Introduction
In 2010, salivary gland carcinoma, a histological
type of salivary gland carcinoma with a morphology similar to
secretory carcinoma of the mammary gland, was reported as mammary
analogue secretory carcinoma (MASC) (1). In 2017, the WHO Classification of Head
and Neck Tumors (4th edition) classified MASC as a secretory
carcinoma (SC), establishing it as a new histologic type of
salivary gland cancer (2). The
histological types of salivary gland carcinoma are diverse and
often difficult to diagnose. Before the disease classification was
established as SC, it was most likely classified as acinic cell
carcinoma or cystadenocarcinoma (3). In addition, for the diagnosis of SC,
it is crucial to identify the ETV6-NTRK3 fusion gene (EN gene) by
molecular biological search in addition to immunohistochemical
stains such as S100 and mammaglobin (1).
SC accounts for only 5% of all salivary gland
malignancies, and the parotid gland is the predilection site. It is
most common in people in their 40s, with a male-to-female ratio of
1.4:1 (4). SC presents as a
painless, slow-growing mass (2),
and the main treatment is surgical resection. SC is considered a
low-grade malignant tumor because the prognosis of SC is generally
good, with a 10-year survival rate of 95% and a disease-free
survival rate of 89%, although isolated cases of cervical lymph
node metastasis and distant metastasis have been reported (4).
To the best of our knowledge, there have been no
previous reports of SCs with complete cystic lesions originating
from minor salivary glands of the buccal mucosa. Herein, we report
a case of SC of minor salivary gland origin in the buccal mucosa,
which was suspected to be a mucocele on preoperative imaging
examination.
Case report
A 46-year-old man visited the Department of Oral and
Maxillofacial Surgery at Saiseikai Senri Hospital in October 2022
with a chief complaint of swelling of the right buccal mucosa. He
had been aware of the swelling for approximately 18 months.
However, he left it untreated because it was painless. As the
swelling gradually increased in size, he opted to visit the
Department of Oral and Maxillofacial Surgery at Saiseikai Senri
Hospital. The patient's current medical history included
hypertension and cerebral infarction, and his only medication was
an antihypertensive drug. He was 169.5 cm tall and weighed 86.7 kg,
with a Body Mass Index of 30.2. On physical examination, there was
