Introduction
von Willebrand factor (VWF) is a large, complex
glycoprotein, predominantly synthesized in endothelial cells (ECs)
and megakaryocytes (1,2). VWF is released via synthetic pathways
or regulatory mechanisms associated with secretory storage and
subsequent discharge (3,4). Although platelets also release VWF,
plasma VWF mostly originates from ECs (5). A considerable quantity of VWF within
ECs is compartmentalized in Weibel-Palade bodies, from which it is
released into the vascular lumen in response to a range of stimuli
(6,7). Once in the bloodstream, the primary
function of VWF is to facilitate hemostasis. This is primarily
accomplished by its strong interaction with platelet receptor
glycoprotein Ib (GPIb) and various constituents of the
subendothelial connective tissue (8,9).
Furthermore, VWF binds to another clotting protein, factor VIII,
and serves as its carrier in the blood circulation (10). Previous studies have demonstrated
that VWF is a pivotal regulator in multiple biological processes.
Specifically, VWF has been identified to contribute to the
modulation of angiogenesis (11),
inflammatory responses (12), cell
proliferation dynamics (13) and
apoptotic mechanisms (14).
The EC monolayer serves a critical function as a
regulatory gateway for the ingress and egress of metastatic tumor
cells. Disseminated tumor cells secrete an array of factors that
directly instigate the activation of ECs, which is defined by the
upregulation of distinct adhesion receptors and a concurrent
increase in vascular permeability, thereby facilitating the
transendothelial migration of tumor cells (15–19).
In a study conducted by Bauer et al (20), malignant melanoma cells were
demonstrated to induce EC activation, a phenomenon validated in
controlled in vitro environments and within living
organisms. The initiation of EC activation culminates in the
increased secretion of VWF and the subsequent formation of
ultra-large VWF multimers on the surface of the ECs (20). Previous studies have consistently
indicated that VWF enhances the attachment of melanoma and colon
cancer cells to the endothelium under conditions of shear stress.
This process is critical in promoting the metastasis of these tumor
types (21–23).
Cancer is a growing burden on global health systems
(24). It was forecast that in 2023
there would be ~1.96 million new cancer cases and 610,000
cancer-associated fatalities in the United States (25). Furthermore, it has been predicted
that by 2040 there will be ~28.4 million new cancer cases
worldwide, representing a 47% increase compared with the number of
cases reported in 2020 (26).
Evidence suggests that notable increases in VWF plasma levels occur
in patients with various types of tumors (27,28).
Wang et al (29) observed a
significant increase in the VWF plasma levels of patients with
colorectal cancer compared with healthy individuals. Moreover,
another study of colorectal cancer indicated a direct association
between heightened plasma VWF levels and tumor progression to
advanced stages, as well as the presence of metastases (30). Notably, patients with colorectal
cancer whose VWF levels were low exhibited a significantly extended
survival time compared with those whose VWF levels were high
(29). In addition, Yang et
al (31) found that vascular
endothelial growth factor derived from cancer cells promotes the
metastasis of gastric adenocarcinoma. Research has also shown that
VWF facilitates the adhesion between tumor cells and ECs, and
assists in the recruitment of platelets to the tumor
microenvironment (32). This leads
to the formation of tumor-platelet aggregates, promoting the
hematogenous dissemination of cancer.
However, findings contradictory to the
aforementioned results have also been reported. Meschengieser et
al (33) observed that patients
with myeloproliferative tumors exhibited lower VWF levels compared
with healthy individuals. In addition, Von Willebrand disease (VWD)
is a condition typically caused by mutations in the VWF gene, which
lead to reduced quantities or abnormal quality of VWF in the plasma
(34). Franchini et al
(34) analyzed the VWF levels in
patients with VWD who also had various types of cancer, including
liver cancer, breast cancer, non-Hodgkin lymphoma and acute myeloid
leukemia, and identified no statistically significant differences
in the data when comparing patients with metastatic cancer to those
without.
The aim of the present meta-analysis was to assess
whether VWF is consistently elevated in patients with cancer,
determine its association with cancer metastasis and thereby
evaluate its potential as an effective cancer biomarker.
Materials and methods
Search strategy
A systematic examination of the literature was
undertaken, encompassing various databases including The Cochrane
Library (https://www.cochranelibrary.com/library), PubMed
(https://www.ncbi.nlm.nih.gov/pubmed),
Web of Science (https://www.webofscience.com), China National
Knowledge Infrastructure (https://www.cnki.net) and Wanfang Data (http://www.wanfangdata.com), to ensure a thorough
analysis. The study included case-control investigations published
from database inception until March 3, 2023, which presented
findings regarding plasma VWF concentrations in patients with
cancer compared with individuals without the condition. These
studies were systematically identified and evaluated for inclusion.
Only studies published in Chinese or English were considered for
inclusion in the present study. Both free text and (Mesh) keywords
were utilized, including: ‘von Willebrand Factor’, ‘von Willebrand
protein’, ‘VWF’, ‘neoplasm’, ‘tumor’, ‘cancer’, ‘cancerization’,
‘cysts’, ‘cancerous’ and ‘neurofibromas’. To identify additional
potentially relevant research, the citation lists of notable
reviews and studies were manually searched.
Study selection
The titles, summaries and whole texts of the chosen
studies were checked by two independent reviewers. If authors had
published multiple works using the same sample data from the same
institution, only the most recent or most comprehensive work was
included. The inclusion criteria were as follows: i) All patients
with cancer were diagnosed using the gold standard test of
histological examination; and ii) case-control studies that
included patients with and without cancer. Case reports, reviews,
abstracts from conferences, letters and comments were excluded, as
were studies using cells or animals, studies without access to
data, duplicate papers and studies using healthy volunteers as
controls.
Data extraction
Using a standardized form, two reviewers
independently retrieved data from the included studies. Several key
details from each study were systematically collected, including
the surname of the first author, year of publication, demographics
and geographical location of the study population, and ethnicities
of the participants. In addition, the mean plus standard mean
difference (SMD) or standard error of the mean of plasma VWF
concentrations were recorded, along with the units used for VWF
measurements. Any disparities between the two reviewers were
addressed through discussion or, when deemed essential, by
soliciting the perspective of a third reviewer.
Quality assessment
The methodological quality of each included
non-randomized and observational study was independently assessed
by two reviewers using the Newcastle-Ottawa Scale (NOS). This scale
was used to evaluate various aspects of the design and execution of
each study. Studies achieving a score of ≥7 were classified as high
quality, those with a score of 6 were deemed to be medium quality,
whereas those with a score of ≤5 were deemed low quality.
Statistical analysis
RevMan software (version 5.4; The Cochrane
Collaboration; http://community.cochrane.org/) was utilized to
calculate a pooled SMD and 95% confidence interval (CI) using a
random-effects model. This approach was chosen as the included
studies used a variety of measurement units. By employing SMD and
95% CI, the results were standardized across different units, such
as %, IU/Dl, IU/l and IU/ml, facilitating a more coherent and
meaningful comparison of the pooled effects. P<0.05 was
considered to indicate a statistically significant result. Using
the inverse-variance approach, the studies were weighted, with
higher weights assigned to studies with larger sample sizes.
