Introduction
Ovarian cancer (OC) is a complex and challenging
disease that affects women worldwide. As the fifth most common
cancer in women (1), it poses
several significant health risks, ranging from late diagnosis to
chemoresistance, and contributes to ~150,000 global deaths per
year, mental health problems and a financial burden for patients
(2). OC is not a singular entity;
it comprises several histological subtypes, each with distinct
characteristics. Epithelial (E)OC is the most common type,
accounting for 85–90% of OCs (3,4). It
arises from the cells that cover the outer surface of the ovary.
EOC includes subtypes such as serous, mucinous, endometrioid and
clear cell carcinoma. Hereditary factors also serve a role, with
family histories of ovarian or breast cancer increasing the risk.
The association between OC and mutations in the BRCA genes,
particularly BRCA1 and BRCA2, highlights the genetic contribution
to the etiology of the disease. Individuals with familial histories
of breast or OC carrying BRCA1/2 mutations have elevated risks,
emphasizing the importance of genetic testing and precision
medicine approaches for targeted risk assessment and management
(5–7). This risk increases with age, as the
median age of diagnosis is ~63 years (8).
The primary challenge in OCs pertains to its
diagnosis, which is characterized by an absence of efficient early
detection methods as the symptoms of OC are notoriously subtle and
are often referred to as ‘stealthy assailant’ (4). This leads to patients being diagnosed
in advanced stages (9), rendering
surgical intervention more complex due to widespread metastasis. If
effective early-stage detection is achievable, the survival rate
can potentially increase to 70%. However, with an estimated
early-stage detection rate of only 20%, late-stage detection with
advanced cancer notably lowers the survival rate to 35% for most
patients (10).
The current standard treatment for OC includes
surgery and chemotherapy, alongside emerging therapies like
anti-angiogenic agents, poly(ADP-ribose) polymerase inhibitors and
immunotherapeutic approaches. To mitigate morbidity rates, a
strategy involving pre-sensitization of cancer cells with standard
therapy has been investigated, particularly effective with
platinum-based agents due to its heightened response in relapsed
cases, addressing drug resistance. Second-line chemotherapy
selection depends on tumor sensitivity to platinum derivatives,
often incorporating carboplatin or cisplatin in combination with
other drug therapy (such as paclitaxel and gemcitabine) (11). However, the emergence of
chemotherapy resistance, especially during disease recurrence,
remains a critical challenge. Given the inherent heterogeneity of
OC, a precision medicine approach aims to identify specific
mutations and customize treatment strategies accordingly. This
tailored approach aims to address the challenges of resistance and
enhance treatment efficacy in the context of the molecular
diversity of OC (12).
Within the last decades, several biomolecular
studies focused on trying to address the aforementioned challenges.
Following their first discovery in 1993 (13), microRNAs (miRNAs/miRs) have been
studied in many diseases, including OC, to understand biological
mechanisms of diseases as well as improving the diagnosis and
treatment of patients. miRNAs are small RNA molecules that regulate
gene expression and can act as oncogenes (promoting cancer) or
tumor suppressors (inhibiting cancer) in OC. Research on miRNAs has
reported that deregulation of miRNAs is one of the pathogenesis
processes found in several types of OC. Furthermore, dysregulation
of specific miRNAs has been associated with OC development,
progression and chemoresistance (12).
miRNAs have dual roles in OC management: i) The
miRNA expression profile can be used for diagnostic and prognostic
purposes, forming the basis of a personalized medicine approach. By
identifying specific miRNA signatures, clinicians can tailor
treatment plans to improve outcomes and minimize exposure to
ineffective therapies (12,14); and ii) miRNAs themselves are being
explored as therapeutic agents. Synthetic miRNAs, such as miRNA
mimics and inhibitors, have shown promise in preclinical studies.
These synthetic miRNAs can restore normal gene expression patterns,
inhibit tumor growth and enhance the sensitivity of cancer cells to
chemotherapeutic agents like cisplatin. Studies have concluded that
targeting miRNAs can be a viable therapeutic strategy in OC. By
modulating miRNA activity, researchers aim to correct the aberrant
gene expression that drives cancer progression and resistance.
Therefore, the miRNA profile not only aids in selecting effective
therapies but also forms the basis for developing miRNA-targeted
treatments. This dual approach shows the potential of miRNA
research to revolutionize OC therapy, leading to better patient
outcomes (12).
The present review aimed to assess the current
research landscape concerning OC treatment within the past decades,
and the outcomes of the review are categorized into the following
distinct dimensions: i) Understanding the role of miRNAs in
influencing treatment outcomes; ii) harnessing miRNAs to improve
therapy outcomes; and iii) miRNA-enhanced adjunctive strategies for
therapy outcomes. In the former dimension, the review identified
the underlying mechanisms of miRNAs that have been elucidated to
contribute to therapy, addressing both their impacts on treatment
efficacy and the development of resistance. In the two latter
dimensions, a review of previously published studies that centered
around miRNAs and OC revealed potential avenues for enhancing the
current treatment strategies in the clinical practice.
Materials and methods
A systematic search was performed by first searching
with keywords that were built around two key terms, namely:
‘Ovarian cancer’ and ‘miR’. More detailed terminology was
elaborated on for each term, which further combined to construct
comprehensive search keywords, allowing for a broader background
while maintaining the study's emphasis (Table SI). To guarantee optimal studies
that might suit the aims of the current investigation, high
false-positive search results were tolerated, in which
‘therapy’-related terms were not included in the search keywords.
The keywords were then used in the PubMed database (https://punmed.ncbi.nlm.nih.gov/), using the
PubMed Advanced Search Builder tool. Subsequently, the titles and
abstracts of the eligible studies were retrieved from the
database.
The systematic review process was as follows: First,
two independent reviewers scanned titles and abstracts by applying
the following inclusion criteria: i) A focus on human OC; ii) use
of and a focus on miRNAs; and iii) a focus on chemotherapy.
Subsequently, the full report of all studies that passed the first
screening were reviewed. The inclusion criteria for the second
screening were as follows: i) Use of synthetic miRNAs; and ii) an
available full text in the English language. All discrepancies
between two reviewers were resolved by discussions to reach an
agreement. The reasons for the exclusion of studies is summarized
in Table SII. Finally, the
following information was extracted from all the studies that
passed the second screening: Year of publication, country of first
author, key findings, tissues/cell lines, therapy type and miR of
interest (including its regulatory functions). The reporting and
workflow of the present systematic review study followed the
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
reporting guidelines (15).
