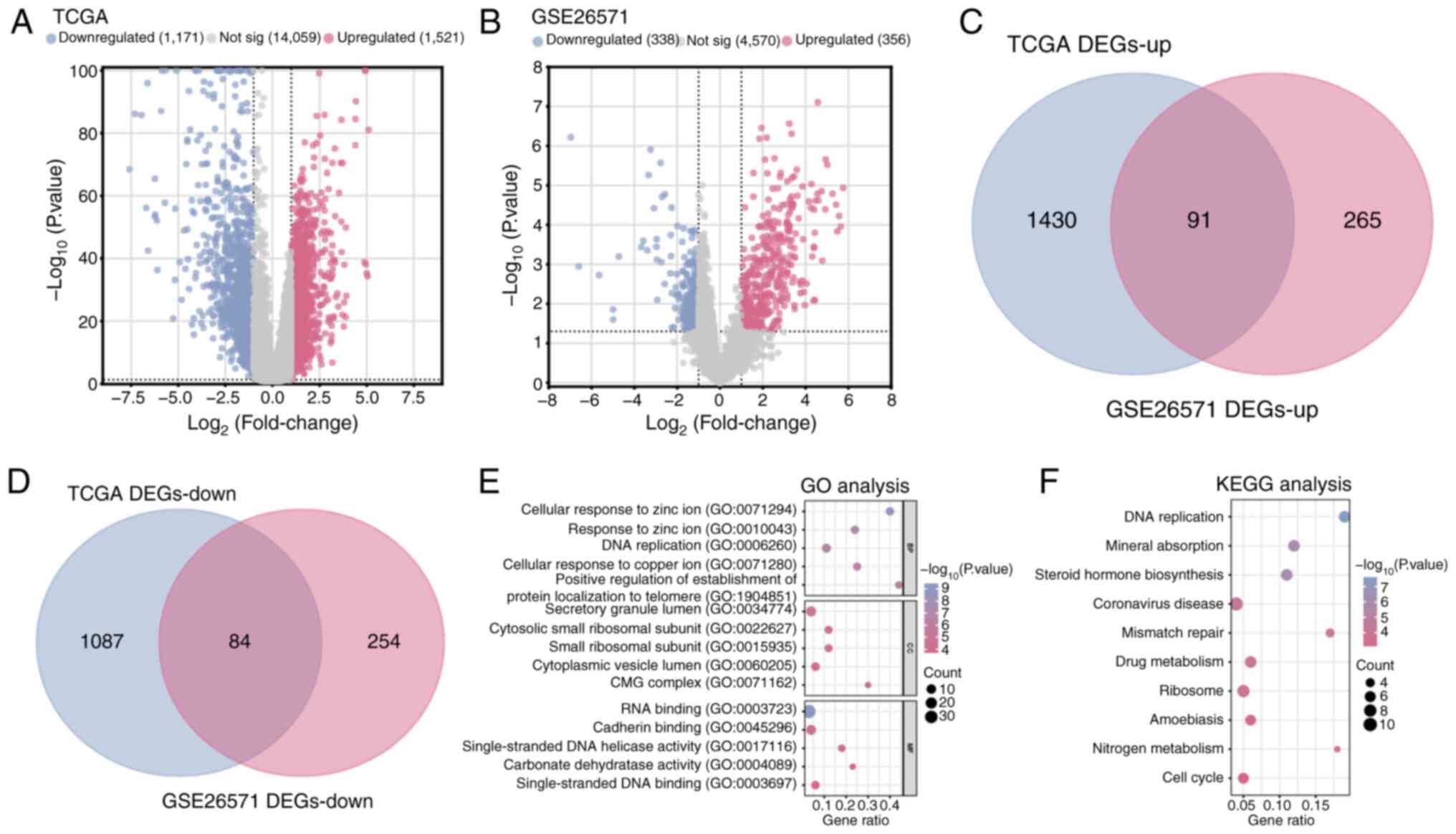
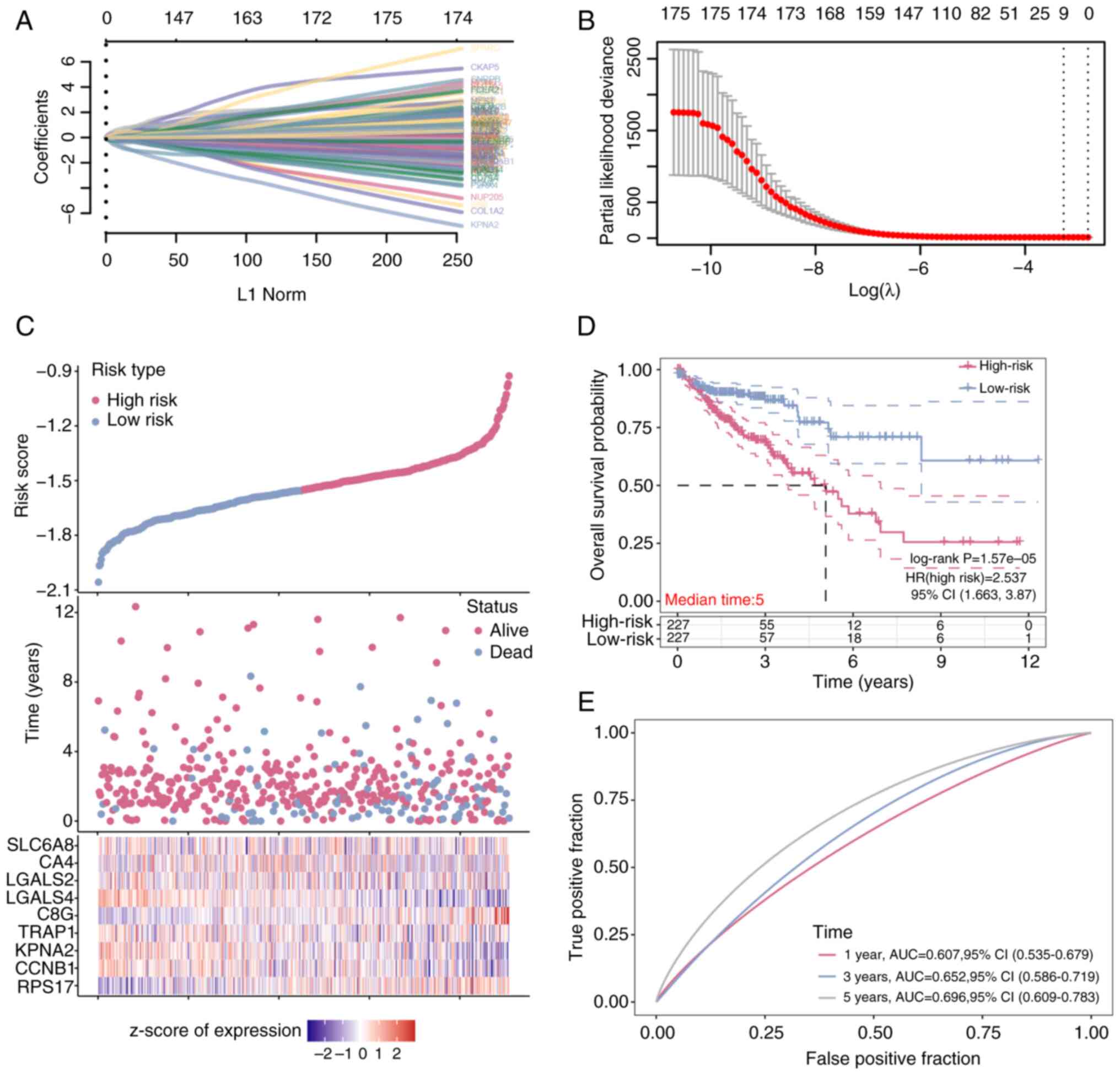
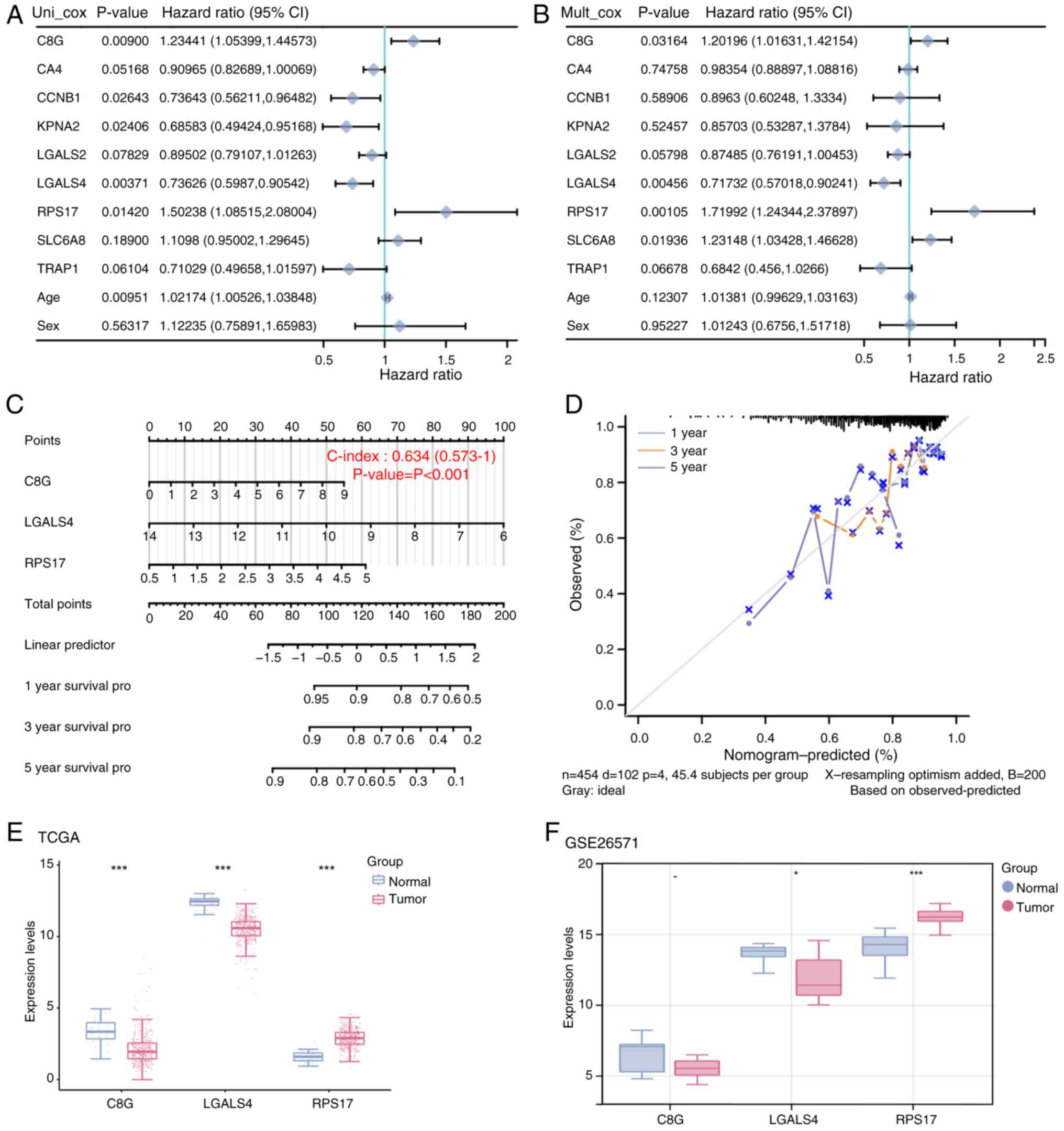