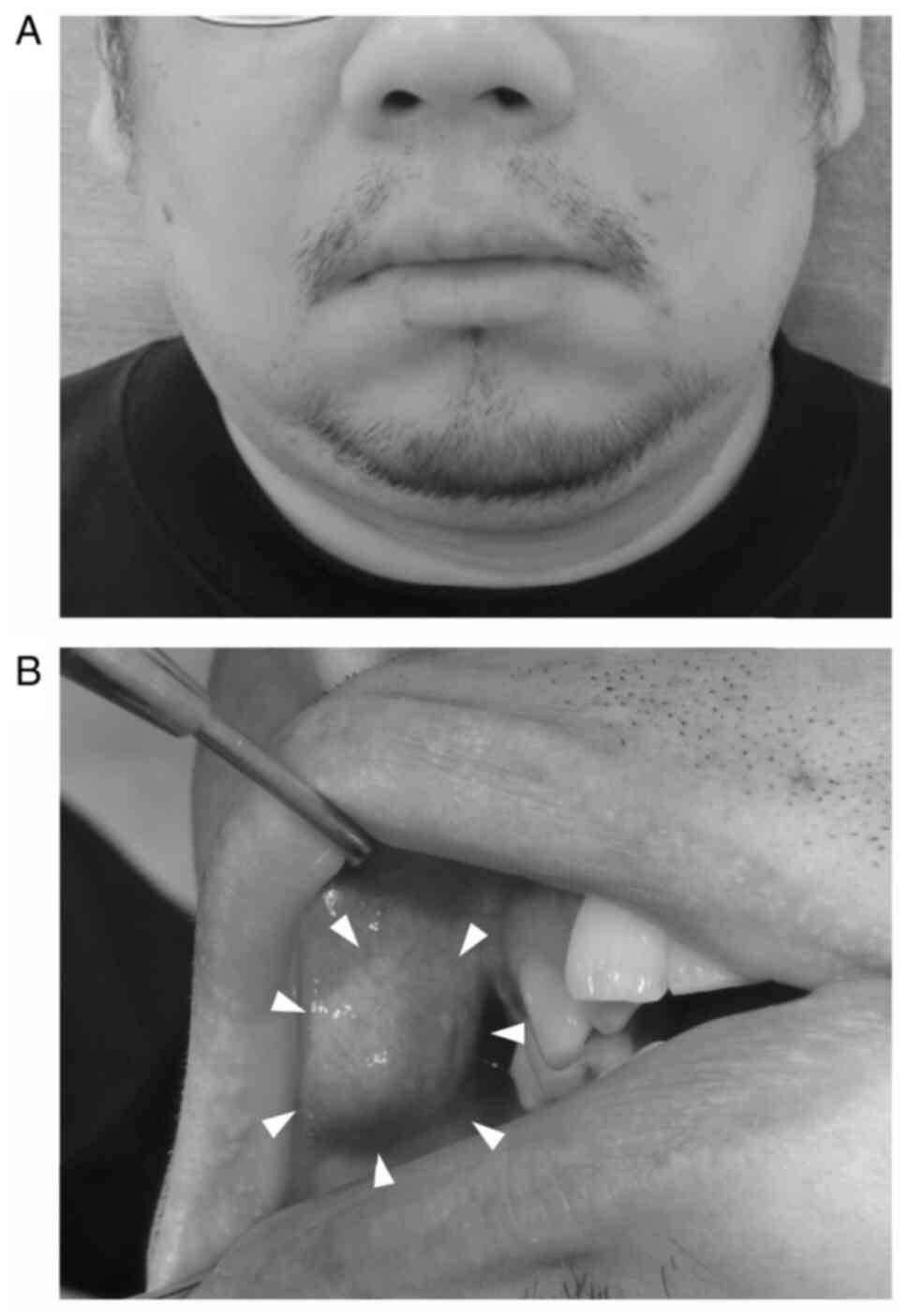
no obvious facial swelling or perceptual abnormalities (Fig. 1A). Intraoral examinations revealed a
submucosal lesion of approximately 20 mm in diameter located
anteriorly inferior to the parotid papilla in the right buccal
mucosa (Fig. 1B). The lesion was
dark blue, elastic hard, painless, submucosally mobile, and did not
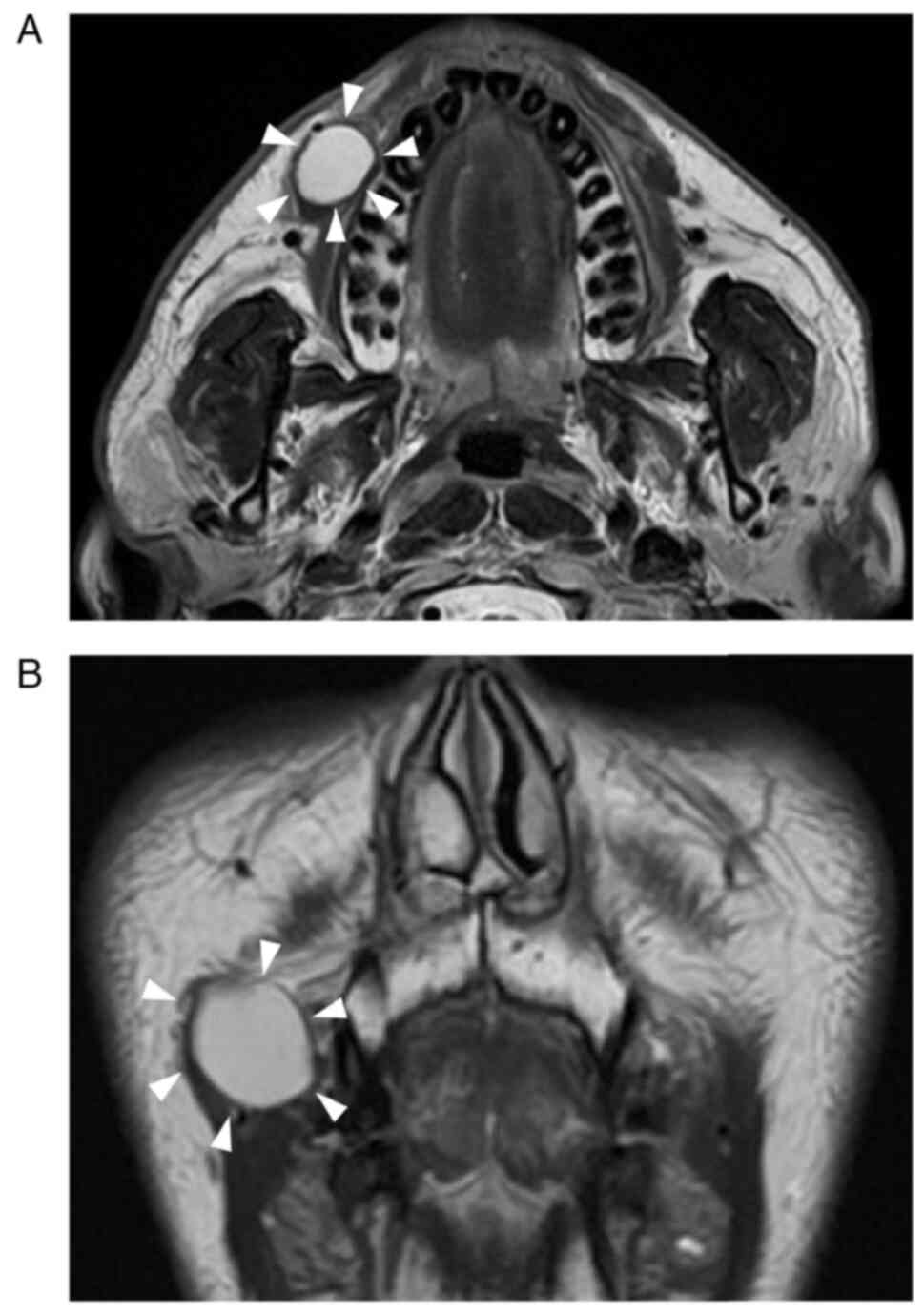
fade under pressure. Magnetic Resonance (MR) T2-weighted imaging
showed a high-signal area with a clear boundary and uniform
interior, approximately 18 mm in diameter, within the right buccal
mucosa (Fig. 2). Based on our
findings, the clinical diagnosis of a mucocele of minor salivary
gland origin in the right buccal mucosa was made. However, given
the size of the lesion and the length of time since the onset of
subjective symptoms, we considered the possibility that it was a
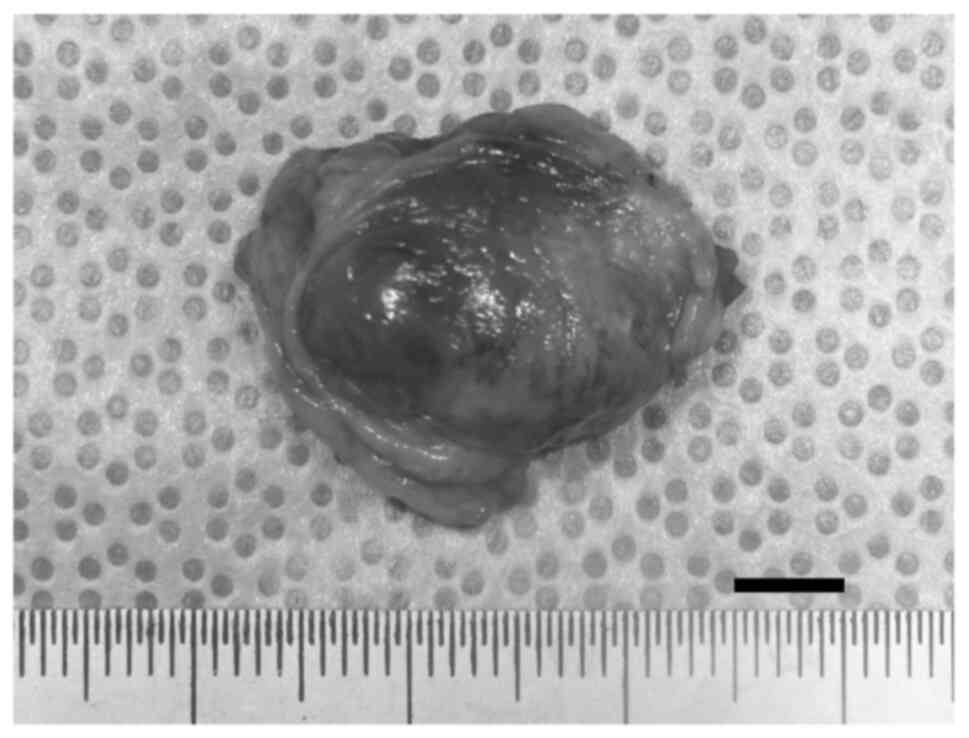
malignancy tumor. Therefore, although we did not perform fine
needle aspiration cytology (FNAC) before surgery, the lesion,
including a portion of the surrounding tissue, was resected under
general anesthesia in the month after his initial consultation
(Fig. 3).
