Introduction
Glioma is among the most common and lethal malignant
brain tumors in humans (1). Despite
advancements in surgical, radiotherapeutic and chemotherapeutic
treatments, the survival of glioma patients remains very poor, and
the majority of patients die within 2 years of diagnosis due to
cancer progression (2). According
to the World Health Organization (WHO) grading criteria, glioma is
classified into four grades (I–IV), and survival rate has been
reported to worsen with increasing grade; the 5-year survival rate
is 30–70% in patients with grade I/II, compared with 3% in patients
with grade IV disease (3). However,
the detailed mechanisms underlying these differences in survival
rates between different tumor grades remain obscure. Therefore, it
is necessary to better understand the molecular mechanisms of
glioma progression and thus develop novel therapeutic
strategies.
Recently, studies have suggested that the
epithelial-mesenchymal transition (EMT) plays an important role in
cancer progression (4,5). EMT is the biological process in which
cells are converted from an epithelial phenotype into a
mesenchymal-like phenotype, which is involved in development and
tissue regeneration. The hallmarks of EMT are decreased expression
of epithelial markers (E-cadherin, β-catenin, etc.) and increased
expression of mesenchymal markers (vimentin, N-cadherin, etc.), as
well as changes in cell morphology (6). Numerous studies have confirmed that
EMT is involved in the invasion and metastasis of cancers,
including glioma. Patients with glioma that exhibit EMT-related
changes typically have decreased survival times, and glioma cells
that undergo EMT have stronger migratory and invasive potentials
(7–9). Hence, further exploration of the
association between EMT and glioma is critical for clarifying the
mechanism of glioma progression.
BAF53a (also known as ACTL6A or ARP4) is a subunit
of the Brg/Brm-associated factor (BAF) complex, which is crucial
for embryonic development and maintenance of stemness in stem or
progenitor cells (10–12). Therefore, BAF53a may also be
associated with EMT. Recently, studies have reported that the
aberrant expression of BAF53a is correlated with the progression of
rhabdomyosarcoma, osteosarcoma, hepatocellular carcinoma and head
and neck squamous cell carcinoma (HNSCC) (13–16).
Based on its biological function in stem and progenitor cells,
BAF53a is considered to promote cancer progression via EMT.
However, the role of BAF53a in the invasion and metastasis of
glioma remains unclear to date.
In the present study, we report for the first time
that a high level of BAF53a expression in glioma specimens is
associated with poor prognosis, and that the overexpression of
BAF53a can promote the proliferation and invasion of glioma cells.
Furthermore, BAF53a expression was found to be significantly
associated with the expression of EMT markers, which indicates that
BAF53a may promote the metastasis of glioma through EMT. Taken
together, these findings preliminarily indicate that BAF53a may be
a novel prognostic marker and promising therapeutic target for
glioma.
Materials and methods
Patient specimens and cell
cultures
The 121 glioma tissue samples were randomly obtained
from the Department of tissue, Xiangya Hospital between January
2009 and December 2012. All the gliomas were pathology confirmed by
2 independent pathologists according to the 2007 WHO
classification. All patients eligible for this study acceptted
surgery and were regularly followed-up, and collected detailed
clinicopathological data and survival data. Magnetic resonance
imaging (MRI) or contrast-enhanced MRI was performed every 6
months. OS was defined as the time from the surgery to the death or
the last follow-up visit. PFS was defined as the time from the
surgery to the first evidence of recurrence, progression, or death.
All patients signed a written informed consent. The study was
approved by the ethics committee of Xiangya Hospital in accordance
with the Declaration of Helsinki.
The human primary glioma cell line U87 was obtained
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China), and cultured in DMEM (Gibco, NY, USA) with 10%
fetal bovine serum (Gibco) at 37°C in a humidified atmosphere of 5%
CO2.
