Introduction
The combination of atezolizumab (Atez), a humanized
immunoglobulin G1 monoclonal antibody targeting programmed
death-ligand 1 (PD-L1), and bevacizumab (BV), a monoclonal antibody
that targets vascular endothelial growth factor (VEGF) and inhibits
angiogenesis and tumor growth, is the recommended first-line
treatment for patients with unresectable hepatocellular carcinoma
(HCC) with Child-Pugh A (CP-A) liver function (1–4).
Lenvatinib (LEN), a multitarget tyrosine kinase inhibitor, is often
used as second-line treatment. LEN targets vascular endothelial
growth factor receptors 1, 2, and 3, fibroblast growth factor (FGF)
receptors 1 through 4, platelet-derived growth factor receptor α,
rearranged during transfection, and stem cell factor receptor.
Moreover, LEN also inhibits the formation of vessel-like luminal
structures by vascular endothelial cells induced by VEGF and
FGF.
Atez is an immune checkpoint inhibitor (ICI) that
binds to cell surface PD-L1 and suppresses its function. ICIs
produce unique side effects of immune-related adverse events in
various organs. These include interstitial lung disease, colitis,
hypothyroidism, liver damage, skin rash, vitiligo, hypophysitis,
type I diabetes, renal dysfunction, myasthenia gravis, peripheral
neuropathy, myositis, and uveitis. Adverse immune events generally
occur several months after drug administration; however, the timing
of their appearance varies widely. For example, regarding Atez/BV,
the onset of thyroid dysfunction was reported to be 93.5 (range
13–419) days in the IMbrave150 study (1). Therefore, the possibility of the
influence of secondary treatment on first-line treatment cannot be
ruled out. When LEN is selected as a second-line treatment in
patients with CP-A liver function, it is important to maintain its
safety and continue treatment.
LEN is a standard therapeutic agent for HCC, but the
high incidence of adverse events (AEs) is a problematic aspect of
LEN treatment (5–8), as these AEs may necessitate treatment
discontinuation. The most common any-grade AEs of LEN were
hypertension (42%), diarrhea (39%), decreased appetite (34%),
decreased weight (31%), and fatigue (30%) during first-line LEN
treatment (1st LEN) (6). The
incidence of hypothyroidism was 16.4% (6). Regarding the safety of using LEN as a
second-line treatment (2nd LEN), Yoo et al (9), Aoki et al (10), and Hiraoka et al (11) reported significant antitumor
efficacy with acceptable safety. However, they did not compare the
AEs of LEN when used as first- or second-line treatment. Comparing
the frequency and appearance of AEs in first- and second-line
treatments, will contribute to managing AEs in the 2nd-line
treatment.
In this study, we investigated the safety of LEN in
second-line therapy by comparing the AEs of LEN in second-line
therapy after first-line Atez/BV treatment for unresectable liver
cancer, with those of LEN in first-line therapy.
Patients and methods
Patients and evaluations
A total of 53 patients with CP-A unresectable liver
cancer treated at the Ogaki Municipal Hospital between April 2018
and September 2023 were retrospectively evaluated. Patients who
received Atez/BV as first-line therapy and LEN as second-line
therapy or those who received LEN as first-line therapy were
included. Patient characteristics, treatment duration, AEs, and
relative dose intensity (RDI) were analyzed. Data were analyzed
using electronic and pharmacy service records. AEs were evaluated
according to the Common Terminology Criteria for Adverse Events,
version 5.0 (12), and the most
severe grades during chemotherapy were reported. Personal
information was protected in the aggregated data. This study was
approved by the Institutional Review Board of the Ogaki Municipal
Hospital (Ogaki, Japan; approval number: 20220728-17-h). The need
for informed consent was waived due to the retrospective nature of
the study.
Treatment protocol
Atez was administered intravenously at 1,200 mg for
60 min on the first day. If the first dose was well-tolerated, the
duration of the second infusion was shortened to 30 min. BV was
administered intravenously at a dose of 15 mg/kg for 90 min on the
first day. If no problems were encountered, the durations of the
second and third infusions were shortened to 60 and 30 min,
respectively. This procedure was repeated every 21 days.
The LEN dose was based on body weight; the initial
dose was 12 mg/day for those weighing ≥60 kg and 8 mg/day for those
weighing <60 kg. During the 28-day cycle, dose adjustment,
including reduction to 8 or 4 mg/day, 4 mg every other day, or
interruption, was allowed for LEN treatment based on AEs (6,13). In
patients who experienced unacceptable drug-related AEs, the LEN
dose was reduced or treatment was interrupted according to the
manufacturer's instructions. Dose reduction or temporary
interruption of LEN was maintained until the AE severity dropped to
grade 1 or 2. In cases where dose reduction was maintained, the
reduced doses administered were 20, 14, 10, 8, or 4 mg once
daily.
Statistical analysis
To test whether the variances of two populations
were equal regarding patient characteristics and RDI the F-test was
performed. The Mann-Whitney U test or Fisher's exact probability
test was used to compare patient characteristics, AEs, and RDI. The
change in AE grade from first line to second line treatment was
compared with the Wilcoxon signed-rank test. Kaplan-Meier and
log-rank tests were used to compare treatment durations.