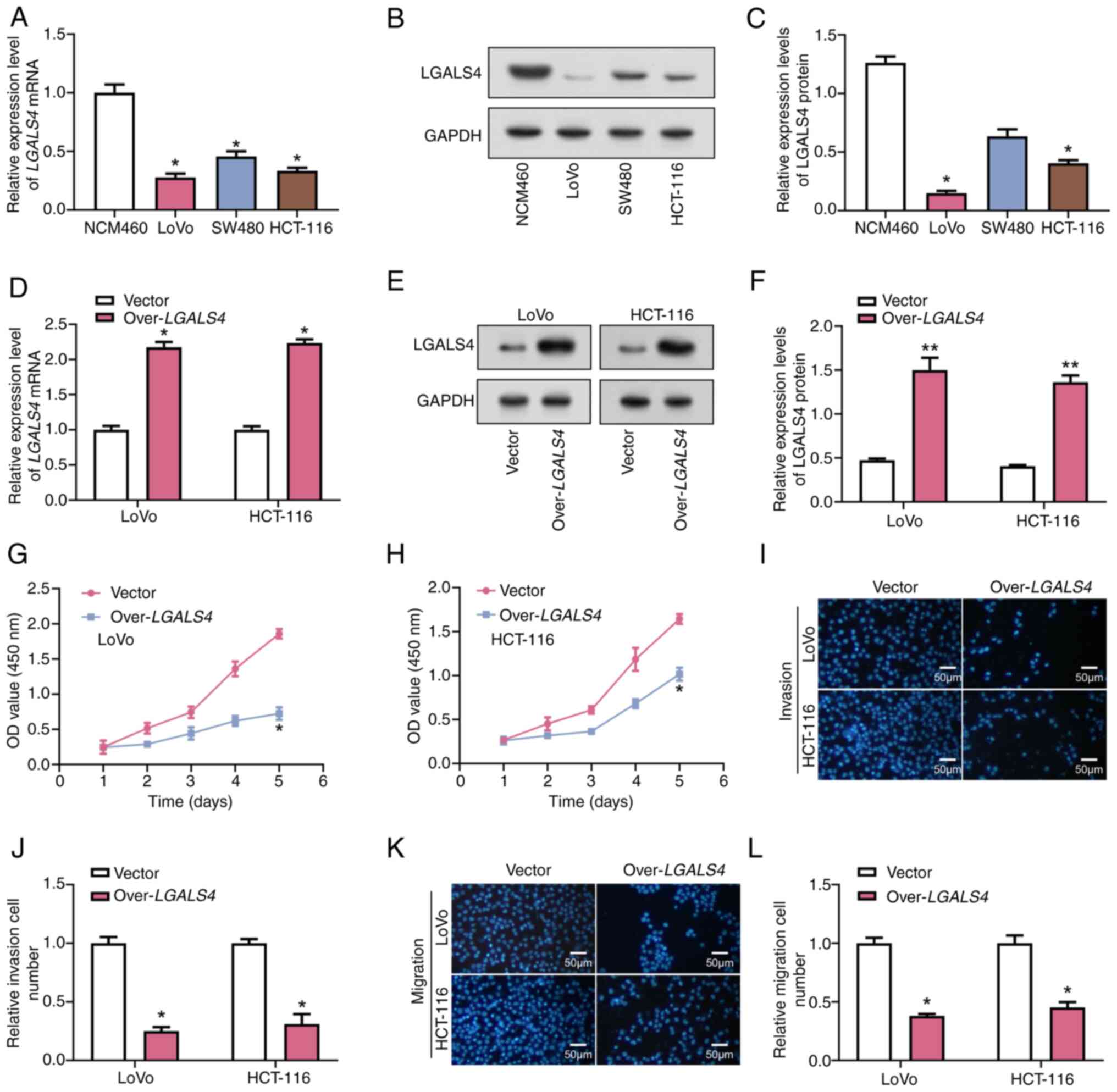
|
1
|
Roshandel G, Ghasemi-Kebria F and
Malekzadeh R: Colorectal cancer: Epidemiology, risk factors, and
prevention. Cancers. 16:15302024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parmar S and Easwaran H: Genetic and
epigenetic dependencies in colorectal cancer development.