Ethnicity-specific subgroup analyses were also performed. A Begg's
funnel plot was constructed to compare and assess publication bias
among the studies.
Results
Study characteristics
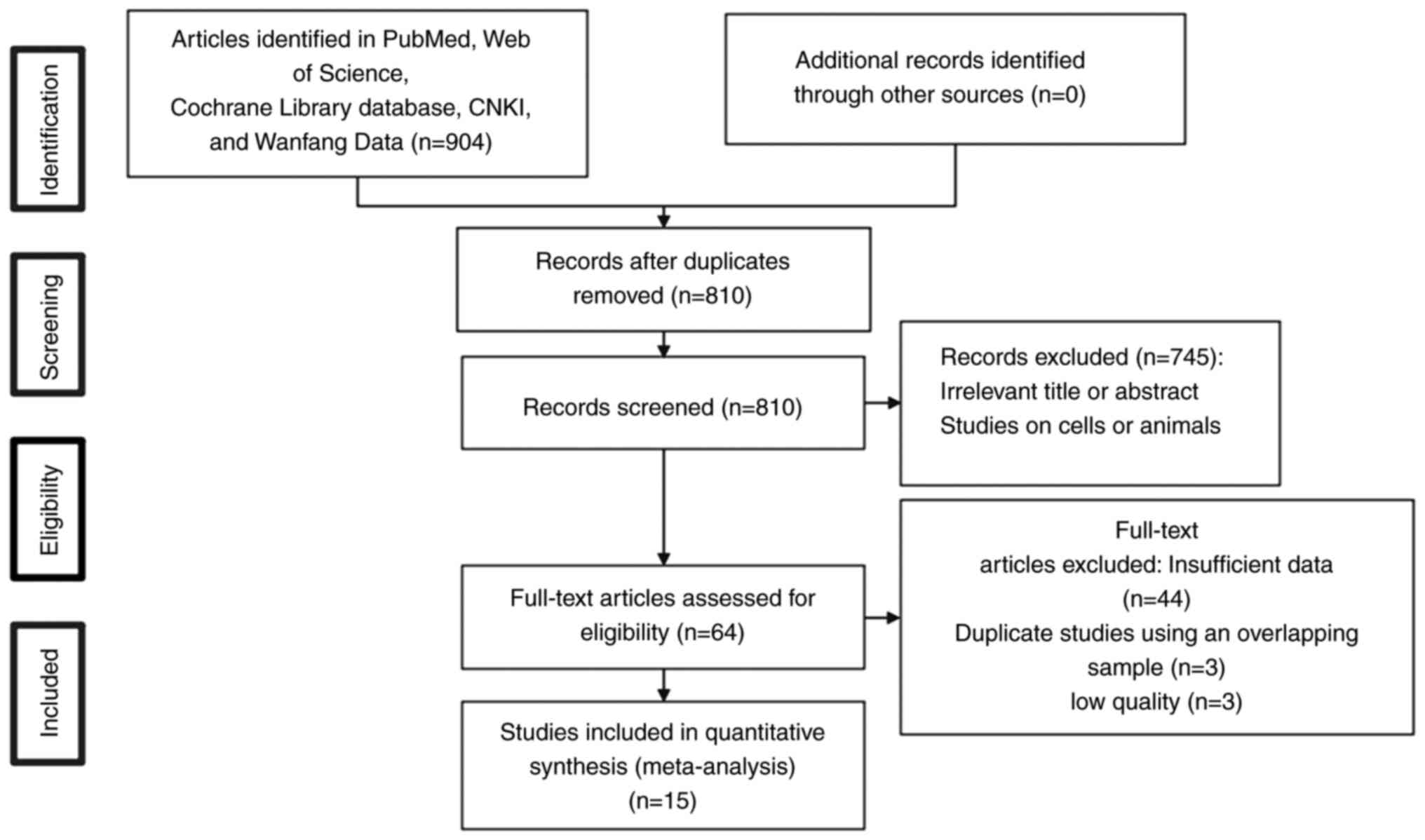
Fig. 1 shows the
study selection procedure. A total of 15 studies encompassing 1,403
individuals were included in the present meta-analysis (27–30,33,35–44).
Table I provides a summary of the
characteristics of the studies, all of which were published between
1987 and 2022. Of the included studies, four focused on breast
cancer (27,41,42,44),
three on colorectal cancer (29,30,37),
two on prostate cancer (28,35),
two on mixed cancer (39,40), two on leukemia (acute lymphoblastic
leukemia and chronic myeloid leukemia) (33,36),
one on non-small cell lung cancer (38) and one on bladder cancer (43). All included studies that assessed
VWF expression in cancer tissues were deemed to be of high quality
based to their NOS scores (vide infra).
 | Table I.Characteristics of the studies
included in the meta-analysis. |
Table I.
Characteristics of the studies
included in the meta-analysis.