The resected lesion was grossly cyst-like, with
yellowish-brown serous fluid. Hematoxylin-and-eosin (H&E)
staining revealed a cystic lesion covered with epithelium (Fig. 4A). The surface of the cystic lesion
was predominantly lined with a single cuboidal or cylindrical
epithelium layer (Fig. 4B). In some
areas, clusters of cells with a pale, foamy cytoplasm invading the
fibrous wall were observed (Fig.
4C). Additionally, in some areas, hobnail or papillary growth
was evident (Fig. 4D). The
cytoplasm was pale, foamy, and vacuolated. Nuclear atypia was not
prominent (Fig. 4E). Periodic acid
Schiff (PAS) staining revealed that the epithelial cells were
negative for both cytoplasmic mucin and cytoplasmic zymogen
granules, which are morphological characteristics of mucoepidermoid
carcinomas and acinic cell carcinomas, respectively (Fig. 4F). Immunohistochemical analysis
using cytokeratin 7 (CK7) and macrophage marker CD163 was performed
to rule out the invasive nature of the lesion; clusters of cells
invading the fibrous wall were CK7-negative (Fig. 4G) and CD163-positive indicating that
the foamy cells were mucophages (Fig.
4H). These histological analyses suggested that the cystic
lesion was non-invasive, and differential diagnosis included both
benign neoplastic lesions and malignant lesions, such as
cystadenoma, intraductal carcinoma, and SC. Immunohistochemical
staining showed that the epithelial cells were positive for CK7
(Fig. 4I), S100 (Fig. 4J), and mammaglobin (Fig. 4K), and negative for p63 and DOG1
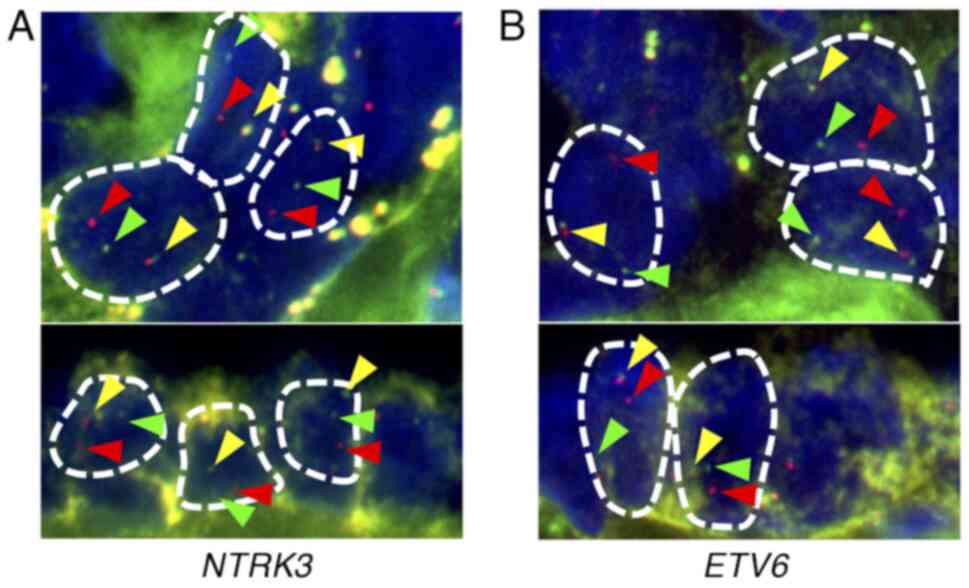
(not shown). Thereafter, we indirectly confirmed the presence of
the ETV6-NTRK3 fusion using NTRK3 break-apart
fluorescence in situ hybridization (FISH) (Fig. 5A), ETV6 break-apart FISH
(Fig. 5B), and pan-TRK
immunohistochemistry (Fig. 4L).