RNA isolation and real-time PCR
Total RNA of glioma tissues and cells were isolated
with TRIzol reagent (Invitrogen, CA, USA) following the
manufacturer's instructions. The concentration and purity of all
RNA samples were determined by NanoDrop Spectrophotometer (NanoDrop
Technologies, TX, USA). Then, the cDNA was synthesized by reverse
transcription of total RNA using cDNA synthesis kit (Roche Life
Sciences, Switzerland), and real-time PCR reaction was conducted
using the SYBR Green PCR kit (Roche Life Sciences) and performed on
ABI PRISM 7100 Sequence Detection system (Applied Biosystems, CA,
USA) following the manufacturer's instructions. GAPDH served as
control. The target mRNA expression was calculated using the
2−∆∆Ct method. Each experiment was repeated 3 times. The
primers were as follows: BAF53a (forward,
5′-CCAGGTCTCTATGGCAGTGTAA-3′; reverse,
5′-CGTAAGGTGACAAAAGGAAGGTA-3′); GAPDH (forward,
5′-GTCTCCTCTGACTTCAACAGCG-3′; reverse,
5′-ACCACCCTGTTGCTGTAGCCAA-3′); E-cadherin (forward,
5′-GCCTCCTGAAAAGAGAGTGGAAG-3′; reverse,
5′-TGGCAGTGTCTCTCCAAATCCG-3′); vimentin (forward,
5′-AGGCAAAGCAGGAGTCCACTGA-3′; reverse,
5′-ATCTGGCGTTCCAGGGACTCAT-3′).
Protein extraction and western
blotting
Fresh tissues or cells were dissolved by RIPA lysis
buffer (Invitrogen) and PMSF (Zhongshan Goldenbridge Biotechnology,
Shanghai, China). Then isolated by centrifugation and quantified by
Bradford Protein assay kit (Beyotime Institute of Biotechnology,
Shanghai, China). Next the protein was separated by 10% SDS-PAGE
and transferred to the PVDF membrane (Sigma, MO, USA). The
membranes were blocked with 5% calf serum and incubated with
appropriate primary antibody, followed by incubation with secondary
antibody. Lastly, the signal was detected using enhanced
chemiluminescence regents (Thermo Scientific, MA, USA) and
photographed by camera and image processing system (Bio-Rad, CA,
USA).
Immunohistochemistry
The glioma tissues were embedded in paraffin, and
sectioned into slides with a thickness of 4 µm. Then, sections were
dewaxed in xylene and rehydrated in graded ethanol. Antigen
retrieval was accomplished using citrate buffer at pH 6.0. Then 3%
H2O2 was used to block endogenous peroxidase
activity, and goat serum incubation was used to block the
non-specific antigen binding, followed by incubation with
appropriate concentration of primary antibody at 4°C overnight.
Then, the sections were incubated with the corresponding secondary
antibody for 30 min at room temperature after rinsing three times
in PBS. The antigen-antibody interactions was detected by DAB and
counterstained by hematoxylin, followed by dehydrated in graded
ethanols and mounted. The results were evaluated by two independent
pathologists blinded to the study. The staining intensity of BAF53a
was scored according to the percentage of positive stained tumor
cells, as negative (−) <5% of tumor cells stained positive, (+)
0–25%, (++) 26–75%, >76% of tumor cells stained positive.
Lentiviral vectors production and
cellular transduction
Full-length human BAF53a overexpression clone
lentivirus and short hairpin RNAs (shRNA) lentivirus and their
control vectors were constructed by a biotechnology company
(GeneChem, Shanghai, China). Cells were cultured in 6-well plates
before transfection until 80–90% confluency within 24 h. Then the
lentivirus were transfected into cells according to the
manufacturer's instructions. Puromycin (2 µg/ml) was used to select
stable clones if necessary. After 48–72-h transfection, the cells
were harvested for subsequent assays. Transfection efficiency was
assessed by fluorescence microscope, real-time PCR and western
blotting.
Reagents
Mitomycin-C and puromycin were purchased from
ApexBio, Technology LLC A4452, A3740; ApexBio, TX, USA). The
Matrigel matrix was purchased from Corning Life Sciences (354248;
Corning, MA, USA). The primary antibodies against BAF53a
(sc-137062), E-cadherin (sc-71007), vimentin (sc-80975), and
β-actin (sc-130300) were purchased from Santa Cruz Biotechnology
(CA, USA).
Proliferation assays
The proliferation potential was measured by MTT
assay and colony formation assay. For MTT assays, the cells were
grown into 96-well plates with a density of 5×103
cells/well. Each day 6 wells of each group were added 100 µl MTT
(0.5 mg/ml; Sigma) reagent per well and incubated at 37°C for 4 h.
