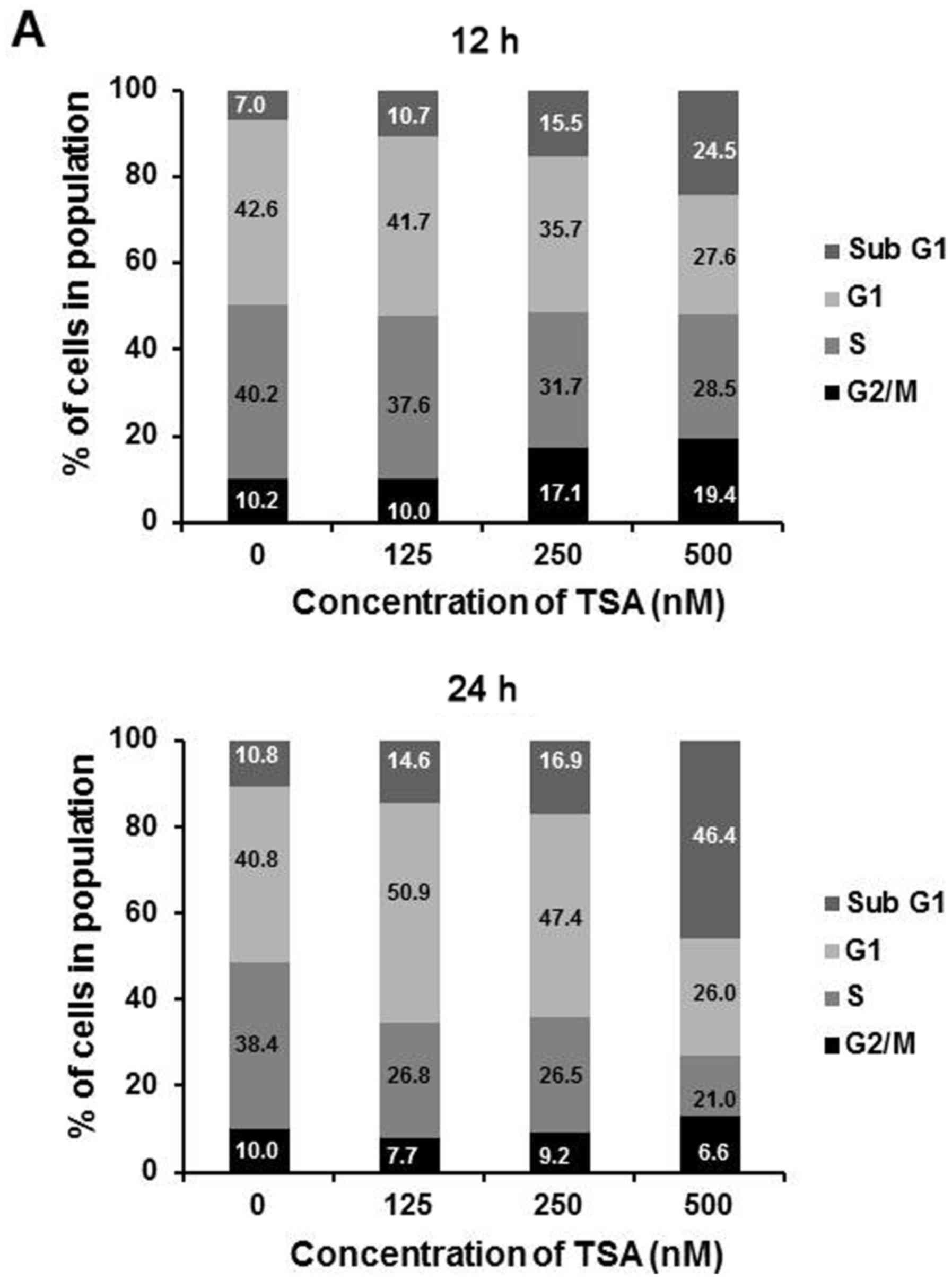
|
1
|
Stewart BW and Wild C: International
Agency for Research on Cancer and World Health Organization: World
Cancer Report. 2014.
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Avritscher EB, Cooksley CD, Grossman HB,
Sabichi AL, Hamblin L, Dinney CP and Elting LS: Clinical model of
lifetime cost of treating bladder cancer and associated
complications. Urology. 68:549–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ocker M and Schneider-Stock R: Histone
deacetylase inhibitors: Signalling towards p21cip1/waf1.
Int J Biochem Cell Biol. 39:1367–1374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hitomi T, Matsuzaki Y, Yokota T, Takaoka Y
and Sakai T: p15INK4b in HDAC inhibitor-induced growth
arrest. FEBS Lett. 554:347–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosato RR and Grant S: Histone deacetylase
inhibitors: Insights into mechanisms of lethality. Expert Opin Ther
Targets. 9:809–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Batty N, Malouf GG and Issa JP: Histone
deacetylase inhibitors as anti-neoplastic agents. Cancer Lett.