Results and Discussion
Systematic search results
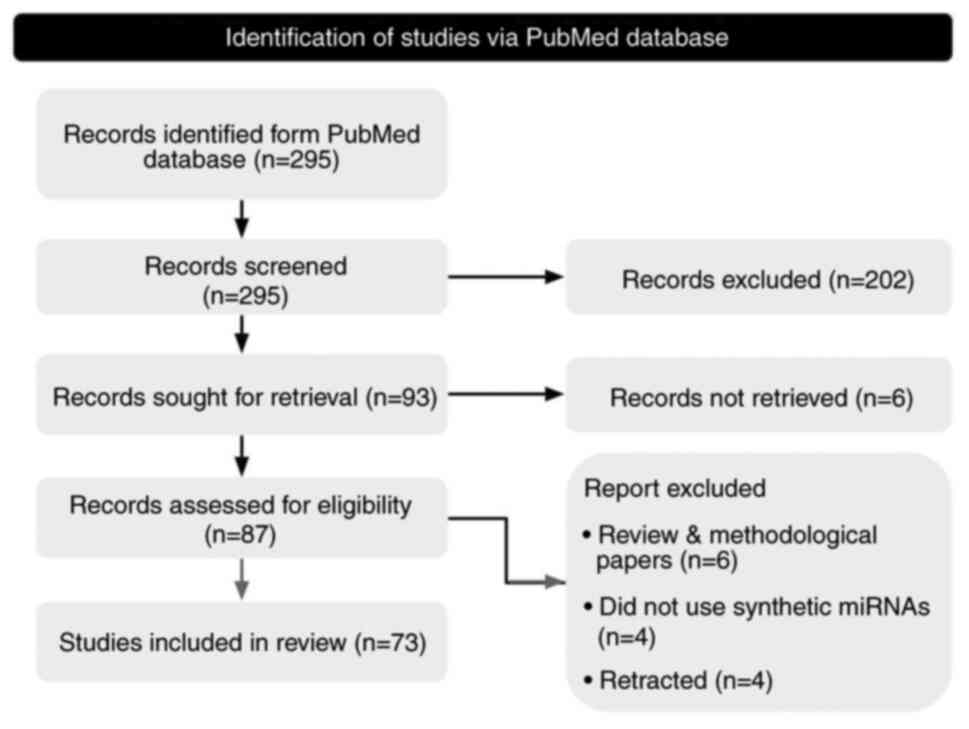
As of July 2023, the keyword search resulted in 295
publications from the PubMed repository. The two-step screening
process resulted in 73 eligible publications for information
extraction (Fig. 1). All eligible
studies exclusively used tissue samples and/or cell lines. However,
this outcome was not predetermined; namely, sample types were not
specifically defined during the search process as one of selection
criteria. Notably, there was a relatively low annual publication
count on the topic of interest, in particular the association of
miRNAs with ovarian cancer chemotherapy (Fig. S1), averaging only two publications
per year. The countries of the first authors were also mostly
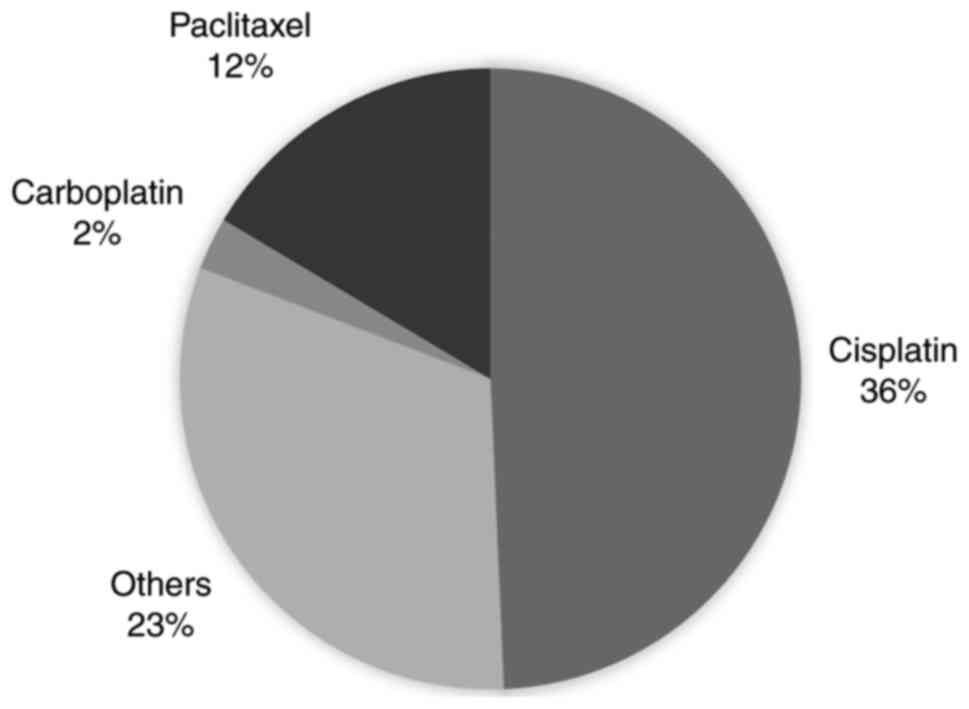
centralized to China and the United States (Fig. S2). Furthermore, 36% of the eligible
studies focused on cisplatin therapy (Fig. 2). Cisplatin is a potent chemotherapy
drug used in the treatment of several cancers, including OC. It is
part of a class of drugs known as platinum-containing compounds and
it exerts its anticancer effects by binding to and damaging the DNA
in cancer cells. This damage interferes with the ability of the
cells to divide and grow, ultimately leading to cell death
(16).