Differences were considered statistically significant at P<0.05.
All analyses were performed using EZR software (version 1.30,
Saitama Medical Center, Jichi Medical University, Saitama, Japan),
which is a graphical user interface for R software (The R
Foundation for Statistical Computing, Vienna, Austria) (14).
Results
Patient characteristics
The 1st LEN and 2nd LEN groups comprised 39 and 13
patients, respectively. Patient characteristics are summarized in
Table I. The median age of the
patients in the 1st LEN and the 2nd LEN group was 77 (range=58-88)
years and 73 (range=64-85) years, respectively. Patients differed
significantly in terms of sex and history of transcatheter arterial
chemoembolization between the 1st LEN and 2nd LEN groups.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | 1st LEN | 2nd LEN | P-value |
|---|
| Patients, n | 39 | 13 |
|
| Age, years |
|
|
|
| Median
(range) | 77 (58–88) | 73 (64–85) | 0.547a |
| Sex, n |
|
|
|
|
Male/female | 36/3 | 5/8 |
<0.005b,c |
| Height, cm |
|
|
|
| Median
(range) | 161 (127–171) | 155 (145–169) | 0.063a |
| Weight, kg |
|
|
|
| Median
(range) | 60 (40–88) | 58 (52–90) | 0.726a |
| Body surface area,
kg/m2 |
|
|
|
| Median
(range) | 1.66 (1.17–2.01) | 1.58 (1.45–2.01) | 0.505a |
| Creatinine clearance,
ml/min |
|
|
|
| Median
(range) | 61.9
(40.9–134.0) | 77.8
(28.4–133.0) | 0.369a |
| Cause of
hepatocellular carcinoma, n |
|
|
|
| Hepatitis
B virus | 6 | 4 | 0.183b |
| Hepatitis
C virus | 15 | 2 | 0.125b |
| Non-B
non-C | 18 | 3 | 0.142b |
| Performance status,
n |
|
|
|
| 0 | 31 | 10 | 0.845b |
| 1 | 7 | 3 | 0.685b |
| 2 | 1 | 0 | 0.559b |
| Post history of
transcatheter arterial chemoembolization, n |
|
|
|
|
Yes | 34 | 8 | 0.042b,c |
Adverse events
The proportion of patients showing an increase in
aspartate aminotransferase/alanine aminotransferase (AST/ALT)
levels was greater in the 2nd LEN (76.9%) than in the 1st LEN
(46.2%) group (P=0.016). Hypothyroidism was more common in the 2nd
LEN (46.2%) than in the 1st LEN (12.8%) group (P=0.011). The major
AEs for the 1st LEN and 2nd LEN groups are summarized in Table II. No differences were observed in
other AEs between 1st LEN and 2nd LEN groups. The major AEs of
1st-line Atez/BV treatment in patients who were able to transition
to LEN as 2nd-line treatment are summarized in Table III.
 | Table II.Adverse events. |
Table II.
Adverse events.
|
| 1st LEN (n=39) | 2nd LEN (n=13) |
|
|---|
|
|
|
|
|
|---|
|
| Grade, n |
| Grade, n |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Events | 1 | 2 | 3 | 4 | All grades (%) | 1 | 2 | 3 | 4 | All grades (%) | P-value |
|---|
| Leucopenia | 5 | 7 | 1 | 0 | 13 (33.3) | 2 | 1 | 1 | 0 | 4 (30.8) | 0.864 |
| Neutropenia | 2 | 6 | 3 | 0 | 11 (28.2) | 3 | 1 | 0 | 0 | 4 (30.8) | 0.860 |
| Platelet count
decreased | 17 | 10 | 1 | 0 | 28 (71.8) | 5 | 3 | 1 | 0 | 9 (69.2) | 0.860 |
| Aspartate
aminotransferase/alanine aminotransferase increased | 17 | 1 | 0 | 0 | 18 (46.2) | 7 | 0 | 3 | 0 | 10 (76.9) | 0.016a |
| Blood bilirubin
increased | 10 | 6 | 2 | 0 | 18 (46.2) | 2 | 3 | 0 | 0 | 5 (38.5) | 0.629 |