280:192–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J and Zhong Q: Histone deacetylase
inhibitors and cell death. Cell Mol Life Sci. 71:3885–3901. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minucci S and Pelicci PG: Histone
deacetylase inhibitors and the promise of epigenetic (and more)
treatments for cancer. Nat Rev Cancer. 6:38–51. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mottamal M, Zheng S, Huang TL and Wang G:
Histone deacetylase inhibitors in clinical studies as templates for
new anticancer agents. Molecules. 20:3898–3941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoon S and Eom GH: HDAC and HDAC
inhibitor: From cancer to cardiovascular diseases. Chonnam Med J.
52:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vanhaecke T, Papeleu P, Elaut G and
Rogiers V: Trichostatin A-like hydroxamate histone deacetylase
inhibitors as therapeutic agents: Toxicological point of view. Curr
Med Chem. 11:1629–1643. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Beneden K, Mannaerts I, Pauwels M, Van
den Branden C and van Grunsven LA: HDAC inhibitors in experimental
liver and kidney fibrosis. Fibrogenesis Tissue Repair. 6:12013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamashita Y, Shimada M, Harimoto N,
Rikimaru T, Shirabe K, Tanaka S and Sugimachi K: Histone
deacetylase inhibitor trichostatin A induces cell-cycle
arrest/apoptosis and hepatocyte differentiation in human hepatoma
cells. Int J Cancer. 103:572–576. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noh EJ, Lim DS, Jeong G and Lee JS: An
HDAC inhibitor, trichostatin A, induces a delay at G2/M
transition, slippage of spindle checkpoint, and cell death in a
transcription-dependent manner. Biochem Biophys Res Commun.
378:326–331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cohen HY, Lavu S, Bitterman KJ, Hekking B,
Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM and Sinclair
DA: Acetylation of the C terminus of Ku70 by CBP and PCAF controls
Bax-mediated apoptosis. Mol Cell. 13:627–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Medina V, Edmonds B, Young GP, James R,
Appleton S and Zalewski PD: Induction of caspase-3 protease
activity and apoptosis by butyrate and trichostatin A (inhibitors
of histone deacetylase): Dependence on protein synthesis and
synergy with a mitochondrial/cytochrome c-dependent pathway.
Cancer Res. 57:3697–3707. 1997.PubMed/NCBI
|
|
19
|
Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE,
Lee SW, Moon EJ, Kim HS, Lee SK, Chung HY, et al: Histone
deacetylases induce angiogenesis by negative regulation of tumor
suppressor genes. Nat Med. 7:437–443. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deroanne CF, Bonjean K, Servotte S, Devy
L, Colige A, Clausse N, Blacher S, Verdin E, Foidart JM, Nusgens
BV, et al: Histone deacetylases inhibitors as anti-angiogenic
agents altering vascular endothelial growth factor signaling.
Oncogene. 21:427–436. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshikawa M, Hishikawa K, Marumo T and
Fujita T: Inhibition of histone deacetylase activity suppresses
epithelial-to-mesenchymal transition induced by TGF-beta1 in human
renal epithelial cells. J Am Soc Nephrol. 18:58–65. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu LT, Chang HC, Chiang LC and Hung WC:
Histone deacetylase inhibitor up-regulates RECK to inhibit MMP-2
activation and cancer cell invasion. Cancer Res. 63:3069–3072.
2003.PubMed/NCBI
|
|
23
|
West AC and Johnstone RW: New and emerging
HDAC inhibitors for cancer treatment. J Clin Invest. 124:30–39.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park SJ, Kim MJ, Kim HB, Sohn HY, Bae JH,
Kang CD and Kim SH: Trichostatin A sensitizes human ovarian cancer
cells to TRAIL-induced apoptosis by down-regulation of
c-FLIPL via inhibition of EGFR pathway. Biochem
Pharmacol. 77:1328–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen CS, Weng SC, Tseng PH, Lin HP and
Chen CS: Histone acetylation-independent effect of histone
deacetylase inhibitors on Akt through the reshuffling of protein
phosphatase 1 complexes. J Biol Chem. 280:38879–38887. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu JY, Tsai KW, Li YZ, Chang YS, Lai YC,
Laio YH, Wu JD and Liu YW: Anti-bladder-tumor effect of baicalein
from Scutellaria baicalensis Georgi and its application in
vivo. Evid Based Complement Alternat Med.