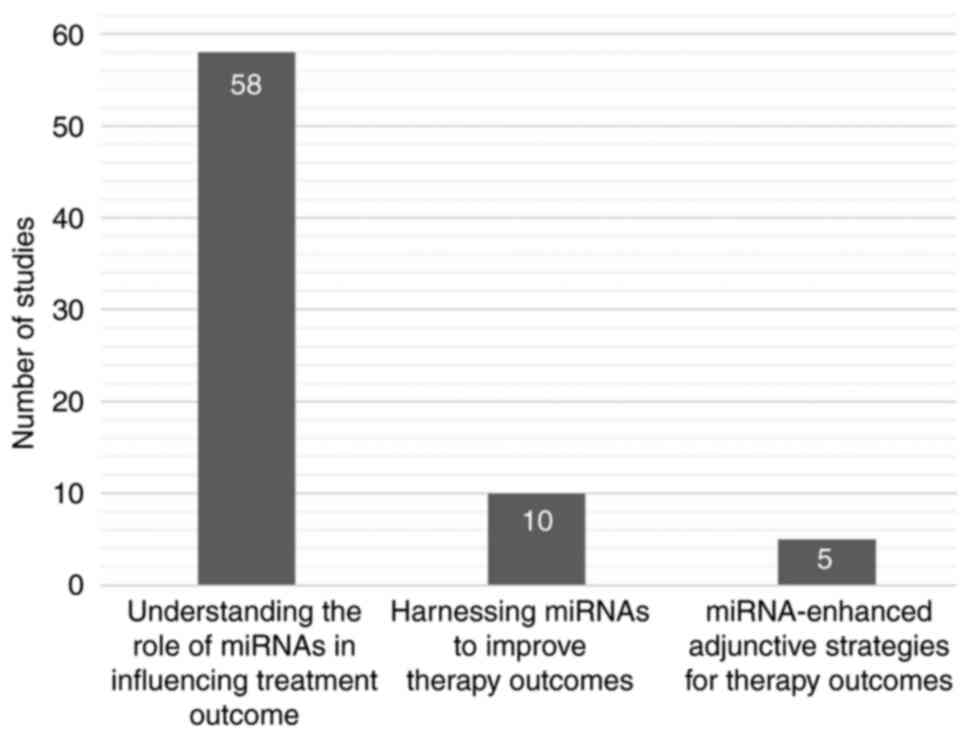
There was a notable proportion of studies amongst
the eligible studies that focused on understanding the role of
miRNAs in influencing treatment outcomes (79.45%; Fig. 3), indicating substantial unknown
aspects regarding treatment outcomes in OC from the perspective of
miRNAs. This hypothesis was strengthened by the wide variety of
miRNAs studied in the eligible studies, in which there were
cumulatively 66 miRNAs investigated. A total of nine of those
miRNAs were identified by the Human Gene Database (17), which were associated with OC,
although they were not specific to the disease. Moreover, four
miRNAs were not reported to be associated with any diseases
(Table SIII).
Understanding the role of miRNAs in
influencing treatment outcome
OC is known for its high mortality rate, often due
to chemoresistance. This resistance significantly limits the
effectiveness of treatment and often leads to the recurrence of the
disease and poor patient outcomes (10,18).
Table SIV presents the eligible
studies that evaluated the association between miRNAs and therapy
outcomes in OC, with a focus on studies that used miRNAs to assess
why specific therapeutic interventions yielded suboptimal
results.
miR-21 is a well-known miR that is associated with
therapy resistance in OC. It is known as oncomiR in several other
cancers, including cervical, colorectal and breast cancer (19). Abnormally highly expressed miR-21
may regulate drug resistance via augmented apoptotic pathways
(20). As such, whether through
natural compounds like icariin or by modulating c-MYB expression,
targeting miR-21 may hold promise for enhancing the efficacy of
therapies, particularly against drug resistance in OC. Icariin was
assessed by Li et al (21)
as a potential alternative therapy for OC. Icariin, the primary
bioactive compound present in epimedium, a traditional Chinese
medicinal herb, demonstrates diverse pharmacological properties
including bolstered immune function, anticancer potential,
cardiovascular enhancement and modulation of endocrine activity
(21). Moreover, icariin has been
reported to suppress miR-21 expression in OC cells, although the
precise mechanisms underlying modulation of miR-21 expression by
icariin remains unknown (21).
Another study on cisplatin-resistant therapy indicated that miR-21
serves a crucial role in c-MYB-induced cisplatin resistance
(22). c-MYB is a transcription
factor protein encoded by the MYB gene and dysregulation of c-MYB
has been implicated in several cancers, including OC, where it
contributes to tumor progression and chemotherapy resistance
(22). Silencing c-MYB has been
reported to lead to reduced miR-21 levels, decreased
epithelial-mesenchymal transmission (EMT), lowered cisplatin
resistance and increased β-catenin phosphorylation (22).
Cisplatin stands as the prominent platinum-based
therapy entwined with miR regulation, based on the results of the
present systematic review, which revealed that 36% of the total
eligible studies focused on cisplatin therapy. The emergence of
cisplatin resistance poses a formidable challenge to effective
treatment. The following miRNAs were mentioned due to their pivotal
roles in orchestrating this resistance phenomenon: i) A high
expression of miR-93 has been reported to be associated with
cisplatin resistance and it can target genes that regulate
apoptosis. L-tetrahydropalmatine (L-THP; an anticancer compound
obtained mainly from genera Stephania and Corydalis)
has been reported to suppress miR-93 expression whilst increasing
PTEN levels, a pivotal tumor suppressor in OC (23). Furthermore, PTEN small interfering
(si)RNA-treated cells increased survival, which was reversed by the
AKT inhibitor Triciribine. L-THP enhanced OC cell sensitivity to
