Introduction
The synchronous occurrence (within 6 months) of two
neoplasms is rarely observed, particularly acute myeloid leukemia
(AML) coexisting with breast cancer (BC) [with actual incidence
rates of <1% (1)], which is also
associated with a poor prognosis [the survival time varies from 1
month to 3 years] (2–8). The prognosis of patients with
synchronous occurrence of AML and BC is closely related to the risk
factors of AML, and in terms of the long-term survival of the
patient, the solution in such a case is based primarily on the
treatment of AML, while placing the management of BC on hold
(9). B-cell lymphoma-2 (BCL-2)
expression is high not only in leukemia stem cells in AML, but also
in solid tumors, including BC (10,11).
Venetoclax is an oral selective BCL-2 inhibitor widely used in the
treatment of hematological malignancies (12). In such cases, venetoclax inhibits
BCL-2 to promote tumor cell apoptosis (13,14),
to simultaneously combat the coexistence of AML and BC. The present
study describes a rare case of AML coexisting with metastatic BC
(mBC), focusing on the treatment options with the use of venetoclax
combined with other regimens to achieve an effective response in
both AML and BC.
Case report
In March 2021, a 50-year-old female patient
presented to another hospital (The Affiliated Hospital of Hebei
University, Baoding, China) with progressive fatigue that had
persisted for 1 week. At 3 months prior to admission, the patient
had noted a 20-mm mass in the left breast; however, the patient
refused to be hospitalized. A complete blood count indicated a
white blood cell count of 22×109/l (normal range,
3.5–9.5×109/l), a hemoglobin concentration of 92 g/l
(normal range, 115–150 g/l) and a platelet count of
42×109/l (normal range, 125–300×109/l). A
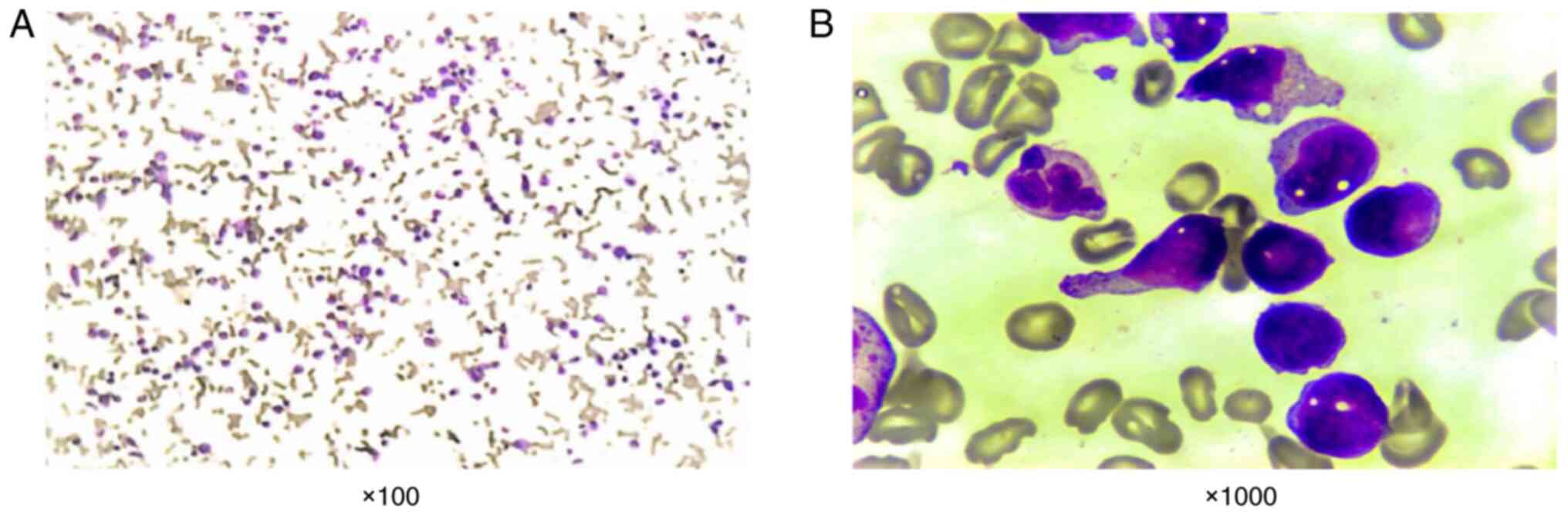
large number of monoblastic cells, which accounted for 66% of bone
marrow nucleated cells, were observed in a bone marrow smear
analysis (Fig. 1).
Immunophenotyping revealed that the blast cells were
CD34+, HLA-DR+, CD123+,
CD38+, CD117+, CD7+,
CD11b+, CD13+ and CD33+, which
appeared with myeloid blasts (Fig.
2). The patient harbored a complex karyotype (CK), as well as a
monosomal karyotype (MK)
{44,XX,t(4;16)(q11;p13),-15,-17,add(17)(p11),-19,-21,+mar1,+mar2[5]/44,idem
del(5)(q31)[2]/46,XX[9]} (Fig. 3),
without any molecular and next-generation sequencing abnormalities.
Based on the World Health Organization 2016 criteria (15), the patient was diagnosed with AML
(AML-not otherwise specified; poor-risk). There was a mass in the
upper outer quadrant of the left breast of the patient and a
palpable left axillary node was present. An ultrasound revealed a
39×32-mm mass (3 o'clock); however, the patient did not consent to
a biopsy of the breast mass. These aforementioned results were
provided by a relative of the patient.