Gastroenterol Report. 10:goac0352022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu J, Feng Q, Kim JH and Zhu Y: Combined
effect of healthy lifestyle factors and risks of colorectal
adenoma, colorectal cancer, and colorectal cancer mortality:
Systematic review and meta-analysis. Front Oncol. 12:8270192022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong MC, Huang J, Lok V, Wang J, Fung F,
Ding H and Zheng ZJ: Differences in incidence and mortality trends
of colorectal cancer worldwide based on sex, age, and anatomic
location. Clin Gastroenterol Hepatol. 19:955–966.e61. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao H, Ming T, Tang S, Ren S, Yang H, Liu
M, Tao Q and Xu H: Wnt signaling in colorectal cancer: Pathogenic
role and therapeutic target. Mol Cancer. 21:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satelli A, Rao PS, Thirumala S and Rao US:
Galectin-4 functions as a tumor suppressor of human colorectal
cancer. Int J Cancer. 129:799–809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michalak M, Golde V, Helm D, Kaltner H,
Gebert J and Kopitz J: Combining Recombinase-mediated cassette
exchange strategy with quantitative proteomic and Phosphoproteomic
analyses to inspect intracellular functions of the tumor suppressor
galectin-4 in colorectal cancer cells. Int J Mol Sci. 23:64142022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang GL, Pan LL, Huang T and Wang JH: The
transcriptome difference between colorectal tumor and normal
tissues revealed by single-cell sequencing. J Cancer. 10:5883–5890.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheraghi-Shavi T, Jalal R and Minuchehr Z:
TGM2, HMGA2, FXYD3, and LGALS4 genes as biomarkers in acquired
oxaliplatin resistance of human colorectal cancer: A systems
biology approach. PLoS One. 18:e02895352023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chandel NS: Glycolysis. Cold Spring Harb
Perspect Biol. 13:a0405352021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jaiswara PK, Gupta VK, Rawat SG, Tiwari
RK, Sonker P, Maurya RP and Kumar A: Targeting of Aerobic
Glycolysis: An Emerging Therapeutic Approach Against Colon Cancer.
Colon Cancer Diagnosis and Therapy. Vol 2. Springer; pp. 225–244.
2021, View Article : Google Scholar
|
|
13
|
Zuo S, Wu L, Wang Y and Yuan X: Long
non-coding RNA MEG3 activated by vitamin D suppresses glycolysis in
colorectal cancer via promoting c-Myc degradation. Front Oncol.
10:2742020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu Y, Wu G, Yan W, Zhan H and Sun P:
miR-146b-5p regulates cell growth, invasion, and metabolism by
targeting PDHB in colorectal cancer. Am J Cancer Res.
7:11362017.PubMed/NCBI
|
|
15
|
Zhu J, Wang S, Bai H, Wang K, Hao J, Zhang
J and Li J: Identification of five glycolysis-related gene
signature and risk score model for colorectal cancer. Front Oncol.
11:5888112021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong Y, Lei J, Zhao J, Lu Q, Feng Y, Qiao
T, Xin S, Han Y and Jiang T: A gene-based survival score for lung
adenocarcinoma by multiple transcriptional datasets analysis. BMC
Cancer. 20:10462020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He R, Zhang M, He L, Huang J, Man C, Wang
X, Lang Y and Fan Y: Integrated analysis of Necroptosis-related
genes for prognosis, immune microenvironment infiltration, and drug
sensitivity in colon cancer. Front Med (Lausanne). 9:8452712022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou S, Li L, Hou H, Zhou T and Zhou H:
Establishment of nomogram to predict overall survival and
cancer-specific survival of local tumor resection in patients with
colorectal cancer liver metastasis with unresectable metastases: A
large population-based analysis. Discover Oncol. 15:3152024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S, Su T, Zhang Y, Lee A, He J, Ge Q
and Wang L, Si J, Zhuo W and Wang L: Fusobacterium nucleatum
promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7.