Thus, the patient was diagnosed with SC. The surgical margins were
negative.
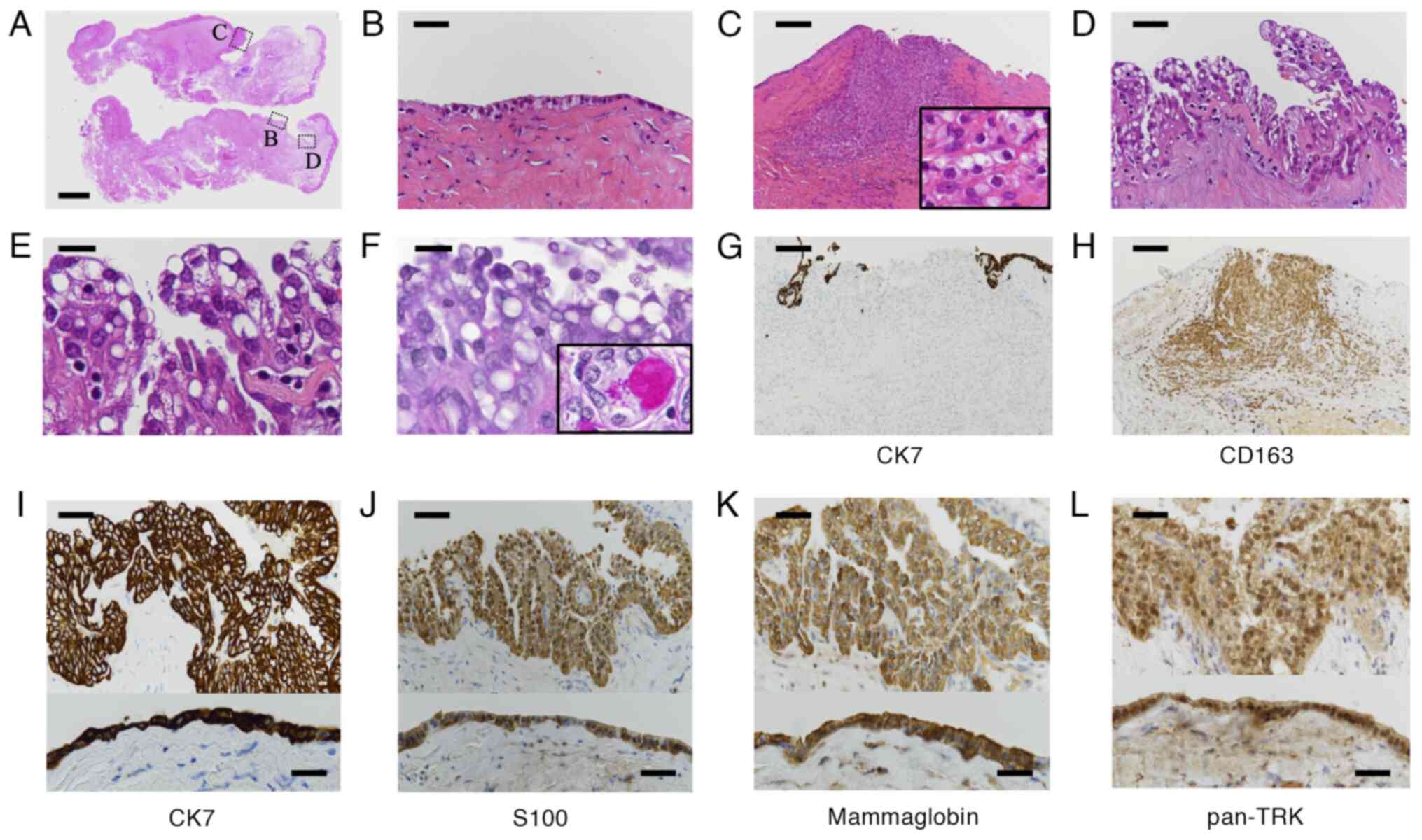 | Figure 4.Microscopic findings of the surgically
resected tumor. (A) Loupe image of the resected tissue. The lesion
was covered with cuboidal or cylindrical epithelium with focal
hobnail or papillary growth. Each of the dashed line squares (B-D)
are also shown at higher magnification. (B) Component of the cystic
lesion covered with flat cuboidal or cylindrical epithelium. (C)
Clusters of cells invading the fibrous wall. Inset, higher
magnification (×400) of the cells. The invading cells had pale and
foamy cytoplasm. (D) Component of the cystic lesion covered with
epithelium showing hobnail and papillary growth. (E) Higher
magnification of (D). The cytoplasm of the epithelium was pale,
foamy and vacuolated. Nuclear atypia was not prominent. (F) PAS
staining. Epithelial cells were negative for both cytoplasmic mucin
and zymogen granules. Inset: Positive control (magnification,
×400), cytoplasmic mucin and zymogen granules in a normal acinar
cell. (G and H) Immunohistochemistry of clusters of cells invading
the fibrous wall using anti-CK7 and CD163 [same area as in (C)].