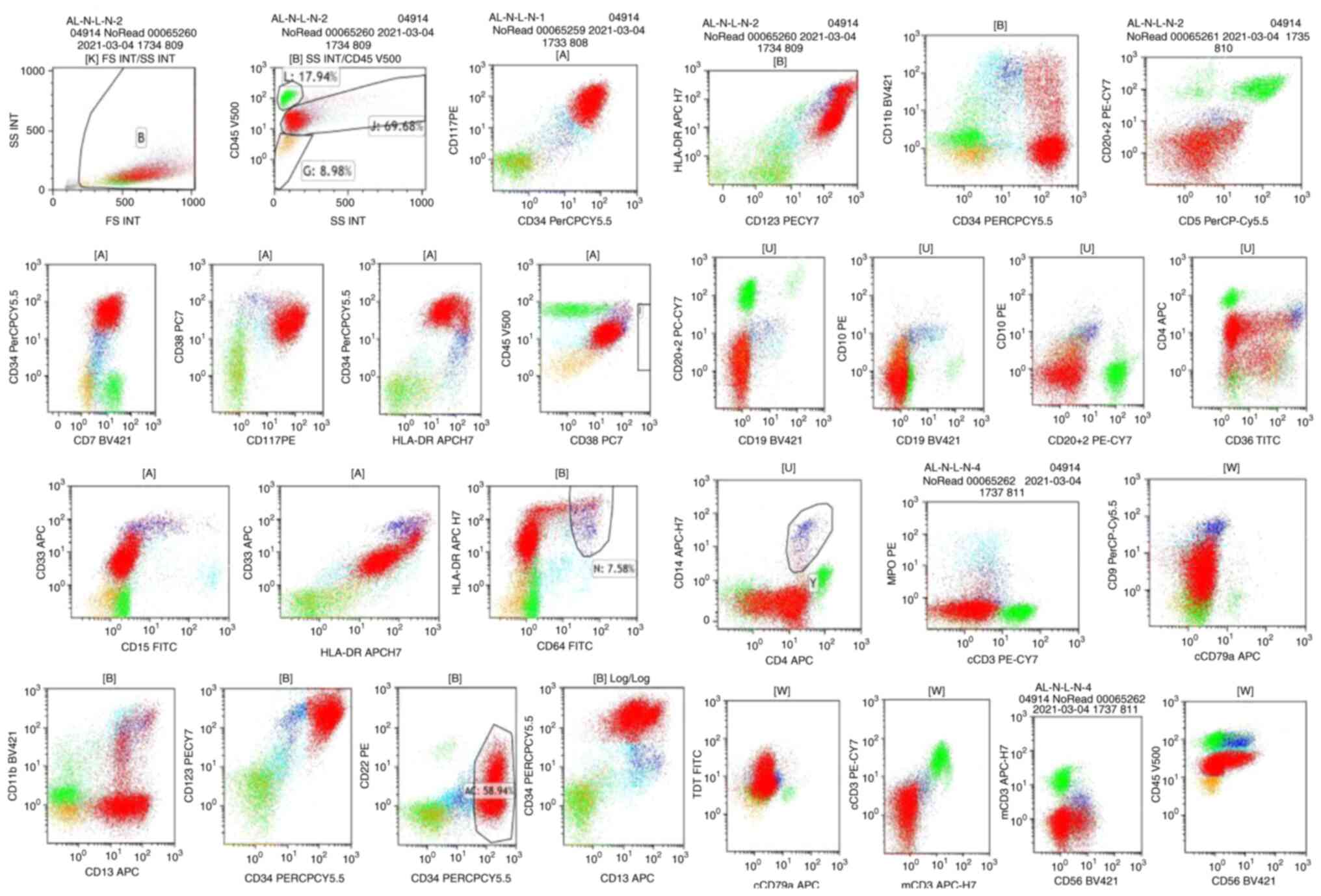 | Figure 2.Flow cytometry analysis of a bone
marrow aspirate. Bone marrow flow cytometry indicates an acute
myeloid leukemia diagnosis: CD34+, HLA-DR+,
CD123+, CD 38+, CD117+,
CD7+, CD11b+, CD13+,
CD33+, CD5−, CD3− and
CD19−. |
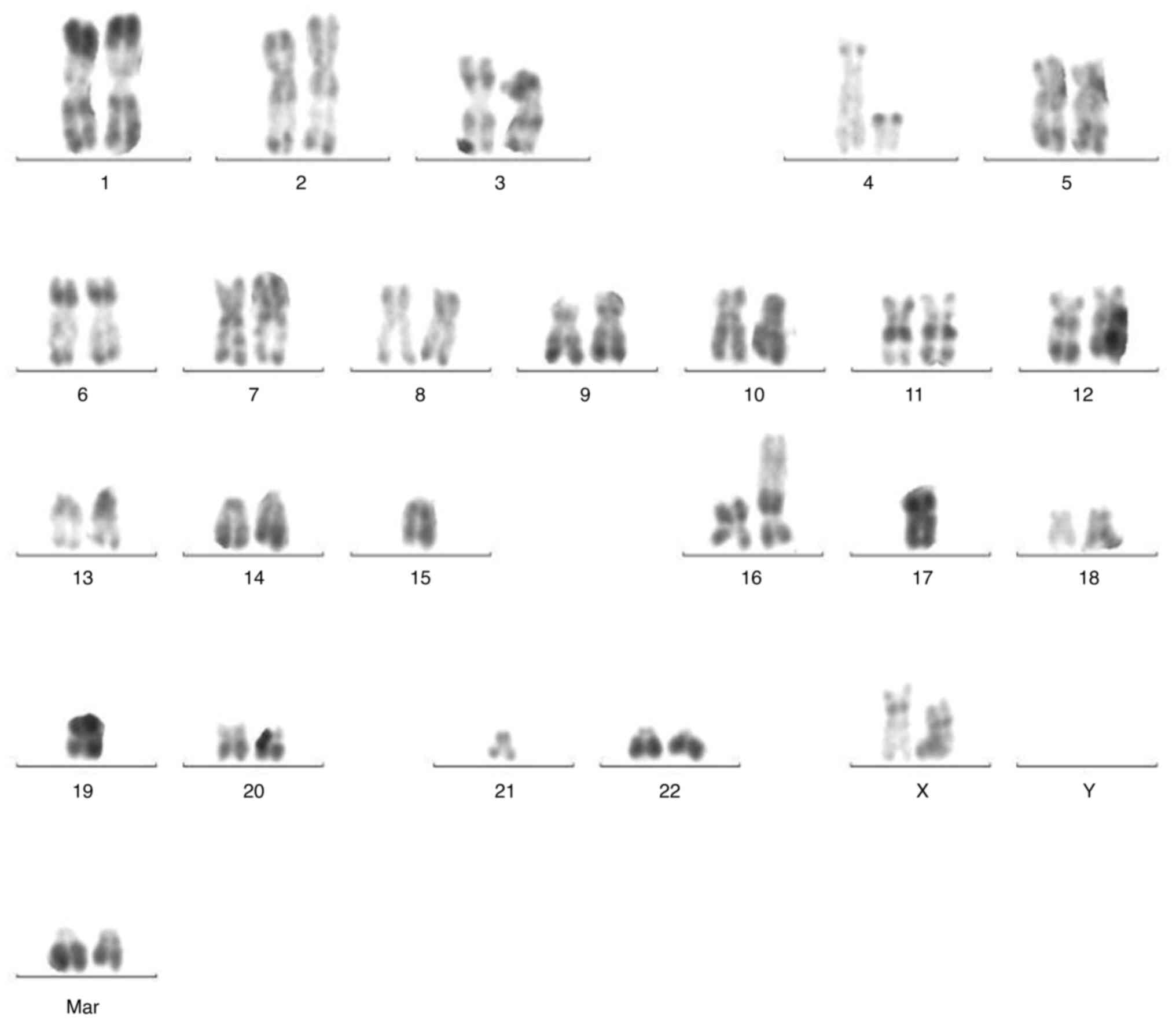 | Figure 3.G-banded karyotype of bone marrow
cells demonstrated a complex karyotype and a monosomal karyotype
{44,XX,t(4:16)(q11;p13),-15,-17,add(17)(p11),-19,-21,+mar1,+mar2[5]/44,idem
del(5)(q31)[2]/46,XX[9]}. |
An induction chemotherapeutic regimen consisting of
azacitidine (AZA; 75 mg/m2, days 1–7) + CAG [aclarubicin
at 20 mg, days 4–7; cytarabine at 20 mg twice a day, days 4–14;
granulocyte-colony stimulating factor (G-CSF) at 150 µg, days 4–14]
was administered, and 21 days following the commencement of
therapy, the patient did not achieve hypoplasia with 15% residual
blasts. Moreover, the mass in the left breast exhibited rapid
growth, which was confirmed by a subsequent ultrasound. The patient
was then admitted to the Department of Hematology of The Second
Hospital (Hebei Medical University, Shijiazhuang, China) for
further diagnosis and therapy in April, 2021.
A subsequent peripheral blood cell count indicated a
white blood cell count of 0.7×109/l, a hemoglobin
concentration of 58 g/l and a platelet count of
53×109/l. To evaluate the effect of the previous cycle
of combination chemotherapy, another bone marrow smear analysis was
performed. For this, the bone marrow smear was naturally dried,
then 2–3 drops of Wright-Giemsa staining solution was added to the
entire specimen smear at room temperature for 1–2 min. An equal
amount of 0.01 mol/l sodium dihydrogen phosphate solution was added
to the slide, the slide was gently shaken to thoroughly mix with
the Wright-Giemsa staining solution and the sample was incubated at
room temperature for 3–5 min. The smear was washed with double
distilled water, blotted dry and examined under a light microscope.