Gut Microbes. 11:511–525. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J, Xiao Y, Liu B, Pan S, Liu Q, Shan Y,
Li S, Qi Y, Huang Y and Jia L: Exosomal MALAT1 sponges miR-26a/26b
to promote the invasion and metastasis of colorectal cancer via
FUT4 enhanced fucosylation and PI3K/Akt pathway. J Exp Clin Cancer
Res. 39:542020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang Q, Chen J, Di Z, Yuan W, Zhou Z, Liu
Z, Han S, Liu Y, Ying G, Shu X and Di M: TM4SF1 promotes EMT and
cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal
cancer. J Exp Clin Cancer Res. 39:2322020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aslan M, Hsu EC, Liu S and Stoyanova T:
Quantifying the invasion and migration ability of cancer cells with
a 3D Matrigel drop invasion assay. Biol Methods Protoc.
6:bpab0142021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mao Z, Xiao H, Shen P, Yang Y, Xue J, Yang
Y, Shang Y, Zhang L, Li X, Zhang Y, et al: KRAS(G12D) can be
targeted by potent inhibitors via formation of salt bridge. Cell
Discov. 8:52022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen H, Chen H, Zhang J, Wang Y, Simoneau
A, Yang H, Levine AS, Zou L, Chen Z and Lan L: cGAS suppresses
genomic instability as a decelerator of replication forks. Sci Adv.
6:eabb89412020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Zhang X, Wang W, Liu T, Ren J, Chen
S, Lu T, Tie Y, Yuan X, Mo F, et al: Ammonia promotes the
proliferation of bone marrow-derived mesenchymal stem cells by
regulating the Akt/mTOR/S6k pathway. Bone Res. 10:572022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Acharjee A, Agarwal P, Nash K, Bano S,
Rahman T and Gkoutos GV: Immune infiltration and prognostic and
diagnostic use of LGALS4 in colon adenocarcinoma and bladder
urothelial carcinoma. Am J Transl Res. 13:113532021.PubMed/NCBI
|
|
28
|
Principe M, Borgoni S, Cascione M,
Chattaragada MS, Ferri-Borgogno S, Capello M, Bulfamante S,
Chapelle J, Di Modugno F, Defilippi P, et al: Alpha-enolase (ENO1)
controls alpha v/beta 3 integrin expression and regulates
pancreatic cancer adhesion, invasion, and metastasis. J Hematol
Oncol. 10:162017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding L, Cao J, Lin W, Chen H, Xiong X, Ao
H, Yu M, Lin J and Cui Q: The roles of cyclin-dependent kinases in
cell-cycle progression and therapeutic strategies in human breast
cancer. Int J Mol Sci. 21:19602020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun L, Suo C, Li ST, Zhang H and Gao P:
Metabolic reprogramming for cancer cells and their
microenvironment: Beyond the Warburg Effect. Biochim Biophys Acta
Rev Cancer. 1870:51–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fang Y, Shen ZY, Zhan YZ, Feng XC, Chen
KL, Li YS, Deng HJ, Pan SM, Wu DH and Ding Y: CD36 inhibits
β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4
to repress colorectal tumorigenesis. Nat Commun. 10:39812019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang M, Song H, Yang S, Zhang Y, Tian Y,
Wang Y and Liu D: Deciphering the antibacterial mechanisms of
5-fluorouracil in Escherichia coli through biochemical and
transcriptomic analyses. Antibiotics. 13:5282024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malki A, ElRuz RA, Gupta I, Allouch A,
Vranic S and Al Moustafa AE: Molecular mechanisms of colon cancer
progression and metastasis: Recent insights and advancements. Int J
Mol Sci. 22:1302020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zou Y, Liu FY, Liu H, Wang F, Li W, Huang
MZ, Huang Y, Yuan XQ, Xu XY, Huang OP and He M: Frequent POLE1 p.
S297F mutation in Chinese patients with ovarian endometrioid
carcinoma. Mutat Res. 761:49–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zurlo D, Leone C, Assante G, Salzano S,
Renzone G, Scaloni A, Foresta C, Colantuoni V and Lupo A:
Cladosporol a stimulates G1-phase arrest of the cell cycle by
up-regulation of p21waf1/cip1 expression in human colon carcinoma
HT-29 cells. Mol Carcinog. 52:1–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Patil R, Sontakke T, Biradar A and Nalage
D: Zinc: An essential trace element for human health and beyond.