cisplatin by modulating the miR-93/PTEN/AKT pathway (23); ii) in cisplatin-resistant OC cells,
miR-1271 is often overexpressed. It can target tumor suppressors
and cell cycle regulators like p53 and cyclin B1, promoting cell
survival and reducing apoptosis. This, in turn, makes cancer cells
more resistant to the cytotoxic effects of cisplatin (24); iii) miR-302 is known to influence
cisplatin resistance through multiple mechanisms. It can affect
drug transporters and drug-metabolizing enzymes. In certain cases,
miR-302 can downregulate copper transporters such as solute carrier
family 31 member 1, leading to decreased intracellular cisplatin
accumulation. This limits the access of the drug to its target DNA
(25); iv) miR-138-5p appears to
influence cisplatin resistance through its ability to regulate
cellular senescence and stemness. The miR was reported to be
downregulated in cisplatin resistance cell-lines. Increasing
miR-138-5p levels (either by inducing HOX transcript antisense RNA
siRNA or by its mimics) improved chemosensitivity and reduced
enhancer of zeste 2 polycomb repressive complex 2 subunit and
sirtuin 1 expression, which are key players in cisplatin resistance
(26); and v) high expression of
miR-149-3p in OC tissues has been reported in cisplatin-resistant
OC cells. Knockdown of miR-149-3p inhibited cisplatin resistance
and malignant traits in OC cells. Mechanistically, miR-149-3p
targeted cyclin dependent kinase inhibitor 1A and TIMP
metallopeptidase inhibitor 2 to promote cisplatin resistance and
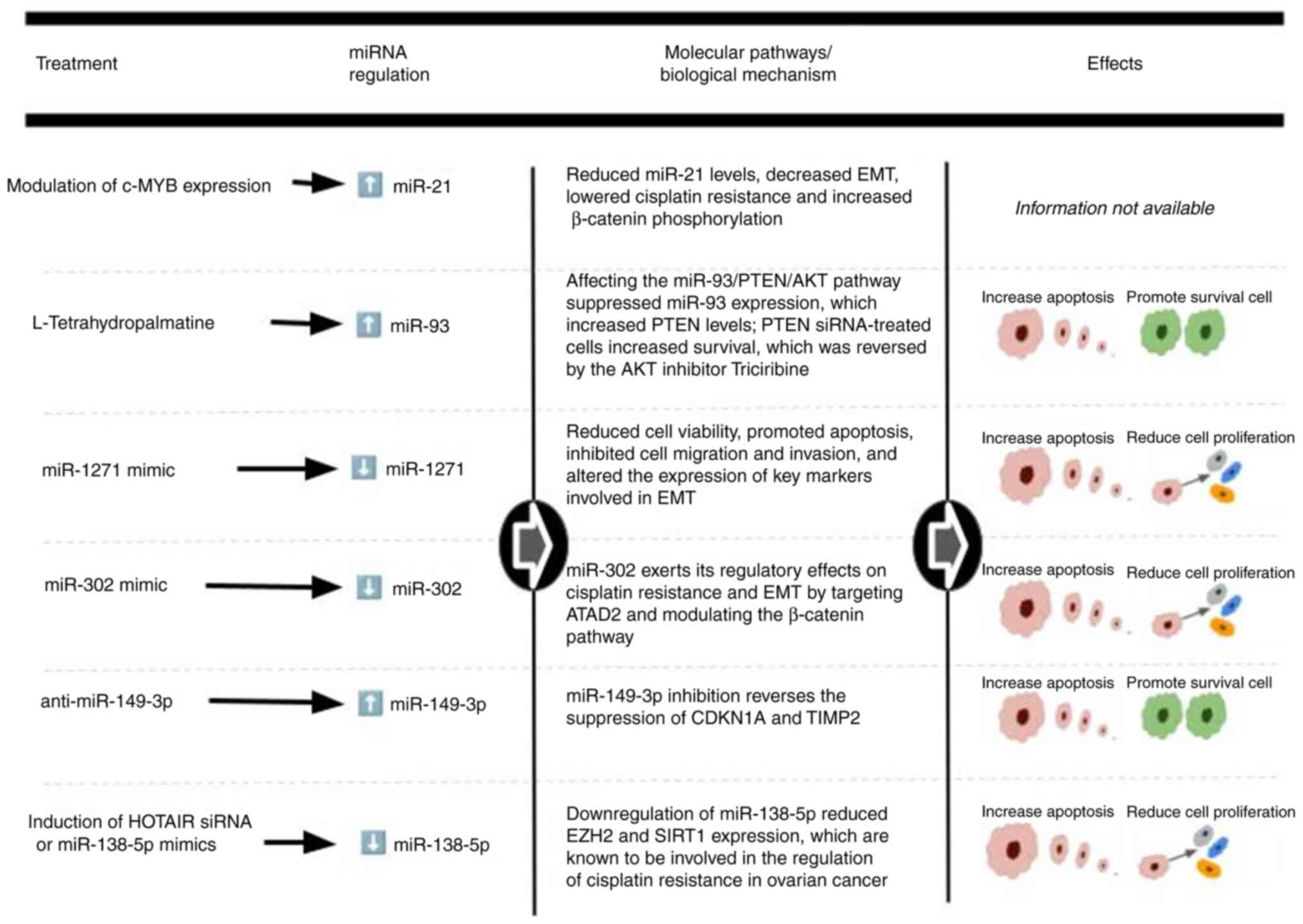
EMT in OC (27). Fig. 4 summarizes key insights on cisplatin
resistance from the eligible studies presented in this
sub-section.
Notably, there appears to be potential associations
between the aforementioned miRNAs, indicating overlapping molecular
pathways or biological processes affected in OC. Table I provides a comparative analysis of
the effectiveness of those miRNAs: miR-21 and miR-93 are associated
with cisplatin resistance by regulating apoptosis pathways in
several types of cancer; miR-1271 and miR-149-3p are also
associated with cisplatin resistance in ovarian cancer cells by
modulating multiple pathways involved; and miR-302 and miR-138-5p
are associated with drug transport in cancer cells through the stem
pathway and several other pathways. However, the aforementioned
miRNAs require further experimental validation to ensure their
significance and clinical implications, which further may offer
promise for optimizing therapy outcomes in OC management.
 | Table I.Summary of potential associations and
comparative analysis of the effectiveness of certain microRNAs in
enhancing therapy outcomes. |
Table I.
Summary of potential associations and
comparative analysis of the effectiveness of certain microRNAs in
enhancing therapy outcomes.
| miR | Potential
association | Comparative
analysis |
|---|
| miR-21 and
miR-93 | Associated with
cisplatin resistance by regulating apoptotic pathways | Targeting miR-21
may offer broader applicability in enhancing therapy outcomes due
to its involvement in multiple cancer types |
| miR-1271 and
miR-149-3p | Associated with
cisplatin resistance in ovarian cancer cells | Targeting
miR-149-3p may offer broader implications for overcoming cisplatin
resistance and malignant traits in ovarian cancer cells due to its
modulation of multiple pathways involved in drug resistance and
epithelial-mesenchymal transition |
| miR-302 and
miR-138-5p | Influenced
cisplatin resistance, albeit through distinct mechanisms; whilst
miR-302 affects drug transporters, miR-138-5p regulates cellular
senescence and stemness | Targeting
miR-138-5p may offer potential advantages in overcoming cisplatin
resistance by modulating stemness-related pathways, thereby
sensitizing cancer cells to chemotherapy-induced apoptosis.