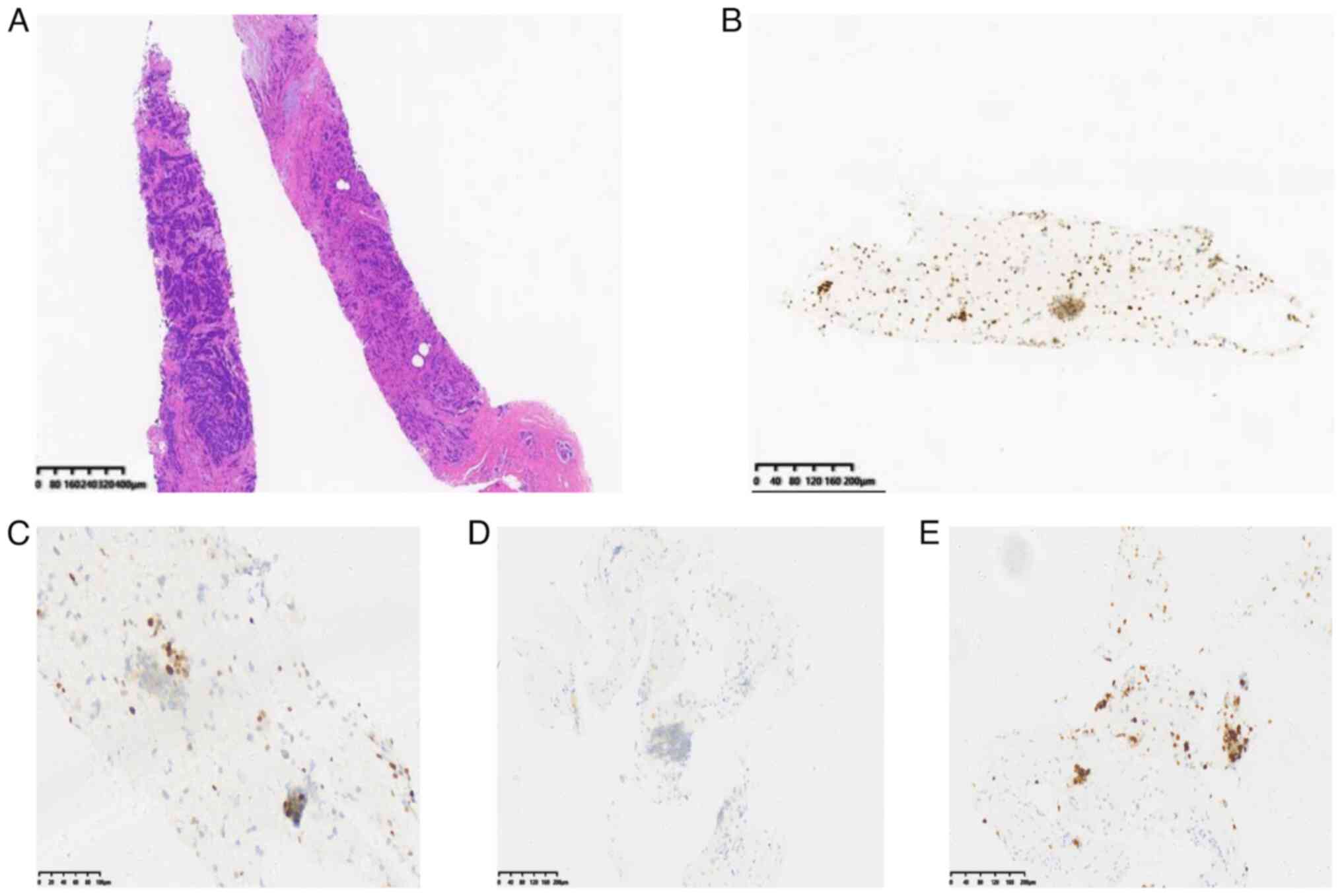
The bone marrow smear analysis revealed 58% monoblastic cells
(Fig. 4A-D) on the 28th day
following the commencement of chemotherapy. According to the
response criteria definitions for AML, the patient had not achieved
complete remission (CR) or partial remission (PR). An ultrasound
revealed that the mass in the left breast was 62×43-mm (3 o'clock)
with ipsilateral axillary lymphadenopathy, and evidence of bone
destruction was confirmed by a subsequent bone scan. An
ultrasound-guided core needle biopsy confirmed a diagnosis of
invasive carcinoma. For this, the puncture tissues were immersed in
formaldehyde solution (4%) and fixed overnight at 4°C. The next
day, the tissues were dehydrated and embedded in paraffin wax. The
embedded material was then cut into 5-µm sections and the sections
were dewaxed with xylene, rehydrated through an alcohol gradient
(100, 95, 90, 80 and 70%; 5 min each), then washed with distilled
water. H&E staining was performed at room temperature; slices
were dyed with Harris hematoxylin for 3–8 min and washed with tap
water, then incubated in eosin dye solution for 1–3 min and washed
with tap water. The sections were successively immersed in 95%
alcohol, anhydrous ethanol and xylene for 5 min each to dehydrate
the sample. The sample was dried and sealed with neutral gum.
Antigens were extracted at 100°C before immunohistochemical
staining. Endogenous peroxidase activity was then blocked with 3%
hydrogen peroxide, after washing with phosphate buffered saline
(PBS) twice for 5 min each. The sample was incubated with normal
goat serum [5%; Yeason Biotechnology (Shanghai) Co., Ltd.] at 37°C
for 30 min to block non-specific background staining. Next, the
sections were incubated with anti-estrogen receptor (ER; cat. no.
ab16660; 0.5 µg/ml; Abcam), anti-progesterone receptor (cat. no.
ab32085; 1 µg/ml; Abcam), anti-human epidermal growth factor
receptor 2 (HER2; cat. no. ab16662; 1 µg/ml; Abcam) and anti-Ki-67
