Introduction
Chronic myelogenous leukemia (CML) is one of the
common types of chronic leukemia in China that occurs in all age
groups (1). Though the traditional
therapeutic drugs such as hydroxycarbamide and interferon can
improve the symptoms in patients within a certain period, they can
neither cure this disease, nor block the disease progression
(2). An overwhelming majority of
patients will enter the acceleration phase or the blastic phase
after a period of chronic phase, and finally die of complications
such as hematopoietic failure or infection. The fusion gene bcr/abl
is the genetic and molecular biological feature of CML, and its
product Bcr/Abl kinase is the key factor of pathogenesis. Since
Bcr/Abl kinase possesses strong tyrosine kinase activity, the
tyrosine kinase inhibitors (TKIs) such as imatinib serve as the
first-line therapeutic drugs for leukemia applied in clinic
(3). It is found during treatment
that imatinib shows excellent therapeutic effect on the chronic
phase of CML; however, resistance occurs in some patients. As
demonstrated in research, Bcr/Abl kinase can activate multiple
intracellular signaling pathways, for instance, Ras/MAPK, Jun/STAT5
and PI3K/Akt/mTOR, and thus influence the cell cycle distribution
and cell apoptosis, leading to the genesis of CML.
MicroRNA (miRNA) is a type of single stranded
non-coding microRNA that is ~20 nt in length, and it mainly exerts
its posttranscriptional regulatory function through degrading its
target gene or inhibiting its translation by complete or incomplete
pairing with the target gene 3′-UTR (4). In-depth research on the mechanisms of
the production and precise action of miRNA demonstrated that miRNA
plays significant roles in the development regulation,
differentiation, proliferation, cell apoptosis and metabolism
(5). The disordered expression of
miRNA may participate in the genesis and development of a tumor,
including some hematological tumors. Because miRNA can directly
regulate the expression of some oncogenes or tumor suppressor genes
after transcription, it can exert functions that are similar to
tumor suppressor genes or oncogenes in the body; for instance,
miR-424 can induce apoptosis by targeting oncogene BCL-2; while
miR-21 negatively regulates the expression of the tumor suppressor
gene PTEN and promotes cell proliferation and invasion (5,6).
Matrix metalloproteinase-2 and −9 (MMP-2 and MMP-9)
have the important function of hydrolyzing gelatin, type I, IV, V,
VII and X collagens as well as elastin (7). Their corresponding tissue inhibitors
of metalloproteinase (TIMP) are TIMP-2 and TIMP-1, respectively
(8). It has been reported that many
solid tumors, such as human breast cancer, endometrial cancer,
brain tumor, colorectal cancer and pancreatic cancer, are
associated with high gelatinase expression (9). A recent study indicated that TIMP-2 is
closely related to the genesis and development of hematological
tumor diseases, such as leukemia and myelodysplastic syndrome
(10).
The common biological feature of a tumor is the
uncontrolled growth, the major molecular mechanism of which is the
inhibited cell malignant proliferation and apoptosis (11). The multiple pathological mechanisms
that are involved in the transformation, proliferation and
apoptosis of malignant tumors are correlated with the regulation of
signaling pathways, among which, RaS/Raf/ERK signal transduction
cascade pathway is one of the important pathways. The extracellular
signal-regulated kinase (ERK) is a subgroup of the mitogen actived
protein kinase (MAPK) family, which can be divided into ERKl and
ERK2 and is generally termed as ERKl/2 (11). ERKl/2 takes part in cell
proliferation, differentiation, transformation and apoptosis after
being activated by numerous growth factors and cytokines. Recent
research suggests that the excessively activated ERKl/2 is related
to the genesis of the tumor (11).
ERK is associated with the abnormal expression or enhanced activity
in multiple tumor tissues as well as tumor cell lines, such as
liver cancer, breast cancer, oral squamous cell carcinoma and
prostate cancer (12).
Tumor is a common malignancy, however, the
pathogenesis is still unclear (13). With the increasing in-depth research
on tumors, more and more signaling pathways that play important
regulatory effects on the growth and development of organism are
proved to be correlated with tumors, among which there are PI3K/Akt
signaling pathway, JAK/STAT signaling pathway, MAPK signaling
pathway and Wnt/Notch signaling pathway (14). PI3K/Akt signaling pathway is the
most extensively studied, it plays an important role in the
development of leukemia (15).
Numerous studies have demonstrated that EpsS, which participates in
EGFR signaling pathway, Rac signaling pathway, PI3K/Akt signaling
pathway and mitosis signaling pathway, also plays an important role
in the cell cycle and apoptosis (16). The purpose of this study was to
investigate the cancer mediation effect of miRNA-301a on apoptosis
of CML and its possible mechanism.
Materials and methods
Ethics statement
Bone marrow and peripheral blood samples were
evaluated from 42 CML patients and 8 normal volunteer at Department
of Hematology, The First Affiliated Hospital of Nanchang University
from 2012, September to 2012, December. All patients and volunteer
were obtained from the clinical trials and biomedical ethics
special committee of The First Affiliated Hospital of Nanchang
University. Every two months, we made a followed-up of the
patients.
Quantitative real-time PCR
Total RNA was extracted from CML patients or normal
volunteer samples or cell lines using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA). Total RNA (1 µg) was synthesized into
complementary DNA (cDNA) using reverse transcriptase (Epicentre,
Madison, WI, USA). A SYBR Green PCR kit (Takara, Dalian, China) was
used in quantitative real-time PCR. The primers for miR-301a were:
forward, 5′-GGCAGTGCAATAGTATTGT-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′. The relative miR-301a expression level was
determined by using the 2−ΔΔCT method.
Cells and reagents
The K562 cells were cultured in RPMI-1640 (Hyclone,
Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco,
Grand Island, NY, USA) in an incubator maintained at 37°C in an
atmosphere containing 5% CO2.
Plasmids transfection
miRNA-301a, si-TIMP2 and negative plasmids were
purchased from Genechem (Shanghai, China). K562 cells were
transfected using Lipofectamine 2000 transfection reagent
(Invitrogen).
Cytotoxicity assay
The K562 cells were seeded in 96-well plates at
1×105 cells per well, and 20 µl 3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
solution (5 mg/ml) was added to each well and incubated at 37°C for
4 h. After removing the medium, 100 µl of DMSO (Sigma) was added to
solubilize the crystals and the absorbance was measured using
scanning multiwell spectrophotometer (Model 550, Bio-Rad, Hercules,
CA, USA) at 450 nm.
Flow cytometric analysis
The K562 cells were seeded in 6-well plates at
2×106 cells per well and washed twice with
phosphate-buffered saline (PBS). K562 cells were incubated with
Annexin V-FITC and PI (BD Biosciences) for 15 min in the dark.
Apoptosis rate was analyzed using FACScan flow cytometry and
CellQuest analysis software (BD Biosciences, Franklin Lakes, NJ,
USA).