Food Health. 5:132023. View Article : Google Scholar
|
|
37
|
Haase H and Rink L: Zinc signals and
immune function. Biofactors. 40:27–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pelletier J, Thomas G and Volarević S:
Ribosome biogenesis in cancer: New players and therapeutic avenues.
Nat Rev Cancer. 18:51–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Villalobo A: Ca2+ signaling and Src
functions in tumor cells. Biomolecules. 13:17392023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zurlo D, Assante G, Moricca S, Colantuoni
V and Lupo A: Cladosporol A, a new peroxisome
proliferator-activated receptor γ (PPARγ) ligand, inhibits
colorectal cancer cells proliferation through β-catenin/TCF pathway
inactivation. Biochim Biophys Acta. 1840:2361–2372. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu C, Hong H, Zhang S, Zong Y, Ma J, Lu A,
Sun J and Zheng M: Identification of key genes and pathways
involved in microsatellite instability in colorectal cancer. Mol
Med Rep. 19:2065–2076. 2019.PubMed/NCBI
|
|
42
|
Günther J and Galuska SP: A brief history
of galectin evolution. Front Immunol. 14:11473562023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ferlizza E, Solmi R, Miglio R, Nardi E,
Mattei G, Sgarzi M and Lauriola M: Colorectal cancer screening:
Assessment of CEACAM6, LGALS4, TSPAN8 and COL1A2 as blood markers
in faecal immunochemical test negative subjects. J Adv Res.
24:99–107. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Watanabe M, Takemasa I, Kaneko N, Yokoyama
Y, Matsuo EI, Iwasa S, Mori M, Matsuura N, Monden M and Nishimura
O: Clinical significance of circulating galectins as colorectal
cancer markers. Oncol Rep. 25:1217–1226. 2011.PubMed/NCBI
|
|
45
|
Zhou J, Belov L, Chapuis P, Chan C,
Armstrong N, Kaufman KL, Solomon MJ, Clarke SJ and Christopherson
RI: Surface profiles of live colorectal cancer cells and tumor
infiltrating lymphocytes from surgical samples correspond to
prognostic categories. J Immunol Methods. 416:59–68. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Passarella S, Schurr A and Portincasa P:
Mitochondrial transport in glycolysis and gluconeogenesis:
Achievements and perspectives. Int J Mol Sci. 22:126202021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gupta R and Gupta N, Gupta R and Gupta N:
Glycolysis and gluconeogenesis. Fundamentals of bacterial
physiology and metabolism. 267–287. 2021. View Article : Google Scholar
|
|
48
|
Zhou L, Yu X, Li M, Gong G, Liu W, Li T,
Zuo H, Li W, Gao F and Liu H: Cdh1-mediated Skp2 degradation by
dioscin reprogrammes aerobic glycolysis and inhibits colorectal
cancer cells growth. EBioMedicine. 51:1025702020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou CF, Li XB, Sun H, Zhang B, Han YS,
Jiang Y, Zhuang QL, Fang J and Wu GH: Pyruvate kinase type M2 is
upregulated in colorectal cancer and promotes proliferation and
migration of colon cancer cells. IUBMB Life. 64:775–782. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao J, Li J, Hassan W, Xu D, Wang X and
Huang Z: Sam68 promotes aerobic glycolysis in colorectal cancer by
regulating PKM2 alternative splicing. Ann Transl Med. 8:4592020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sethy C and Kundu CN: 5-Fluorouracil
(5-FU) resistance and the new strategy to enhance the sensitivity
against cancer: Implication of DNA repair inhibition. Biomed
Pharmacother. 137:1112852021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zou X, Liang J, Sun J, Hu X, Lei L, Wu D
and Liu L: Allicin sensitizes hepatocellular cancer cells to
anti-tumor activity of 5-fluorouracil through ROS-mediated
mitochondrial pathway. J Pharmacol Sci. 131:233–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zou K, JU JH and Xie H: Pretreatment with
insulin enhances anticancer functions of 5-fluorou-racil in human
esophageal and colonic cancer cells. Acta Pharmacol Sin.