(cat. no. ab15580; 1 µg/ml; Abcam) at room temperature for 30 min.
The slices were washed with PBS and incubated with secondary
antibody (HRP marker; cat. no. ab6721; 1 µg/ml; Abcam) at room
temperature for 20 min. DAB substrate staining was used.
Representative images were collected using an optical microscope
and analyzed with ImageJ (v.1.8.0; National Institutes of Health;
http://imagej.net/software/imagej/)
(16). Immunohistochemistry yielded
positive results for the ER and progesterone receptor (80 and 10%,
respectively), with a high Ki67 proliferation index (40%) and
negative expression of HER2 (Fig.
5). Fluorescence in situ hybridization for the HER2 gene
also yielded negative results using the PathVysion HER-2 DNA probe
kit (Abbott) as previously described (17). These results indicated a luminal
B-like (HER2−) type BC [staging T3N1M1, according to the
American Joint Committee on Cancer TNM staging system (18)] diagnosis.
After obtaining informed consent, the patient began
an adjuvant chemotherapy treatment regimen with pegylated liposomal
doxorubicin at 40 mg (day 1, per 21 days for two cycles) and
albumin-bound paclitaxel at 200 mg (days 1 and 8, per 21 days for
two cycles) to treat mBC. The patient was also treated with
standard AZA at 75 mg/m2 subcutaneously for 7 days, in
combination with oral venetoclax for AML (based on a
pharmacokinetic test, from a dose of 100–200 mg/day, the dosing of
which must be adjusted in the setting of the concomitant
administration of the CYP3A4 inhibitor, voriconazole (200 mg twice
daily for 4 weeks). Tumor lysis syndrome was monitored during a
3–5-day dose ramp-up of venetoclax, and G-CSF was also administered
(300 µg per day for 3 weeks) to reduce the risk of infections and
other cytopenia-related adverse events with the venetoclax-based
combinations. When the white blood cell and platelet counts of the
patient were normal (white blood cell count, 5.6×109/l;
platelet count, 218×109/l) a bone marrow aspiration was
performed on day 28 (Fig. 4E-H).