However, combination strategies targeting both miRNAs may provide
synergistic effects in combating cisplatin resistance by
simultaneously modulating multiple pathways involved in drug
resistance |
Harnessing miRNAs to improve therapy
outcomes
The present review explored studies in which miRs
were used to improve standard treatment outcomes via the
transfection of anti-miRs or mimic-miRs. This pursuit aimed to both
enhance treatment effectiveness and gain more insights into
alternative instances where therapeutic interventions failed to
achieve the intended outcomes, by either reinstating miR expression
in tumor suppressor genes or by inhibiting the activity of
oncogenic miRNAs, referred to as ‘mimic-miRs’ and ‘anti-miRs’,
respectively. By assessing the scientific evidence, the present
review aimed to present how miRNA-based interventions offer their
potential for optimizing therapeutic strategies. The present review
identified 10 studies that reported that mimic- and/or anti-miRs
could help improve therapy outcomes, and cumulatively ten miRNAs of
interest were discussed (Table
II). Notably, none of the studies were registered in a clinical
trials database. Moreover, cisplatin and paclitaxel were the most
common type of therapies used in the experimental studies (Fig. 2).
 | Table II.List of observed miRNAs from studies
focused on improving therapy outcomes through miRNAs and other
substances. |
Table II.
List of observed miRNAs from studies
focused on improving therapy outcomes through miRNAs and other
substances.
| A, Downregulated
miR can improve therapy sensitivity |
|---|
|
|---|
| First author/s,
year | miRNA of
interest | anti-miR | mimic-miR | Type of
therapy | (Refs.) |
|---|
| Jin and Wei,
2015 | miR-23a | AntagomiR-23a | x | Cisplatin | (28) |
| Rupaimoole et
al, 2016 | miR-630 | Anti-miR
630a | miR-630 mimics | Anti-vascular
endothelial growth factor antibody and anti-miR-630 | (43) |
| García-Vázquez
et al, 2018 | let-7d-3p | let-7d-3p inhibitor
and let-7d-3p antagomiR | x | Carboplatin and
paclitaxel | (30) |
| Ye et al,
2011 | miR-376c | anti miR-376c | x | Cisplatin | (44) |
| Echevarría-Vargas
et al, 2014 | miR-21 | miR-21
inhibitors | x | Cisplatin | (45) |
| Vandghanooni et
al, 2018 |
| anti miR-21 | x | Cisplatin | (46) |
| Suardi et
al, 2020 | miR-324-5p | Antagonist
miR-324-5pa | x | Chitosan | (47) |
|
| B, Upregulated
miR can improve therapy sensitivity |
|
| First author/s,
year | miRNA of
interest |
anti-miR |
mimic-miR | Type of
therapy | (Refs.) |
|
| Wu et al,
2016 | miR-873 | anti-miR-873 | miR-630 mimics | Cisplatin and
paclitaxel | (35) |
| Bieg et al,
2019 | miR-424-3p | x | miR-424-3p
mimics | Cisplatin | (36) |
| Sun et al,
2015 | miR-186 | anti-miR-186 | x | Cisplatin and
paclitaxel | (37) |
| Suardi et
al, 2020 | miR-155-5p | x | miR-155-5p
mimics | Chitosan | (47) |
Jin and Wei (28)
indicated that when miR-23a inhibition was coupled with cisplatin
treatment in OC cells, there was a significant increase in the
effectiveness of cisplatin in limiting cell proliferation. miR-23a
had been previously reported to be upregulated in a
chemotherapy-resistant OC cell and this led to a marked increase in
the rate of apoptosis in cancer cells. However, this effect was not
observed when cells were treated with cisplatin alone, emphasizing
the role of miR-23a in suppressing cell death and promoting drug
resistance (29). Another study
reported how the downregulated miR let-7d-3p could improve therapy
outcomes (30). let-7d-3p is a miR
associated with tumor progression and chemotherapy resistance in
OC, where it is reported to be upregulated. let-7d-3p is the
passenger strand and used to be considered ‘non-functional’
(30). Moreover, it is part of the
let-7 family, which is comprised of 13 members that are highly
conserved across species. let-7 is a key regulator of
differentiation, pluripotency and apoptosis in eukaryotic cells,
and has a role in cell cycle deregulation, cell division,
proliferation, angiogenesis and apoptosis (31). Thus, let-7 can be used as a
molecular tool and marker in cancer therapy (31–34).
Previous studies have reported that miR-let-7d-3p can be used as a
diagnostic biomarker for non-invasive screening of ovarian cancer
and its precursors (31–34). To observe the potential of the miR
to enhance treatment outcomes, García-Vázquez et al
(30) used a two-fold strategy: i)
let-7d-3p was inhibited using an antagomiR, an antagonist designed
to suppress the activity of miRNAs. This inhibition led to a marked
decrease in cell proliferation and the activation of apoptosis.
These outcomes suggest that inhibiting let-7d-3p with the antagomiR
sensitizes OC cells to therapy and increases their susceptibility
to cell death; and ii) carboplatin was introduced. let-7d-3p
affects the Ras pathway, which is associated with therapy
resistance; therefore, targeting let-7d-3p with an antagomiR may
modulate key cellular processes and signaling pathways, including
ErbB and hypoxia-inducible factor-1, which are implicated in tumor
progression and drug resistance (30). As such, the antagomiR for let-7d-3p
inhibited the activity of carboplatin, leading to reduced cell
proliferation and increased apoptosis. Suppressing let-7d-3p using
an antagomiR sensitized the OC cells to carboplatin and enhanced
the effectiveness of the treatment by promoting apoptosis. This
two-pronged approach, using an antagomiR and chemotherapy, offers a
promising strategy to improve therapy outcomes and combat the
challenges of drug resistance in OC (30–34).
The present review also investigated studies that
focused on reinstating the expression of miRNAs which are
associated as tumor suppressor genes, indicating that the injection
of mimic-miR to OC cells (possibly in combination with another
treatment) may yield an improved therapy outcome (35). miR-873 mimics were introduced into
OC cells and the results revealed that the overexpression of
miR-873 led to a marked increase in the sensitivity of the cells to
cisplatin and paclitaxel. In another study, researchers aimed to
enhance therapy outcomes for OC by using miR-424-3p mimics. This
miR-based approach targeted the expression of galectin-3, an
anti-apoptotic protein that is often overexpressed in OC and
associated with resistance to chemotherapy, especially cisplatin
(28). miR-424-3p mimics were
transfected into OC cells to assess its impact on the expression of
galectin-3. The results demonstrated that miR-424-3p mimics notably
suppressed the expression of galectin-3 at the protein level. This
downregulation of galectin-3 is critical as this protein is
associated with inhibiting apoptosis, a process that serves a
central role in the response of the cell to chemotherapy. Following
that, the cell viability and proliferation rates decreased,
indicating a reduced capacity for the cells to grow and divide.