28:721–730. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zuo T, Zhang Z, Jiang P, Zhang R, Ni D,
Yuan Y and Zhang S: Characterization of chikusetsusaponin IV and V
induced apoptosis in HepG2 cancer cells. Mol Biol Rep.
49:4247–4255. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Raeisi E and Mir LM: 2-NBDG, a fluorescent
analogue of glucose, as a marker for detecting cell
electropermeabilization in vitro. J Membr Biol. 245:633–642. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin HC, Chen YJ, Wei YH, Lin HA, Chen CC,
Liu TF, Hsieh YL, Huang KY, Lin KH, Wang HH and Chen LC: Lactic
acid fermentation is required for NLRP3 inflammasome activation.
Front Immunol. 12:6303802021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Akram M: Mini-review on glycolysis and
cancer. J Cancer Educ. 28:454–457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mondal P, Tiwary N, Sengupta A, Dhang S,
Roy S and Das C: Epigenetic reprogramming of the glucose metabolic
pathways by the chromatin effectors during cancer. Subcell Biochem.
100:269–336. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou Q, Li J, Pang J, Fan F, Li S and Liu
H: Gefitinib inhibits glycolysis and induces programmed cell death
in non-small cell lung cancer cells. Nan Fang Yi Ke Da Xue Xue Bao.
40:884–892. 2020.(In Chinese). PubMed/NCBI
|
|
60
|
Zu XY, Zhang QH, Liu JH, Cao RX, Zhong J,
Yi GH and Pizzorno G: ATP citrate lyase inhibitors as novel cancer
therapeutic agents. Recent Pat Anticancer Drug Discov. 7:154–167.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zuo Xm, Sun Hw, Fang H, Wu Y, Shi Q and Yu
YF: miR-4443 targets TRIM14 to suppress metastasis and energy
metabolism of papillary thyroid carcinoma (PTC) in vitro. Cell Biol
Int. 45:1917–1925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shah K and Kazi JU:
Phosphorylation-dependent regulation of WNT/Beta-catenin signaling.
Front Oncol. 12:8587822022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jung YS and Park JI: Wnt signaling in
cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and
the destruction complex. Exp Mol Med. 52:183–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
van Zuylen WJ, Rawlinson WD and Ford CE:
The Wnt pathway: A key network in cell signalling dysregulated by
viruses. Rev Med Virol. 26:340–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zou Y, Liu L, Meng J and Dai M: Circular
RNA circ_0068464 combined with microRNA-383 regulates Wnt/β-catenin
pathway to promote the progression of colorectal cancer.
Bioengineered. 13:5113–5125. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zou J, Huang Y, Chen Y, Wu Z, Xie H, Zhou
H and Xing C: FOXC2-induced circCASK aggravates colorectal cancer
progression by upregulating SIX1 expression. IUBMB Life.
75:659–672. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhou Y, Gu H, Shao B, Zhang S, Pall H,
Peixoto RD, Mok SRS and Zhu G: Glycolysis-related gene
dihydrolipoamide acetyltransferase promotes poor prognosis in
hepatocellular carcinoma through the Wnt/β-catenin and PI3K/Akt
signaling pathways. Ann Transl Med. 10:12402022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhou J, Su CM, Chen HA, Du S, Li CW, Wu H,
Tsai SH and Yeh YT: Cryptanshinone inhibits the glycolysis and
inhibits cell migration through PKM2/β-catenin axis in breast
cancer. Onco Targets Ther. 13:8629–8639. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wenxuan L, Liu L, Zhang L, Qiu Z, Wu Z and
Deng W: Role of gonadally synthesized steroid hormones in the
colorectal cancer microenvironment. Front Oncol. 13:13238262023.
View Article : Google Scholar : PubMed/NCBI
|