Subsequently, the absorbance values were measured at 570 nm. For
colony formation assays, 2×103 cells/well were seeded
into 6-well plates and cultured for 14 days. Then the cell colonies
were dyed with crystal violet and counted. The results were
recorded as mean ± SD of three repeats.
Migration and invasion assays
The migration potential was evaluated by
wound-healing and Transwell assay. The cells were cultured into
6-well plates at a density of 2×105 cells/well, when
grew to 90% confluence, cells were incubated with mitomycin-C (10
µg/ml) for 1 h to suppress proliferation, and starved in serum-free
medium for 24 h. When cells were confluent monolayer artificial
wound was scraped by a 10-µl pipette tip. The migration gap of the
wound was assessed after 24 or 48 h. The migration potential was
evaluated by Transwell system. The cells treated with mitomycin-C
(10 µg/ml) for 1 h at 37°C in serum-free medium were placed into
them with a density of 1×105 cells/insert. The DMEM with
10% FBS was added into the lower chamber. After incubation for
24–48 h, the cells in the upper membrane of insert were removed and
the cells adhering to the lower membrane of the inserts were
stained with crystal violet and counted by microscope after
rinsing. Then the invasion potential was evaluated by
Transwell-Matrigel system. The culture upper inserts were coated
with Matrigel, the subsequence processes were carried out as
Transwell assay. All the experiments were performed in
triplicates.
Statistical analysis
Statistical analysis was conducted with SPSS version
18.0 (SPSS, Inc., Chicago, IL, USA). Student's two-tailed t-test
was used to determine the statistical significance of measurement
data. Survival curves were performed by the Kaplan-Meier method and
compared by the log-rank test. Univariate and multivariate Cox
regression model was used for survival analysis. The continuous
data are shown as the mean ± SEM. A P-value of <0.05 was
considered statistically significant.
Results
BAF53a is highly expressed in glioma
specimens
To examine the role of BAF53a in glioma, we
initially detected the protein expression of BAF53a in tumor
tissues by immunohistochemistry (IHC). BAF53a was predominantly
expressed in the nucleus. We defined high BAF53a expression as
strongly (+++) or moderately (++) positive staining, and low BAF53a
expression as weakly positive (+) or negative staining (Fig. 1A). BAF53a was positively expressed
in the majority of glioma tissues (102/121), of which 79 exhibited
high expression (Table I).
Additionally, BAF53a mRNA expression was examined in 10 randomly
selected cases of glioma and 10 normal brain tissues obtained from
resection following trauma. These results indicated that BAF53a
mRNA expression levels in glioma tissues were significantly higher
than those in normal brain tissues (P<0.01; Fig. 1B).
 | Table I.BAF53a expression and
clinicopathologic features of 121 glioma cases. |
Table I.
BAF53a expression and
clinicopathologic features of 121 glioma cases.
|
|
| BAF53a
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Total n (121) | Low (42) | High (79) | P-value |
|---|
| Sex |
|
|
|
|
|
Female | 53 | 17 | 36 |
|
| Male | 68 | 25 | 43 | 0.591 |
| Age (years) |
|
|
|
|
| ≤50 | 72 | 29 | 43 |
|
|
>50 | 49 | 13 | 36 | 0.119 |
| Tumor size (cm) |
|
|
|
|
| ≤4 | 66 | 25 | 41 |
|
|
>4 | 55 | 17 | 38 | 0.423 |
| Necrosis |
|
|
|
|
|
Absence | 71 | 26 | 45 |
|
|
Presence | 50 | 16 | 34 | 0.599 |
| KPS score |
|
|
|
|
|
≤90 | 82 | 31 | 51 |
|
|
>90 | 39 | 11 | 28 | 0.299 |
| WHO grade |
|
|
|
|
| I and
II | 53 | 24 | 29 |
|
| III and
IV | 68 | 18 | 50 | 0.031 |
Subsequently, the expression profile of BAF53a was
explored using the Oncomine™ database (https://www.oncomine.org). The results showed that the
gene copy number of BAF53a in astrocytoma was higher than that in
normal brain tissue (Sun brain: fold change, 2.460; P<0.01;
Fig. 1C). Furthermore, the BAF53a
gene copy number in glioblastoma was significantly higher than that
in normal brain tissue (TCGA brain: fold change, 11.006; P<0.01;
Murat brain: fold change, 5.298; P=0.002; Fig. 1D). These results enable us to
speculate that BAF53 may be involved in the progression of
glioma.