| Anemia | 13 | 3 | 2 | 0 | 18 (46.2) | 4 | 3 | 0 | 0 | 7 (53.8) | 0.631 |
| Diarrhea | 5 | 0 | 1 | 0 | 6 (15.4) | 3 | 1 | 0 | 0 | 4 (30.8) | 0.244 |
| Nausea | 3 | 2 | 0 | 0 | 5 (12.8) | 1 | 0 | 0 | 0 | 1 (7.7) | 0.616 |
| Vomiting | 2 | 0 | 0 | - | 2 (5.1) | 0 | 0 | 0 | - | 0 | 0.405 |
| Fatigue | 11 | 4 | 1 | - | 16 (41.0) | 6 | 2 | 0 | - | 8 (61.5) | 0.199 |
| Proteinuria | 0 | 11 | 4 | - | 15 (38.5) | 2 | 0 | 3 | - | 5 (38.5) | 1 |
| Anorexia | 11 | 2 | 1 | 0 | 14 (35.9) | 5 | 0 | 0 | 0 | 5 (38.5) | 0.868 |
| Edema in limbs | 7 | 0 | 0 | - | 7 (17.9) | 5 | 0 | 0 | - | 5 (38.5) | 0.128 |
| Hypertension | 8 | 4 | 2 | 0 | 14 (35.9) | 1 | 0 | 0 | 0 | 1 (7.7) | 0.052 |
|
Hand-foot-syndrome | 4 | 4 | 0 | - | 8 (20.5) | 4 | 1 | 0 | - | 5 (38.5) | 0.196 |
| Hoarseness | 1 | 0 | 0 | - | 1 (2.6) | 0 | 0 | 0 | - | 0 | 0.560 |
| Rash | 1 | 0 | 0 | 0 | 1 (2.6) | 0 | 0 | 0 | 0 | 0 | 0.560 |
| Musculoskeletal and
connective tissue disorder | 2 | 0 | 0 | 0 | 2 (5.1) | 0 | 0 | 0 | 0 | 0 | 0.405 |
| Hypothyroidism | 2 | 3 | 0 | 0 | 5 (12.8) | 3 | 3 | 0 | 0 | 6 (46.2) | 0.011a |
| Epistaxis | 1 | 0 | 0 | 0 | 1 (2.6) | 0 | 0 | 0 | 0 | 0 | 0.560 |
| Oral pain | 2 | 0 | 0 | - | 2 (5.1) | 0 | 0 | 0 | - | 0 | 0.405 |
| Dizziness | 1 | 0 | 0 | - | 1 (2.6) | 0 | 0 | 0 | - | 0 | 0.560 |
| Abdominal pain | 2 | 0 | 0 | - | 2 (5.1) | 0 | 0 | 0 | - | 0 | 0.405 |
 | Table III.Adverse events of atezolizumab plus
bevacizumab as 1st-line. |
Table III.
Adverse events of atezolizumab plus
bevacizumab as 1st-line.
|
| Grade, n |
|
|---|
|
|
| All grades |
|---|
| Events | 1 | 2 | 3 | 4 | (%) |
|---|
| Leucopenia | 2 | 1 | 1 | 0 | 4 (30.8) |
| Neutropenia | 3 | 1 | 0 | 0 | 4 (30.8) |
| Platelet count
decreased | 5 | 4 | 1 | 0 | 10 (76.9) |
| Aspartate
aminotransferase/alanine | 5 | 0 | 3 | 0 | 8 (61.5) |
| aminotransferase
increased |
|
|
|
|
|
| Blood bilirubin
increased | 2 | 4 | 0 | 0 | 6 (46.2) |
| Anemia | 4 | 2 | 0 | 0 | 6 (46.2) |
| Diarrhea | 3 | 1 | 0 | 0 | 4 (30.8) |
| Nausea | 1 | 0 | 0 | 0 | 1 (7.7) |
| Fatigue | 6 | 1 | 0 | - | 7 (53.8) |
| Proteinuria | 2 | 1 | 1 | - | 4 (30.8) |
| Anorexia | 6 | 0 | 0 | 0 | 6 (46.2) |
| Edema in limbs | 5 | 0 | 0 | - | 5 (38.5) |
| Hypertension | 2 | 0 | 0 | 0 | 2 (15.4) |
|
Hand-foot-syndrome | 3 | 1 | 0 | - | 4 (30.8) |
| Stomatitis | 1 | 0 | 0 | 0 | 1 (7.7) |
| Hypothyroidism | 4 | 0 | 0 | 0 | 4 (30.8) |
Hypothyroidism was observed in 30.8% of these
patients. In comparison, hypothyroidism was seen in 11.1% (4/36) of
all patients who received 1st-line Atez/BV, including the patients
who were able to transition to LEN as 2nd-line treatment and those
who were not. AEs with an incidence of more than 50% were reduced
platelet count (76.9%) and increased AST/ALT (61.5%) in 1st-line
Atez/BV treatment in patients who were able to transition to LEN as
2nd-line treatment.
Subgroup analysis of adverse events by
sex
Table IV shows
subgroup analysis of AEs by sex. In 2nd LEN, hypothyroidism was
more common in females (75%) than in males (0%). There were no
differences by sex in other AEs.
 | Table IV.Subgroup analysis of adverse events
by sex. |
Table IV.
Subgroup analysis of adverse events
by sex.