Invading cells were (G) negative for CK7 and (H) positive for
CD163. (I-L) Immunohistochemistry against (I) anti-CK7, (J)
anti-S100, (K) anti-mammaglobin and (L) anti-pan-TRK. The upper
panel shows an area with papillary growth in (D), and the lower
panel shows an area with flat epithelium in (B). (A-E) H&E
staining images. Scale bar, 2 mm (A), 25 µm (B, D and I-L), 50 µm
(C, G and H) or 10 µm (E and F). CK7, cytokeratin 7; TRK, tyrosine
receptor kinase; PAS, periodic acid Schiff. |
Contrast-enhanced CT and FDG-PET/CT performed after
the diagnosis of SC showed no cervical lymph node metastasis or
distant metastasis (Fig. S1).
Ultimately, the patient was diagnosed with stage I secretory cancer
(pT1N0M0). Because more than 1 month had passed before the
diagnosis was confirmed, the margins of the resected lesion were
negative, and the wound was completely epithelialized, no
additional resection was performed. One year and 4 months
postoperatively, no local recurrence, cervical lymph node
metastasis, distant metastasis has been observed.
Discussion
Cystic salivary gland diseases that occur in the
buccal submucosa and other minor salivary gland areas include
mucoceles; ductal, epidermoid, dermoid, and lymphoepithelial cysts;
cystadenoma; cystic polymorphous adenoma; and intraductal,
low-grade mucoepidermoid, and acinic cell carcinomas (5–8). In
this case, because MRI T2-weighted imaging performed in the
preoperative examination showed a high-signal image with clear
boundaries and a homogeneous interior, and the lesion was painless
and mobile, a non-neoplastic mucocele was suspected and excision
was performed. Subsequently, H&E staining ruled out
non-neoplastic lesions, and immunostaining results led to strongly
suspected SC. Break-apart FISH analysis confirmed the presence of
the EN gene. Furthermore, the cystic epithelium was positive for
pan-TRK staining, which supported the presence of the NTRK gene,
and this case was diagnosed as SC (Figs. 4 and 5) (9–12).
Differentiating SC from acinic cell carcinoma is sometimes
difficult, and it is important to confirm the presence of Zymogen
granules by immunostaining for DOG1 and PAS staining if needed
(13).
SC may present with a mucocele-like appearance on
MRI, and FNAC is considered when salivary gland cystic disease is
suspected. One of the limitations of this study is that we did not
perform FNAC, which would have been useful for preoperative
diagnosis (14). In total, four
cases of SC with suspected cystic disease on preoperative imaging
examination have been reported in the past (Table I) (15–18).
Of the two patients who underwent preoperative FNAC, the EN gene
was detected in one case, and the diagnosis of SC was made. In
contrast, the other patient was not diagnosed with malignant
lesions. The other two patients who did not undergo FNAC had
preoperatively suspected cystic lesions or benign tumors.
Therefore, performing FNAC does not guarantee preoperatively
diagnosis of SC. The preoperative diagnosis of salivary gland
malignancies is very difficult to make because of the infrequency
of salivary gland malignancies themselves, the rarity of SC, and
the frequent absence of pain symptoms that characterize many
malignancies. For this reason, the possibility of malignancy must
always be considered when salivary gland disease is suspected. FNAC
for salivary gland disease has a sensitivity of 89–100% and
specificity of 85–92% (19), and
some reports indicate that FNAC for salivary gland cystic disease
in particular is more accurate than for all salivary gland lesions
(14).
 | Table I.SC with cystic lesion suspected on
preoperative imaging. |
Table I.