BAF53a expression is associated with
poor prognosis in patients with glioma
We next explored the detailed associations between
BAF53a protein expression (determined by IHC) and the
clinicopathological features and prognosis of glioma patients. The
results indicated that high BAF53a expression was significantly
correlated with WHO grade (P=0.031; Table I). Survival analysis showed that the
BAF53a low expression group had favorable overall survival (OS)
[median OS time, 23.0 (95% CI, 11.6–34.4) vs. 12.0 (95% CI,
8.9–15.1) months; P<0.001; Fig.
2A] and progression-free survival (PFS) [median PFS time, 18.7
(95% CI, 10.6–26.8) vs. 8.0 (95% CI, 6.7–9.3) months, P<0.001;
Fig. 2B] compared with the BAF53a
high expression group. Furthermore, univariate and multivariate Cox
regression analyses indicated that BAF53a expression was an
independent prognostic factor for the OS and PFS of glioma patients
(HR=1.752, P=0.013 and HR=1.874, P 0.008, respectively; Tables II and III). These results indicate that BAF53a
may be useful as a prognostic marker for patients with glioma.
 | Table II.The univariate and multiple Cox
regression analyses of overall survival (OS) in glioma
patients. |
Table II.
The univariate and multiple Cox
regression analyses of overall survival (OS) in glioma
patients.
|
| Univariable
analysis | Multivariable
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (male vs.
female) | 1.322
(0.492–2.485) | 0.251 |
|
|
| Age (≤50 vs. >50
years) | 1.183
(0.642–1.934) | 0.426 |
|
|
| Tumor size (≤4 vs.
>4 cm) | 0.613
(0.382–1.267) | 0.137 |
|
|
| Necrosis (absence
vs. presence) | 0.714
(0.433–1.162) | 0.325 |
|
|
| KPS score (≤90 vs.
>90) | 0.682
(0.310–1.254) | 0.185 |
|
|
| WHO grade (III and
IV vs. I and II) | 3.126
(1.643–5.233) |
<0.001 | 2.304
(1.192–3.542) | 0.002 |
| BAF53a expression
(high vs. low) | 1.937
(1.326–3.174) | 0.006 | 1.752
(1.226–2.638) | 0.013 |
 | Table III.The univariate and multiple Cox
regression analyses of progression-free survival (PFS) in glioma
patients. |
Table III.
The univariate and multiple Cox
regression analyses of progression-free survival (PFS) in glioma
patients.
|
| Univariable
analysis | Multivariable
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (male vs.
female) | 1.167
(0.643–1.823) | 0.572 |
|
|
| Age (≤50 vs. >50
years) | 1.094
(0.735–1.541) | 0.714 |
|
|
| Tumor size (≤4 vs.
>4 cm) | 0.582
(0.221–1.142) | 0.095 | 0.623
(0.334–1.095) | 0.142 |
| Necrosis (absence
vs. presence) | 0.802
(0.455–1.372) | 0.483 |
|
|
| KPS score (≤90 vs.