|
|
1st
LEN |
2nd
LEN |
|---|
|
|
|
|
|---|
|
| Male (n=36) | Female (n=3) |
| Male (n=5) | Female (n=8) |
|
|---|
|
|
|
|
|
|
|
|
|---|
|
| Grade, n |
| Grade, n |
|
| Grade, n |
| Grade, n |
|
|
|---|
|
|
| All grades |
| All grades |
|
| All grades |
| All grades |
|
|---|
| Events | 1 | 2 | 3 | 4 | (%) | 1 | 2 | 3 | 4 | (%) | P-value | 1 | 2 | 3 | 4 | (%) | 1 | 2 | 3 | 4 | (%) | P-value |
|---|
| Leucopenia | 4 | 6 | 1 | 0 | 11 (30.6) | 1 | 1 | 0 | 0 | 2 (66.7) | 0.253 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 4 (50.0) | 0.105 |
| Neutropenia | 2 | 6 | 2 | 0 | 10 (27.8) | 0 | 0 | 1 | 0 | 1 (33.3) | 1.253 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 4 (50.0) | 0.105 |
| Platelet count
decreased | 16 | 8 | 1 | 0 | 25 (69.4) | 1 | 2 | 0 | 0 | 3 (100) | 0.545 | 2 | 0 | 1 | 0 | 3 (60.0) | 3 | 3 | 0 | 0 | 6 (75.0) | 1 |
| Aspartate | 17 | 1 | 0 | 0 | 18 (50.0) | 0 | 0 | 0 | 0 | 0 | 0.235 | 4 | 0 | 1 | 0 | 5 (100) | 3 | 0 | 2 | 0 | 5 (62.5) | 0.231 |
|
aminotransferase/alanine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
aminotransferase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| increased |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Blood
bilirubin | 9 | 6 | 2 | 0 | 17 (47.2) | 1 | 0 | 0 | 0 | 1 (33.3) | 1 | 0 | 1 | 0 | 0 | 1 (20.0) | 2 | 2 | 0 | 0 | 4 (50.0) | 0.565 |
| increased |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Anemia | 12 | 2 | 2 | 0 | 16 (44.4) | 1 | 1 | 0 | 0 | 2 (46.2) | 0.586 | 2 | 1 | 0 | 0 | 3 (60.0) | 2 | 2 | 0 | 0 | 4 (50.0) | 1 |
| Diarrhea | 5 | 0 | 1 | 0 | 6 (16.7) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 (20.0) | 3 | 0 | 0 | 0 | 3 (37.5) | 1 |
| Nausea | 3 | 2 | 0 | 0 | 5 (13.9) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 (12.5) | 1 |
| Vomiting | 2 | 0 | 0 | - | 2 (5.6) | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 0 | - |
| 0 | 0 | 0 | - |
| 1 |
| Fatigue | 11 | 4 | 0 | - | 15 (41.7) | 0 | 0 | 1 | - | 1 (33.3) | 1 | 2 | 1 | 0 | - | 3 (60.0) | 4 | 1 | 0 | - | 5 (62.5) | 1 |
| Proteinuria | 0 | 9 | 4 | - | 13 (36.1) | 0 | 2 | 0 | - | 2 (46.2) | 0.547 | 1 | 0 | 2 | - | 3 (60.0) | 1 | 0 | 1 | - | 2 (25.0) | 0.293 |