Moreover, apoptosis was enhanced, with the cells treated with
miR-424-3p mimics and cisplatin demonstrating a marked increase in
apoptosis compared with the control group. Another similar study
that assessed the enhancement of platinum-based-therapy through
mimic-miRs, reported similar results, although they used miR-186 as
their main object of observation (33).
miRNA-enhanced adjunctive strategies
for therapy outcomes
Finally, the present study investigated adjunctive
substances that have the potential to enhance therapy outcomes in
OC, with a focus on their interactions with anti- and mimic miRs.
The aim was to provide insights into emerging strategies that
leverage miRNAs in conjunction with other compounds to optimize the
effectiveness of OC treatments. For example, nanoparticles have
been used for targeted miR therapy as drug delivery systems for
miRNA-based cancer therapy (36–38)
(Table III). These nanoparticles
were engineered to efficiently deliver anti-miR or miR-mimics
payloads to OC cells, specifically either targeting overexpressed
oncogenic miRNAs or reinstating miR expression in tumor suppressor
genes. The five studies presented in Table III demonstrated innovative
approaches to OC therapy by emphasizing the use of nanotechnology
and miRNA-based strategies to target oncogenic miRNAs, sensitize
drug-resistant cells, and enhance the precision and effectiveness
of treatment.
 | Table III.Observed miRNAs from studies which
focused on improving therapy outcomes through microRNAs and other
substances. |
Table III.
Observed miRNAs from studies which
focused on improving therapy outcomes through microRNAs and other
substances.
| First author/s,
year | miRNA of
interest |
anti-/mimic-miRs | Substance | (Refs.) |
|---|
| Bertucci et
al, 2019 | miR-21 | anti-miR-21 | CGKRK-pSiNP | (38) |
| Javanmardi et
al, 2020 |
|
| PEG2k-CMPEI-ss | (39) |
| Vandghanooni et
al, 2020 | miR-214 | anti-miR-214 | Ap-CIS-PCL NPs | (40) |
| Gandham et
al, 2022 | miR-let-7b | miR-let-7b
mimics | Hyaluronic
acid-based nanoparticle | (41) |
| Zhao et al,
2022 | miR-484 | miR-484 mimics | RGD-modified
exosomes | (42) |
miR-21 serves a role in therapy resistance in OCs,
indicating its potential to regulate drug resistance via apoptosis.
Furthermore, it serves an important role in the oncogenic process
as indicated by its association with the high proliferation, low
apoptosis, high invasion and metastatic potential of cancer cells.
Whilst evaluating the potential of anti-miR-21 as a therapeutic
strategy for countering the oncogenic effects of miR-21 in OC,
studies have concurrently explored the enhancement of treatment
outcomes through the efficient nanoparticle-based delivery of
anti-miR-21 to cancer cells whilst minimizing off-target effects.
Biodegradable porous silicon nanoparticles were engineered to
encapsulate an anti-miR-21 locked nucleic acid payload (38). Additionally, these nanoparticles
displayed a tumor-homing peptide that enabled targeted
distribution. This targeting peptide, CGKRK, ensured that the
nanoparticles accumulated primarily in the tumor environment. CGKRK
is a tumor-tracking peptide discovered by phage display techniques
and it shows high selective binding affinity to neovascular
endothelial cells and tumor tissue, but not to non-tumorigenic
cells (39). Another nanoparticle,
redox-sensitive, Polyethylene (PEG)-shielded carboxymethyl
polyethylenimine (PEI) nanogels were used as a delivery system for
anti-miR-21 (39). These nanogels
were designed to efficiently deliver the anti-miR-21 to the OC
cells by modifying branched PEI, which involved PEGylation
(PEG2k-PEI) for steric shielding, redox-sensitive crosslinking for
nanogel synthesis (PEG2k-PEI-ss nanogels) and carboxymethylation
(PEG2k-CMPEI-ss) to modulate the properties of the polymer. Another
study aimed to develop a novel polymeric drug delivery system (DDS)
using star-shaped glucose-core polycaprolactone-polyethylene glycol
(Glu-PCL-PEG) block copolymer nanoparticles. This system was used
to deliver cisplatin and locked nucleic acid anti-miR-214 to OC
cells. The study confirmed that nucleolin-mediated endocytosis of
the targeted polymeric DDS containing both cisplatin and
anti-miR-214 A2780 R cells led to enhanced apoptosis (40).
Other studies with a specific focus on addressing
relapse and multidrug resistance issues have assessed let-7b
(41) and miR-484 (42). Both studies recognized tumor
suppressors with the capacity to target a range of oncogenes and
chemoresistance-related genes. let-7b has an important role in
growth and proliferation and induces apoptosis of cancer cells by
inhibiting cell cycle progression through pathways such as CYP2J2
regulation and Wnt/B-catenin pathway. Downregulation of let-7b
levels in clinical OC has been linked to chemotherapy resistance,
heightened proliferation, invasion and relapse in EOCs. The
restoration of let-7b expression was pivotal as it can
significantly heighten sensitivity to chemotherapy whilst
inhibiting oncogenic pathways and thwarting chemoresistance
mechanisms (41). To ensure
effective delivery of let-7b to tumor cells, innovative
nanoparticle systems (namely, hyaluronic acid-based nanoparticles
and Glu-PCL-PEG nanoparticles) were used to act as versatile
delivery vehicles adept at transporting nucleic acids like miRNA.
The delivery of let-7b to tumor cells involved using HA-PEI as a
delivery vehicle, which is a versatile system known for efficient
nucleic acid delivery, including miR (41). The strategic combination of let-7b
with these nanoparticles represents a multifaceted approach aimed
at reprogramming the miR profile within tumor cells.
Another study that also assessed a tumor suppressor
miRNA, provided mechanistic insights into how the delivery of
miR-484 via exosomes impacted the tumor vasculature and
chemotherapy sensitization (42).
It revealed that miR-484 may serve a role in vessel normalization,
which enhances the response of cancer cells to chemotherapy-induced
apoptosis. Mechanistically, miR-484 may achieve this effect by
simultaneously inhibiting the expression of VEGF-A in cancer cells
and its corresponding receptors in endothelial cells. The research
demonstrates how miR-484 and exosomal delivery contributed to
vascular normalization and chemotherapy sensitization in OC.
