase contrast light microscope (DMI8; Leica Microsystems GmbH) was
used to capture images of the representative plates. ImageJ
software (version 1.52; National Institutes of Health) and its
wound healing assay macro was used to measure wound healing. All
experiments were performed in triplicate and data are expressed as
the mean ± SD.
Cell cycle analysis
After overnight incubation, ~7.5×105
cells/well were plated in 100-mm plates, serum starved for 24 h and
then treated with 500 µg/ml GEOCYDO for 16, 24 or 48 h at 37°C. PBS
was used to wash the cells, which were then fixed in cold 70%
ethanol for 30 min at 4°C. After centrifugation at 850 × g for 8
min at 4°C, cells were washed twice with cold PBS and cell pellets
were dissolved in 500 µl cold PBS. To ensure only DNA was stained,
cells were digested for 30 min at 37°C with 100 µg/ml RNase A.
Propidium iodide (50 µg/ml) was then used to stain cells for 30 min
at room temperature and the cells were then analyzed by flow
cytometry (FACSCanto; BD Biosciences) using FACSDiva software
(version 6.1.3; BD Biosciences). A total of 20×104
events were recorded for each sample and the percentage of cell
fractions in all cell cycle phases was calculating. All experiments
were performed in triplicate and data are expressed as the mean ±
SD.
Western blot analysis
Protein extracts were obtained from cells treated
with GEOCYDO (500 µg/ml) for 16, 24 or 48 h at 37°C as previously
described (11). Total cell
lysates (20 µg) were separated on 4–15% Tris-glycine gels by
SDS-PAGE (Bio-Rad Laboratories, Inc.) at 100 V and proteins were
then transferred to PVDF membranes (Bio-Rad Laboratories, Inc.).
The membranes were blocked in 5% milk in TBS/5% Tween at room
temperature for 1 h and were then probed overnight at 4°C with the
specific primary antibodies, and then with horseradish
peroxidase-conjugated secondary antibodies (1:10,000; cat. no.
A6154; MilliporeSigma) for 1 h at room temperature according to the
manufacturer's indications. The primary antibodies used for western
blotting include: Anti-cyclin E (cat. no. sc-481; Santa Cruz
Biotechnology, Inc), anti-cyclin A (cat. no. sc-596; Santa Cruz
Biotechnology, Inc.), anti-cyclin B1 (cat. no. sc-245; Santa Cruz
Biotechnology, Inc.), anti-p21 (cat. no. 2947; Cell Signaling
Technology, Inc.), anti-p27 (cat. no. sc-528; Santa Cruz
Biotechnology, Inc.) and anti-β-actin (cat. no. 3700; Cell
Signaling Technology, Inc.), which was used as a loading control,
at the concentrations suggested by manufacturers (1:1,000). Clarity
western ECL (Bio-Rad Laboratories, Inc.) was used to detect protein
bands and the blots were semi-quantified with ImageJ software. All
experiments were performed in triplicate and data are expressed as
the mean ± SD.
Statistical analysis
Graph Pad Prism 6.0 (GraphPad Software, Inc.)
analysis was used to evaluate the difference between control and
treatment groups. One-way ANOVA was used to evaluate the
significance of the differences among means. Dunnett's multiple
comparison test with Bonferroni post hoc correction was used to
assess the significance between each treatment group and the
control group. P≤0.05 was considered to indicate a statistically
significant difference.
Results
GEOCYDO affects cell viability
To analyze the bioactivity of GEOCYDO on
mesothelioma cells, the present study first determined, in a
dose-response curve at 24 h, the amount of extract that had a
lethal effect on MSTO cells. Results revealed that GEOCYDO had a
IC50 of ~500 µg/ml in MSTO cells (Fig. 1A). Therefore, a concentration of
500 µg/ml was used for subsequent experiments. By contrast, the
same concentration of GEOCYDO had no effect on wild-type Met-5A
mesothelial cells (Fig. 1B). The
present study also analyzed if the effects of GEOCYDO were
increased over the time. As shown in Fig. 1C, cell viability decreased from 16
to 48 h; it was reduced by 75% in MSTO, 70% in NCI and 80% in Mes2
cells at 48 h compared with in the vehicle-treated cells.