| Anorexia | 11 | 2 | 1 | 0 | 14 (38.9) | 0 | 0 | 0 | 0 | 0 | 0.540 | 1 | 0 | 0 | 0 | 1 (20.0) | 4 | 0 | 0 | 0 | 4 (50.0) | 0.565 |
| Edema in limbs | 7 | 0 | 0 | - | 7 (19.4) | 0 | 0 | 0 | - | 0 | 1 | 2 | 0 | 0 | - | 2 (40.0) | 3 | 0 | 0 | - | 3 (37.5) | 1 |
| Hypertension | 8 | 3 | 2 | 0 | 13 (36.1) | 0 | 1 | 0 | 0 | 1 (33.3) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 (12.5) | 1 |
|
Hand-foot-syndrome | 4 | 4 | 0 | - | 8 (22.2) | 0 | 0 | 0 | - | 0 | 1 | 1 | 1 | 0 | - | 2 (40.0) | 3 | 0 | 0 | - | 3 (37.5) | 1 |
| Hoarseness | 1 | 0 | 0 | - | 1 (2.8) | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | - | 0 | 1 |
| Rash | 1 | 0 | 0 | 0 | 1 (2.8) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Musculoskeletal and
connective tissue disorder | 2 | 0 | 0 | 0 | 2 (5.6) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hypothyroidism | 2 | 3 | 0 | 0 | 5 (13.9) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 6 (75.0) | 0.021a |
| Epistaxis | 1 | 0 | 0 | 0 | 1 (2.8) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Oral pain | 2 | 0 | 0 | - | 2 (5.6) | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | - | 0 | 1 |
| Dizziness | 1 | 0 | 0 | - | 1 (2.8) | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | - | 0 | 1 |
| Abdominal pain | 2 | 0 | 0 | - | 2 (5.6) | 0 | 0 | 0 | - | 0 | 1 | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | - | 0 | 1 |
The effects of lenvatinib treatment on
the treatment duration
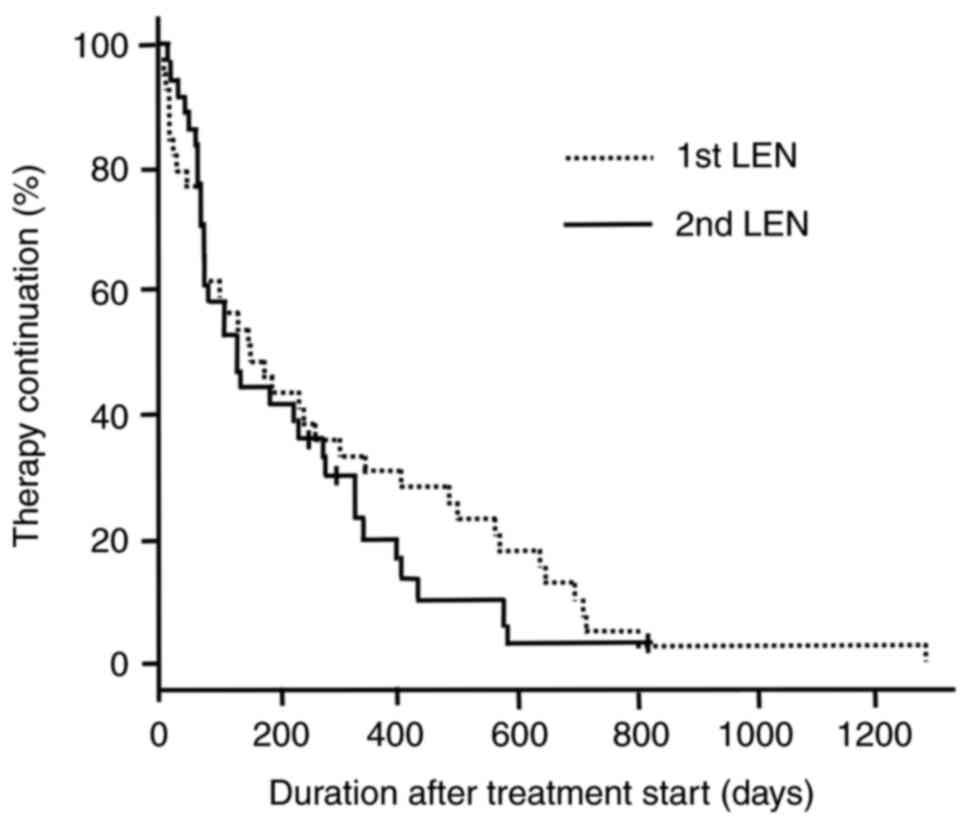
Fig. 1 shows
Kaplan-Meier survival curves of the duration of treatment with LEN
for all patients. The median treatment durations for patients in
the 1st LEN and 2nd LEN groups were 151.0 [95% confidence interval
(CI), 77–303] days, and 128.5 (95% CI, 68–270) days, respectively
(log-rank test, P=0.385).
RDI of lenvatinib in the treatment
groups
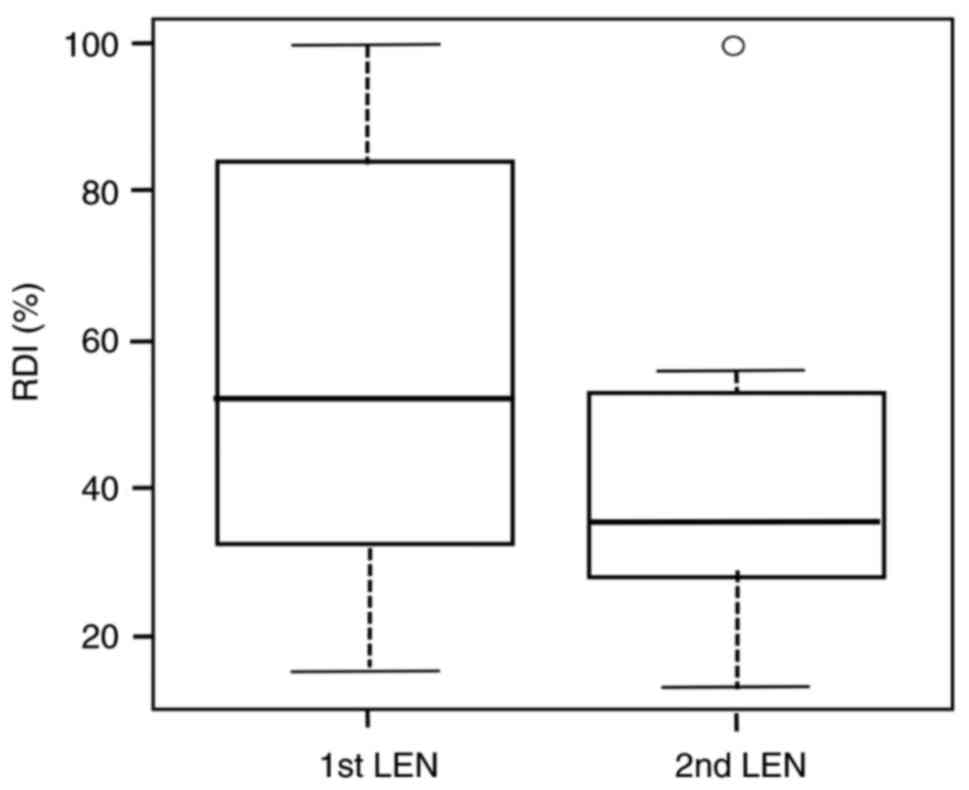
The RDIs of LEN for the 1st LEN and 2nd LEN groups
are shown in Fig. 2. The median
RDIs in the 1st LEN and 2nd LEN groups were 52.0 and 35.5%,
respectively. There was no significant difference in the median RDI
between 1st LEN and 2nd LEN (P=0.081).