Western blotting
The K562 cells were seeded in 6-well plates at
2×106 cells per well and washed twice with PBS. Cells
were lysed using RIPA buffer containing a protease and phosphatase
inhibitor mixture (Roche). Total protein was quantified with the
BioRad Dc protein assay kit (Bio-Rad, Richmond, CA, USA). Protein
was loaded onto 6–10% sodium and electro-transferred to
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). Membranes were incubated with the primary antibody anti-Bax,
anti-Bcl-2, anti-TIMP2, anti-phosphorylation-ERK1/2,
anti-phosphorylation-AKT and GAPDH overnight at 4°C, followed by
incubation with the anti-rabbit secondary antibody for 1 h at room
temperature. Bands were detected by enhanced chemiluminescence
(ECL) detection system (Bio-Rad Laboratories).
Statistical analysis
The results are reported as means ± standard
deviation (SD). Student's t-test, one-way analysis of variance
(ANOVA) and the Dunnett-test were used to assess statistical
significance. Values of p<0.05 were considered statistically
significant.
Results
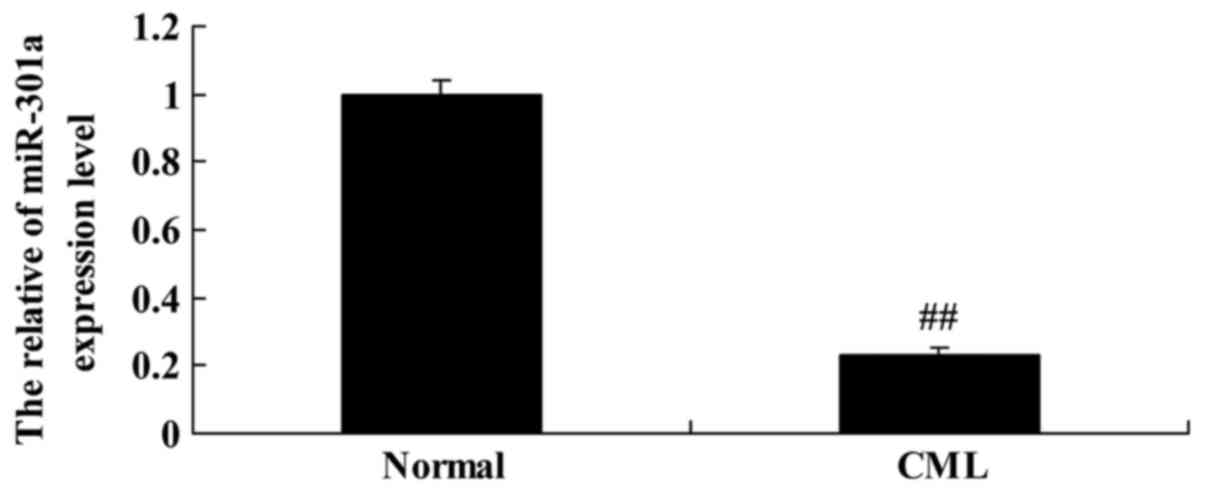
The expression of miRNA-301a in
patient with CML
To explore the expression level of miR-301a in CML
patients, we performed quantitative real-time PCR on miRNA-301a
expression. Interestingly, we found that miR-301a expression level
of CML patients were lower than that of the normal group (Fig. 1).
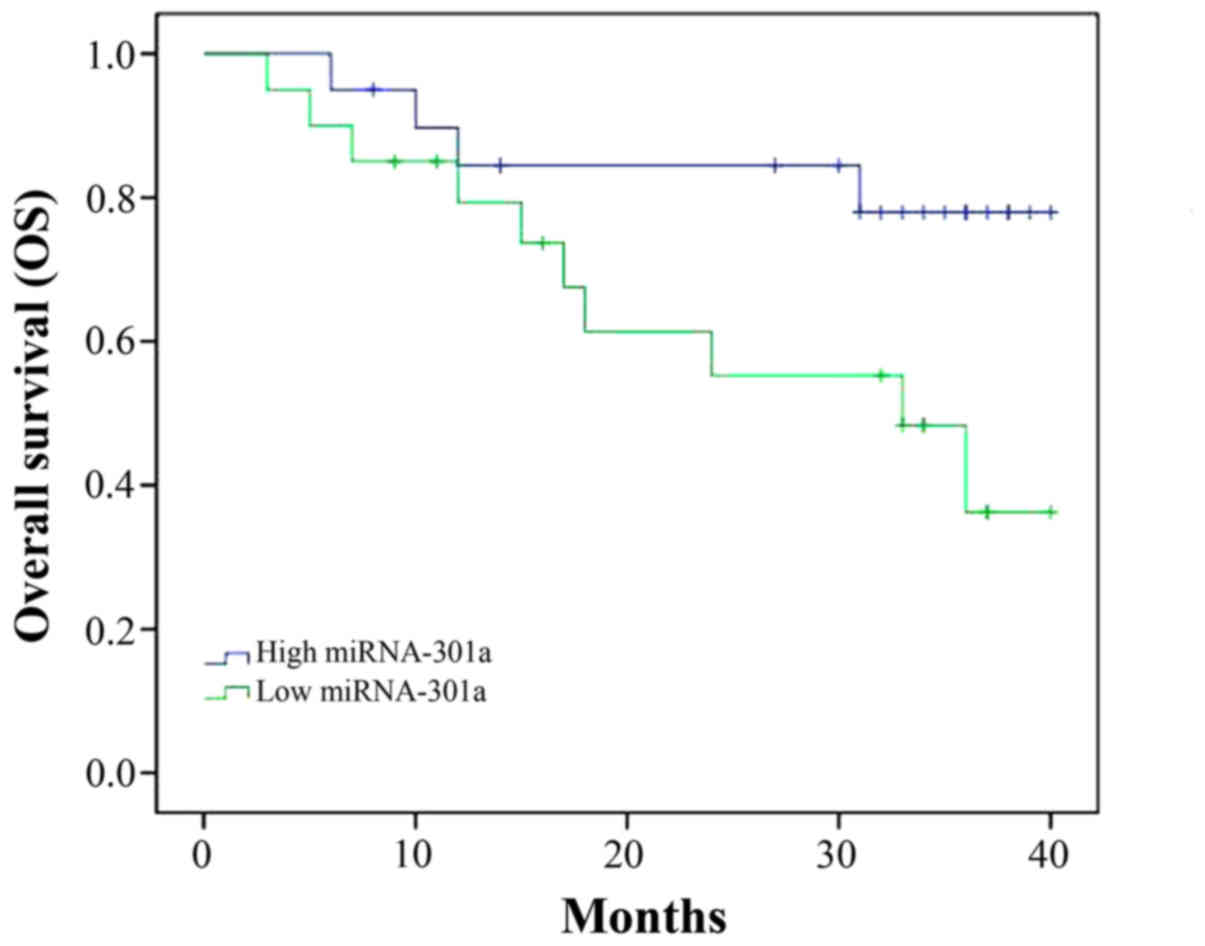
Overall survival (OS) of patient with
CML
We followed up the patient with CML and analyzed the
OS of patient with CML and miRNA-301a expression. As shown in
Fig. 2, OS of CML patient with high
miRNA-301a expression was superior to that of CML patient with low
miRNA-301a expression.