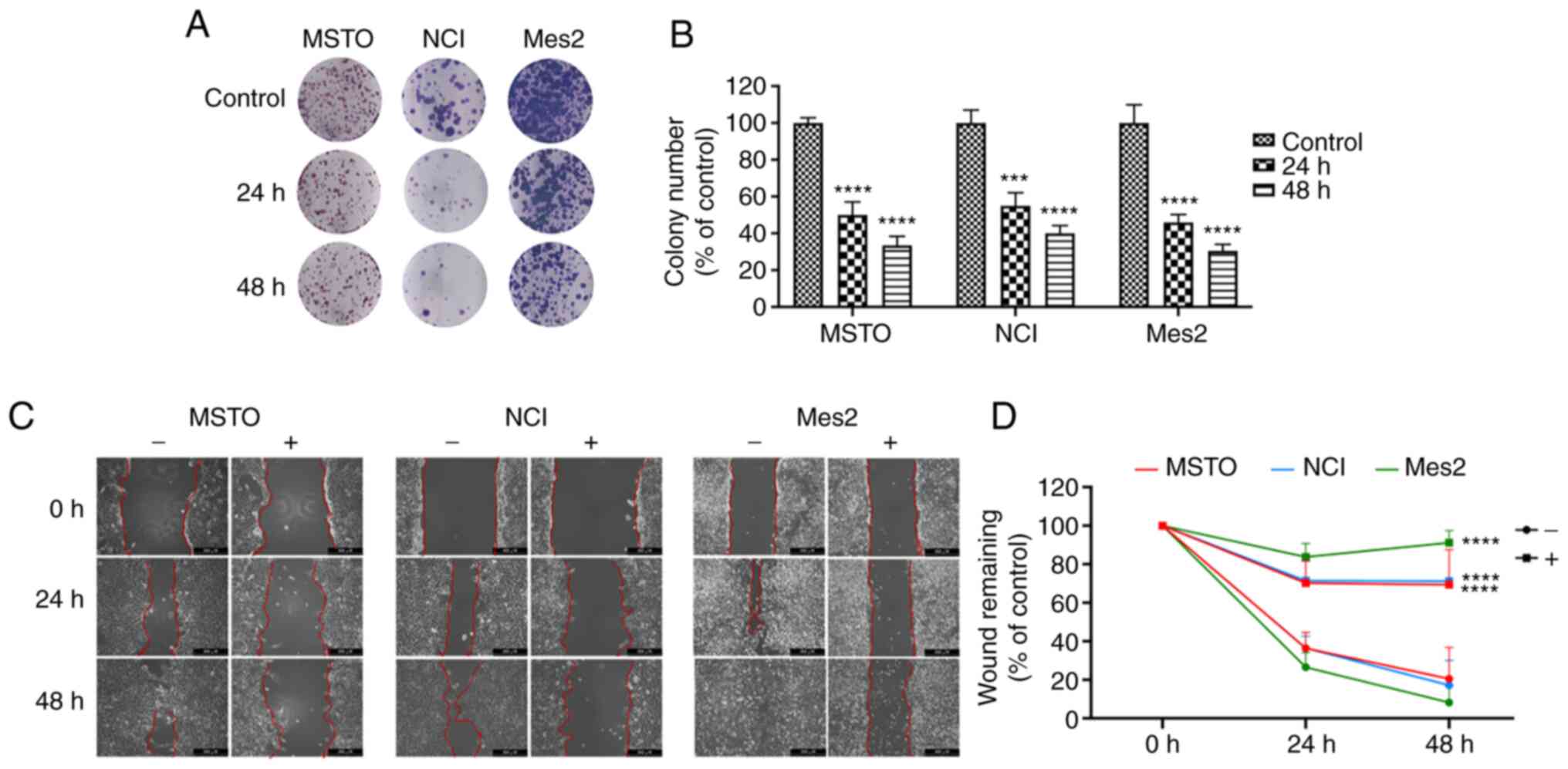
Subsequently, cell proliferation and migration were
analyzed to evaluate the anticancer potential of GEOCYDO. The
results clearly showed that GEOCYDO was able to impair cell
proliferation, reducing cellular self-renewal ability and long-term
proliferative potential in all of the cell lines tested. Notably,
treatment with 500 µg/ml GEOCYDO inhibited colony formation after
24 and 48 h, and the ability to produce colonies was reduced by
~50% after 24 h, and by 65, 60 and 70% after 48 h in MSTO, NCI and
Mes2 cells, respectively (Fig. 2A and
B). In addition, to measure the migratory capability of cells,
a wound-healing assay was performed, where scratched cells were
treated with GEOCYDO. The results showed that GEOCYDO significantly
inhibited the ability of cells to close the gap in all of the cell
lines (Fig. 2C and D). By
contrast, in the untreated cells, the wound gap was closed at the
end of the treatment. Notably, this experiment does not completely
distinguish if GEOCYDO treatment affects cell migration or
proliferation, since it was not performed in low serum conditions;
thus, proliferation inhibition was analyzed in more detail.
GEOCYDO extract induces
G0/G1 cell cycle arrest
To explore the mechanisms underlying the inhibition
of mesothelioma cells induced by GEOCYDO, cell cycle distribution
was analyzed by flow cytometry. Cells were analyzed following
treatment with GEOCYDO for 16, 24 or 48 h, in order to analyze cell
proliferation modifications. As shown in Figs. 3 and S1, untreated MSTO cells exhibited a
typical cell cycle distribution over time, whereas GEOCYDO
treatment induced a cell cycle arrest at 24 and at 48 h, as shown
by an increased percentage of the cell population in the
G0/G1 phase. Notably, alongside the increase
in the G0/G1 cell population, there was a
corresponding reduction in the population of cells in S and
G2/M phases. Similar results were obtained with NCI and
Mes2 cells (Fig. 3 and Fig. S3). Specifically, the percentage of
cells arrested in G0/G1 phase was increased
after 24 and 48 h in all of the cell lines analyzed; conversely, a
slight decrease in the percentage of cells in S and G2/M
phases was found.
To assess the effects of GEOCYDO, the expression
levels of different cyclins and of two CDK inhibitors (CDKIs), p21
and p27, which are crucial for cell cycle progression, were
detected. In particular, the expression levels of cyclin E, which
is required for cell cycle G1/S transition, of cyclin A,
which is needed for the G2/M transition, and of cyclin
B1, which is the mitotic cyclin, were detected. The analysis
indicated a decrease in the expression levels of cyclin E in MSTO,
NCI and Mes2 cells, which was in agreement with the observed cell
cycle arrest (Fig. 4). Considering
that cyclin E can regulate the passage between phase G1
and S, these results confirmed that GEOCYDO blocked the transition
between those two cell cycle phases. Cyclin A exhibited a similar
decreased expression in MSTO and NCI cells, whereas it was not
modulated in Mes2 treated cells compared with control cells. By
contrast, cyclin B1 expression was decreased at 16 and 24 h in MSTO
and NCI treated cells compared with in the control cells, and at
all timepoints in Mes2 treated cells compared with in the control
cells. The differences in the expression of cyclin B1 between the
different cell lines could be related to the tumorigenicity of the
cell lines. Finally, the decreased expression level of cyclins was
accompanied by an increase in the expression levels of p21 and p27
in MSTO and NCI treated cells compared with in the control cells,
and of p27 in Mes2 treated cells compared with in the control
cells.