With the clearance of blasts (<5%), the response to AML
treatment was defined as CR with incomplete hematological recovery
(CRi). The treatment response to BC was defined as PR as the mass
in the left breast was notably smaller (only 20×20 mm in size). Due
to economic reasons, the patient refused an allogeneic
hematopoietic stem cell transplantation (allo-HSCT); thus, the
patient was then treated with another cycle of chemotherapy without
dose modifications. However, the patient succumbed due to septic
shock from neutropenia following the third cycle of
chemotherapy.
Discussion
Synchronous multiple cancer, defined as a cancer
diagnosed simultaneously with another cancer or diagnosed within 6
months, is uncommon. The risk of a patient diagnosed with BC being
diagnosed with a co-existing second cancer is 2–3% (1), and the synchronous occurrence of BC
and AML is rare. The present study described the case of a female
patient presenting with the co-existence of AML and BC. To date and
to the best of our knowledge, only 11 cases of a synchronous
occurrence of AML and BC have been described in the literature
(Table I) (2–8). The
simultaneous diagnosis of AML and BC is associated with an
extremely poor prognosis with standard therapies. Specifically,
only 4 such cases have reached a CR for AML (4,6–8) and
only 1 patient who achieved a CR for both diseases was able to
undergo an allo-HSCT (8), which is
currently regarded as the only recommended therapeutic approach
aimed at achieving long-term disease-free survival for patients
with AML (9). Of the 11 patients
identified in the literature, 4 cases survived for as little as 1
week to 1.5 months, 2 patients survived for >3 years and 9
patients succumbed due to AML. To date, the median overall survival
(OS) time of patients with mBC is only ~37 months (19), and clinicians typically treat AML
first before BC due to the poor outcomes associated with AML. In
the present study, the authors aimed to select the appropriate
therapeutic strategy for the two malignancies simultaneously.
 | Table I.Cases of synchronous occurrence of BC
and AML in the literature. |
Table I.
Cases of synchronous occurrence of BC
and AML in the literature.
| Author, year | Sex/Age, years | Interval | Diagnosis (type of
BC) | Morphology of
AML | Phenotype of
AML | Genetics of
AML | Karyotype of
AML | Therapy for
AML/BC | Response to AML/BC
therapy | Outcome | (Refs.) |
|---|
| Softic, 1964 | F/84 | Simultaneous | NA | NA | NA | NA | NA | None | NA | Death | (2) |
| Carey et al,
1967 (case 1) | F/57 | 2 months | Ductal cell
carcinoma | NA | NA | NA | NA | Cyclophosphamide of
AML; radical mastectomy of BC | NR | Died during
induction in 43 days. | (3) |
| Carey et al,
1967 (case 6) | F/55 | Simultaneous | NA | M3 | NA | NA | NA | None | NA | Death | (3) |
| Rosner et
al, 1978 (case 18) | F/58 | 3 months | NA | NA | NA | NA | NA | 6-mercaptopu-rine,
methotrexate, vincristine, and prednisone of AML radical mastectomy
of BC | CR for AML lasting
1 year | Died for diabetic
coma and gastrointestinal hemorrhage in CR of AML | (4) |
| Rosner et
al, 1978 (case 23) | F/59 | 6 months | NA | NA | NA | NA | NA | Combination
chemotherapy of AML; radical mastectomy of BC | HR for AML | Death after HR | (4) |
| Ershler et
al, 1982 (case 6) | F/64 | 6 months | NA | NA | NA | NA | 45, banding not
possible | Hydroxyurea for
AML; cyclophosphamide for BC | PR for AML; NA for
BC | Died after 3
months | (5) |
| Ershler et
al, 1982 (case 8) | F/65 | Simultaneous | NA | M4 | NA | NA | NA | Cytarabine for AML;
none for BC | NR for AML | Died during
induction in 9 days | (5) |
| Mishra et
al, 2004 | F/38 | 1 month | Infiltrating duct
carcinoma | M1 | CD13+,
CD33+, HLA-DR+, CD7+ | p53 mutation in
both AML and BC | 46, XX | DA of AML; modified
radical mastectomy of BC | CR for AML | Died following
intracerebral hemorrhage | (6) |
| Hu et al,
2016 | F/57 | Simultaneous | Invasive | M4 ductal
carcinoma | CD117+,
CD34+, CD33+, CD11c+,
CD13+ | NPM1 and CEBPA
mutations | 47, XX, +11 | DA/CAG for AML;
tamoxifen/TEC/MRM for BC | NR/CR for AML;
progressed/stable for BC | Alive in CR; stable
BC | (7) |
| Ballotta et
al, 2020 | F/40 | 1 month | Invasive poorly
differentiated ductal carcinoma | NA | CD34+,
CD117+, HLA-DR+, CD4+,
CD7+, CD13, TdT+, CD33+/− | Negative | 46, XX | ICE/G-CLAC/HSCT of
AML; right quadrantectomy/DC/tamoxifen of BC. | NR/CR of AML; CR of
BC. | Alive in CR for AML
and BC after allo-HSCT | (8) |
| Ballotta et
al, 2020 | NA | Simultaneous | NA | M2 | CD34+/−,
CD117+, CD7+, HLA-DR+,
CD13+, CD33+ | MLL
self-fusion | 46, XX | NA | RD | Died of AML after
13 months | (8) |
| Present case | F/50 | 3 months | Invasive
carcinoma | M5 | CD34+,
HLA−DR+, CD123+, CD38+,
CD117+, CD7+, CD11b+,
CD13+, CD33+ | Negative | Complex
karyotype | AZA + CAG/VA for
AML | NR/CRi for AML; PR
for BC | Died of septic
shock | - |
Patients with ER+ mBC who are
HER2− and who are not suitable to receive treatments
such as quadrantectomy, local radiotherapy and HER2 inhibitors, are
recommended classical endocrine therapy with or without molecular
therapies (20–22). However, acquired resistance to
hormonal therapies occurs in 30–50% of cases, which has been
confirmed to occur via the PI3K-AKT-mTOR, cyclin
D1-CDK4/6-retinoblastoma protein, BCL-2-p53-MDM2, ER1 and other
cell-signaling pathways (23).