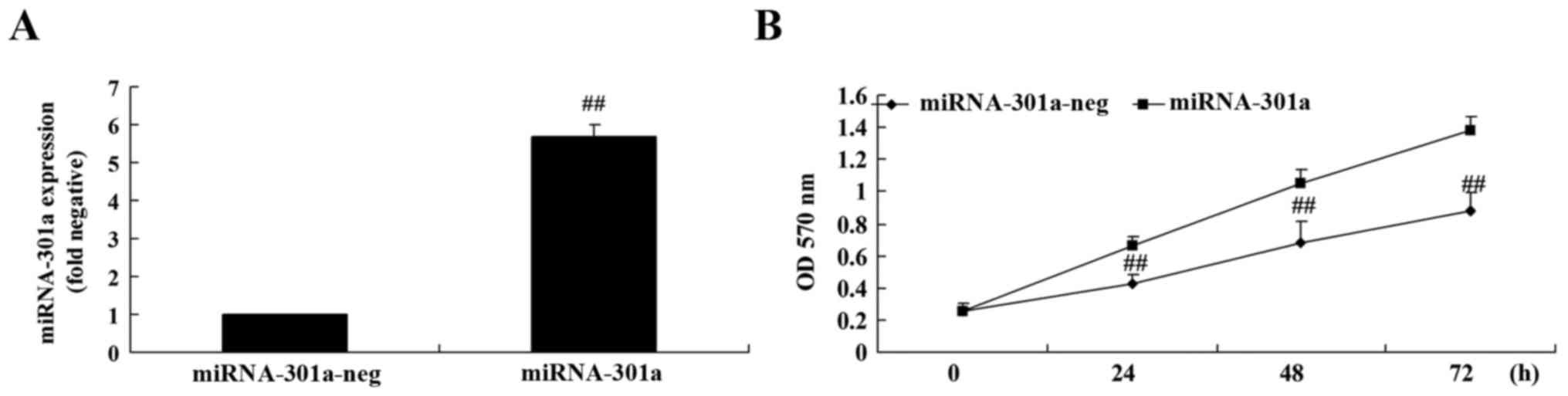
Upregulation of miRNA-301a affects
cell proliferation of K562 cells
The upregulation of miRNA-301a on cell proliferation
of K562 cells was investigated using MTT assay. Fig. 3A shows that miRNA-301a plasmids
significantly increased miRNA-301a expression in K562 cells,
compared with the negative control group. Then, we found that
miRNA-301a upregulation significantly accelerated cell
proliferation of K562 cells, compared with the negative control
group (Fig. 3B).
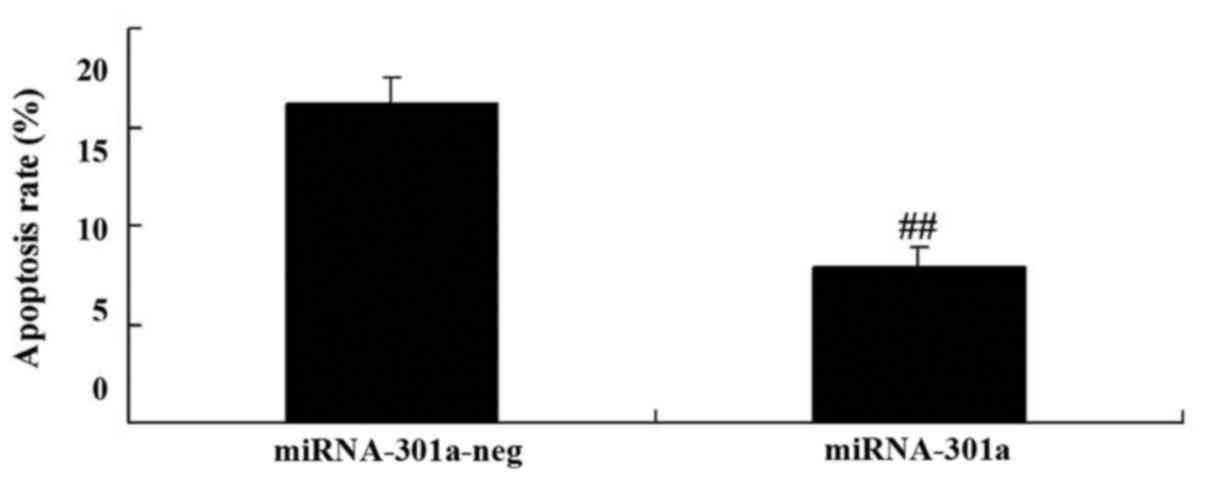
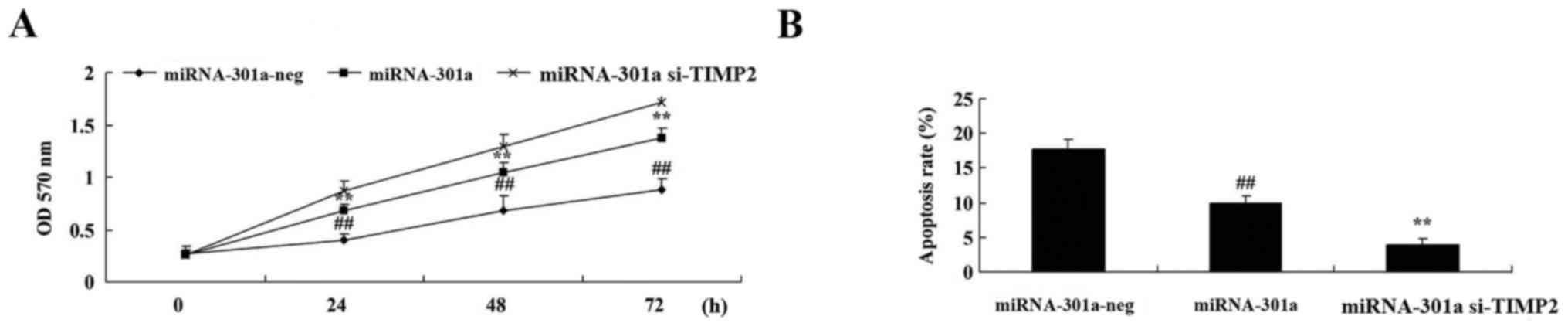
Upregulation of miRNA-301a affects
apoptosis of K562 cells
To evaluate whether the upregulation of miRNA-301a
affect apoptosis of K562 cells, apoptosis was investigated using
western blotting. Apoptosis of K562 cells was reduced by
upregulation of miRNA-301a expression, compared with negative
control group (Fig. 4).