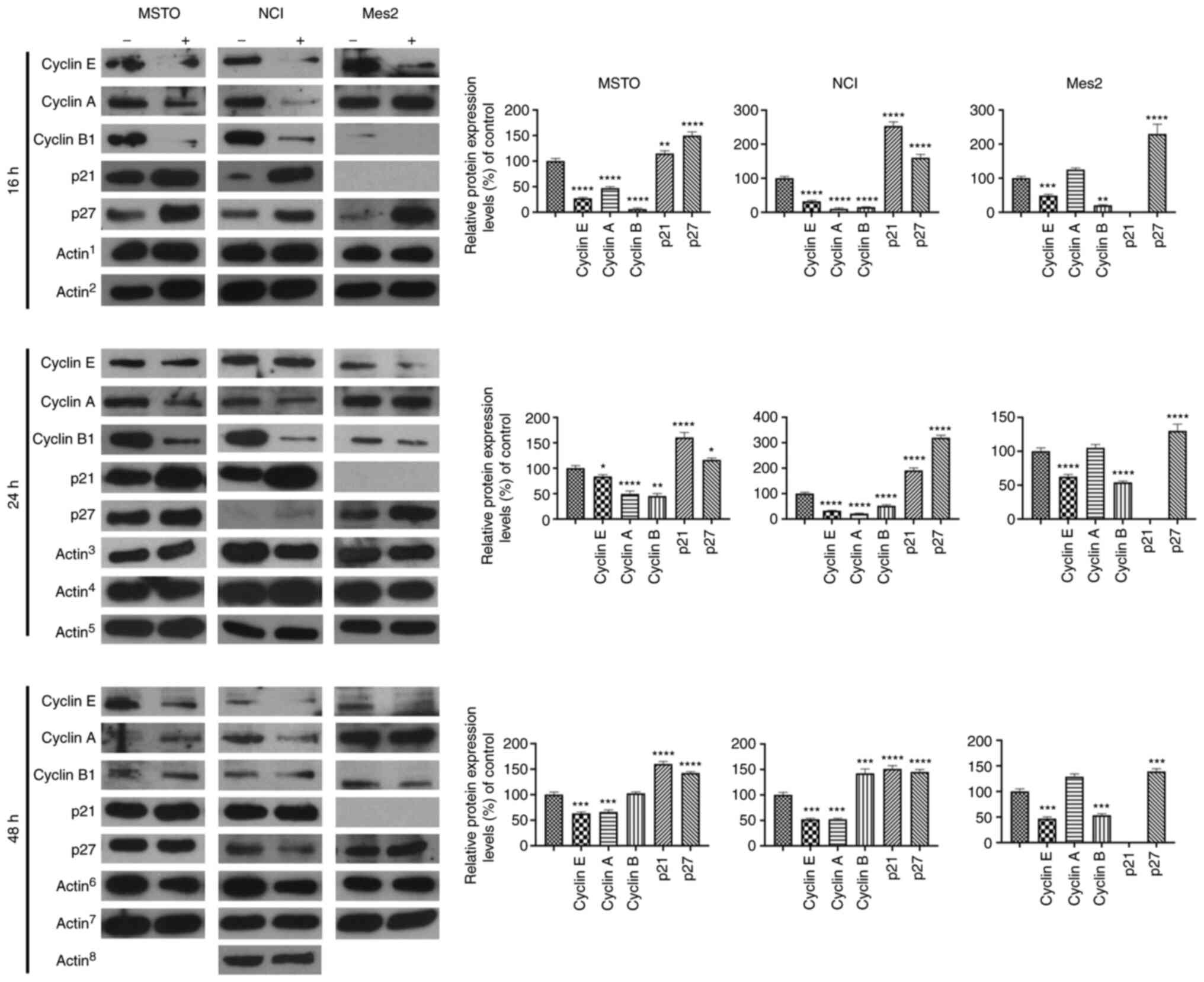 | Figure 4.GEOCYDO induces cell cycle arrest at
G0/G1 phase through modulation of cyclins and
CDK inhibitors. Western blot analysis of the expression levels of
cyclin E, cyclin A, cyclin B1, p21 and p27 16 h, 24 or 48 h after
treatment with GEOCYDO. β-actin expression was used as a loading
control. Actin1 refers to the control for cyclin E,
cyclin B1 and p21; Actin2 refers to the control for
cyclin A and p27; Actin3 refers to the control for
cyclin E, cyclin B1 and cyclin A (for NCI cells); Actin4
refers to the control for p21; Actin5 refers to the
control for p27 and cyclin A (for MSTO and Mes2 cells);
Actin6 refers to the control for cyclin E, cyclin B1 and
p21; Actin7 refers to the control for p27 and cyclin A
(for MSTO and Mes2 cells); Actin8 refers to the control
for cyclin A (for NCI cells). Histograms represent the relative
expression levels relative to the control. All the controls were
set at 100%. Data are presented as the mean ± standard deviation
(n=3). *P<0.05, **P<0.01, ***P<0.005, ****P<0.001 vs.