| First author,
year | Groups | Ethnicity | Cancer type | Sample size | Mean VWF | (Refs.) |
|---|
| Ablin et al,
1988 | Healthy/cancer | Non-Chinese | Prostate | 8/18 | 1.36±0.61/4.33±2.34
IU/ml | (35) |
| Athale et
al, 2010 | Healthy/cancer | Non-Chinese | Acute
lympho-blastic leukemia | 13/17 | 1.14±0.48/1.89±0.61
IU/ml | (36) |
| Blann et al,
2001 | Healthy/cancer | Non-Chinese | Breast | 41/41 | 99±20/121±29
IU/dl | (27) |
| Blann et al,
2011 | Healthy/cancer | Non-Chinese | Prostate | 27/31 | 118±26/137±20
IU/dl | (28) |
| Damin et al,
2002 | Healthy/cancer
metastatic | Non-Chinese | Colorectal | 87/75/16 |
150.2±58.1/230.6±96/276±117.2 IU/dl | (37) |
| Dhami et al,
2022 | Healthy/cancer | Non-Chinese | Breast | 11/44 | 89.1±8.8/217±13
IU/dl | (44) |
| Gil-Bazo et
al, 2005 |
Healthy/cancer/metastatic | Non-Chinese | Colorectal | 20/14/12 |
98.2±46.2/102.8±40.7/190±85.3 IU/dl | (30) |
| Guo et al,
2018 |
Healthy/cancer/metastatic | Chinese | Non-small cell
lung | 102/119/64 |
1,019.9±789.4/1,583.5±787.7/1,812.3±675.5
IU/l | (38) |
| Mannucci et
al, 2003 |
Healthy/cancer/metastatic | Non-Chinese | Mixed | 49/29/20 |
114±37/170±103/266±177% | (39) |
| Meschengieser et
al, 1987 | Healthy/cancer | Non-Chinese | Chronic myeloid
leukemia | 11/14 | 0.28±0.11/0.23±0.12
U/l | (33) |
| Pépin et al,
2016 | Healthy/cancer | Non-Chinese | Mixed | 140/20 | 242±158/326±158
IU/ml | (40) |
| Röhsig et
al, 2001 |
Healthy/cancer/metastatic | Non-Chinese | Breast | 27/128/15 |
130.6±45/170.7±78/170.7±78 IU/dl | (41) |
| Wang et al,
2005 |
Healthy/cancer/metastatic | Chinese | Colorectal | 22/40/86 |
10.1±27/241.3±68.2/266.1±91.3% | (29) |
| Yigit et al,
2008 |
Healthy/cancer/metastatic | Non-Chinese | Breast | 100/100/65 |
78.19±43.69/99.49±47.27/105.09±48.02% | (42) |
| Ziętek et
al, 1996 |
Healthy/cancer/metastatic | Non-Chinese | Bladder
carcinoma | 35/20/31 |
98±42/106±51/194±41% | (43) |
Association between the VWF expression
level in cancer and health
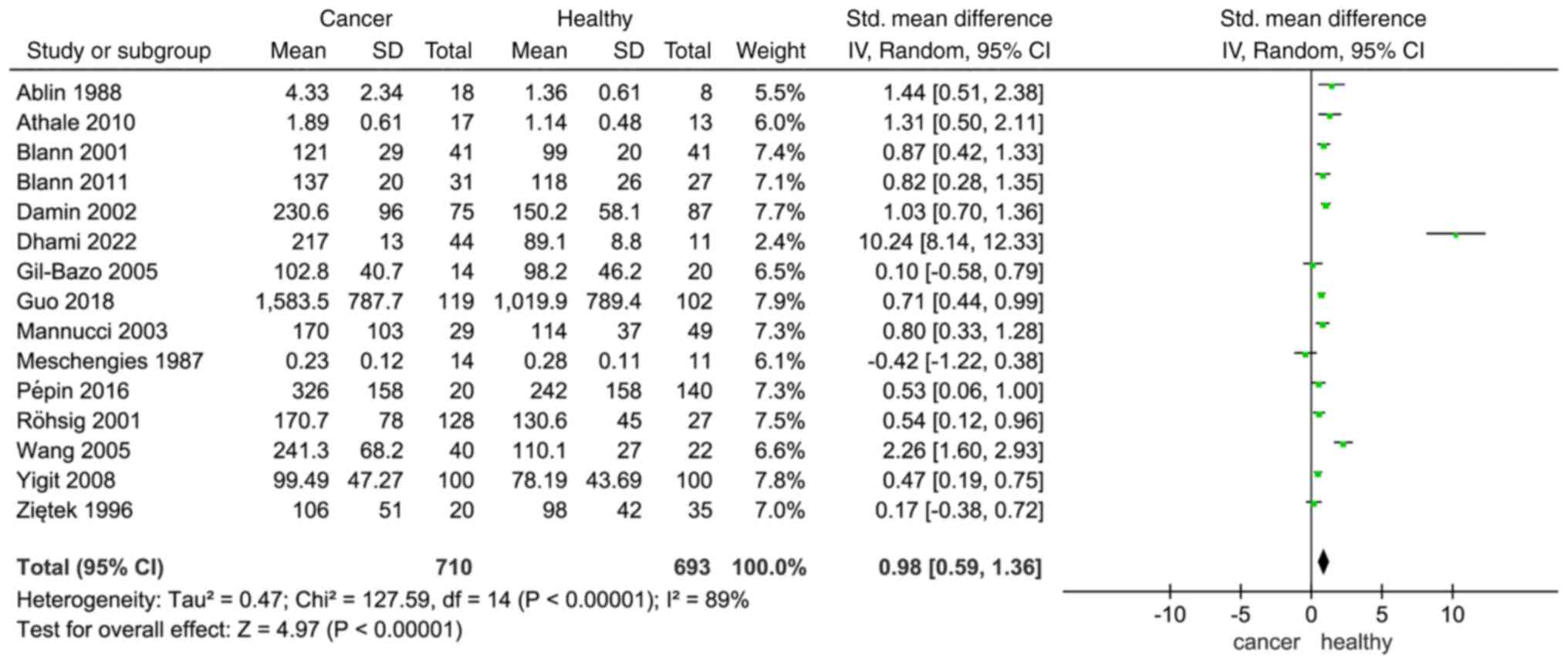
A cumulative meta-analysis was conducted on the
selected studies to understand how VWF affects patients with cancer
and healthy individuals. The results indicated a differential
expression of VWF between individuals diagnosed with cancer and
their healthy counterparts, based on an analysis of all 15 studies
and 1,403 participants (SMD, 0.98; 95% CI, 0.59–1.36; Fig. 2). Since significant heterogeneity
was detected (I2=89%; P<0.00001), the random-effects
model was used. The expression of VWF in different types of tumors
may be the reason for this heterogeneity.
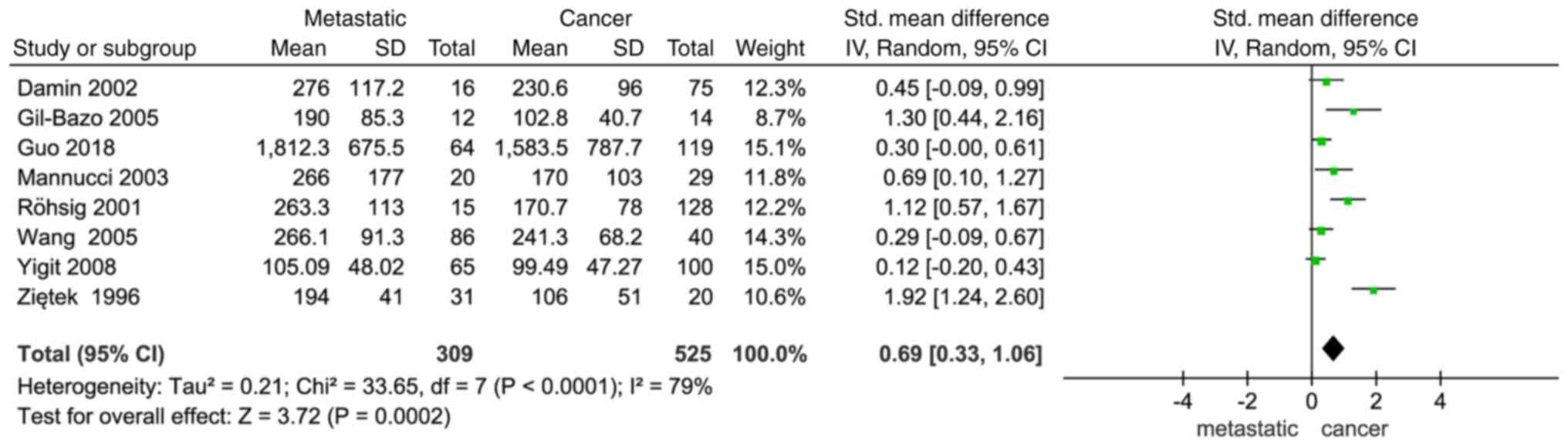
Association between the VWF expression
level and metastasis
VWF expression and metastasis were found to exhibit
a significant association (SMD, 0.69; 95%CI, 0.33–1.06; P=0.0002;
Fig. 3). Additionally, the results
revealed a notable difference in the occurrence of metastatic
cancer between the two groups, with a higher VWF expression level
in patients with cancer indicating a higher risk of developing
metastatic disease.