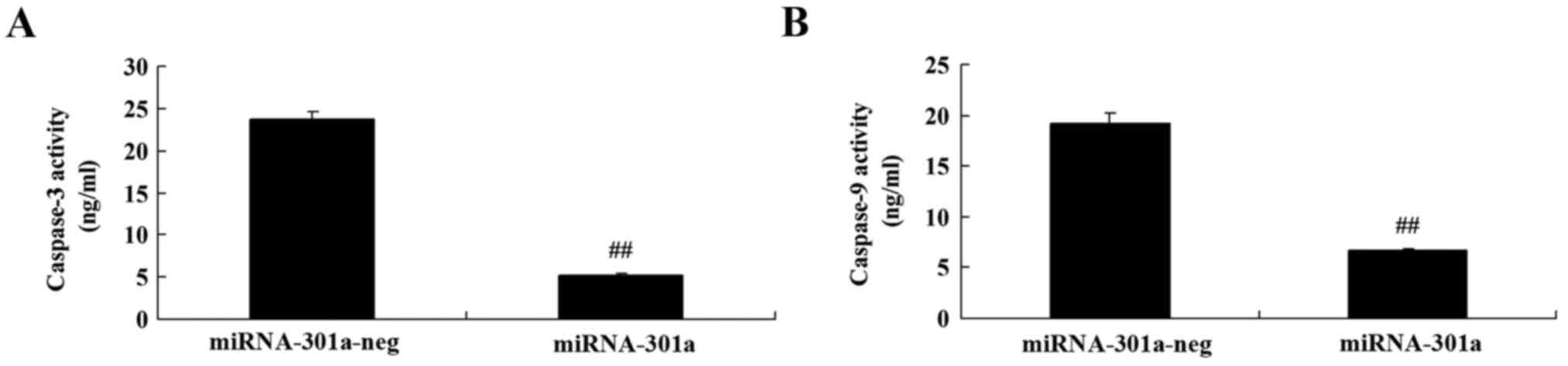
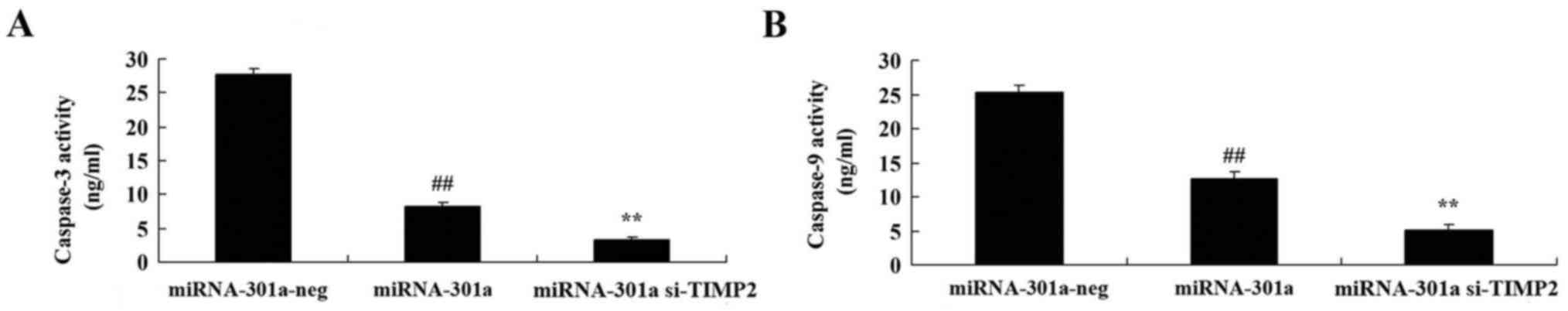
Upregulation of miRNA-301a affects
caspase-3 and −9 activity of K562 cells
The effect of miRNA-301a upregulation on caspase-3
and −9 activity of K562 cells, was measured using ELISA kits. As
shown in Fig. 5, upregulation of
miRNA-301a significantly decreased caspase-3 and −9 activity of
K562 cells, compared with negative control group.
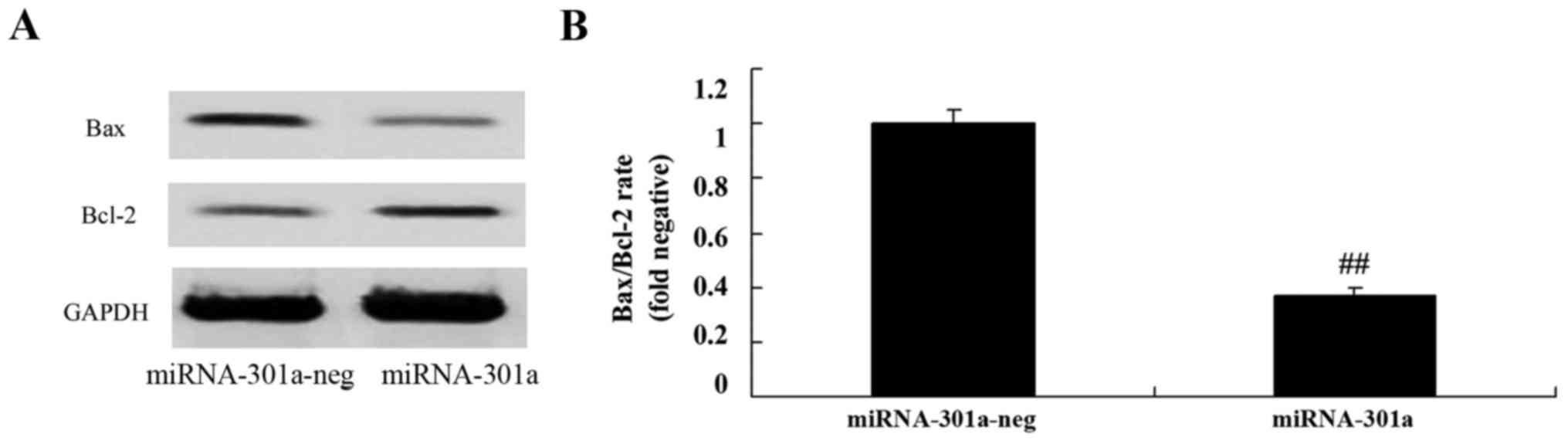
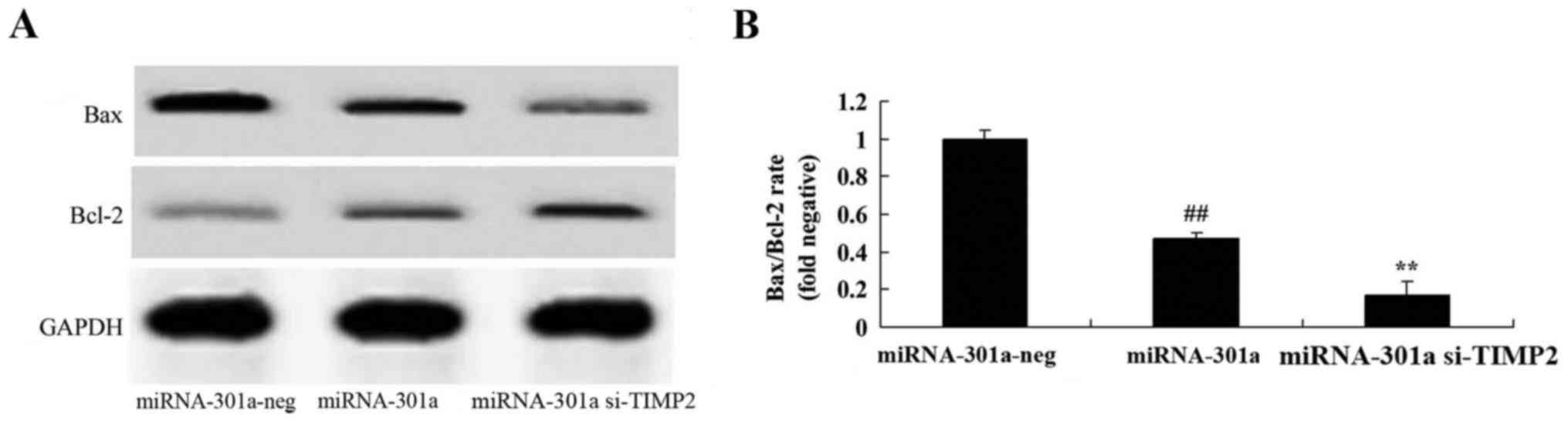
Upregulation of miRNA-301a affects
Bax/Bcl-2 rate of K562 cells
We observed the upregulation of miRNA-301a on
Bax/Bcl-2 rate of K562 cells. We found that the upregulation of
miRNA-301a significantly suppressed Bax/Bcl-2 rate of K562 cells,
compared with negative control group (Fig. 6).