control. MSTO, MSTO-211H; NCI, NCI-H2452; Mes2, Ist-Mes2; GEOCYDO,
Geodia cydonium extract. |
Analysis of GEOCYDO and SPE
fractions
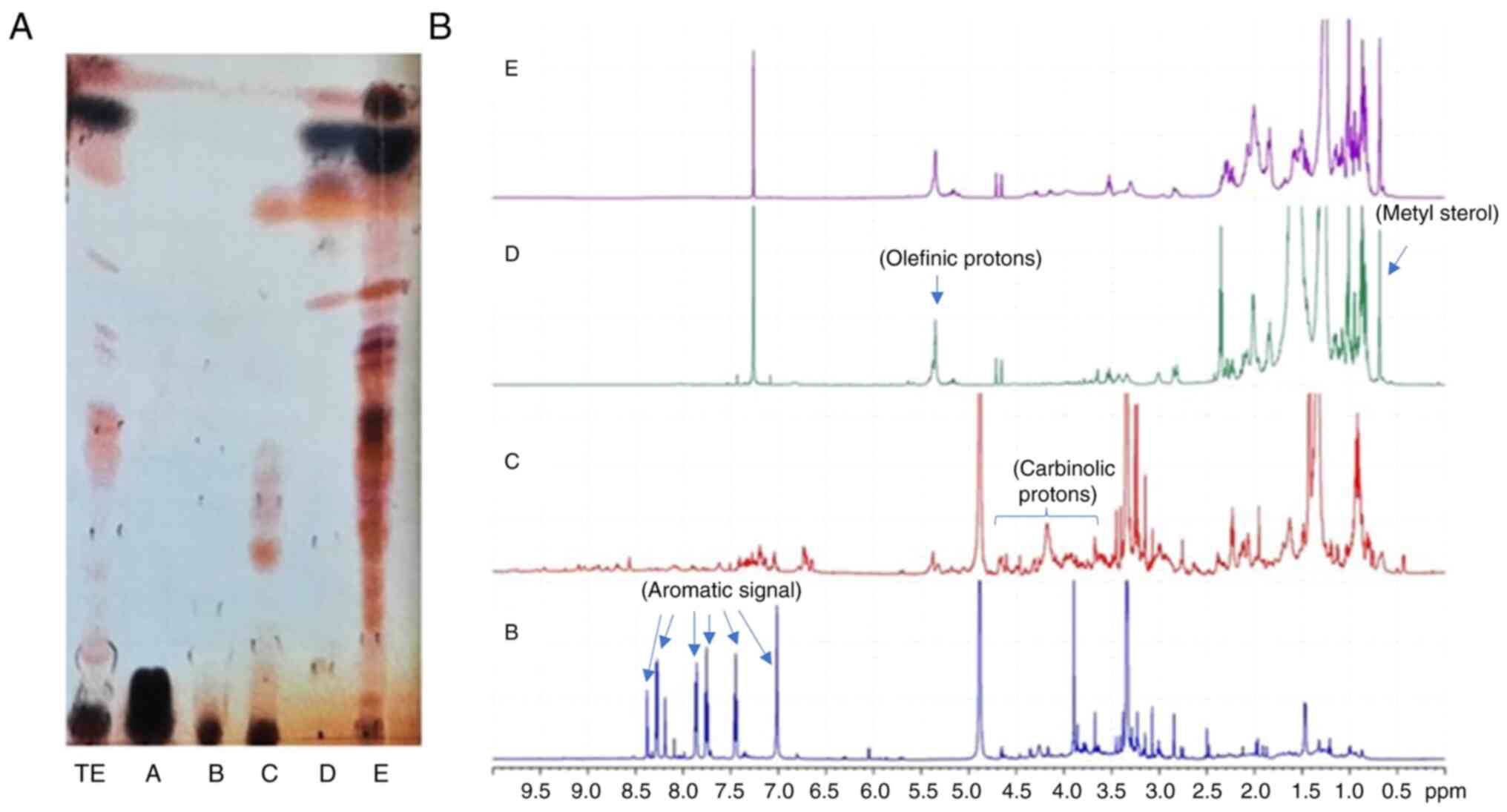
Spectroscopic analysis of GEOCYDO indicated that the
active methanolic extract was very rich in metabolites. Proton
signals in the region at low field showed the presence of aromatic
compounds (blue arrows in Fig. 5),
whereas the abundance of methyl singlets in the region between 2.7
and 3.7 ppm suggested the occurrence of heteroatom-containing
compounds (purple arrows in Fig.
5). Fractionation of GEOCYDO by SPE-HRX column led to five new
samples: Salts were eluted in fraction A, whereas the enriched
fractions B-E, contained different classes of metabolites. As shown
in Fig. 6, according to the
expected resolution of the method (12), chemical analysis of the SPE
fractions clearly indicated the presence of water-soluble
metabolites in fraction A (mainly sugars); nucleosides and
nitrogen-containing compounds in fraction B; complex lipids,
including sphingolipids, lipopeptides, glycolipids and
phospholipids in fraction C; sterols, terpenes, alcohols and fatty
acids in fraction D; and triglycerides and neutral lipids in
fraction E.