Sensitivity analysis
A sensitivity analysis was conducted by sequentially
excluding each study to assess its impact on the overall results.
Across all studies, no significant impact was observed on the
pooled outcomes, underscoring the strength and reliability of the
results of the meta-analysis.
Quality evaluation
The quality of the included studies was assessed
using the NOS and the results revealed that 13 studies were of high
quality and 2 were of medium quality (Table II) (27–30,33,35–44).
The average rating assigned to the 15 studies was 7.4. The quality
of a further three studies was low (score of 5), so they were
eliminated from the meta-analysis (18,45,46).
 | Table II.Quality assessment of the included
studies based on Newcastle-Ottawa Scale scores. |
Table II.
Quality assessment of the included
studies based on Newcastle-Ottawa Scale scores.
|
| Scores |
|
|---|
|
|
|
|
|---|
| First author,
year | Section | Comparability | Exposure | Total | (Refs.) |
|---|
| Ablin et al,
1988 | 3 | 2 | 3 | 8 | (35) |
| Athale et
al, 2010 | 3 | 2 | 3 | 8 | (36) |
| Blann et al,
2001 | 4 | 1 | 2 | 7 | (27) |
| Blann et al,
2011 | 3 | 2 | 3 | 8 | (28) |
| Damin et al,
2002 | 2 | 1 | 3 | 6 | (37) |
| Dhami 2022 | 3 | 3 | 3 | 9 | (44) |
| Gil-Bazo et
al, 2005 | 4 | 2 | 3 | 9 | (30) |
| Guo et al,
2018 | 4 | 1 | 2 | 7 | (38) |
| Mannucci et
al, 2003 | 3 | 1 | 3 | 7 | (39) |
| Meschengieser et
al, 1987 | 3 | 2 | 2 | 7 | (33) |
| Pépin et al,
2016 | 2 | 1 | 3 | 6 | (40) |
| Röhsig et
al, 2001 | 3 | 1 | 3 | 7 | (41) |
| Wang et al,
2005 | 3 | 2 | 3 | 8 | (29) |
| Yigit et al,
2008 | 3 | 1 | 3 | 7 | (42) |
| Ziętek et
al, 1996 | 2 | 2 | 3 | 7 | (43) |
| John et al,
2020 | 2 | 1 | 2 | 5 | (18) |
| Knöfler et
al, 2020 | 2 | 1 | 2 | 5 | (46) |
| Lehrer et
al, 2019 | 1 | 1 | 3 | 5 | (45) |
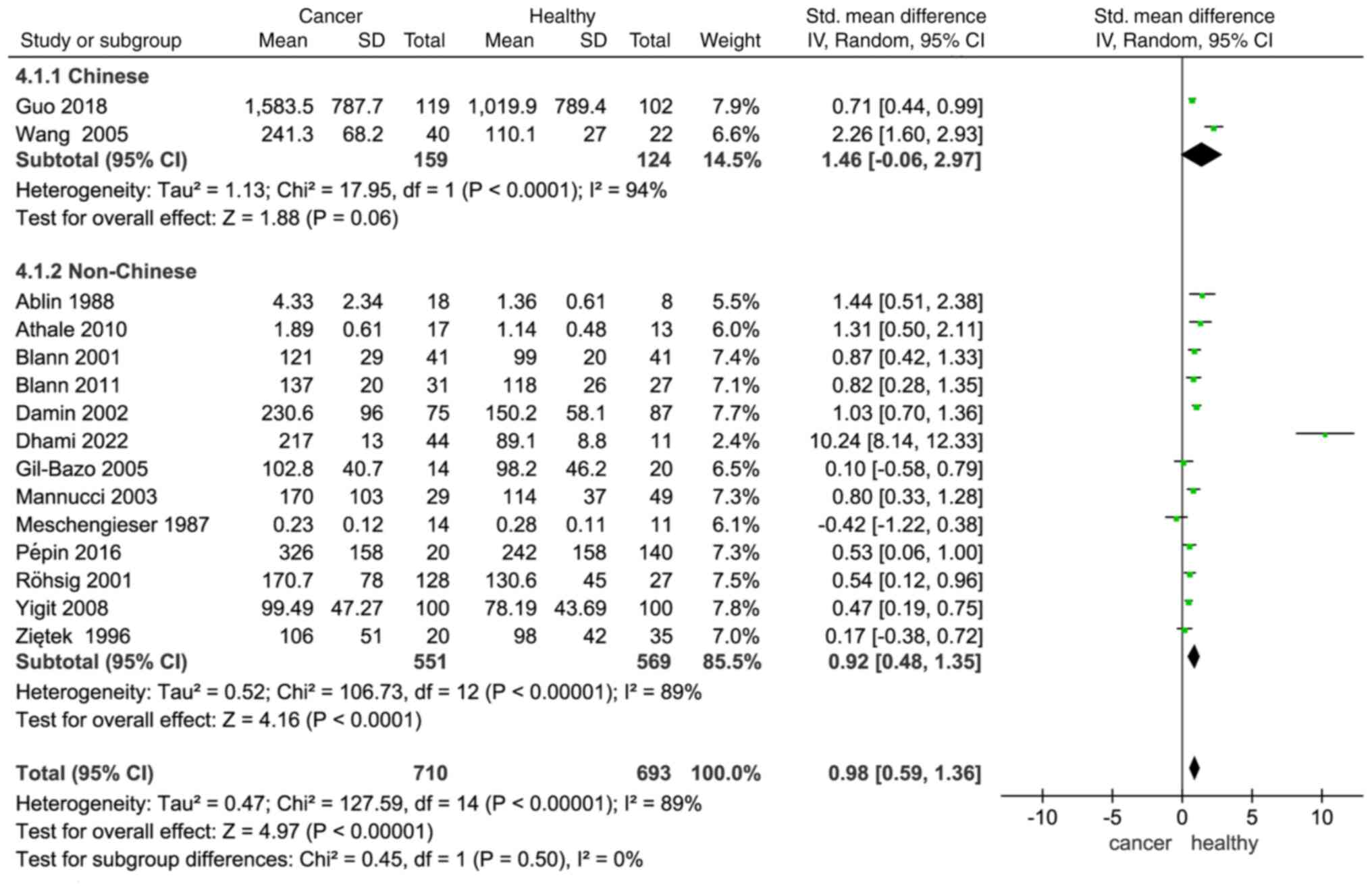
Subgroup analysis
In a study by Conlan et al (47), VWF levels were found to be higher in
black individuals than in white individuals, indicating that there
are ethnic differences in VWF levels. In a subgroup analysis
categorized by ethnicity (Chinese vs. non-Chinese), plasma VWF
levels were higher in patients with cancer compared with healthy
individuals in both subgroups (Chinese: SMD, 1.46; −0.06–2.97;
non-Chinese: SMD, 0.92; 1.48–0.35; Fig.