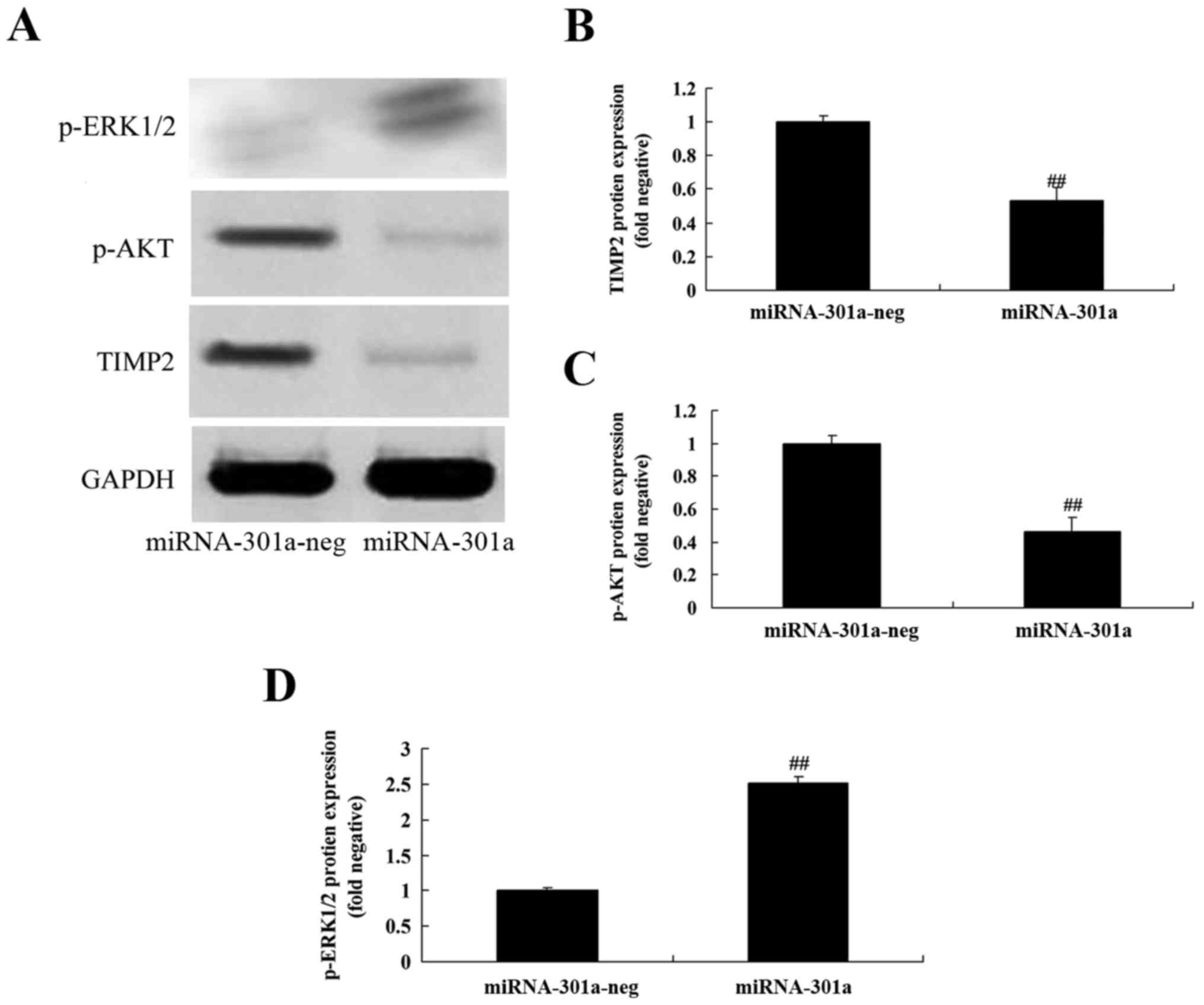
Upregulation of miRNA-301a affects
TIMP2 protein expression of K562 cells
To evaluate whether the upregulation of miRNA-301a
affects TIMP2 protein expression of K562 cells, western blotting
was performed to examine the level of TIMP2 protein of K562 cells
with upregulation of miRNA-301a. TIMP2 protein expression of K562
cells was significantly decreased by upregulation of miRNA-301a,
compared with negative control group (Fig. 7A and B).
Upregulation of miRNA-301a affect on
Akt protein expression of K562 cells
Akt is an anti-apoptotic protein, we inspected p-Akt
protein expression of K562 cells after miRNA-301a transfection. As
shown in Fig. 7A and C,
upregulation of miRNA-301a significantly suppressed p-Akt protein
expression of K562 cells, compared with negative control group.
Upregulation of miRNA-301a on ERK
protein expression of K562 cells
We elucidated whether the upregulation of miRNA-301a
affects the mechanism apoptosis of K562 cells, p-ERK protein
expression was detected using western blotting. The results of
western blotting showed that upregulation of miRNA-301a
significantly advanced p-ERK protein expression of K562 cells,
compared with negative control group (Fig. 7A and D).