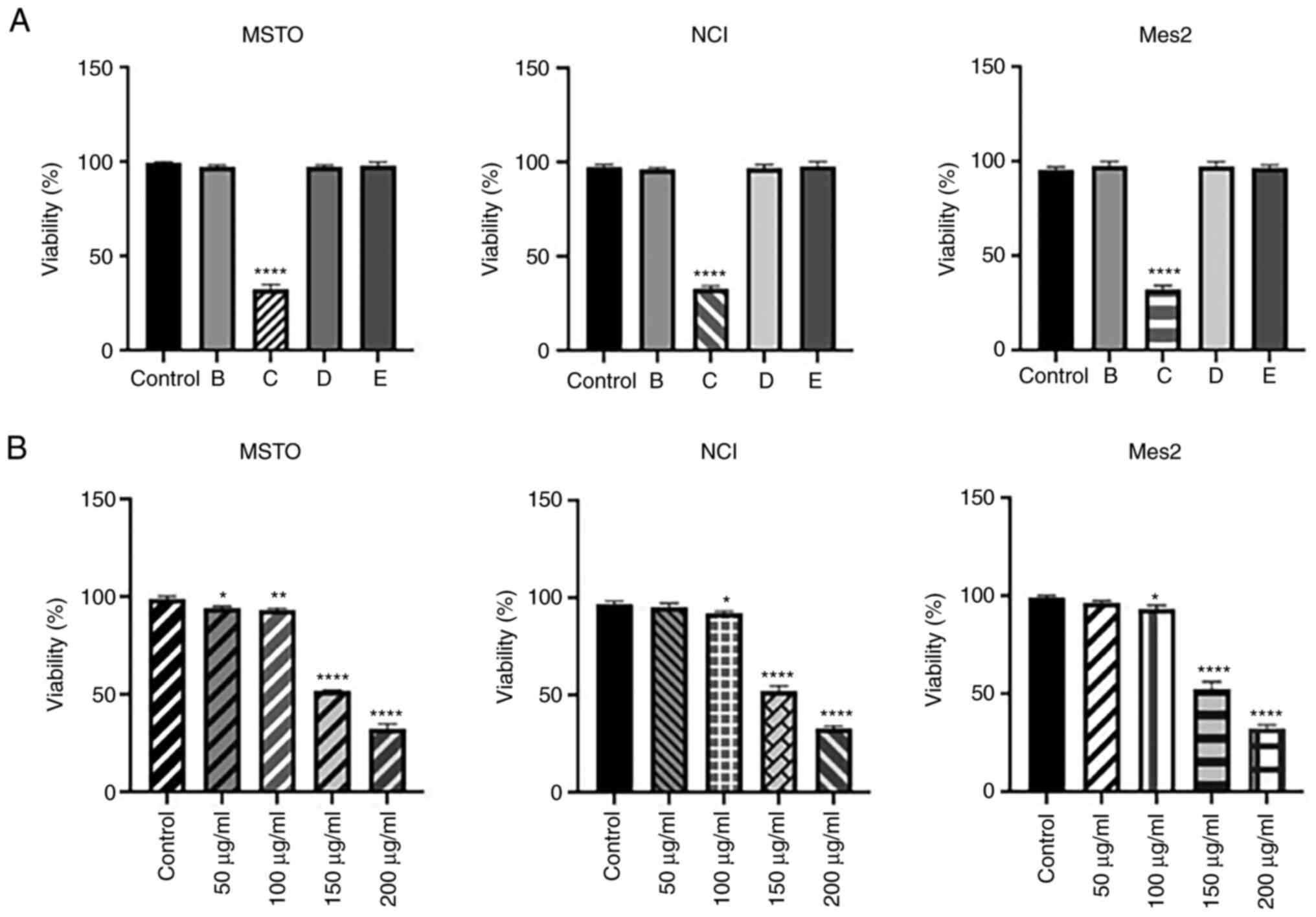
To evaluate the bioactivity of SPE fractions, MSTO,
NCI and Mes2 cells were treated with 200 µg/ml of the four enriched
samples (B-E) for 24 h; this concentration was selected as this
concentration of the total extract did not affect cell viability.
Cell viability analysis indicated that only fraction C was able to
decrease viability in all cell lines (Fig. 7A). Subsequent analysis using
various concentrations of fraction C (50–200 µg/ml) for 24 h
confirmed its strong cytotoxicity on all mesothelioma cell lines
analyzed, lowering the IC50 of GEOCYDO to ~150 µg/ml
(Fig. 7B).
Preliminary 1H-NMR and TLC analysis of
the enriched fraction C indicated a prevalence in this fraction of
polar minor metabolites (Fig. 6).
However, although the composition of fraction C is very complex and
produced a crowded NMR spectra with several overlapping signals,
these data are consistent with the presence of molecules with
cyclodepsipeptide or a macrolide skeleton (Figs. S2 and S3). In detail, spectroscopic data of
this fraction revealed a chemical signature with several methine
groups between 4 and 5 ppm, coupled with carbon between 50 and 60
ppm that could be diagnostic of amino acid skeletons, and various
methine and methylene signals between 3.5 and 4.10 ppm coupled with
oxygen-bearing carbon between 62 and 75 ppm (Fig. S1). In the HMBC spectrum (Fig. S2), these signals also showed
correlations with ester functions at 170–175 ppm. In addition to
methoxy moieties, the NMR spectra supported the presence of a
carbonyl group below 210 ppm (Fig.
S2), several methyl groups (1H NMR signals at 0.57,
0.76, 0.82, 1.03 ppm; 13C NMR signals at 25.3, 21.1,
15.9, 19.8 ppm), and various down-shifted protons between 6.5 and
7.3 ppm of conjugated unsaturated systems (Fig. S1). Unfortunately, the amount of
fraction C available was not enough to complete the
characterization of the active metabolite.
Discussion
To the best of our knowledge, the present study was
the first to describe the in vitro antiproliferative effect
of GEOCYDO extract on mesothelioma, a rare but very aggressive
cancer characterized by high chemoresistance (10,14).
Mesothelioma displays a long latency period (30–40 years) and a
generally unfavorable outcome (10). Despite several reports in the
literature on marine sponges (2,16–18),
few studies have assessed the biotechnological applications of one
of the major sources of bioactive natural products, G.
cydonium. Our group previously reported that a methanolic
extract from this sponge had an anti-inflammatory and pro-apoptotic
effect on breast cancer cell lines (8,9).
The results of the present study clearly indicated
that GEOCYDO was able to affect mesothelioma cancer properties
acting on cell proliferation in a time-dependent manner.
Cytofluorimetric analysis revealed that GEOCYDO induced cell cycle
arrest at G0/G1 phase, which may induce
inhibition of proliferation in mesothelioma. The present findings
were confirmed by expression analysis of proteins involved in cell
cycle arrest. Notably, specific alterations in the expression
levels of cyclins A, E and B1, and of CDKIs p21 and p27, were
detected (19).
Cell cycle progression is a finely tuned event
regulated by protein kinase complexes containing cyclins and CDKs
(20). In response to DNA damage,
cell proliferation undergoes arrest until DNA is repaired and
correctly replicated. Cell cycle arrest can occur at two specific
checkpoints of the cell cycle: G1/S and G2/M
(21). It is known that cyclin E
represents the key molecule of the G1/S checkpoint. The binding of
endogenous CDKis, such as p21 and p27, regulates the activity of
the complex cyclin E-CDK2 (22).