4).
Publication bias
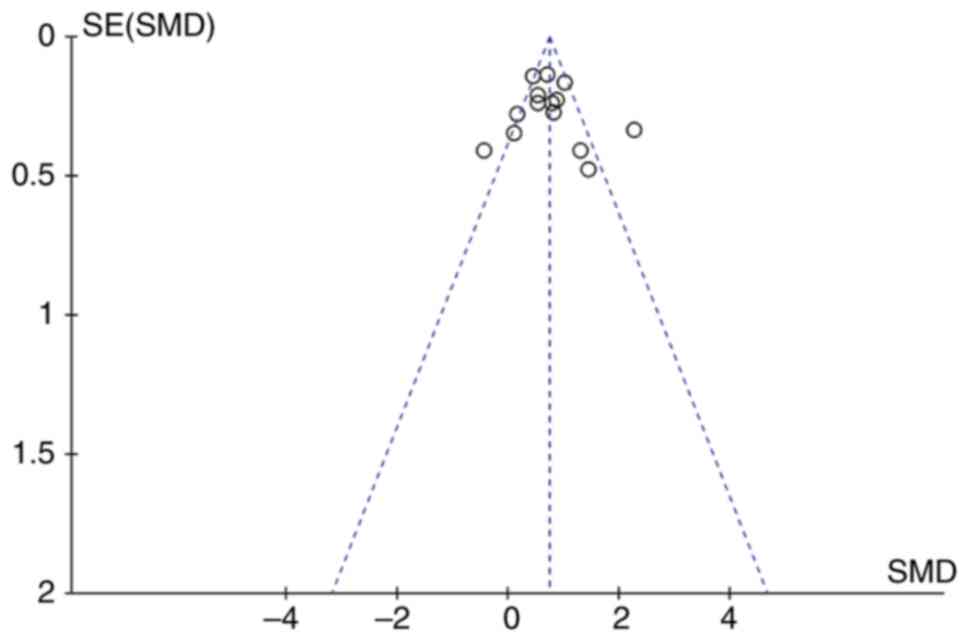
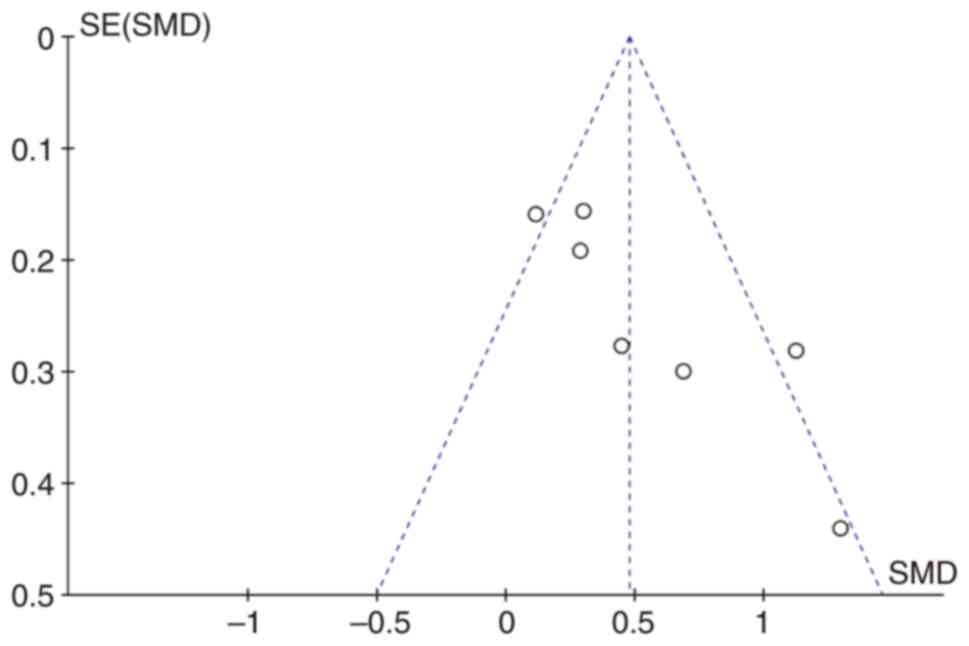
Begg's funnel plots were constructed to evaluate
publication bias. The almost symmetrical funnel plots showed no
significant evidence of asymmetry for VWF in patients with cancer
and healthy individuals (Fig. 5) or
with metastatic and non-metastatic cancer (Fig. 6).
Discussion
The present meta-analysis incorporated data from 15
studies, comprising a combined sample of comprising 710 patients
with cancer and 693 healthy controls. The comprehensive analysis of
SMD revealed that the plasma VWF levels of patients with cancer
were significantly higher than those of healthy controls.
Furthermore, patients with metastatic cancer displayed notably
elevated levels of VWF compared with those with non-metastatic
cancer. The results of the sensitivity analysis underscored the
reliability of the combined findings. In summary, the results of
the present study suggest that plasma VWF levels are a reliable
indicator of a patient's predisposition to cancer development.
The clinical studies that were selected cover
different types of cancer, each with its own distinct behaviors and
characteristics. There may be concerns about including different
types of cancer in a single meta-analysis. Including different
types of cancer in a single meta-analysis may affect the final
accuracy of the results. However, the decision to include multiple
cancer types was based on the objective of exploring and
identifying common biomarkers or therapeutic responses that might
transcend specific cancer typologies. By analyzing a broader
spectrum of cancers, the aim was to provide insights that could
potentially apply to multiple forms of the disease, which may be
particularly valuable for the development of generalized
therapeutic strategies or diagnostic tools. Additionally, all
analyses were carefully adjusted for cancer type as a covariate to
mitigate heterogeneity and provide more accurate insights across
different cancer types.
There has been a growing understanding of the
association between angiogenesis and the hemostasis cascade, and
their roles in the progression and spread of tumors within the
bloodstream of patients afflicted by various types of cancer
(48–50). Numerous patients with cancer exhibit
imbalances in coagulation and fibrinolysis systems, often
manifested as dysfunctions in ECs and platelets (51,52).
VWF is a marker specific to ECs and an indicator of endothelial
dysfunction (53,54). In addition, increased expression
levels of VWF have been detected in the lung adenocarcinoma tissues
of patients with cancer (55). VWF
is considered to mediate the binding of tumor cells to platelets,
thereby facilitating their systemic dissemination (30). Previous studies have demonstrated
that VWF can act as a diagnostic biomarker in a multitude of
disease contexts (56–58). The results of the present study
validated the association between plasma VWF levels and cancer,
implying the possible diagnostic and prognostic significance of VWF
in the context of cancer. Additionally, in the four studies of
patients with breast cancer, it was unanimously observed that the
plasma VWF levels in patients with cancer were higher compared with
those in the healthy control group (27,41,42,44).