Grade change of adverse events from
first-line atezolizumab plus bevacizumab to second-line
lenvatinib
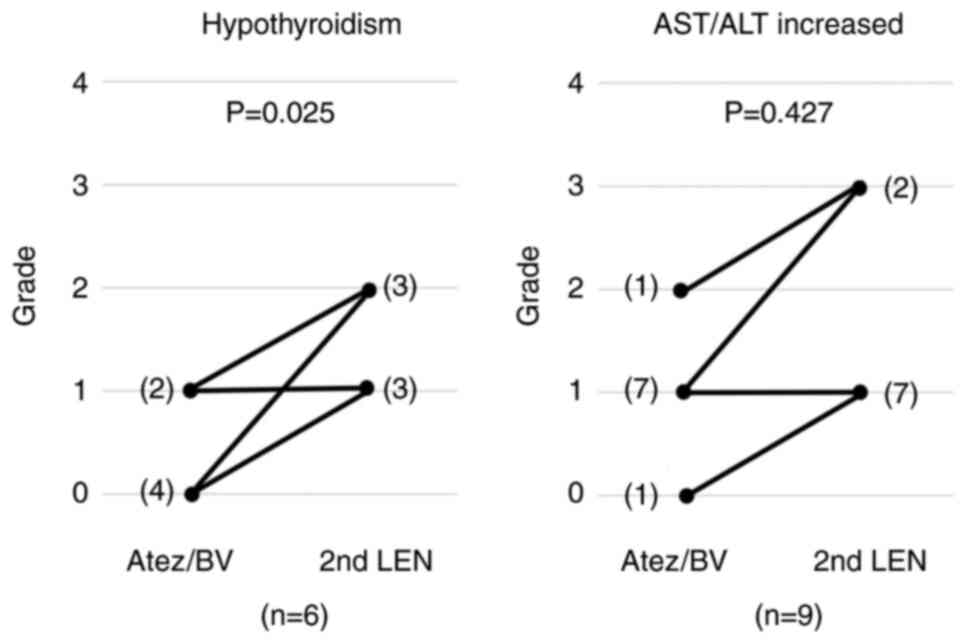
In patients with hypothyroidism or elevated AST/ALT
levels with second-line LEN, changes in the grade of AEs from
first-line treatment to second-line treatment are shown in Fig. 3. Grade 1 (three patients) and grade
2 (three patients) hypothyroidism was observed with the 2nd LEN.
All six patients with hypothyroidism receiving 2nd LEN were women.
In these six patients, during the first-line Atez/BV treatment, the
AE grade was 0 in four patients and grade 1 in two patients. There
was a significant increase in the hypothyroidism grade from Atez/BV
as the first-line treatment to 2nd LEN (P=0.025). Grade 1 (seven
patients) and grade 3 (two patients) elevated AST/ALT levels were
observed in the 2nd LEN. For these nine patients, when receiving
Atez/BV as the first-line treatment the AE grade was 0 in one
patient, 1 in seven, and 2 in one. The change in the AE grade for
increased AST/ALT levels was not significantly different between
first-line Atez/BV and 2nd LEN treatment (P=0.427).
Discussion
In this study, the safety of using LEN as
second-line treatment was clarified by comparing the AEs of the 2nd
LEN administered after the first-line Atez/BV treatment, with the
AEs of 1st LEN in patients with unresectable liver cancer. While
2nd LEN may be as effective as 1st LEN, the incidence of
hypothyroidism was higher with 2nd LEN compared to 1st LEN.
In this study, when comparing 1st LEN and 2nd LEN,
elevated AST/ALT levels and hypothyroidism were more common in the
2nd LEN group. The incidences of hypothyroidism were 12.8% in the
1st LEN and 46.2% in the 2nd LEN groups. Kudo et al reported
that the incidence of hypothyroidism when LEN was used as
first-line treatment was 16% (6),
while Ogushi et al reported that it was 25.4% (7). Thus, for 1st LEN, the incidence rate
in this study was similar to or lower than that previously
reported.
Lee et al reported the risk of developing
hypothyroidism using ICIs (15).
The incidence of hypothyroidism in patients who received both an
angiogenesis inhibitor (AI) and an ICI was 4.4 times higher than
that in patients treated with ICI alone. In other words, they
showed a synergistic effect in patients who received multiple
doses, which may be associated with thyroid dysfunction. Therefore,
when using agents such as AIs in conjunction with ICI treatment,
special attention should be paid to treatment-related side effects
(15). The timing of onset of
adverse immune events caused by ICIs is highly variable (1). Tyrosine kinase inhibitors such as LEN
have been reported to cause thyroid dysfunction by acting on the
hypothalamic-pituitary-thyroid system (16,17).
Therefore, it is predicted that after Atez/BV, LEN will also be
expected to exacerbate the incidence of hypothyroidism in patients
treated with drugs that are potentially associated with thyroid
dysfunction.