Furthermore, the induction of p21 and p27 arrests cell cycle in the
G1 phase, thus inhibiting the cells from entering the S
phase for replication (23). It is
known that cyclin E overexpression can promote cancer progression,
whereas its downregulation, by limiting cell cycle progression to
the G0/G1 phase, can decrease and inhibit
tumor cell proliferation (24,25).
Similarly, p21 and p27 act as tumor suppressors by controlling cell
cycle progression and cell proliferation (26–28);
decreased expression levels of p21 and p27 have been detected in
various types of human cancer (29–31).
Finally, the efficacy of GEOCYDO in Mes2 cells that do not express
p21, can be ascribed to the activity of p27 that regulates cell
cycle progression through decreasing p21 expression (32).
The ability of GEOCYDO to induce G1/S
cell cycle arrest was confirmed by protein analysis, showing a
downregulation in cyclin E, cyclin A and cyclin B1 expression, and
a concomitant activation of p21 and p27. A hallmark of cancer is
represented by uncontrolled and rapid cell division (2); therefore, the inhibition of cell
cycle progression may be a powerful anticancer approach.
Natural marine compounds are known to exert
anticancer activity in vitro and in vivo (33,34),
as well as in clinical settings (35), by acting on the cell cycle
(36). Several extracts have been
described to induce cell cycle perturbations in various tumor cell
lines. Extracts from the Lissodendorix sponge have been
described to act by preventing microtubule assembly (37–39).
The antiproliferative activity of an extract from the Negombata
magnifica sponge has been related to specific
G0/G1 and G2/M cell cycle block
(40). Furthermore, marine sponge
compounds derived from Jaspis stellifera and Monanchora
viridis have been reported to induce cell cycle arrest through
the reduction of cyclin D1 expression (2,41).
These findings are in agreement with the present findings, which
demonstrated that GEOCYDO blocked the transition between phase
G1 and S, as indicated by the decrease in the expression
levels of cyclin E, the key protein that regulates this
passage.
Finally, to the best of our knowledge, the
preliminary results from spectroscopic analysis indicated for the
first time that GEOCYDO contained different active metabolites. The
results are particularly promising since, to date, the knowledge of
secondary metabolites derived from members of the genus
Geodia (class, Demospongiae; family, Geodiidae) remains
limited. The bioactivity of GEOCYDO was initially tested using the
TE, after which the TE was fractionated and the different fractions
were analyzed to identify the active fraction; the results revealed
that fraction C was responsible for the bioactivity of GEOCYDO.
Molecular networking analysis of the bioactive
GEOCYDO extract revealed that it may contain different molecules,
such as nucleosides and amino acids, which are currently providing
lead compounds for new drugs as main constituents (9). In particular, previous studies have
reported that different nucleosides exert antiviral, anticancer and
hypertensive effects (42,43); and that some amino acids have a
high specificity against cancer cells (44). Moreover, GEOCYDO was shown to
contain 3-hydroxyquinaldic acid, a chromophore present in natural
antitumor agents that is required for DNA intercalation being able
to binds duplex (45). In
addition, the SPE fraction C exerted the strongest activity on
mesothelioma cancer cell lines, as revealed by the IC50
value, suggesting this fraction may be enriched in a specific
molecule that is responsible of the observed effect. Although
fractionation of the extract highlighted the general
characteristics of the compounds that are likely responsible for
the activity of the extract, fraction C is still a complex mixture
of metabolites; therefore, further chemical purifications are
required to isolate and characterize the active compound.
The present results are novel since, despite the
large number of marine compounds used as drug candidates in various
types of cancer, to the best of our knowledge, none have assessed
their effects on mesothelioma. The results of the present study
also suggested that new molecules from marine organisms could be
further investigated for novel treatment of this type of cancer,
which is characterized by high chemoresistance.
In conclusion, GEOCYDO extract from the marine
sponge G. cydonium could be considered a novel candidate as
a potential antitumor drug for human malignant mesothelioma. This
is a very important step in development of alternative and more
effective therapies to cure mesothelioma, considering that to date
the standard therapies, including chemotherapy, surgery and
radiotherapy, have produced unsatisfactory outcomes (46).
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the Dr Laura Pisapia
(IGB-CNR FACS Core Facility); Dr Maria Rosaria Aletta (IGB-CNR) and
Dr Chiara Nobile (IBBR-CNR) for bibliographic assistance; and Dr
Valentina Brasiello (IBBR-CNR) for editing assistance. The authors
would also like thank Dr. Enrico Gallocchio (‘Parco Sommerso di
Baia’, Naples), Dr Francesco Terlizzi and Dr Marco Cannavacciuolo
(Fishing Service of Stazione Zoologica Anton Dohrn) for providing
Geodia cydonium; and Dr Davide Caramiello (Marine Organisms
Core Facility, Stazione Zoologica) for his technical support in
sponge maintenance.