SC with cystic lesion suspected on
preoperative imaging.
| First author/s,
year | Case | Sex | Age, years | Location | MR image | Size | Image diagnosis | Biopsy | FNAC | Treatment | Recurrence | (Refs.) |
|---|
| Gupta et al,
2019 | 1 | Male | 65 | Left parotid | Unilocular | 6.2 cm diameter | Cyst | No | No | Resection | No | (15) |
| Helmy et al,
2020 | 2 | Female | 11 | Right parotid | Unilocular | 2.2×2.2 cm | Cyst | No | SC | Resection | No | (16) |
| Black et al,
2020 | 3 | Male | 18 | Right parotid | Unilocular | 2.5 cm diameter | Cyst | No | Benign tumor or
cyst | Resection | No | (17) |
| Shibata et al,
2020 | 4 | Female | 59 | Left Stensen's
duct | Unilocular | 2.1×2.0×2.3 cm | Stensen's duct
cyst | No | No | Resection | No | (18) |
| Present study | 5 | Male | 46 | Right buccal
mucosa | Unilocular | 1.8 cm diameter | Mucocele | No | No | Resection | No | - |
While there have been reports of the macrocystic
form of SC in major salivary glands, there have been no reports
thereof in minor salivary glands of the buccal mucosa, as in the
present case. The reports of macrocystic SC in major salivary
glands showed that tumor cells lining the lumen of the cyst may be
reduced or not infiltrate the surrounding area, thus making it
difficult to distinguish them from mucoceles or ductal cysts on
histopathologic H&E staining; immunohistochemical findings
targeting S-100 and mammaglobin and molecular biological searches
targeting the EN gene are therefore important for diagnosis
(13). Our case demonstrated the
presence of the macrocystic form of SC in minor salivary glands.
Both major and minor salivary glands may show mucocele-like images
on MR imaging and it is therefore important to always include
clinical and histopathological investigations for malignancy in
cystic lesions in the minor salivary glands, without excluding the
possibility of neoplastic lesions. The patient currently has no
signs of recurrence and metastasis, and the prognosis is considered
good. However, further studies are needed to accumulate more
cases.
In conclusion, in this report, we described a case
of SC, of minor salivary gland origin, in the buccal mucosa and
reviewed related literature. The unique characteristics and
treatment considerations highlighted in this case contribute
valuable insights to the field of oral oncology.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Motomu Tsuji
(Department of Pathology, Saiseikai Senri Hospital, Suita-shi,
Japan) for assisting with the diagnosis.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The manuscript was produced and reviewed by all
authors collectively. TC and YM confirm the authenticity of all the
raw data. TC, ATN, YU, TK, KW, SK, YM and NU designed the study.
TC, ATN, YU, KW, SK, YM and NU wrote the manuscript. TC, KW and TK
were involved in patient care. YU and KH were the pathologists in
charge of the case. All authors agreed to be held accountable for
all aspects of the work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient has been informed that there is no risk
to their anonymity in association with the publication of this
report. Written informed consent was obtained from the patient for
the publication of the present case report.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CK7
|
cytokeratin 7
|
|
FISH
|
fluorescence in situ
hybridization
|
|
FNAC
|
fine needle aspiration cytology
|
|
MASC
|
mammary analogue secretory
carcinoma
|
|
PAS
|
periodic acid Schiff
|
|
SC
|
secretory carcinoma
|
References
|
1
|
Skálová A, Vanecek T, Sima R, Laco J,
Weinreb I, Perez-Ordonez B, Starek I, Geierova M, Simpson RHW,
Passador-Santos F, et al: Mammary analogue secretory carcinoma of
salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto
undescribed salivary gland tumor entity. Am J Surg Pathol.
34:599–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Naggar AK, Chan JKC, Grandis JR, Takata
T and Slootweg PJ: World Health Organization Classification of Head
and Neck Tumours. 4th edition. IARC Press; Lyon: 2017
|
|
3
|
Chiosea SI, Griffith C, Assaad A and
Seethala RR: Clinicopathological characterization of mammary
analogue secretory carcinoma of salivary glands. Histopathology.