In this study, when Atez/BV was used as the 1st-line
treatment and LEN as the 2nd-line treatment, the incidence of
hypothyroidism in 2nd LEN was 46.2%. According to Finn et
al, the incidence of hypothyroidism when Atez/BV was used as
the first-line treatment was 10.6% (1). Although not shown in the results, the
rate in the present study was 11.1%, which is similar. In other
words, in this study, the incidence of hypothyroidism was not high,
even when Atez/BV was used as first-line treatment. Furthermore,
the incidence during 1st LEN was 12.8 and 46.2% with 2nd LEN, which
was high. In contrast, Muto et al (18) reported that when Atez/BV was used as
the 1st-line treatment and LEN was used as the 2nd-line treatment,
the incidence of hypothyroidism in patients receiving 2nd LEN was
15%. There is a clear difference to the results of this study.
However, it should be noted that the number of patients in both
studies was small (Muto et al n=20, the current study n=13).
Furthermore, in this study, there were differences in sex between
patients in the 1st LEN and 2nd LEN groups. In this study, all six
patients with hypothyroidism receiving 2nd LEN were women.
Hypothyroidism is more common in women than men. Therefore, women
receiving treatment with 2nd LEN should be closely monitored for
hypothyroidism.
In this study, four of the six patients (66.7%) who
had hypothyroidism with 2nd LEN did not have hypothyroidism during
the Atez/BV first-line treatment. In addition, one of the two
patients with grade 1 hypothyroidism during first-line Atez/BV
treatment showed deterioration to grade 2. Thus, hypothyroidism
increased with 2nd LEN after first-line Atez/BV treatment. These
results suggest that tyrosine kinase inhibitors may cause thyroid
dysfunction when the hypothalamus-pituitary-thyroid system is
damaged by the synergistic effect from the combined use of
angiogenesis inhibitors and ICIs.
The incidence of increased AST/ALT levels was 46.2%
with 1st LEN and 76.9% with 2nd LEN. In contrast, eight of the nine
patients (88.9%) who had an increase in AST/ALT during 2nd LEN
already had an increase in AST/ALT during the first-line Atez/BV
treatment. In addition, 3/9 patients (33.3%) showed worsening from
the first-line treatment or a new increase in AST/ALT with the 2nd
LEN. Thus, no significant increase in AST/ALT levels was observed
from first-line Atez/BV treatment to 2nd LEN. Of the 13 patients
receiving 2nd LEN, 7 out of 12 patients (58.3%) who discontinued
treatment did so due to decreased performance status (results not
shown). Therefore, the reason AST/ALT increased more with 2nd LEN
than 1st LEN was considered to be the worsening of the patient's
condition.
In this study, the treatment duration was similar
for both 1st LEN and 2nd LEN, and the RDI was also statistically
similar. Therefore, the onset of side effects was not affected by
the administration period or RDI.
The limitations of this study include the limited
sample size and retrospective nature of the study, which was
conducted at a single institution. High hypothyroidism during LEN
use after Atez/BV treatment may be due to immune-related factors or
anti-VEGF therapy. The onset timing and risk factors for
immune-related AEs are not clear, and cases have been reported
where they occurred several months after the end of treatment
(19). The median (range) first
onset of hypothyroidism has been reported as 93.5 (13–419) days, with considerable variation
(1,20). Furthermore, it has also been
reported that patients with HCC may have a higher incidence of
thyroid dysfunction when using both LEN and Atez/BV than those
treated with just one of the drugs (21). However, due to the small sample
size, a final conclusion could not be drawn. Hypothyroidism may
also be influenced by factors other than treatment. Future studies
should address these limitations. In particular, it is necessary to
examine sex-based differences in incidence and severity.
Additionally, durvalumab plus tremelimumab combination therapy and
durvalumab monotherapy have been approved in Japan for the
treatment of unresectable HCC. It is worth investigating whether
similar results will be found when using these ICIs.
In clinical practice, patients receiving LEN as
second-line therapy after treatment with Atez/BV are at increased
risk of hypothyroidism. However, further research is required to
confirm this hypothesis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MK contributed to the study design, collected and
provided the data, was the principal author of the report, and is
the guarantor of this article. MK and MG confirm the authenticity
of all the raw data. ShY, MG, SaY, HT and EU contributed to the
study design, reviewed the manuscript, and supervised the drafting
of the report and submission process. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of the Ogaki Municipal Hospital (Ogaki, Japan;
approval number 20220728-17-h). The requirement for informed
consent was waived because of the retrospective study design.