>90) | 0.713
(0.386–1.197) | 0.224 |
|
|
| WHO grade (III and
IV vs. I and II) | 4.075
(1.825–7.437) |
<0.001 | 3.194
(1.426–5.273) |
<0.001 |
| BAF53a expression
(high vs. low) | 2.056
(1.274–3.312) | 0.003 | 1.874
(1.411–2.942) | 0.008 |
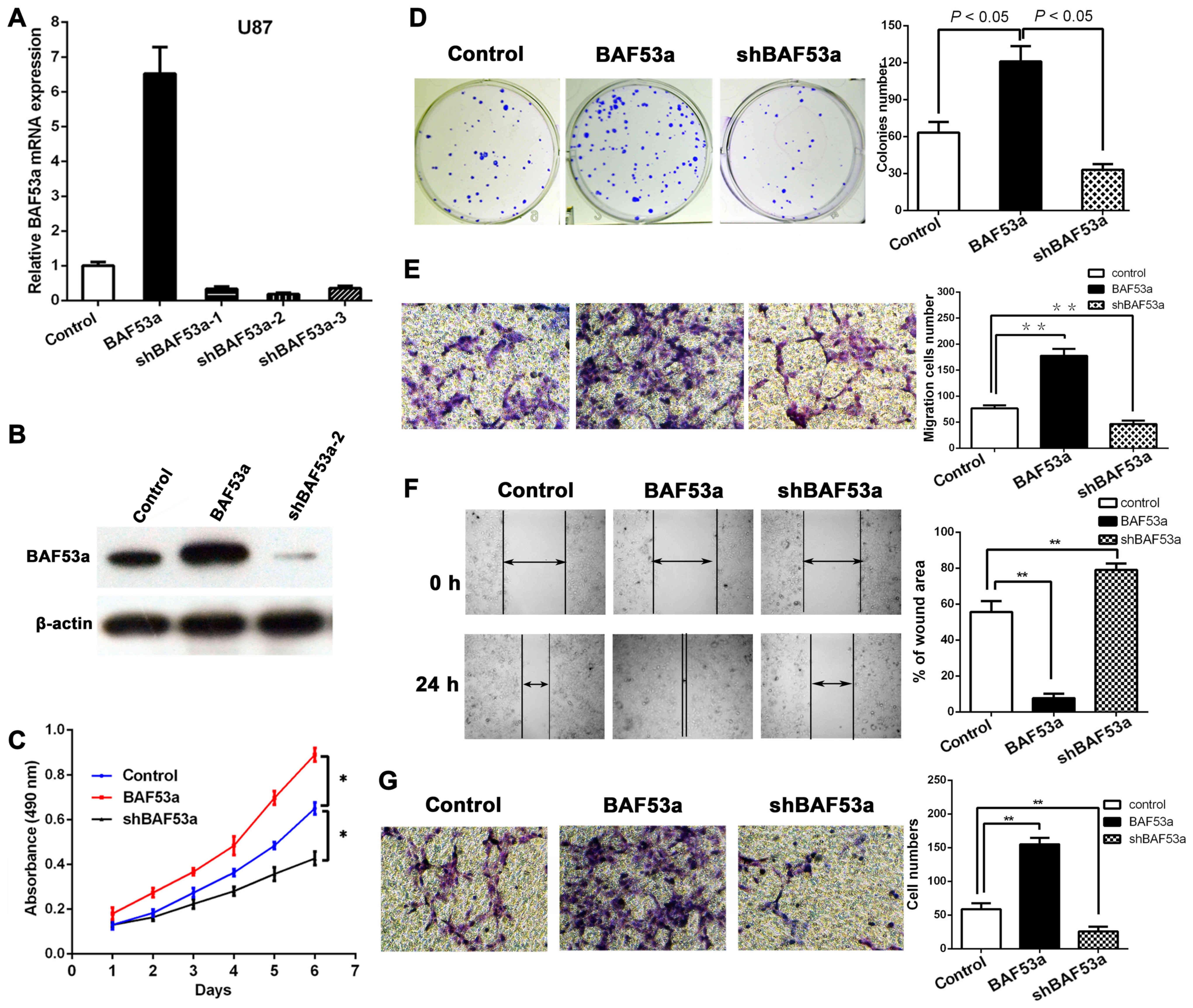
BAF53a promotes the proliferation of
glioma cells in vitro
In order to study the functional role of BAF53a in
glioma, stable BAF53a-overexpressing U87BAF53a and
BAF53a-knockdown U87shBAF53a cells and control cells
transfected with empty vector were established. The transfection
efficiency in each cell type was confirmed by qPCR and western
blotting (Fig. 3A and B).
Subsequently, the cells were subjected to MTT and colony formation
assays. The MTT assay revealed that BAF53a overexpression could
markedly increase U87 cell growth, while BAF53a knockdown had the
opposite effect as compared with control cells (P<0.05,
respectively; Fig. 3C). The colony
formation assay also showed that the U87BAF53a cells
were significantly larger and greater in number than the control
cells, while U87shBAF53a cells exhibited the opposite
characteristics (P<0.05, respectively; Fig. 3D). These results indicated that
BAF53a could promote the proliferation of glioma cells.