Funding
This research was partially funded by CNR project NUTR-AGE
(grant nos. FOE-2019 and DSB.AD004.271). Francesco Di Meo's PhD
fellowship in Biology is supported by MIUR project PON ‘Dottorati
Innovativi con caratterizzazione industriale’ 2017–2018. Roberta
Esposito was supported by a PhD (PhD in Biology, University of
Naples Federico II) fellowship funded by the Photosynthesis 2.0
project of the Stazione Zoologica Anton Dohrn. Rossana Cuciniello
was supported by a PhD (PhD in Biology XXXVI cycle, University of
Naples Federico II) fellowship funded by CNR/IRCCS Neuromed. Nadia
Ruocco was supported by a research grant ‘Antitumor Drugs and
Vaccines from the Sea (ADViSE)’ project (PG/2018/0494374).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC, MC, SF and AF conceptualized the study. FDM, RC,
MA and GF performed cellular and molecular biology experiments. GN,
AF, NR and RE performed chemical extraction. FDM, RC, MA, GF, NR
and RE performed data analysis. SC, MC, NR, RE, FDM and RC were
responsible for original draft preparation. FDM, RE, RC, GF, MA,
NR, GN, AF, SF, SC and MC confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Skropeta D, Pastro N and Zivanovic A:
Kinase inhibitors from marine sponges. Mar Drugs. 9:2131–2154.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bailon-Moscoso N, Cevallos-Solorzano G,
Romero-Benavides JC and Orellana MI: Natural compounds as
modulators of cell cycle arrest: Application for anticancer
chemotherapies. Curr Genomics. 18:106–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blunt JW, Copp BR, Keyzers RA, Munro MH
and Prinsep MR: Marine natural products. Nat Prod Rep. 31:160–258.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mehbub MF, Lei J, Franco C and Zhang W:
Marine sponge derived natural products between 2001 and 2010:
Trends and opportunities for discovery of bioactives. Mar Drugs.
12:4539–4577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Romano G, Costantini M, Sansone C,
Lauritano C, Ruocco N and Ianora A: Marine microorganisms as a
promising and sustainable source of bioactive molecules. Mar
Environ Res. 128:58–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giordano D, Costantini M, Coppola D,
Lauritano C, Pons LN, Ruocco N, di Prisco G, Ianora A and Verde C:
Biotechnological applications of bioactive peptides from marine
sources. Adv Microb Physiol. 73:171–220. 2018.
Malve H: Exploring
the ocean for new drug developments: Marine pharmacology. J Pharm
Bioallied Sci 8, 83–91, 2016. View Article : Google Scholar
|
|
7
|
Costantini S, Romano G, Rusolo F, Capone
F, Guerriero E, Colonna G, Ianora A, Ciliberto G and Costantini M:
Anti-inflammatory effects of a methanol extract from the marine
sponge geodia cydonium on the human breast cancer MCF-7 cell line.
Mediators Inflamm. 2015:2049752015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Costantini S, Guerriero E, Teta R, Capone
F, Caso A, Sorice A, Romano G, Ianora A, Ruocco N, Budillon A, et
al: Evaluating the effects of an organic extract from the
mediterranean sponge geodia cydonium on human breast cancer cell
lines. Int J Mol Sci. 18:21122017. View Article : Google Scholar
|
|
9
|
Crispi S, Cardillo I, Spugnini EP, Citro
G, Menegozzo S and Baldi A: Biological agents involved in malignant
mesothelioma: Relevance as biomarkers or therapeutic targets. Curr
Cancer Drug Targets. 10:19–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baldi A, Piccolo MT, Boccellino MR,
Donizetti A, Cardillo I, La Porta R, Quagliuolo L, Spugnini EP,
Cordero F, Citro G, et al: Apoptosis induced by piroxicam plus
cisplatin combined treatment is triggered by p21 in mesothelioma.
PLoS One. 6:e235692011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cutignano A, Nuzzo G, Ianora A, Luongo E,
Romano G, Gallo C, Sansone C, Aprea S, Mancini F, D'Oro U and
Fontana A: Development and application of a novel SPE-method for
bioassay-guided fractionation of marine extracts. Mar Drugs.
13:5736–5749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calcabrini C, Catanzaro E, Bishayee A,
Turrini E and Fimognari C: Marine sponge natural products with
anticancer potential: An updated review. Mar Drugs. 15:3102017.
View Article : Google Scholar
|
|
13
|
Di Meo F, Filosa S, Madonna M, Giello G,
Di Pardo A, Maglione V, Baldi A and Crispi S: Curcumin C3
complex®/Bioperine® has antineoplastic
activity in mesothelioma: An in vitro and in vivo analysis. J Exp
Clin Cancer Res. 38:3602019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kauanova S, Urazbayev A and Vorobjev I:
The frequent sampling of wound scratch assay reveals the
‘Opportunity’ window for quantitative evaluation of cell
motility-impeding drugs. Front Cell Dev Biol. 9:6409722021.
View Article : Google Scholar
|
|
15
|
Wellington KD, Cambie RC, Rutledge PS and
Bergquist PR: Chemistry of sponges. 19. Novel bioactive metabolites
from Hamigera tarangaensis. J Nat Prod. 63:79–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sipkema D, Franssen MC, Osinga R, Tramper
J and Wijffels RH: Marine sponges as pharmacy. Mar Biotechnol (NY).
7:142–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Varijakzhan D, Loh JY, Yap WS, Yusoff K,
Seboussi R, Lim SHE, Lai KS and Chong CM: Bioactive compounds from
marine sponges: Fundamentals and applications. Mar Drugs.
19:2462021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malumbres M and Barbacid M: To cycle or
not to cycle: A critical decision in cancer. Nat Rev Cancer.
1:222–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim S and Kaldis P: Cdks cyclins and CKIs:
Roles beyond cell cycle regulation. Development. 140:3079–3093.
2013. View Article : Google Scholar
|
|
20
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bresnahan WA, Boldogh I, Ma T, Albrecht T
and Thompson EA: Cyclin E/Cdk2 activity is controlled by different
mechanisms in the G0 and G1 phases of the cell cycle. Cell Growth
Differ. 7:1283–1290. 1996.PubMed/NCBI
|
|
22
|
Harper JW, Elledge SJ, Keyomarsi K,
Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley
L and Swindell E: Inhibition of cyclin-dependent kinases by p21.
Mol Biol Cell. 6:387–400. 1995. View Article : Google Scholar
|
|
23
|
Lodén M, Nielsen NH, Roos G, Emdin SO and
Landberg G: Cyclin E dependent kinase activity in human breast
cancer in relation to cyclin E, p27 and p21 expression and
retinoblastoma protein phosphorylation. Oncogene. 18:2557–2566.