In comparison with other studies, the research by Dhami et
al (44) in 2022 appears as an
outlier in the meta-analysis. The subjects included in that study
were patients with metastatic breast cancer. Considering that VWF
may be a potential risk factor for tumor metasatsis, the study may
be an outlier as a result of the patients having a more severe
illness. The weight of the study is only 2.4%, so it does not
markedly impact the overall results. Furthermore, three of these
studies noted that plasma VWF levels in patients with breast cancer
were significantly higher in the advanced stages of disease
compared with the early stages, and that this was associated with
tumor staging (41,42,44).
However, a study by Blann et al (27) found no significant differences in
the plasma VWF levels among different histological types or stages
of breast cancer. In the studies examining patients with colorectal
cancer, plasma VWF levels were significantly higher in the patients
with cancer than in the healthy individuals, and VWF was indicated
to promote the distant metastasis of colorectal cancer (29,30,37).
The primary function of VWF is to initiate the blood
clotting process by enabling platelets to adhere to damaged blood
vessel walls in response to vascular injuries. VWF also serves as a
transporter for factor VIII (59).
In a study by Yigit et al (42), an investigation of patients with
breast cancer and healthy individuals demonstrated that the
patients with breast cancer exhibited elevated plasma levels of
factor VIII and VWF compared with the healthy control group. VWF,
secreted by ECs under the influence of thrombin, vasoactive amines
and various cytokines, is an adhesive glycoprotein with the ability
to effectively bind to tumor cells and platelets, potentially
contributing to the formation of microthrombi. VWF also prolongs
tumor cell survival by protecting the cells from immune system
attacks, turbulence and frictional forces (60). The aggregation of platelets and
tumor cells promotes the metastatic process by facilitating the
adhesion of tumor cells and their subsequent migration through
vascular walls (61). In addition,
cadmium, a well-known carcinogen, increases VWF expression and
secretion in ECs (62,63).
Metastasis entails a cascade of events, including
modifications in cellular interactions, the formation of new blood
vessels, degradation of the extracellular matrix, evasion of immune
surveillance and adhesion to the surrounding matrix (64). The interactions of tumor cells with
the sub-endothelial matrix are crucial for metastasis. The tumor
cells release thrombin, which induces the production of VWF in ECs
and thereby promotes tumor cell adhesion (65,66).
The glycoproteins GPIb and GPIIb/IIIa expressed by tumor cells
(67) may facilitate tumor
cell-platelet binding by interaction with plasma VWF, thus
promoting the metastasis process. Furthermore, this interaction
leads to heterotypic cell aggregation, which reduces the
recognition of tumor cells by the immune system and increases their
ability to bind to the lining of blood vessels, such that it
surpasses that of individual tumor cells (68).
In summary, the present meta-analysis involved the
synthesis of data concerning plasma VWF levels in individuals with
and without cancer, with a focus on comparing the observed
variances. Each study included in the meta-analysis underwent
evaluation using the NOS. However, certain limitations should be
acknowledged. First, the number of studies eligible for the
meta-analysis was comparatively limited. Second, the studies
employed varied methodologies and measurement units for the plasma
VWF levels, introducing potential inconsistencies. Third, while
prior research indicates an association between blood type and VWF
levels (55,58), the absence of specific blood type
data in the included studies precluded a detailed subgroup analysis
in this context. Additionally, due to the current research on VWF
being conducted predominantly at the cellular and animal level,
clinical cases concerning the expression of VWF in tumors are
extremely limited.
In conclusion, the results of the present
meta-analysis revealed that individuals with cancer demonstrated
significantly upregulated plasma VWF levels compared with healthy
individuals. Furthermore, plasma VWF levels were significantly
elevated in patients with metastatic cancer compared with patients
with non-metastatic cancer. These findings suggest that VWF may
serve as a promising biomarker for the diagnosis of cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
JL and LQ contributed to the design and
conceptualization of the study. XW, XZ and CZ collected and
analyzed the data. XW wrote the original draft of the text, while
the other contributors provided feedback on earlier drafts. All
authors read and approved the final version of the manuscript. XW,
XZ and CZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
VWF
|
von Willebrand factor
|
|
SMD
|
standard mean difference
|
|
CI
|
confidence interval
|
|
EC
|
endothelial cell
|
References
|
1
|
Sharda AV, Barr AM, Harrison JA, Wilkie
AR, Fang C, Mendez LM, Ghiran IC, Italiano JE and Flaumenhaft R:
VWF maturation and release are controlled by 2 regulators of
Weibel-Palade body biogenesis: exocyst and BLOC-2. Blood.
136:2824–2837. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z and Li W: Formation and function
of Weibel-Palade bodies. Yi Chuan. 31:882–888. 2009.(In Chinese).
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holthenrich A and Gerke V: Regulation of
von-willebrand factor secretion from endothelial cells by the
annexin A2-S100A10 complex. Int J Mol Sci. 19:17522018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sadler JE: von Willebrand factor assembly
and secretion. J Thromb Haemost. 7 (Suppl 1):S24–S27. 2009.
View Article : Google Scholar
|
|
5
|
Kanaji S, Fahs SA, Shi Q, Haberichter SL
and Montgomery RR: Contribution of platelet vs endothelial VWF to
platelet adhesion and hemostasis. J Thromb Haemost. 10:1646–1652.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mourik M and Eikenboom J: Lifecycle of
Weibel-Palade bodies. Hamostaseologie. 37:13–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naß J, Terglane J and Gerke V: Weibel
Palade bodies: Unique secretory organelles of endothelial cells
that control blood vessel homeostasis. Front Cell Dev Biol.
9:8139952021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prasannan N and Scully M: Novel
antiplatelet strategies targeting VWF and GPIb. Platelets.
32:42–46. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pierre-Louis O, Resiere D, Alphonsine C,
Dantin F, Banydeen R, Dubois MD, Mehdaoui H and Neviere R:
Increased binding of von willebrand factor to sub-endothelial
collagen may facilitate thrombotic events complicating bothrops
lanceolatus envenomation in humans. Toxins (Basel). 15:4412023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson AR: Structure and function of the
factor VIII gene and protein. Semin Thromb Hemost. 29:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lenting PJ, Casari C, Christophe OD and
Denis CV: von Willebrand factor: The old, the new and the unknown.