BAF53a promotes the migration and
invasion of glioma cells in vitro
Wound healing and Transwell assays were used to
assess the migratory ability of U87 cells expressing varying levels
of BAF53a. The results showed that BAF53a-overexpressing
U87BAF53a cells exhibited increased migration and faster
wound healing capacities, whereas U87shBAF53a cells
exhibited decreased migration and slower wound healing capacities
when compared with control cells (Fig.
3E and F). A Transwell-Matrigel chamber assay accordingly
showed that U87BAF53a cells had a greater capacity for
invasion across the Matrigel membrane compared with the control
cells, whereas U87shBAF53a cells had reduced invasive
ability (Fig. 3G). These results
indicated that BAF53a could promote the invasion capacity of glioma
cells and potentially facilitate the metastasis of glioma.
BAF53a expression is associated with
EMT in glioma
As previous studies have reported a possible link
between BAF53a and EMT in other physiological processes and cancer
progression, we aimed to explore this association in glioma. The
expression levels of two EMT markers, E-cadherin and vimentin, were
detected in cells expressing different levels of BAF53a. The qPCR
and western blotting results showed decreased expression of the
epithelial marker E-cadherin and increased expression of the
mesenchymal marker vimentin in U87BAF53a cells. By
contrast, the opposite pattern of expression was demonstrated in
U87shBAF53a cells (Fig. 4A and B).
Alterations in cellular morphology assessed by phase contrast
microscopy also revealed that U87BAF53a cells had more
pseudopodia and were elongated, indicating a mesenchymal phenotype,
whereas U87shBAF53a cells had an oval or
cobblestone-like appearance (Fig.
4C). E-cadherin and vimentin expression were also detected in
glioma tissues by IHC. The results revealed that E-cadherin
expression was frequently absent in glioma, whereas vimentin was
consistently expressed (Fig. 4D).
The Spearman rank correlation coefficient indicated that BAF53a
expression was positively correlated with the expression of
vimentin (r=0.384, P<0.001) and negatively correlated with the
expression of E-cadherin (r=−0.367, P<0.001) (Fig. 4E). Taken together, these results
suggest that BAF53a may promote glioma progression via EMT.
Discussion
The findings of the present study revealed that
BAF53a is a potential valuable prognostic marker for glioma
patients, and that it may promote glioma cell proliferation,
migration and invasion and be associated with EMT.
Notably, the present study demonstrated the
functional role of BAF53a in promoting glioma progression. The
subunits of the BAF complex are known to serve important roles in
many physiological and pathological processes in various cell types
and organs. At present, the association of BAF53a with cancer
development and progression is attracting increasing attention. In
2002, a study demonstrated that BAF53a was critical for the
oncogenic activity of c-Myc (17).
Additionally, studies of rhabdomyosarcoma, hepatocellular carcinoma
and squamous cell carcinoma have also provided evidence that BAF53a
is essential for cancer invasion and metastasis, and is associated
with poor prognosis (13,15,16).
Studies have also demonstrated that the subunits of the BAF
complex, including ARID2, ARID5B and BAF47, play important roles in
a variety of cancer types (18–20).
These results are consistent with those of the present study of
glioma, which showed that BAF53a may be a cancer-promoting
gene.
According to previous studies, BAF53a knockdown can
significantly impair neural stem/progenitor cell proliferation
capacity and their differentiation into neurons (12,21).
Another study showed that BAF53a was overexpressed in fibroblasts
and blocked the neuronal conversion of fibroblasts (22). A study by Krasteva et al also
found that the deletion of BAF53a resulted in embryonic lethality
and proliferation defects of hematopoietic stem cells (11). Furthermore, a recent study by Bao
et al showed that ectopic expression of BAF53a promoted the
proliferation and progenitor state of epidermal cells (23). These studies suggest that BAF53a can
control differentiation and proliferation in embryonic and adult
stem cells, as well as during embryogenesis. It is well established
that cancer progression shares many similar events with
embryogenesis, such as cell growth, differentiation and migration.