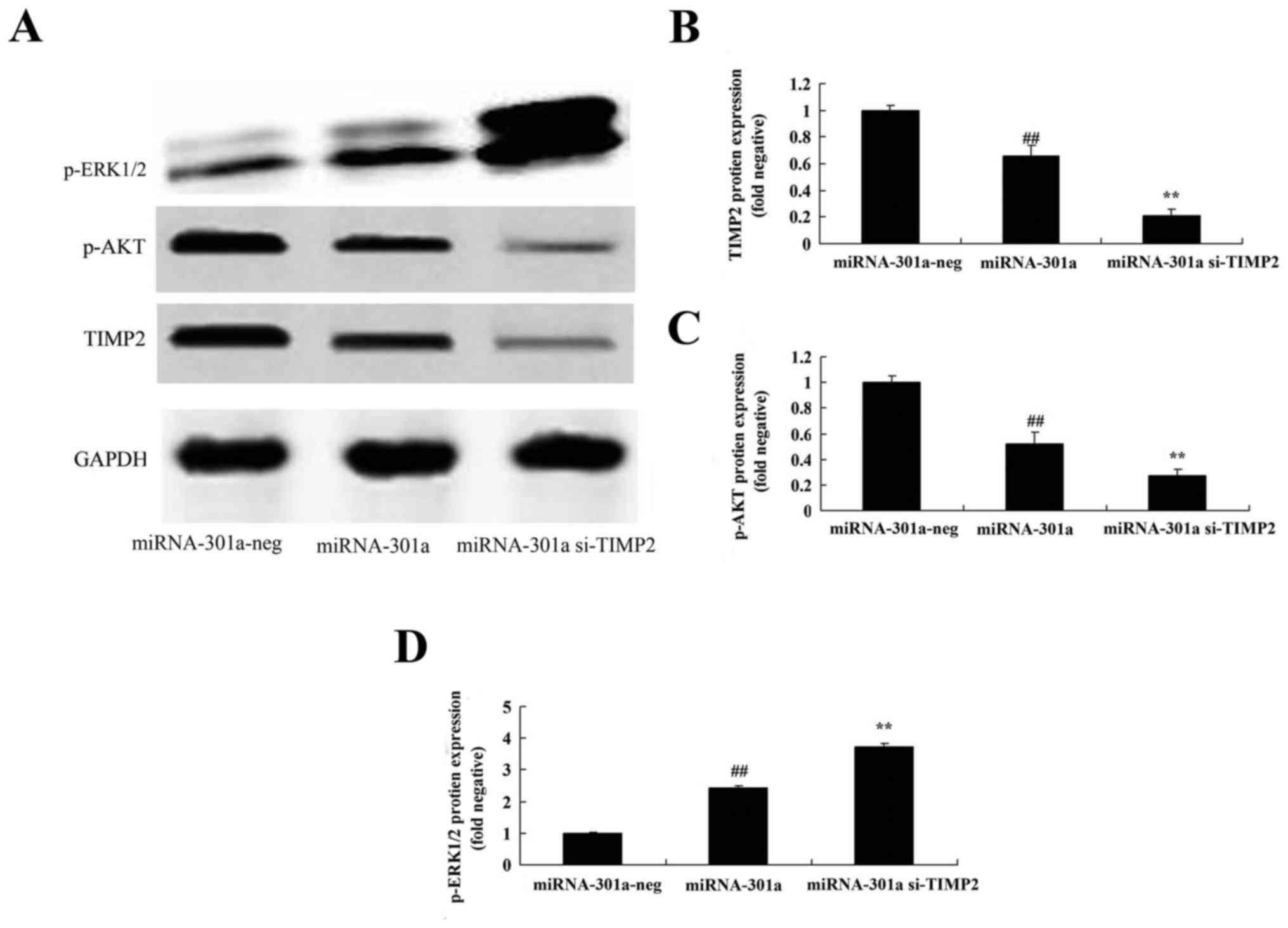
Downregulation of TIMP2 expression
influences the effect of miRNA-301a on TIMP2 protein expression of
K562 cells
We explored the TIMP2 expression of miRNA-301a on
cell growth of CML, and transfected si-TIMP2 into K562 cells. As
shown in Fig. 8A and B, si-TIMP2
effectively suppressed the TIMP2 protein expression of K562 cells,
compared with upregulation of miRNA-301a expression in K562
cells.
Downregulation of TIMP2 expression
influences the effect of miRNA-301a on cell proliferation and cell
apoptosis of K562 cells
We further examined downregulation of TIMP2
expression of miRNA-301a on cell proliferation and cell apoptosis
of K562 cells. We found that the downregulation of TIMP2 expression
effectively increased cell proliferation and inhibited cell
apoptosis of K562 cell transfection with upregulation of
miRNA-301a, compared with upregulation of miRNA-301a expression in
K562 cells (Fig. 9).
Downregulation of TIMP2 expression
influences the effect of miRNA-301a on caspase-3 and −9 activity of
K562 cells
We transfected K562 cells with si-TIMP2 and
determine caspase-3 and −9 activity of K562 cells with upregulation
of miRNA-301a. Results from ELISA assay showed that downregulation
of TIMP2 expression inhibited caspase-3 and −9 activity of K562
cells with upregulation of miRNA-301a, compared with upregulation
of miRNA-301a expression in K562 cells (Fig. 10).
Downregulation of TIMP2 expression
influences the effect of miRNA-301a on Bax/Bcl-2 rate of K562
cells
To determine whether TIMP2 would affect K562 cell
apoptosis with upregulation of miRNA-301a, Western blotting was
performed to analyze the protein expression of Bax and Bcl-2 as
shown in Fig. 11. The protein
expression of Bax/Bcl-2 rate was significantly decreased by
downregulation of TIMP2 in K562 cells with upregulation of
miRNA-301a, compared with upregulation of miRNA-301a expression in
K562 cells.
Downregulation of TIMP2 expression
influences the effect of miRNA-301a on Akt protein expression of
K562 cells
The TIMP2-mediated signaling was examined to
elucidate the Akt activation of K562 cells with upregulation of
miRNA-301a. After miRNA-301a transfection, the downregulation of
TIMP2 expression significantly suppressed p-Akt protein expression
of K562 cells with upregulation of miRNA-301a, compared with
upregulation of miRNA-301a expression in K562 cells (Fig. 8A and C).
Downregulation of TIMP2 expression
influences the effect of miRNA-301a on ERK protein expression of
K562 cells
We further elucidate whether downregulation of TIMP2
expression influenced the effect of miRNA-301a on ERK protein
expression of K562 cells. As shown in Fig. 8A and D, after miRNA-301a
transfection, the downregulation of TIMP2 expression significantly
enhanced p-ERK protein expression of K562 cells with upregulation
of miRNA-301a, compared with upregulation of miRNA-301a expression
in K562 cells.