Limitations
Despite the comprehensive nature of this systematic
review, several limitations may affect the generalizability and
applicability of the findings. First, this study was limited to the
PubMed database, potentially excluding relevant studies indexed in
other databases, such as Scopus, Web of Science or Embase. Second,
the included studies varied widely in their methodologies, miRNAs
investigated and therapeutic interventions used. This heterogeneity
makes it challenging to draw definitive conclusions and compare
results across studies. Finally, while the review highlighted
associations between miRNAs and treatment outcomes, detailed
mechanistic insights were often lacking. Understanding the precise
molecular mechanisms is crucial for the development of effective
therapeutic strategies.
Conclusion
The present systematic review highlights the role of
miRNAs in enhancing OC treatment outcomes. Half of the selected
studies, which focused on understanding the impact of miRNAs on
treatment outcomes, highlighted the presence of numerous unanswered
questions, indicating significant unknowns about OC therapy from
the perspective of miRNAs and the need for more comprehensive and
ongoing research in this area. The present analysis reveals
potential associations between miRNAs that may imply those miRNAs
affect similar molecular pathways or biological processes in OC.
However, further research is needed to confirm their significance
and clinical implications. Furthermore, the innovative use of
nanotechnology for targeted miR delivery represents a significant
advancement in treatment precision. The findings of the present
review demonstrate the intricate relationship between miRNAs and
therapy outcomes, providing valuable insights for future research
directions and the development of miRNA-based therapeutic
interventions in OC management.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HMARP designed the study, formulated the strategy
for the literature search, performed the review and information
extraction, confirmed the authenticity of the data, wrote and
reviewed the article. PWN performed a systematic search, reviews
and information extraction, confirmed the authenticity of the data,
drafted and wrote the article. HP and SMH analyzed the data,
drafted and reviewed the article. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Webb PM and Jordan SJ: Epidemiology of
epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol.
41:3–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shao C, Guo H, Chen L, Chen J, Wang L and
Wang H: Prognostic factors and clinic-pathologic characteristics of
ovarian tumor with different histologic subtypes-a SEER database
population study of 41,376 cases. Transl Cancer Res. 12:1937–1950.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lavoro A, Scalisi A, Candido S, Zanghì GN,
Rizzo R, Gattuso G, Caruso G, Libra M and Falzone L: Identification
of the most common BRCA alterations through analysis of germline
mutation databases: Is droplet digital PCR an additional strategy
for the assessment of such alterations in breast and ovarian cancer
families? Int J Oncol. 60:582022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sekine M, Nishino K and Enomoto T:
Differences in ovarian and other cancers risks by population and
BRCA mutation location. Genes (Basel). 12:10502021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vos S, van Diest PJ and Moelans CB: A
systematic review on the frequency of BRCA promoter methylation in
breast and ovarian carcinomas of BRCA germline mutation carriers:
Mutually exclusive, or not? Crit Rev Oncol Hematol. 127:29–41.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurman RJ and Shih IM: The dualistic model
of ovarian carcinogenesis: Revisited, revised, and expanded. Am J
Pathol. 186:733–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doubeni CA, Doubeni AR and Myers AE:
Diagnosis and management of ovarian cancer. Am Fam Physician.
93:937–944. 2016.PubMed/NCBI
|
|
10
|
Chandra A, Pius C, Nabeel M, Nair M,
Vishwanatha JK, Ahmad S and Basha R: Ovarian cancer: Current status
and strategies for improving therapeutic outcomes. Cancer Med.
8:7018–7031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortez AJ, Tudrej P, Kujawa KA and
Lisowska KM: Advances in ovarian cancer therapy. Cancer Chemother
Pharmacol. 81:17–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alshamrani AA: Roles of microRNAs in
ovarian cancer tumorigenesis: Two decades later, what have we
learned? Front Oncol. 10:10842020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee RC, Feinbaum RL and Ambrost V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinose Y, Sawada K, Nakamura K and Kimura
T: The role of microRNAs in ovarian cancer. Biomed Res Int.
2014:2493932014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC and Mulrow CD: PRISMA_2020_flow_diagram_new_SRs_v1
[Internet]. BMJ. 3722017.Available from:. http://www.prisma-statement.org/PRISMAStatement/FlowDiagram
|
|
16
|
Aldossary SA: Review on pharmacology of
cisplatin: Clinical use, toxicity and mechanism of resistance of
cisplatin. Biomed Pharmacol J. 12:7–15. 2019. View Article : Google Scholar
|
|
17
|
Stelzer G, Rosen N, Plaschkes I, Zimmerman
S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et
al: The GeneCards suite: From gene data mining to disease genome
sequence analyses. Curr Protoc Bioinformatics. 54:1.30.1–1.30.33.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S, Li MY, Liu Y, Vlantis AC, Chan
JYK, Xue L, Hu BG, Yang S, Chen MX, Zhou S, et al: The role of
microRNA in cisplatin resistance or sensitivity. Expert Opin Ther
Targets. 24:885–897. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Zeng G and Jiang Y: The emerging
Roles of miR-125b in cancers. Cancer Manag Res. 12:1079–1088. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan JK, Blansit K, Kiet T, Sherman A,
Wong G, Earle C and Bourguignon LY: The inhibition of miR-21
promotes apoptosis and chemosensitivity in ovarian cancer. Gynecol
Oncol. 132:739–744. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Jiang K and Zhao F: Icariin
regulates the proliferation and apoptosis of human ovarian cancer
cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol
Rep. 33:2829–2836. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XY, Li YF, Ma H and Gao YH:
Regulation of MYB mediated cisplatin resistance of ovarian cancer
cells involves miR-21-wnt signaling axis. Sci Rep. 10:68932020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong J, Xing C, Wang LY, Xie SS and Xiong
WD: L-Tetrahydropalmatine enhances the sensitivity of human ovarian
cancer cells to cisplatin via microRNA-93/PTEN/Akt cascade. J BUON.