Molecular-based therapies, including CDK4/6 inhibitors (such as
palbociclib/ribociclib/abemaciclib) (24), an mTORC1 inhibitor (everolimus)
(25), an α isoform-specific PI3K
inhibitor (alpelisib) (26) and a
BCL-2 inhibitor (venetoclax) (27)
have been demonstrated to have potential efficacy as adjuvant
therapies in patients with advanced-stage BC. Moreover, regardless
of the molecular subtype, chemotherapy still represents a
fundamental option for patients with mBC. In the present study,
according to the pathological results, the patient accepted
adjuvant chemotherapy, combining pegylated liposomal doxorubicin
with albumin-bound paclitaxel. Furthermore, BCL-2 inhibitor
molecular therapy was administered to the patient for BC and AML
simultaneously.
BCL-2 expression is high in the leukemia stem cells
of AML (28). Venetoclax, an oral
selective BCL-2 inhibitor first approved for the treatment of
chronic lymphocytic leukemia, can lead to the rapid initiation of
AML cell apoptosis (13). Despite
the modest efficacy of single-agent treatment with either
venetoclax or hypomethylating agents (HMAs) (29), the rationale for combining
venetoclax with HMAs was provided by the potential of BCL-2
inhibitors to sensitize AML cells to HMAs (30). In two pivotal clinical trials, the
rates of CR plus CRi were 54 and 67% in patients treated with
venetoclax plus low-dose cytarabine (LDAC) or HMAs, and the median
OS time was 10.4 and 17.5 months, respectively (31,32).
Due to these results, the U.S. Food and Drug Administration
approved the use of venetoclax in combination with LDAC or HMAs for
older or unfit patients newly diagnosed with AML and for patients
who have relapsed/refractory AML.
The overall frequency of a CK in AML, the most
unfavorable prognostic factor in AML with a well-known poor
outcome, has been shown to occur in 10–14% of patients in prior
studies and has been reported to occur in up to 23% of older
patients with AML (33,34). Patients with AML with a CK are
generally resistant to conventional induction and consolidation and
only 10–40% achieve CR and tend to relapse in a median time of 6–8
months (35). The MK, another
adverse cytogenetic aberration, appears to be a worse prognostic
predictor of a poor outcome, with a 4-year OS rate of 4% compared
with 21% in patients with CK (36).
Patients with AML presenting with the co-existence of MK and CK
achieve an overall remission rate of 28% following treatment with
intensive chemotherapy (37). A few
studies to date have indicated that combination treatment of
venetoclax with HMAs or mono-regimen CPX 351, a liposomal
formulation of cytarabine and daunorubicin, is associated with an
optimal trend for the OS of patients (38,39).
The patient described in the present study diagnosed with adverse
AML with CK and MK, first failed to achieve remission with one
regimen of AZA + CAG; however, the patient achieved an effective
response of CRi following the adjustment of a combination of
venetoclax with AZA.
The increased expression of the anti-apoptotic
protein, BCL-2, has been reported in a number of different solid
tumor histotypes and is also linked to resistance to
chemotherapies, including in renal, breast and thyroid carcinoma,
but not in AML, mature B-cell malignancies and lymphoid
malignancies; thus, studies mainly focus on BCL-2 inhibitor
application in solid tumors (10,11).
The expression of BCL-2 in BC is the second highest in a wide range
of tumor histotypes, according to The Human Protein Atlas database
(https://www.proteinatlas.org/). Due to
the upregulation of BCL-2 in BC, particularly in 80% of primary
ER+ BC cases, the molecular mechanisms of venetoclax,
including BC cell proliferation and growth inhibition via the
promotion of apoptosis, cell cycle arrest and autophagy, have been
confirmed via in vitro studies (14,40).
Preclinical data on the combination of venetoclax with tamoxifen to
increase the apoptosis of patient-derived xenograft models of
ER+ BC have led to a phase Ib dose-escalation and
expansion study of venetoclax combined with endocrine therapy in
mBC, which is both ER− and BCL-2+ (27,41).
In the first clinical study, 15 patients were treated orally with
daily tamoxifen (20 mg) and venetoclax (200–800 mg) in the
escalation phase, and in the expansion phase, 24 patients received
800 mg venetoclax as the recommended phase II dose (RP2D) for
neither dose-limiting toxicities nor high-grade adverse events were
observed. For treatment at the RP2D, all 24 patients had measurable
disease with a clinical benefit rate of 75%; the objective response
rate was 54% (1 CR and 12 PR), stable disease was 21% (5 patients)
and the median progression-free survival was not reached at the
time of data analysis (>51 weeks). According to the marked
efficacy and acceptable safety of the combined use of venetoclax
with endocrine therapy, a randomized phase II trial of venetoclax +
fulvestrant versus fulvestrant in ER+, HER2−
locally advanced or mBC is ongoing in five countries, enrolling 100
patients (42). These findings
support the use of venetoclax both as a single agent and in
combination strategies for the further investigation of patients
with BCL-2+ tumors.
To the best of our knowledge, the case described in
the present study is the only one characterized in the literature
by a severe prognosis of AML with CK and MK that achieved CRi of
AML and PR of BC. Venetolax was used as a therapeutic agent for
AML; however, according to the criteria response of BC in the
patient described in the present study and in the reported
literature (27,41), it is conceivable that venetolax
plays an effective role in the treatment of the two malignancies
simultaneously.
In conclusion, it is essential to determine
appropriate treatment measures for patients with co-existing AML
and BC in the era of novel agents. Following the notable results
with the application of venatoclax in AML and BC, respectively,
this oral selective BCL-2 inhibitor may be used for the treatment
of synchronous cancer (such as AML plus BC) to achieve efficacy and
safety. However, further studies with a greater number of cases are
required to clarify and optimize the therapeutic potential and use
of treatment regimens containing venatoclax.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LX was responsible for clinical data collection,
interpretation of the results, drafting the manuscript and
providing final approval of the version to be published. SQ
participated in the design of the study and analyzed patient data.