Patient consent for publication
Written informed consent for publication was
obtained from each patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
1st LEN
|
first-line LEN treatment
|
|
2nd LEN
|
second-line LEN treatment
|
|
AE
|
adverse event
|
|
AST/ALT
|
aspartate aminotransferase/alanine
aminotransferase
|
|
Atez/BV
|
atezolizumab plus bevacizumab
|
|
CI
|
confidence interval
|
|
CP-A
|
Child-Pugh A
|
|
FGF
|
fibroblast growth factor
|
|
HCC
|
hepatocellular carcinoma
|
|
ICI
|
immune checkpoint inhibitor
|
|
LEN
|
lenvatinib
|
|
PD-L1
|
programmed death-ligand 1
|
|
RDI
|
relative dose intensity
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 14:1894–1905. 2020. View Article : Google Scholar
|
|
2
|
Sonbol MB, Riaz IB, Naqvi SAA, Almquist
DR, Mina S, Almasri J, Shah S, Almader-Douglas D, Uson Junior PLS,
Mahipal A, et al: Systemic therapy and sequencing options in
advanced hepatocellular carcinoma: A systematic review and network
meta-analysis. JAMA Oncol. 6:e2049302020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeda S, Namisaki T, Tsuji Y, Fujimoto Y,
Murata K, Enomoto M, Fujinaga Y, Nishimura N, Kitagawa K, Takaya H,
et al: Initial experience with atezolizumab plus bevacizumab for
unresectable hepatocellular carcinoma: A real-world retrospective
study. Anticancer Res. 42:5465–5473. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobayashi K, Nagai H, Matsui T, Matsuda T
and Higai K: Importance of Atezolizumab plus bevacizumab
combination treatment as first-line therapy for immunological
changes in patients with unresectable hepatocellular carcinoma.
Anticancer Res. 43:4601–4609. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 24:1163–1173. 2018. View Article : Google Scholar
|
|
7
|
Ogushi K, Chuma M, Uojima H, Hidaka H,
Numata K, Kobayashi S, Hirose S, Hattori N, Fujikawa T, Nakazawa T,
et al: Safety and Efficacy of lenvatinib treatment in Child-Pugh A
and B patients with unresectable hepatocellular carcinoma in
clinical practice: A multicenter analysis. Clin Exp Gastroenterol.
13:385–396. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwamoto H, Suzuki H, Shimose S, Niizeki T,
Nakano M, Shirono T, Okamura S, Noda Y, Kamachi N, Nakamura T, et
al: weekends-off lenvatinib for unresectable hepatocellular
carcinoma improves therapeutic response and tolerability toward
adverse events. Cancers (Basel). 12:10102020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoo C, Kim JH, Ryu MH, Park SR, Lee D, Kim
KM, Shim JH, Lim YS, Lee HC, Lee J, et al: Clinical outcomes with
multikinase inhibitors after progression on first-line atezolizumab
plus bevacizumab in patients with advanced hepatocellular
carcinoma: A multinational multicenter retrospective study. Liver
Cancer. 10:107–114. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aoki T, Kudo M, Ueshima K, Morita M,
Chishina H, Takita M, Hagiwara S, Ida H, Minami Y, Tsurusaki M and
Nishida N: Exploratory analysis of lenvatinib therapy in patients
with unresectable hepatocellular carcinoma who have failed prior
PD-1/PD-L1 checkpoint blockade. Cancers (Basel). 12:30482020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiraoka A, Kumada T, Tada T, Hirooka M,
Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E,
Fukunishi S, et al: Lenvatinib as second-line treatment after
atezolizumab plus bevacizumab for unresectable hepatocellular
carcinoma: Clinical results show importance of hepatic reserve
function. Oncology. 101:624–633. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Cancer Institute, . US Department
Of Health And Human Services: Common terminology criteria for
adverse events (CTCAE). Version 5.0, 2017. https://ctepCancer. Gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf
|
|
13
|
Tamai T, Hayato S, Hojo S, Suzuki T,
Okusaka T, Ikeda K and Kumada H: Dose finding of lenvatinib in
subjects with advanced hepatocellular carcinoma based on population
pharmacokinetic and exposure-response analyses. J Clin Pharmacol.
57:1138–1147. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SE, Kim KA, Lee H and Park J: Risk of
developing hypothyroidism with the use of tyrosine kinase
inhibitors and immune checkpoint inhibitors. Cancer Epidemiol.
81:1022652022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Makita N and Iiri T: Tyrosine kinase
inhibitor-induced thyroid disorders: A review and hypothesis.
Thyroid. 23:151–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmadieh H and Salti I: Tyrosine kinase
inhibitors induced thyroid dysfunction: A review of its incidence,
pathophysiology, clinical relevance, and treatment. Biomed Res Int.
2013:7254102013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muto H, Kuzuya T, Kawabe N, Ohno E,
Funasaka K, Nagasaka M, Nakagawa Y, Miyahara R, Shibata T,
Hashimoto S, et al: Clinical outcomes with lenvatinib in patients
previously treated with atezolizumab/bevacizumab for advanced
hepatocellular carcinoma. Anticancer Res. 43:4673–4682. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Otsubo K, Nakatomi K, Furukawa R, Ashida
K, Yoneshima Y, Nakanishi Y and Okamoto I: Two cases of late-onset
secondary adrenal insufficiency after discontinuation of nivolumab.
Ann Oncol. 28:3106–3107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muir CA, Clifton-Bligh RJ, Long GV,
Scolyer RA, Lo SN, Carlino MS, Tsang VHM and Menzies AM: Thyroid
immune-related adverse events following immune checkpoint inhibitor
treatment. J Clin Endocrinol Metab. 18:e3704–e3713. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ichimura T, Ichikura D, Hinata M, Hida N
and Baba T: Thyroid dysfunction with atezolizumab plus bevacizumab
after Lenvatinib in hepatocellular carcinoma: A case series. SAGE
Open Med Case Rep. 11:2050313X2311644882023.PubMed/NCBI
|