1999. View Article : Google Scholar
|
|
24
|
Lodén M, Stighall M, Nielsen NH, Roos G,
Emdin SO, Ostlund H and Landberg G: The cyclin D1 high and cyclin E
high subgroups of breast cancer: Separate pathways in tumorogenesis
based on pattern of genetic aberrations and inactivation of the pRb
node. Oncogene. 21:4680–4690. 2002. View Article : Google Scholar
|
|
25
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: Cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar
|
|
26
|
Chu IM, Hengst L and Slingerland JM: The
Cdk inhibitor p27 in human cancer: Prognostic potential and
relevance to anticancer therapy. Nat Rev Cancer. 8:253–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kreis NN, Louwen F and Yuan J: The
multifaceted p21 (Cip1/Waf1/CDKN1A) in cell differentiation,
migration and cancer therapy. Cancers (Basel). 11:12202019.
View Article : Google Scholar
|
|
28
|
Baldi A, De Luca A, Esposito V, Campioni
M, Spugnini EP and Citro G: Tumor suppressors and cell-cycle
proteins in lung cancer. Patholog Res Int. 2011:6050422011.
|
|
29
|
Bachs O, Gallastegui E, Orlando S, Bigas
A, Morante-Redolat JM, Serratosa J, Fariñas I, Aligué R and Pujol
MJ: Role of p27 Kip1 as a transcriptional regulator.
Oncotarget. 9:26259–26278. 2018. View Article : Google Scholar
|
|
30
|
Razavipour SF, Harikumar KB and
Slingerland JM: p27 as a transcriptional regulator: New roles in
development and cancer. Cancer Res. 80:3451–3458. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gallastegui E, Biçer A, Orlando S, Besson
A, Pujol MJ and Bachs O: p27 Kip1 represses the
Pitx2-mediated expression of p21 Cip1 and regulates DNA
replication during cell cycle progression. Oncogene. 36:350–361.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahn JH, Woo JH, Rho JR and Choi JH:
Anticancer activity of gukulenin A isolated from the marine sponge.
Mar Drugs. 17:1262019. View Article : Google Scholar
|
|
33
|
Chikamatsu S, Saijo K, Imai H, Narita K,
Kawamura Y, Katoh T and Ishioka C: In vitro and in vivo antitumor
activity and the mechanism of siphonodictyal B in human colon
cancer cells. Cancer Med. 8:5662–5672. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nastrucci C, Cesario A and Russo P:
Anticancer drug discovery from the marine environment. Recent Pat
Anticancer Drug Discov. 7:218–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khalifa SAM, Elias N, Farag MA, Chen L,
Saeed A, Hegazy MEF, Moustafa MS, El-Wahed AB, Al-Mousawi SM,
Musharraf SG, et al: Marine natural products: A source of novel
anticancer drugs. Mar Drugs. 17:4912019. View Article : Google Scholar
|
|
36
|
Bergamaschi D, Ronzoni S, Taverna S,
Faretta M, De Feudis P, Faircloth G, Jimeno J, Erba E and D'Incalci
M: Cell cycle perturbations and apoptosis induced by
isohomohalichondrin B (IHB), a natural marine compound. Br J
Cancer. 79:267–277. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bitzer J, Grosse T, Wang L, Lang S, Beil W
and Zeeck A: New aminophenoxazinones from a marine Halomonas sp:
Fermentation structure elucidation and biological activity. J
Antibiot (Tokyo). 59:86–92. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sagar S, Esau L, Holtermann K, Hikmawan T,
Zhang G, Stingl U, Bajic VB and Kaur M: Induction of apoptosis in
cancer cell lines by the red sea brine pool bacterial extracts. BMC
Complement Altern Med. 13:3442013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rady HM, Hassan AZ, Salem SM, Mohamed TK,
Esmaiel NN, Ez-El-Arab MA, Ibrahim MA and Fouda FK: Induction of
apoptosis and cell cycle arrest by Negombata magnifica sponge in
hepatocellular carcinoma. Medicinal Chemistry Research. 25:456–465.
2016. View Article : Google Scholar
|
|
40
|
Wang R, Zhang Q, Peng X, Zhou C, Zhong Y,
Chen X, Qiu Y, Jin M, Gong M and Kong D: Stellettin B induces G1
arrest apoptosis and autophagy in human non-small cell lung cancer
A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep.
6:270712016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang RM, Chen YN, Zeng Z, Gao CH, Su X
and Peng Y: Marine nucleosides: Structure, bioactivity synthesis
and biosynthesis. Mar Drugs. 12:5817–5838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rajan R, Sabnani MK, Mavinkurve V, Shmeeda
H, Mansouri H, Bonkoungou S, Le AD, Wood LM, Gabizon AA and La-Beck
NM: Liposome-induced immunosuppression and tumor growth is mediated
by macrophages and mitigated by liposome–encapsulated alendronate.
J Control Release. 271:139–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Negi B, Kumar D and Rawat DS: Marine
peptides as anticancer agents: A remedy to mankind by nature. Curr
Protein Pept Sci. 18:885–904. 2017. View Article : Google Scholar
|
|
44
|
Sheoran A, King A, Velasco A, Pero JM and
Garneau-Tsodikova S: Characterization of tioF, a tryptophan
2,3-dioxygenase involved in 3-hydroxyquinaldic acid formation
during thiocoraline biosynthesis. Mol Biosyst. 4:622–628. 2008.
View Article : Google Scholar
|
|
45
|
Baldi A, De Luca A, Maiorano P, D'Angelo C
and Giordano A: Curcumin as an anticancer agent in malignant
mesothelioma: A Review. Int J Mol Sci. 21:18392020. View Article : Google Scholar
|