J Thromb Haemost. 10:2428–2437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwameis M, Schörgenhofer C, Assinger A,
Steiner MM and Jilma B: VWF excess and ADAMTS13 deficiency: A
unifying pathomechanism linking inflammation to thrombosis in DIC,
malaria, and TTP. Thromb Haemost. 113:708–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishihara J, Ishihara A, Starke RD,
Peghaire CR, Smith KE, McKinnon TAJ, Tabata Y, Sasaki K, White MJV,
Fukunaga K, et al: The heparin binding domain of von Willebrand
factor binds to growth factors and promotes angiogenesis in wound
healing. Blood. 133:2559–2569. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mochizuki S, Soejima K, Shimoda M, Abe H,
Sasaki A, Okano HJ, Okano H and Okada Y: Effect of ADAM28 on
carcinoma cell metastasis by cleavage of von Willebrand factor. J
Natl Cancer Inst. 104:906–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Desch A, Strozyk EA, Bauer AT, Huck V,
Niemeyer V, Wieland T and Schneider SW: Highly invasive melanoma
cells activate the vascular endothelium via an MMP-2/integrin
αvβ5-induced secretion of VEGF-A. Am J Pathol. 181:693–705. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goerge T, Barg A, Schnaeker EM, Poppelmann
B, Shpacovitch V, Rattenholl A, Maaser C, Luger TA, Steinhoff M and
Schneider SW: Tumor-derived matrix metalloproteinase-1 targets
endothelial proteinase-activated receptor 1 promoting endothelial
cell activation. Cancer Res. 66:7766–7774. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goertz L, Schneider SW, Desch A, Mayer FT,
Koett J, Nowak K, Karampinis I, Bohlmann MK, Umansky V and Bauer
AT: Heparins that block VEGF-A-mediated von Willebrand factor fiber
generation are potent inhibitors of hematogenous but not lymphatic
metastasis. Oncotarget. 7:68527–68545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
John A, Robador JR, Vidal-Y-Sy S, Houdek
P, Wladykowski E, Günes C, Bolenz C, Schneider SW, Bauer AT and
Gorzelanny C: Urothelial carcinoma of the bladder induces
endothelial cell activation and hypercoagulation. Mol Cancer Res.
18:1099–1109. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang P, Goodrich C, Fu C and Dong C:
Melanoma upregulates ICAM-1 expression on endothelial cells through
engagement of tumor CD44 with endothelial E-selectin and activation
of a PKCα-p38-SP-1 pathway. FASEB J. 28:4591–4609. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bauer AT, Suckau J, Frank K, Desch A,
Goertz L, Wagner AH, Hecker M, Goerge T, Umansky L, Beckhove P, et
al: von Willebrand factor fibers promote cancer-associated platelet
aggregation in malignant melanoma of mice and humans. Blood.
125:3153–3163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goerge T, Kleinerüschkamp F, Barg A,
Schnaeker EM, Huck V, Schneider MF, Steinhoff M and Schneider SW:
Microfluidic reveals generation of platelet-strings on
tumor-activated endothelium. Thromb Haemost. 98:283–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCarty OJ, Mousa SA, Bray PF and
Konstantopoulos K: Immobilized platelets support human colon
carcinoma cell tethering, rolling, and firm adhesion under dynamic
flow conditions. Blood. 96:1789–1797. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morganti M, Carpi A, Amo-Takyi B,
Sagripanti A, Nicolini A, Giardino R and Mittermayer C: Von
Willebrand's factor mediates the adherence of human tumoral cells
to human endothelial cells and ticlopidine interferes with this
effect. Biomed Pharmacother. 54:431–436. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feinauer MJ, Schneider SW, Berghoff AS,
Robador JR, Tehranian C, Karreman MA, Venkataramani V, Solecki G,
Grosch JK, Gunkel K, et al: Local blood coagulation drives cancer
cell arrest and brain metastasis in a mouse model. Blood.
137:1219–1232. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blann AD, Gurney D, Wadley M, Bareford D,
Stonelake P and Lip GY: Increased soluble P-selectin in patients
with haematological and breast cancer: A comparison with
fibrinogen, plasminogen activator inhibitor and von Willebrand
factor. Blood Coagul Fibrinolysis. 12:43–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blann AD, Balakrishnan B, Shantsila E,
Ryan P and Lip GY: Endothelial progenitor cells and circulating
endothelial cells in early prostate cancer: A comparison with
plasma vascular markers. Prostate. 71:1047–1053. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang WS, Lin JK, Lin TC, Chiou TJ, Liu JH,
Yen CC and Chen PM: Plasma von Willebrand factor level as a
prognostic indicator of patients with metastatic colorectal
carcinoma. World J Gastroenterol. 11:2166–2170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gil-Bazo I, Catalán Goni V, Alonso
Gutiérrez A, Rodríguez Rodríguez J, Páramo Fernández JA, de la
Cámara Gómez J, Hernández Lizoain JL and García-Foncillas López J:
Impact of surgery and chemotherapy on von Willebrand factor and
vascular endothelial growth factor levels in colorectal cancer.
Clin Transl Oncol. 7:150–155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang AJ, Wang M, Wang Y, Cai W, Li Q, Zhao
TT, Zhang LH, Houck K, Chen X, Jin YL, et al: Cancer cell-derived
von Willebrand factor enhanced metastasis of gastric
adenocarcinoma. Oncogenesis. 7:122018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Colonne CK, Favaloro EJ and Pasalic L: The
intriguing connections between von Willebrand factor, ADAMTS13 and
cancer. Healthcare (Basel). 10:5572022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meschengieser S, Blanco A, Woods A,
Maugeri N, Fernandez J, Dupont J and Lazzari MA: Intraplatelet
levels of vWF:Ag and fibrinogen in myeloproliferative disorders.
Thromb Res. 48:311–319. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Franchini M, Di Perna C, Santoro C,
Castaman G, Siboni SM, Zanon E, Linari S, Gresele P, Pasca S,
Coppola A, et al: Cancers in patients with von Willebrand disease:
A survey from the italian association of haemophilia centres. Semin
Thromb Hemost. 42:36–41. 2016.PubMed/NCBI
|
|
35
|
Ablin RJ, Bartkus JM and Gonder MJ:
Immunoquantitation of factor VIII-related antigen (von Willebrand
factor antigen) in prostate cancer. Cancer Lett. 40:283–289. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Athale U, Moghrabi A, Nayiager T, Delva
YL, Thabane L and Chan AKC: von Willebrand factor and thrombin
activation in children with newly diagnosed acute lymphoblastic
leukemia: An impact of peripheral blasts. Pediatr Blood Cancer.
54:963–969. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Damin DC, Rosito MA, Gus P, Roisemberg I,
Bandinelli E and Schwartsmann G: Von Willebrand factor in
colorectal cancer. Int J Colorectal Dis. 17:42–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo R, Yang J, Liu X, Wu J and Chen Y:
Increased von Willebrand factor over decreased ADAMTS-13 activity
is associated with poor prognosis in patients with advanced
non-small-cell lung cancer. J Clin Lab Anal. 32:e222192018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mannucci PM, Karimi M, Mosalaei A,
Canciani MT and Peyvandi F: Patients with localized and
disseminated tumors have reduced but measurable levels of ADAMTS-13
(von Willebrand factor cleaving protease). Haematologica.
88:454–458. 2003.PubMed/NCBI
|
|
40
|
Pépin M, Kleinjan A, Hajage D, Büller HR,
Di Nisio M, Kamphuisen PW, Salomon L, Veyradier A, Stepanian A and
Mahé I: ADAMTS-13 and von Willebrand factor predict venous
thromboembolism in patients with cancer. J Thromb Haemost.