TT and YL obtained medical images of the bone marrow smear, and JZ
and XG gave advice on the treatment of the patient. JZ and XG
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication of the case
report, including the clinical details and images was provided by
the patient's relative.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee J, Park S, Kim SH, Kim J, Ryu J, Park
HS, Kim SI and Park BW: Characteristics and survival of breast
cancer patients with multiple synchronous or metachronous primary
cancers. Yonsei Med J. 56:1213–1220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Softic N: Acute myeloblastic leukemia in
an 84-year-old woman, associated with a breast cancer and a mixed
parotid tumor without metastasis. Nouv Rev Fr Hematol. 4:458–460.
1964.(In French). PubMed/NCBI
|
|
3
|
Carey RW, Holland JF, Sheehe PR and Graham
S: Association of cancer of the breast and acute myelocytic
leukemia. Cancer. 20:1080–1088. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosner F, Carey RW and Zarrabi MH: Breast
cancer and acute leukemia: Report of 24 cases and review of the
literature. Am J Hematol. 4:151–172. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ershler WB, Robins HI, Davis HL, Hafez GR,
Meisner LF, Dahlberg S and Arndt C: Emergence of acute
non-lymphocytic leukemia in breast cancer patients. Am J Med Sci.
284:23–31. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mishra PP, Mahapatra M, Choudhry VP,
Saxena R, Pati H, Dixit A, Anupama R, Bhattacharya J, Chatterjee T
and Dutta P: Synchronous occurrence of breast carcinoma and acute
myeloid leukemia: Case report and review of the literature. Ann
Hematol. 83:541–543. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu G, Mallik DK, Yang W, Hou Y, Cheng Z,
Chen P, Zhu W, Wang H, Shen L, Zhang H and Yang Z: Appropriate
clinical strategies for breast cancer coexisting with acute myeloid
leukemia in the genomic-molecular era: A case report. Breast Care
(Basel). 11:145–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ballotta L, Trisolini SM, Iori AP, Rocca
UL, Micozzi A, Gentile G, De Giacomo T, Guarini A, Foà R and Capria
S: A rare case of coexisting breast cancer and refractory acute
myeloid leukemia. Case Rep Hematol. 2020:88931852020.PubMed/NCBI
|
|
9
|
Litzow MR: The therapy of relapsed acute
leukaemia in adults. Blood Rev. 18:39–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
D'Aguanno S and Del Bufalo D: Inhibition
of anti-apoptotic Bcl-2 proteins in preclinical and clinical
studies: Current overview in cancer. Cells. 9:12872020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maji S, Panda S, Samal SK, Shriwas O, Rath
R, Pellecchia M, Emdad L, Das SK, Fisher PB and Dash R: Bcl-2
antiapoptotic family proteins and chemoresistance in cancer. Adv
Cancer Res. 137:37–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberts AW, Wei AH and Huang DCS: BCL-2
and MCL-1 inhibitors for hematologic malignancies. Blood.
138:1120–1136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lagadinou ED, Sach A, Callahan K, Rossi
RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer
KM, et al: BCL-2 inhibition targets oxidative phosphorylation and
selectively eradicates quiescent human leukemia stem cells. Cell
Stem Cell. 12:329–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alhoshani A, Alatawi FO, Al-Anaz FE,
Attafi IM, Zeidan A, Agouni A, El Gamal HM, Shamoon LS, Khalaf S
and Korashy HM: BCL-2 inhibivtor venetoclax induces
autophagy-associated cell death, cell cycle arrest, and apoptosis
in human breast cancer cells. Onco Targets Ther. 13:13357–13370.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caroline AS, Vaine SR and Kevin WE: NIH
Image to ImageJ: 25 Years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar
|
|
17
|
Oberauner-Wappis L, Loibner M, Viertler C,
Groelz D, Wyrich R and Zatlouka K: Protocol for HER2 FISH
determination on PAXgene-fixed and paraffin-embedded tissue in
breast cancer. Int J Exp Pathol. 97:202–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giuliano AE, Connolly JL, Edge SB,
Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ and
Hortobagyi GN: Breast cancer-major changes in the American Joint
Committee on Cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:290–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gobbini E, Ezzalfani M, Dieras V, Bachelot
T, Brain E, Debled M, Jacot W, Mouret-Reynier MA, Goncalves A,
Dalenc F, et al: Time trends of overall survival among metastatic
breast cancer patients in the real-life ESME cohort. Eur J Cancer.
96:17–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hortbagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Petrakova K, Blackwell
KL, Winer EP, et al: Updated results from MONALEESA-2, a phase III
trial of first-line ribociclib plus letrozole versus placebo plus
letrozole in hormone receptor-positive, HER2-negative advanced
breast cancer. Ann Oncol. 29:1541–1547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Leo A, Jerusalem G, Petruzelka L,
Torres R, Bondarenko IN, Khasanov R, Verhoeven D, Pedrini JL,
Smirnova I, Lichinitser MR, et al: Results of the CONFIRM phase III
trial comparing fulvestrant 250 mg with fulvestrant 500 mg in
postmenopausal women with estrogen receptor-positive advanced
breast cancer. J Clin Oncol. 28:4594–4600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reinert T and Barrios CH: Optimal
management of hormone receptor positive metastatic breast cancer in
2016. Ther Adv Med Oncol. 7:304–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rozeboom B, Dey N and De P: ER+ metastatic
breast cancer: Past, present, and a prescription for an
apoptosis-targeted future. Am J Cancer Res. 9:2821–2831.