Discussion
CML is a common hematological malignant tumor, the
morbidity of which accounts for ~20% among all types of leukemia,
with an annual rate of 10/1,000,000 people affected worldwide
(17). Ph chromosome can be
detected in CML patients, and the resulting bcr-abl fusion gene
encoded P210 Bcr-Abl protein possesses strong tyrosine kinase
activity, which results in the phosphorylation of the protein
itself, as well as many important substrate molecule tyrosine
residues, influencing multiple intracellular signal transduction
pathways such as Ras/MAPK, PI3K/Akt and JAK/STAT, inducing the
malignant transformation of the hematopoietic cells, enhancing the
ability of cells to repair DNA injury, inhibiting apoptosis, and
mediating resistance of CML to multiple cytotoxic antitumor drugs
(18,19). We concluded that miR-301a expression
level of CML patients were lower than that of normal group. Yue
et al revealed that miR-301a promoted invasion of glioma
cells through Wnt/β-catenin pathway (20).
The genesis and development of leukemia is an
extremely complicated process, which involves genetic and
epigenetic changes (4). Upregulated
or downregulated expression of miRNA can be detected in various
leukemia types, such as CML, chronic lymphocytic leukemia, CML and
acute myeloid leukemia (AML) (21).
In CML, the changes in miRNA expression render changes of the
living environment of the tumor cells, thus accelerating malignant
transformation such as tumor cell proliferation, apoptosis
escaping, angiogenesis and invasion (21,22).
Our results demonstrated that the upregulation of miRNA-301a
increased cell proliferation, inhibited apoptosis, caspase-3 and −9
activity, and suppressed Bax/Bcl-2 rate of K562 cells.
MMP-2 exists in the form of zymogen, and the ternary
theory is widely accepted at present in terms of its activation,
which means that the MMP-14 on the cell membrane binds with TIMP-2
to form the compound, and then TIMP-2 will bind with MMP-2 to form
the ternary compound, which renders the easier junction between
MMP-2 and another adjacent active MMP-14 in space; moreover, MMP-2
is also activated, thus forming the active form (2). The results demonstrate that there is
no expression of MMP-2 and MMP-14 in the normal adult bone marrow
mononuclear cells, but someexpression of TIMP-2; all the bone
marrow mononuclear cells in CML patients express TIMP-2, and 90%
express MMP-2 and MMP-14 (23).
Considering that the chronic phase in CML patients is under the
self-balanced status relative to the acute leukemia, while TIMP-2
may be an influencing factor for inhibiting disease progression,
the loss of balance may result in the disability to inhibit MMP-2,
the excessive expression leads to disease progression (24). Here, we showed that upregulation of
miRNA-301a significantly decreased TIMP2 protein expression of K562
cells. Liang et al reported that miR-301a promotes cell
proliferation by directly targeting TIMP2 in multiple myeloma
(25). These findings suggest that
miRNA-301a may function as a novel oncogene in CML contributing to
tumor progression of CML.
P210Bcr-Abl fusion protein induces the
phosphorylation of the tyrosine in multiple intracellular signaling
molecules through its high tyrosine kinase activity, thus
activating multiple signaling pathways such as Ras/MAPK, PI3K/Akt
and JAK/STAT, the activation of these signaling molecules is the
molecular basis of the anti-apoptosis of Bcr-Abl (26). The signaling pathway activation
downstream of Bcr-Abl also participates in the formation of the
resistance to imatinib. CRKL protein is the adaptor protein during
the intracellular signal transduction process, which participates
in signal transduction pathways like Ras/MAPK JAK/STAT PI3K as well
as β1 integrin-mediated signal transduction pathway (27). CRKL gene is abundantly expressed in
hematopoietic cells, while in normal hematopoietic cells, CRKL
protein exists in the form of non-phosphorylated tyrosine. PI3K/Akt
and MAPK/ERK are the two important pathways downstream of Bcr/Abl
(28). The chronic phase and
blastic phase of CML as well as the CML-derived K562 cell lines are
associated with the constitutive activation of the ERK and Akt
kinase (29). The application of
imatinib in K562 cells can inhibit the activity of the ERK and Akt
kinase, and induce cell apoptosis through activating caspase-3.
However, in some primary leukemia cells in the CML blastic phase,
inhibiting the activity of the ERK and Akt kinase can induce
caspase-3 mediated apoptosis, giving rise to the occurrence of
resistance (30). In this study, we
observed that upregulation of miRNA-301a significantly increased
phosphorylation-ERK1/2 and decreased phosphorylation-AKT protein
expression of K562 cell. These results indicate that ERK1/2 and AKT
pathways affected miRNA-301a in CML.
Taken together, we demonstrated for the first time
that miRNA-301a is significantly upregulated in CML. Moreover,
miRNA-301a promotes cell proliferation and inhibits apoptosis of
CML by direct targeting TIMP2. These results suggest that
miRNA-301a may play as an oncogene in CML and might provide helpful
therapeutic strategies for CML in clinical application.
References
|
1
|
Zhou L, Shi H, Jiang S, Ruan C and Liu H:
Deep molecular response by IFN-α and dasatinib combination in a
patient with T315I-mutated chronic myeloid leukemia.
Pharmacogenomics. 17:1159–1163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaudhary AK, Chaudhary S, Ghosh K,
Shanmukaiah C and Nadkarni AH: Secretion and expression of matrix
metalloproteinase-2 and 9 from bone marrow mononuclear cells in
myelodysplastic syndrome and acute myeloid leukemia. Asian Pac J
Cancer Prev. 17:1519–1529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janowska-Wieczorek A, Majka M,
Marquez-Curtis L, Wertheim JA, Turner AR and Ratajczak MZ:
Bcr-abl-positive cells secrete angiogenic factors including matrix
metalloproteinases and stimulate angiogenesis in vivo in Matrigel
implants. Leukemia. 16:1160–1166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Stefano C, Mirone G, Perna S and Marfe
G: The roles of microRNAs in the pathogenesis and drug resistance
of chronic myelogenous leukemia (Review). Oncol Rep. 35:614–624.
2016.PubMed/NCBI
|
|
5
|
Hershkovitz-Rokah O, Modai S,
Pasmanik-Chor M, Toren A, Shomron N, Raanani P, Shpilberg O and
Granot G: Restoration of miR-424 suppresses BCR-ABL activity and
sensitizes CML cells to imatinib treatment. Cancer Lett.
360:245–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang WZ, Pu QH, Lin XH, Liu MY, Wu LR, Wu
QQ, Chen YH, Liao FF, Zhu JY and Jin XB: Silencing of miR-21
sensitizes CML CD34+ stem/progenitor cells to
imatinib-induced apoptosis by blocking PI3K/AKT pathway. Leuk Res.
39:1117–1124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kreisel SH, Stroick M, Griebe M, Alonso A,
Reuter B, Hennerici MG and Fatar M: True effects or bias? MMP-2 and
MMP-9 serum concentrations after acute stroke. Cerebrovasc Dis.
42:352–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu YJ, Neoh CA, Tsao CY, Su JH and Li HH:
Sinulariolide suppresses human hepatocellular carcinoma cell
migration and invasion by inhibiting matrix metalloproteinase-2/−9
through MAPKs and PI3K/Akt signaling pathways. Int J Mol Sci.