14:306–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Röhsig LM, Damin DC, Stefani SD, Castro CG
Jr, Roisenberg I and Schwartsmann G: von Willebrand factor antigen
levels in plasma of patients with malignant breast disease. Braz J
Med Biol Res. 34:1125–1129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yigit E, Gönüllü G, Yücel I, Turgut M,
Erdem D and Cakar B: Relation between hemostatic parameters and
prognostic/predictive factors in breast cancer. Eur J Intern Med.
19:602–607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zietek Z, Iwan-Zietek I, Paczulski R,
Kotschy M and Wolski Z: von Willebrand factor antigen in blood
plasma of patients with urinary bladder carcinoma. Thromb Res.
83:399–402. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dhami SPS, Patmore S, Comerford C, Byrne
CM, Cavanagh B, Castle J, Kirwan CC, Kenny M, Schoen I, O'Donnell
JS and O'Sullivan JM: Breast cancer cells mediate endothelial cell
activation, promoting von Willebrand factor release, tumor
adhesion, and transendothelial migration. J Thromb Haemost.
20:2350–2365. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lehrer S, Green S, Dembitzer FR,
Rheinstein PH and Rosenzweig KE: Increased RNA expression of von
willebrand factor gene is associated with infiltrating lobular
breast cancer and normal PAM50 subtype. Cancer Genomics Proteomics.
16:147–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Knöfler R, Lange BS, Paul F, Tiebel O and
Suttorp M: Bleeding signs due to acquired von Willebrand syndrome
at diagnosis of chronic myeloid leukaemia in children. Br J
Haematol. 188:701–706. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Conlan MG, Folsom AR, Finch A, Davis CE,
Sorlie P, Marcucci G and Wu KK: Associations of factor VIII and von
Willebrand factor with age, race, sex, and risk factors for
atherosclerosis. The atherosclerosis risk in communities (ARIC)
study. Thromb Haemost. 70:380–385. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bick RL: Alterations of hemostasis
associated with malignancy: Etiology, pathophysiology, diagnosis
and management. Semin Thromb Hemost. 5:1–26. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kwaan HC and Lindholm PF: Fibrin and
fibrinolysis in cancer. Semin Thromb Hemost. 45:413–422. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Senger DR: Molecular framework for
angiogenesis: A complex web of interactions between extravasated
plasma proteins and endothelial cell proteins induced by angiogenic
cytokines. Am J Pathol. 149:1–7. 1996.PubMed/NCBI
|
|
51
|
Lip GYH, Chin BSP and Blann AD: Cancer and
the prothrombotic state. Lancet Oncol. 3:27–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sørensen HT, Mellemkjaer L, Steffensen FH,
Olsen JH and Nielsen GL: The risk of a diagnosis of cancer after
primary deep venous thrombosis or pulmonary embolism. N Engl J Med.
338:1169–1173. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Y, Li L, Dong F, Guo L, Hou Y, Hu H,
Yan S, Zhou X, Liao L, Allen TD and Liu JU: Plasma von Willebrand
factor level is transiently elevated in a rat model of acute
myocardial infarction. Exp Ther Med. 10:1743–1749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu J, Kanki Y, Okada Y, Jin E, Yano K,
Shih SC, Minami T and Aird WC: A +220 GATA motif mediates basal but
not endotoxin-repressible expression of the von Willebrand factor
promoter in Hprt-targeted transgenic mice. J Thromb Haemost.
7:1384–1392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xu Y, Pan S, Liu J, Dong F, Cheng Z, Zhang
J, Qi R, Zang Q, Zhang C, Wang X, et al: GATA3-induced vWF
upregulation in the lung adenocarcinoma vasculature. Oncotarget.
8:110517–110529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fan M, Wang X, Peng X, Feng S, Zhao J,
Liao L, Zhang Y, Hou Y and Liu J: Prognostic value of plasma von
Willebrand factor levels in major adverse cardiovascular events: a
systematic review and meta-analysis. BMC Cardiovasc Disord.
20:722020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Peng X, Wang X, Fan M, Zhao J, Lin L and
Liu J: Plasma levels of von Willebrand factor in type 2 diabetes
patients with and without cardiovascular diseases: A meta-analysis.
Diabetes Metab Res Rev. 36:e31932020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang X, Zhao J, Zhang Y, Xue X, Yin J,
Liao L, Xu C, Hou Y, Yan S and Liu J: Kinetics of plasma von
Willebrand factor in acute myocardial infarction patients: A
meta-analysis. Oncotarget. 8:90371–90379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Comerford C, Glavey S, Quinn J and
O'Sullivan JM: The role of VWF/FVIII in thrombosis and cancer
progression in multiple myeloma and other hematological
malignancies. J Thromb Haemost. 20:1766–1777. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Terraube V, Marx I and Denis CV: Role of
von Willebrand factor in tumor metastasis. Thromb Res. 120 (Suppl
2):S64–S70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Suter CM, Hogg PJ, Price JT, Chong BH and
Ward RL: Identification and characterisation of a platelet
GPIb/V/IX-like complex on human breast cancers: Implications for
the metastatic process. Jpn J Cancer Res. 92:1082–1092. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang X, Starodubtseva MN, Kapron CM and
Liu J: Cadmium, von Willebrand factor and vascular aging. NPJ
Aging. 9:112023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang X, Dong F, Wang F, Yan S, Chen X,
Tozawa H, Ushijima T, Kapron CM, Wada Y and Liu J: Low dose cadmium
upregulates the expression of von Willebrand factor in endothelial
cells. Toxicol Lett. 290:46–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fares J, Fares MY, Khachfe HH, Salhab HA
and Fares Y: Molecular principles of metastasis: A hallmark of
cancer revisited. Signal Transduct Target Ther. 5:282020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nierodzik ML, Plotkin A, Kajumo F and
Karpatkin S: Thrombin stimulates tumor-platelet adhesion in vitro
and metastasis in vivo. J Clin Invest. 87:229–236. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Schulze EB, Hedley BD, Goodale D, Postenka
CO, Al-Katib W, Tuck AB, Chambers AF and Allan AL: The thrombin
inhibitor Argatroban reduces breast cancer malignancy and
metastasis via osteopontin-dependent and osteopontin-independent
mechanisms. Breast Cancer Res Treat. 112:243–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nierodzik ML, Kajumo F and Karpatkin S:
Effect of thrombin treatment of tumor cells on adhesion of tumor
cells to platelets in vitro and tumor metastasis in vivo. Cancer
Res. 52:3267–3272. 1992.PubMed/NCBI
|
|
68
|
Floyd CM, Irani K, Kind PD and Kessler CM:
von Willebrand factor interacts with malignant hematopoietic cell
lines: Evidence for the presence of specific binding sites and
modification of von Willebrand factor structure and function. J Lab
Clin Med. 119:467–476. 1992.PubMed/NCBI
|