Therefore, genes that are crucial for embryonic or stem cell
development are often associated with cancer progression.
Accordingly, the present study has verified the hypothesis that
BAF53a can promote glioma progression and lead to poor
prognosis.
When cancer cells undergo EMT, they acquire
increased motility and invasive capacity, as well as resistance to
chemoradiotherapy; EMT thereby promotes metastasis and results in
poor prognosis. In glioma, various genes and non-coding RNAs, such
as AC1MMRY2, MLK4 and miR-200a/b, are reportedly able to induce
EMT, particularly in glioblastoma (24,25).
Notably, the present data indicated that alteration of BAF53a
expression could induce morphological changes in glioma cells, as
well as influencing the expression levels of epithelial and
mesenchymal markers. This suggested that BAF53a may induce EMT in
glioma and function as an EMT-related transcriptional factor. These
results illustrate a novel biological function of BAF53a in glioma,
which is consistent with previous studies of osteosarcoma and HCC
(14,16). Additionally, a recent study in HNSCC
found that BAF53a could activate YAP1, which can induce EMT in
various cancer types, and these findings confirm the role of BAF53a
in EMT (13,26).
EMT inhibitors, such as BGB324 and A83-01, are
designed to target receptors in the cytomembrane; however,
targeting EMT-related genes in the cell nucleus may be a more
precise approach. Therefore, the present results provide the basis
to potentially develop a novel targeted therapy.
The mechanism by which BAF53a induces EMT remains
unclear. Previous studies found that BAF53a could interact with
p63, SALL4, FGF4 and KLF4 to maintain the stemness and
undifferentiated state of stem/progenitor cells, which is important
for the acquisition of mesenchymal characteristics (10,13,23). A
study of HCC showed that BAF53a could regulate the transcription of
SOX2 to further activate the Notch pathway to induce EMT (16). However, the detailed molecular
mechanism regarding the contribution of BAF53a to EMT in glioma
still requires further research.
The present study had certain limitations. First,
the BAF53a expression profile and its prognostic significance were
only retrospectively analyzed in glioma tissues and in a single
institution; this need to be expanded to different cancer types and
validated in an external cohort in the future. Second, the
association between BAF53 and EMT requires further in vitro
and in vivo experiments to support the hypothesis. Third,
further research into the precise molecular mechanisms of BAF53 in
promoting glioma progression and EMT is necessary.
In conclusion, the present study suggests that high
BAF53a expression predicts poor prognosis of glioma patients, and
that BAF53a can promote invasion and EMT of glioma cells.
Therefore, BAF53a may be a useful prognostic marker and potential
promising target for glioma therapeutics.
References
|
1
|
Wen PYKS and Kesari S: Malignant gliomas
in adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen R, Smith-Cohn M, Cohen AL and Colman
H: Glioma subclassifications and their clinical significance.
Neurotherapeutics. 14:284–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Yang H, Gong B, Jiang C and Yang
L: Combined gene expression and protein interaction analysis of
dynamic modularity in glioma prognosis. J Neurooncol. 107:281–288.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alderton GK: Metastasis: Epithelial to
mesenchymal and back again. Nat Rev Cancer. 13:32013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kahlert UD, Maciaczyk D, Doostkam S, Orr
BA, Simons B, Bogiel T, Reithmeier T, Prinz M, Schubert J,
Niedermann G, et al: Activation of canonical WNT/β-catenin
signaling enhances in vitro motility of glioblastoma cells by
activation of ZEB1 and other activators of
epithelial-to-mesenchymal transition. Cancer Lett. 325:42–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Zhang W, Li Y, Alvarez A, Li Z,
Wang Y, Song L, Lv D, Nakano I, Hu B, et al: SHP-2-upregulated ZEB1
is important for PDGFRα-driven glioma epithelial-mesenchymal
transition and invasion in mice and humans. Oncogene. 35:5641–5652.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kahlert UD, Nikkhah G and Maciaczyk J:
Epithelial-to-mesenchymal(-like) transition as a relevant molecular