61:387–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alves LDB, de Melo AC, Farinha TA, de Lima
Araujo LH, Thiago LS, Dias FL, Antunes HS, Amaral Eisenberg AL,
Santos Thuler LC and Cohen Goldemberg D: A systematic review of
secretory carcinoma of the salivary gland: where are we? Oral Surg
Oral Med Oral Pathol Oral Radiol. 132:e143–e152. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takita H, Takeshita T, Shimono T, Tanaka
H, Iguchi H, Hashimoto S, Kuwae Y, Ohsawa M and Miki Y: Cystic
lesions of the parotid gland: Radiologic-pathologic correlation
according to the latest World Health Organization 2017
classification of head and neck tumours. Jpn J Radiol. 35:629–647.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Layfield LJ and Gopez EV: Cystic lesions
of the salivary glands: Cytologic features in fine-needle
aspiration biopsies. Diagn Cytopathol. 27:197–204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pinto A, Nosé V, Rojas C, Fan YS and
Gomez-Fernandez C: Searching for mammary analogue [corrected]
secretory carcinoma of salivary gland among its mimics. Mod Pathol.
27:30–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoang VT, Trinh CT, Nguyen CH, Chansomphou
V, Chansomphou V and Tran TTT: Overview of epidermoid cyst. Eur J
Radiol Open. 6:291–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Michal M, Hrabal P and Skálová A:
Oncocytic cystadenoma of the parotid gland with prominent
signet-ring cell features. Pathol Int. 48:629–633. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weber A, Langhanki L, Schütz A, Gerstner
A, Bootz F, Wittekind C and Tannapfel A: Expression profiles of
p53, p63, and p73 in benign salivary gland tumors. Virchows Arch.
441:428–436. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Palma S: Carcinoma ex pleomorphic
adenoma, with particular emphasis on early lesions. Head Neck
Pathol. 7 (Suppl 1):S68–S76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hung YP, Fletcher CDM and Hornick JL:
Evaluation of pan-TRK immunohistochemistry in infantile
fibrosarcoma, lipofibromatosis-like neural tumour and histological
mimics. Histopathology. 73:634–644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernandez-Prera JC, Holmes BJ, Valentino
A, Harshan M, Bacchi CE, Petersson F, Liu KK, Najfeld V and Wenig
BM: Macrocystic (mammary analogue) secretory carcinoma: An unusual
variant and a pitfall in the differential diagnosis of cystic
lesions in the head and neck. Am J Surg Pathol. 43:1483–1492. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maleki Z, Allison DB, Butcher M, Kawamoto
S, Eisele DW and Pantanowitz L: Application of the Milan system for
reporting salivary gland cytopathology to cystic salivary gland
lesions. Cancer Cytopathol. 129:214–225. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gupta K, Patwa HS, Niehaus AG, Filho GOF
and Lack CM: Mammary analogue secretory carcinoma presenting as a
cystic parotid mass. Radiol Case Rep. 14:1103–1108. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Helmy D, Chang J, Bishop JW, Vong A,
Raslan O and Ozturk A: MR imaging findings of a rare pediatric
parotid tumor: Mammary analogue secretory carcinoma. Radiol Case
Rep. 15:1460–1463. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Black M, Liu CZ, Onozato M, Iafrate AJ,
Darvishian F, Jour G and Cotzia P: Concurrent identification of
novel EGFR-SEPT14 fusion and ETV6-RET fusion in secretory carcinoma
of the salivary gland. Head Neck Pathol. 14:817–821. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shibata E, Morita KI, Kayamori K, Maruiwa
M, Michi Y, Sato Y, Takeuchi K, Ikeda T, Harada H and Yoda T:
Secretory carcinoma around Stensen's duct misdiagnosed as salivary
duct cyst. Int J Clin Exp Pathol. 13:2211–2217. 2020.PubMed/NCBI
|
|
19
|
Rameeza A and Hemalata M: Fine-needle
aspiration cytology of salivary gland lesions. J Oral Maxillofac
Pathol. 26:52–56. 2022. View Article : Google Scholar : PubMed/NCBI
|