2013:5797512013.PubMed/NCBI
|
|
29
|
Hsu YF, Sheu JR, Lin CH, Yang DS, Hsiao G,
Ou G, Chiu PT, Huang YH, Kuo WH and Hsu MJ: Trichostatin A and
sirtinol suppressed survivin expression through AMPK and p38MAPK in
HT29 colon cancer cells. Biochim Biophys Acta. 1820:104–115. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsu YF, Sheu JR, Hsiao G, Lin CH, Chang
TH, Chiu PT, Wang CY and Hsu MJ: p53 in trichostatin A induced C6
glioma cell death. Biochim Biophys Acta. 1810:504–513. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sleiman SF, Langley BC, Basso M, Berlin J,
Xia L, Payappilly JB, Kharel MK, Guo H, Marsh JL, Thompson LM, et
al: Mithramycin is a gene-selective Sp1 inhibitor that identifies a
biological intersection between cancer and neurodegeneration. J
Neurosci. 31:6858–6870. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Newbold A, Falkenberg KJ, Prince HM and
Johnstone RW: How do tumor cells respond to HDAC inhibition? FEBS
J. 283:4032–4046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jänicke RU, Sohn D, Essmann F and
Schulze-Osthoff K: The multiple battles fought by anti-apoptotic
p21. Cell Cycle. 6:407–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vinall RL, Ripoll AZ, Wang S, Pan CX and
deVere White RW: MiR-34a chemosensitizes bladder cancer cells to
cisplatin treatment regardless of p53-Rb pathway status. Int J
Cancer. 130:2526–2538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
da Silva GN, de Camargo EA and Salvadori
DM: Toxicogenomic activity of gemcitabine in two
TP53-mutated bladder cancer cell lines: Special focus on
cell cycle-related genes. Mol Biol Rep. 39:10373–10382. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Warfel NA and El-Deiry WS: p21WAF1 and
tumourigenesis: 20 years after. Curr Opin Oncol. 25:52–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alao JP, Stavropoulou AV, Lam EW, Coombes
RC and Vigushin DM: Histone deacetylase inhibitor, trichostatin A
induces ubiquitin-dependent cyclin D1 degradation in MCF-7 breast
cancer cells. Mol Cancer. 5:82006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Keenan SM, Lents NH and Baldassare JJ:
Expression of cyclin E renders cyclin D-CDK4 dispensable for
inactivation of the retinoblastoma tumor suppressor protein,
activation of E2F, and G1-S phase progression. J Biol
Chem. 279:5387–5396. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rasola A and Bernardi P: The mitochondrial
permeability transition pore and its involvement in cell death and
in disease pathogenesis. Apoptosis. 12:815–833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lim S, Smith KR, Lim ST, Tian R, Lu J and
Tan M: Regulation of mitochondrial functions by protein
phosphorylation and dephosphorylation. Cell Biosci. 6:252016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alao JP, Stavropoulou AV, Lam EW and
Coombes RC: Role of glycogen synthase kinase 3 beta (GSK3beta) in
mediating the cytotoxic effects of the histone deacetylase
inhibitor trichostatin A (TSA) in MCF-7 breast cancer cells. Mol
Cancer. 5:402006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miyamoto S, Murphy AN and Brown JH: Akt
mediates mitochondrial protection in cardiomyocytes through
phosphorylation of mitochondrial hexokinase-II. Cell Death Differ.
15:521–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheng Q, Ling X, Haller A, Nakahara T,
Yamanaka K, Kita A, Koutoku H, Takeuchi M, Brattain MG and Li F:
Suppression of survivin promoter activity by YM155 involves
disruption of Sp1-DNA interaction in the survivin core promoter.
Int J Biochem Mol Biol. 3:179–197. 2012.PubMed/NCBI
|
|
46
|
Glaros TG, Stockwin LH, Mullendore ME,
Smith B, Morrison BL and Newton DL: The ‘survivin suppressants’ NSC
80467 and YM155 induce a DNA damage response. Cancer Chemother
Pharmacol. 70:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li F and Altieri DC: Transcriptional
analysis of human survivin gene expression. Biochem J. 344:305–311.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee HO, Lee JH, Choi E, Seol JY, Yun Y and
Lee H: A dominant negative form of p63 inhibits apoptosis in a
p53-independent manner. Biochem Biophys Res Commun. 344:166–172.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wei S, Chuang HC, Tsai WC, Yang HC, Ho SR,
Paterson AJ, Kulp SK and Chen CS: Thiazolidinediones mimic glucose
starvation in facilitating Sp1 degradation through the
up-regulation of beta-transducin repeat-containing protein. Mol
Pharmacol. 76:47–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duan H, Heckman CA and Boxer LM: Histone
deacetylase inhibitors down-regulate bcl-2 expression and
induce apoptosis in t(14;18) lymphomas. Mol Cell Biol.
25:1608–1619. 2005. View Article : Google Scholar : PubMed/NCBI
|