event in malignant gliomas. Cancer Lett. 331:131–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu W, Fang L, Ouyang B, Zhang X, Zhan S,
Feng X, Bai Y, Han X, Kim H, He Q, et al: Actl6a protects embryonic
stem cells from differentiating into primitive endoderm. Stem
Cells. 33:1782–1793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krasteva V, Buscarlet M, Diaz-Tellez A,
Bernard MA, Crabtree GR and Lessard JA: The BAF53a subunit of
SWI/SNF-like BAF complexes is essential for hemopoietic stem cell
function. Blood. 120:4720–4732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lessard J, Wu JI, Ranish JA, Wan M,
Winslow MM, Staahl BT, Wu H, Aebersold R, Graef IA and Crabtree GR:
An essential switch in subunit composition of a chromatin
remodeling complex during neural development. Neuron. 55:201–215.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saladi SV, Ross K, Karaayvaz M, Tata PR,
Mou H, Rajagopal J, Ramaswamy S and Ellisen LW: ACTL6A is
co-amplified with p63 in squamous cell carcinoma to drive YAP
activation, regenerative proliferation, and poor prognosis. Cancer
Cell. 31:35–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun W, Wang W, Lei J, Li H and Wu Y:
Actin-like protein 6A is a novel prognostic indicator promoting
invasion and metastasis in osteosarcoma. Oncol Rep. 37:2405–2417.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taulli R, Foglizzo V, Morena D, Coda DM,
Ala U, Bersani F, Maestro N and Ponzetto C: Failure to downregulate
the BAF53a subunit of the SWI/SNF chromatin remodeling complex
contributes to the differentiation block in rhabdomyosarcoma.
Oncogene. 33:2354–2362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao S, Chang RM, Yang MY, Lei X, Liu X,
Gao WB, Xiao JL and Yang LY: Actin-like 6A predicts poor prognosis
of hepatocellular carcinoma and promotes metastasis and
epithelial-mesenchymal transition. Hepatology. 63:1256–1271. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park J, Wood MA and Cole MD: BAF53 forms
distinct nuclear complexes and functions as a critical
c-Myc-interacting nuclear cofactor for oncogenic transformation.
Mol Cell Biol. 22:1307–1316. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li M, Zhao H, Zhang X, Wood LD, Anders RA,
Choti MA, Pawlik TM, Daniel HD, Kannangai R, Offerhaus GJ, et al:
Inactivating mutations of the chromatin remodeling gene ARID2 in
hepatocellular carcinoma. Nat Genet. 43:828–829. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kandoth C, Schultz N, Cherniack AD, Akbani
R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, et al:
Cancer Genome Atlas Research Network: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kadoch C and Crabtree GR: Reversible
disruption of mSWI/SNF (BAF) complexes by the SS18-SSX oncogenic
fusion in synovial sarcoma. Cell. 153:71–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoo AS, Staahl BT, Chen L and Crabtree GR:
MicroRNA-mediated switching of chromatin-remodelling complexes in
neural development. Nature. 460:642–646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoo AS, Sun AX, Li L, Shcheglovitov A,
Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW and Crabtree
GR: MicroRNA-mediated conversion of human fibroblasts to neurons.
Nature. 476:228–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao X, Tang J, Lopez-Pajares V, Tao S, Qu
K, Crabtree GR and Khavari PA: ACTL6a enforces the epidermal
progenitor state by suppressing SWI/SNF-dependent induction of
KLF4. Cell Stem Cell. 12:193–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SH, Ezhilarasan R, Phillips E,
Gallego-Perez D, Sparks A, Taylor D, Ladner K, Furuta T, Sabit H,
Chhipa R, et al: Serine/threonine kinase MLK4 determines
mesenchymal identity in glioma stem cells in an NF-κB-dependent
manner. Cancer Cell. 29:201–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi Z, Zhang J, Qian X, Han L, Zhang K,
Chen L, Liu J, Ren Y, Yang M, Zhang A, et al: AC1MMYR2, an
inhibitor of dicer-mediated biogenesis of Oncomir miR-21, reverses
epithelial-mesenchymal transition and suppresses tumor growth and
progression. Cancer Res. 73:5519–5531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shao DD, Xue W, Krall EB, Bhutkar A,
Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et
al: KRAS and YAP1 converge to regulate EMT and tumor survival.
Cell. 158:171–184. 2014. View Article : Google Scholar : PubMed/NCBI
|