24:701–708. 2019.PubMed/NCBI
|
|
24
|
Chen Y, Wang L and Zhou J: Effects of
microRNA-1271 on ovarian cancer via inhibition of
epithelial-mesenchymal transition and cisplatin resistance. J
Obstet Gynaecol Res. 45:2243–2254. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ge T, Liu T, Guo L, Chen Z and Lou G:
MicroRNA-302 represses epithelial-mesenchymal transition and
cisplatin resistance by regulating ATAD2 in ovarian carcinoma. Exp
Cell Res. 396:1122412020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Ai H, Fan X, Chen S, Wang Y and
Liu L: Knockdown of long non-coding RNA HOTAIR reverses cisplatin
resistance of ovarian cancer cells through inhibiting
miR-138-5p-regulated EZH2 and SIRT1. Biol Res. 53:182020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J and Liu L: MiR-149-3p promotes the
cisplatin resistance and EMT in ovarian cancer through
downregulating TIMP2 and CDKN1A. J Ovarian Res. 14:1652021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin AH and Wei ZL: Molecular mechanism of
increased sensitivity of cisplatin to ovarian cancer by inhibition
of microRNA-23a expression. Int J Clin Exp Med. 8:13329–13334.
2015.PubMed/NCBI
|
|
29
|
Chhabra R, Dubey R and Saini N:
Cooperative and individualistic functions of the microRNAs in the
miR-23a~27a~24-2 cluster and its implication in human diseases. Mol
Cancer. 9:2322010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
García-Vázquez R, Gallardo Rincón D,
Ruiz-García E, Meneses García A, Hernández De La Cruz ON,
Astudillo-De La Vega H, Isla-Ortiz D, Marchat LA, Salinas-Vera YM,
Carlos-Reyes Á, et al: let-7d-3p is associated with apoptosis and
response to neoadjuvant chemotherapy in ovarian cancer. Oncol Rep.
39:3086–3094. 2018.PubMed/NCBI
|
|
31
|
Zheng H, Zhang L, Zhao Y, Yang D, Song F,
Wen Y, Hao Q, Hu Z, Zhang W and Chen K: Plasma miRNAs as diagnostic
and prognostic biomarkers for ovarian cancer. PLoS One.
8:e778532013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langhe R, Norris L, Saadeh FA,
Blackshields G, Varley R, Harrison A, Gleeson N, Spillane C, Martin
C, O'Donnell DM, et al: A novel serum microRNA panel to
discriminate benign from malignant ovarian disease. Cancer Lett.
356:628–636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kobayashi M, Salomon C, Tapia J, Illanes
SE, Mitchell MD and Rice GE: Ovarian cancer cell invasiveness is
associated with discordant exosomal sequestration of Let-7 miRNA
and miR-200. J Transl Med. 12:42014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chung YW, Bae HS, Song JY, Lee JK, Lee NW,
Kim T and Lee KW: Detection of microRNA as novel biomarkers of
epithelial ovarian cancer from the serum of ovarian cancer
patients. Int J Gynecol Cancer. 23:673–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu DD, Li XS, Meng XN, Yan J and Zong ZH:
MicroRNA-873 mediates multidrug resistance in ovarian cancer cells
by targeting ABCB1. Tumor Biol. 37:10499–10506. 2016. View Article : Google Scholar
|
|
36
|
Bieg D, Sypniewski D, Nowak E and Bednarek
I: MiR-424-3p suppresses galectin-3 expression and sensitizes
ovarian cancer cells to cisplatin. Arch Gynecol Obstet.
299:1077–1087. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun KX, Jiao JW, Chen S, Liu BL and Zhao
Y: MicroRNA-186 induces sensitivity of ovarian cancer cells to
paclitaxel and cisplatin by targeting ABCB1. J Ovarian Res.
8:802015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bertucci A, Kim KH, Kang J, Zuidema JM,
Lee SH, Kwon EJ, Kim D, Howell SB, Ricci F, Ruoslahti E, et al:
Tumor-targeting, MicroRNA-silencing porous silicon nanoparticles
for ovarian cancer therapy. ACS Appl Mater Interfaces.
11:23926–23937. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Javanmardi S, Tamaddon AM, Aghamaali MR,
Ghahramani L and Abolmaali SS: Redox-sensitive, PEG-shielded
carboxymethyl PEI nanogels silencing MicroRNA-21, sensitizes
resistant ovarian cancer cells to cisplatin. Asian J Pharm Sci.
15:69–82. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vandghanooni S, Eskandani M, Barar J and
Omidi Y: Antisense LNA-loaded nanoparticles of star-shaped
glucose-core PCL-PEG copolymer for enhanced inhibition of
oncomiR-214 and nucleolin-mediated therapy of cisplatin-resistant
ovarian cancer cells. Int J Pharm. 573:1187292020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gandham SK, Rao M, Shah A, Trivedi MS and
Amiji MM: Combination microRNA-based cellular reprogramming with
paclitaxel enhances therapeutic efficacy in a relapsed and
multidrug-resistant model of epithelial ovarian cancer. Mol Ther
Oncolytics. 25:57–68. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao Z, Shuang T, Gao Y, Lu F, Zhang J, He
W, Qu L, Chen B and Hao Q: Targeted delivery of exosomal miR-484
reprograms tumor vasculature for chemotherapy sensitization. Cancer
Lett. 530:45–58. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rupaimoole R, Ivan C, Yang D, Gharpure KM,
Wu SY, Pecot CV, Previs RA, Nagaraja AS, Armaiz-Pena GN, McGuire M,
et al: Hypoxia-upregulated microRNA-630 targets Dicer, leading to
increased tumor progression. Oncogene. 35:4312–4320. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ye G, Fu G, Cui S, Zhao S, Bernaudo S, Bai
Y, Ding Y, Zhang Y, Yang BB and Peng C: MicroRNA 376c enhances
ovarian cancer cell survival by targeting activin receptor-like
kinase 7: Implications for chemoresistance. J Cell Sci.
124:359–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Echevarría-Vargas IM, Valiyeva F and
Vivas-Mejía PE: Upregulation of miR-21 in cisplatin resistant
ovarian cancer via JNK-1/c-Jun pathway. PLoS One. 9:e970942014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vandghanooni S, Eskandani M, Barar J and
Omidi Y: AS1411 aptamer-decorated cisplatin-loaded
poly(lactic-co-glycolic acid) nanoparticles for targeted therapy of
miR-21-inhibited ovarian cancer cells. Nanomedicine (Lond).
13:2729–2758. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Suardi RB, Ysrafil Y, Sesotyosari SL,
Martien R, Wardana T, Astuti I and Haryana SM: The effects of
combination of mimic miR-155-5p and antagonist miR-324-5p
encapsulated chitosan in ovarian cancer SKOV3. Asian Pac J Cancer
Prev. 21:2603–2608. 2020. View Article : Google Scholar : PubMed/NCBI
|