2019.PubMed/NCBI
|
|
24
|
Piezzo M, Cocco S, Caputo R, Cianniello D,
Gioia GD, Lauro VD, Fusco G, Martinelli C, Nuzzo F, Pensabene M and
De Laurentiis M: Targeting cell cycle in breast cancer: CDK4/6
inhibitors. Int J Mol Sci. 21:64792020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moreau-Bachelard C, Robert M, Gourmelon C,
Bourbouloux E, Patsouris A, Frenel JS and Campone M: Evaluating
everolimus for the treatment of breast cancer. Expert Opin
Pharmacother. 24:1105–1111. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
André F, Ciruelos EM, Juric D, Loibl S,
Campone M, Mayer IA, Rubovszky G, Yamashita T, Kaufman B, Lu YS, et
al: Alpelisib plus fulvestrant for PIK3CA-mutated, hormone
receptor-positive, human epidermal growth factor
receptor-2-negative advanced breast cancer: Final overall survival
results from SOLAR 1. Ann Oncol. 32:208–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lok SW, Whittle JR, Vaillant F, The CE, Lo
LL, Policheni An, Bergin ART, Desai J, Ftouni S and Gandolfo LC: A
phase Ib dose-escalation and expansion study of the BCL2 inhibitor
venetoclax combined with tamoxifen in ER and BCL2-positive
metastatic breast cancer. Cancer Discov. 9:354–369. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tiribelli M, Michelutti A, Cavallin M, Di
Giusto S, Simeone E, Fanin R and Damiani D: BCL-2 expression in AML
patients over 65 years: Impact on outcomes across different
therapeutic strategies. J Clin Med. 10:50962021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Konopleva M, Pollyea DA, Potluri J, Chyla
B, Hogdal L, Busman T, Mckeegan E, Salem AH, Zhu M, Ricker JL, et
al: Efficacy and biological correlates of response in a phase II
study of venetoclax monotherapy in patients with acute myelogenous
leukemia. Cancer Discov. 6:1106–1117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bogenberger JM, Kornblau SM, Pierceall WE,
Lena R, Chow D, Shi CX, Mantei J, Ahmann G, Gonzales IM, Choudhary
A, et al: BCL-2 family proteins as 5-Azacytidine-sensitizing
targets and determinants of response in myeloid malignancies.
Leukemia. 28:1657–1665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei AH, Strickland SA Jr, Hou JZ, Fiedler
W, Lin TL, Walter RB, Enjeti A, Tiong IS, Savona M, Lee S, et al:
Venetoclax combined with low-dose cytarabine for previously
untreated patients with acute myeloid leukemia: Results from a
phase Ib/II study. J Clin Oncol. 37:1277–1284. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
DiNardo CD, Pratz K, Pullarkat V, Jonas
BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH,
Kantarjian HM, et al: Venetoclax combined with decitabine or
azacitidine in treatment-naive, elderly patients with acute myeloid
leukemia. Blood. 133:7–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Röllig C, Bornhäuser M, Thiede C, Taube F,
Kramer M, Mohr B, Aulitzky W, Bodenstein H, Tischler HJ, Stuhlmann
R, et al: Long-term prognosis of acute myeloid leukemia according
to the new genetic risk classification of the European LeukemiaNet
recommendations: Evaluation of the proposed reporting system. J
Clin Oncol. 29:2758–2765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Döhner H, Estey EH, Amadori S, Appelbaum
FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson
RA, et al: Diagnosis and management of acute myeloid leukemia in
adults: Recommendations from an international expert panel, on
behalf of the European LeukemiaNet. Blood. 115:453–474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mrózek K: Cytogenetic, molecular genetic,
and clinical characteristics of acute myeloid leukemia with a
complex karyotype. Semin Oncol. 35:365–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Breems DA, Van Putten WLJ, De Greef GE,
Van Zelderen-Bhola SL, Gerssen-schoorl KB, Mellink CH, Nieuwint A,
Jotterand M, Hagemeijer A, Beverloo HB and Löwenberg B: Monosomal
karyotype in acute myeloid leukemia: A better indicator of poor
prognosis than a complex karyotype. J Clin Oncol. 26:4791–4797.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wierzbowska A, Wawrzyniak E,
Siemieniuk-Rys M, Kotkowska A, Pluta A, Golos A, Robak T,
Szarawarska M, Jaskowiec A, Duszenko E, et al: Concomitance of
monosomal karyotype with at least 5 chromosomal abnormalities is
associated with dismal treatment outcome of AML patients with
complex karyotype-retrospective analysis of Polish adult leukemia
group (PALG). Leuk Lymphoma. 58:889–897. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Estey EH: Acute myeloid leukemia: 2019
Update on risk-stratification and management. Am J Hematol.
93:1267–1291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Daneshbod Y, Kohan L, Taghadosi V,
Weinberg OK and Arber DA: Prognostic significance of complex
karyotypes in acute myeloid leukemia. Curr Treat Options Oncol.
20:152019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alcon C, Gómez Tejeda Zañudo J, Albert R,
Wagle N, Scaltriti M, Letai A, Samitier J and Montero J: ER+ breast
cancer strongly depends on MCL-1 and BCL-xL anti-apoptotic
proteins. Cells. 10:16592021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vaillant F, Merino D, Lee L, Breslin K,
Pal B, Ritchie ME, Smyth GK, Christie M, Phillipson LJ, Burns CJ,
et al: Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen
receptor-positive breast cancer. Cancer Cell. 24:120–129. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lindeman GJ, Fernando TM, Bowen R, Jezak
KJ, Song X, Decker T, Boyle F, McCune S, Armstrong A, Shannon C, et
al: VERONICA: Randomized phase II study of fulvestrant and
venetoclax in ER-positive metastatic breast cancer post-CDK4/6
inhibitors-efficacy, safety, and biomarker results. Clin Cancer
Res. 28:3256–3267. 2022. View Article : Google Scholar : PubMed/NCBI
|