16:16469–16482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ries C, Loher F, Zang C, Ismair MG and
Petrides PE: Matrix metalloproteinase production by bone marrow
mononuclear cells from normal individuals and patients with acute
and chronic myeloid leukemia or myelodysplastic syndromes. Clin
Cancer Res. 5:1115–1124. 1999.PubMed/NCBI
|
|
10
|
Golubnitschaja O, Yeghiazaryan K, Stricker
H, Trog D, Schild HH and Berliner L: Patients with hepatic breast
cancer metastases demonstrate highly specific profiles of matrix
metalloproteinases MMP-2 and MMP-9 after SIRT treatment as compared
to other primary and secondary liver tumours. BMC Cancer.
16:3572016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen CC, Liu TY, Huang SP, Ho CT and Huang
TC: Differentiation and apoptosis induction by lovastatin and
γ-tocotrienol in HL-60 cells via Ras/ERK/NF-κB and Ras/Akt/NF-κB
signaling dependent down-regulation of glyoxalase 1 and HMG-CoA
reductase. Cell Signal. 27:2182–2190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Wang Y, Liu RH and He X: Novel
triterpenoids isolated from raisins exert potent antiproliferative
activities by targeting mitochondrial and Ras/Raf/ERK signaling in
human breast cancer cells. Food Funct. 7:3244–3251. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chien CM, Lin KL, Su JC, Chuang PW, Tseng
CH, Chen YL, Chang LS and Lin SR: Naphtho[1,2-b]furan-4,5-dione
induces apoptosis of oral squamous cell carcinoma: Involvement of
EGF receptor/PI3K/Akt signaling pathway. Eur J Pharmacol.
636:52–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Q, Huai L, Zhang C, Wang C, Jia Y, Chen
Y, Yu P, Wang H, Rao Q, Wang M, et al: Icaritin induces AML cell
apoptosis via the MAPK/ERK and PI3K/AKT signaling pathways. Int J
Hematol. 97:617–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng D, Wang J, Kong P, Chang C and Li J
and Li J: Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in
patient with acute leukemia via inhibiting the activation of
PI3K/Akt and ERK1/2 pathways. Int J Clin Exp Pathol. 7:2172–2178.
2014.PubMed/NCBI
|
|
16
|
Yi YW, Hong W, Kang HJ, Kim HJ, Zhao W,
Wang A, Seong YS and Bae I: Inhibition of the PI3K/AKT pathway
potentiates cytotoxicity of EGFR kinase inhibitors in
triple-negative breast cancer cells. J Cell Mol Med. 17:648–656.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Becker H, Suciu S, Rüter BH, Platzbecker
U, Giagounidis A, Selleslag D, Labar B, Germing U, Salih HR, Muus
P, et al: Decitabine versus best supportive care in older patients
with refractory anemia with excess blasts in transformation (RAEBt)
- results of a subgroup analysis of the randomized phase III study
06011 of the EORTC Leukemia Cooperative Group and German MDS Study
Group (GMDSSG). Ann Hematol. 94:2003–2013. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakurai M, Mori T, Karigane D, Tozawa K,
Matsuki E, Shimizu T, Yokoyama K, Nakajima H, Kanda Y and Okamoto
S: Unfavorable outcome of chronic myelogenous leukemia in
adolescent and young adults treated with tyrosine kinase
inhibitors. Int J Hematol. 102:342–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iriyama N, Fujisawa S, Yoshida C, Wakita
H, Chiba S, Okamoto S, Kawakami K, Takezako N, Kumagai T, Inokuchi
K, et al: Shorter halving time of BCR-ABL1 transcripts is a novel
predictor for achievement of molecular responses in newly diagnosed
chronic-phase chronic myeloid leukemia treated with dasatinib:
Results of the D-first study of Kanto CML study group. Am J
Hematol. 90:282–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yue X, Cao D, Lan F, Pan Q, Xia T and Yu
H: MiR-301a is activated by the Wnt/β-catenin pathway and promotes
glioma cell invasion by suppressing SEPT7. Neuro-oncol.
18:1288–1296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhutra S, Lenkala D, LaCroix B, Ye M and
Huang RS: Identifying and validating a combined mRNA and microRNA
signature in response to imatinib treatment in a chronic myeloid
leukemia cell line. PLoS One. 9:e1150032014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valleron W, Laprevotte E, Gautier EF,
Quelen C, Demur C, Delabesse E, Agirre X, Prósper F, Kiss T and
Brousset P: Specific small nucleolar RNA expression profiles in
acute leukemia. Leukemia. 26:2052–2060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song JH, Kim SH, Cho D, Lee IK, Kim HJ and
Kim TS: Enhanced invasiveness of drug-resistant acute myeloid
leukemia cells through increased expression of matrix
metalloproteinase-2. Int J Cancer. 125:1074–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aref S, Osman E, Mansy S, Omer N, Azmy E,
Goda T and El-Sherbiny M: Prognostic relevance of circulating
matrix metalloproteinase-2 in acute myeloid leukaemia patients.
Hematol Oncol. 25:121–126. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang B, Yin JJ and Zhan XR: MiR-301a
promotes cell proliferation by directly targeting TIMP2 in multiple
myeloma. Int J Clin Exp Pathol. 8:9168–9174. 2015.PubMed/NCBI
|
|
26
|
Wang H, Jia XH, Chen JR, Wang JY and Li
YJ: Osthole shows the potential to overcome P-glycoprotein-mediated
multidrug resistance in human myelogenous leukemia K562/ADM cells
by inhibiting the PI3K/Akt signaling pathway. Oncol Rep.
35:3659–3668. 2016.PubMed/NCBI
|
|
27
|
Zhang X, Dong W, Zhou H, Li H, Wang N,
Miao X and Jia L: α-2,8-sialyltransferase is involved in the
development of multidrug resistance via PI3K/Akt pathway in human
chronic myeloid leukemia. IUBMB Life. 67:77–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rahmani M, Aust MM, Attkisson E, Williams
DC Jr, Ferreira-Gonzalez A and Grant S: Dual inhibition of Bcl-2
and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in
human myeloid leukemia cells through a GSK3- and Bim-dependent
mechanism. Cancer Res. 73:1340–1351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen C, Chang YC, Lan MS and Breslin M:
Leptin stimulates ovarian cancer cell growth and inhibits apoptosis
by increasing cyclin D1 and Mcl-1 expression via the activation of
the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol.
42:1113–1119. 2013.PubMed/NCBI
|
|
30
|
Guo Y, Li Y, Shan Q, He G, Lin J and Gong
Y: Curcumin potentiates the anti-leukemia effects of imatinib by
downregulation of the AKT/mTOR pathway and BCR/ABL gene expression
in Ph+ acute lymphoblastic leukemia. Int J Biochem Cell
Biol. 65:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|