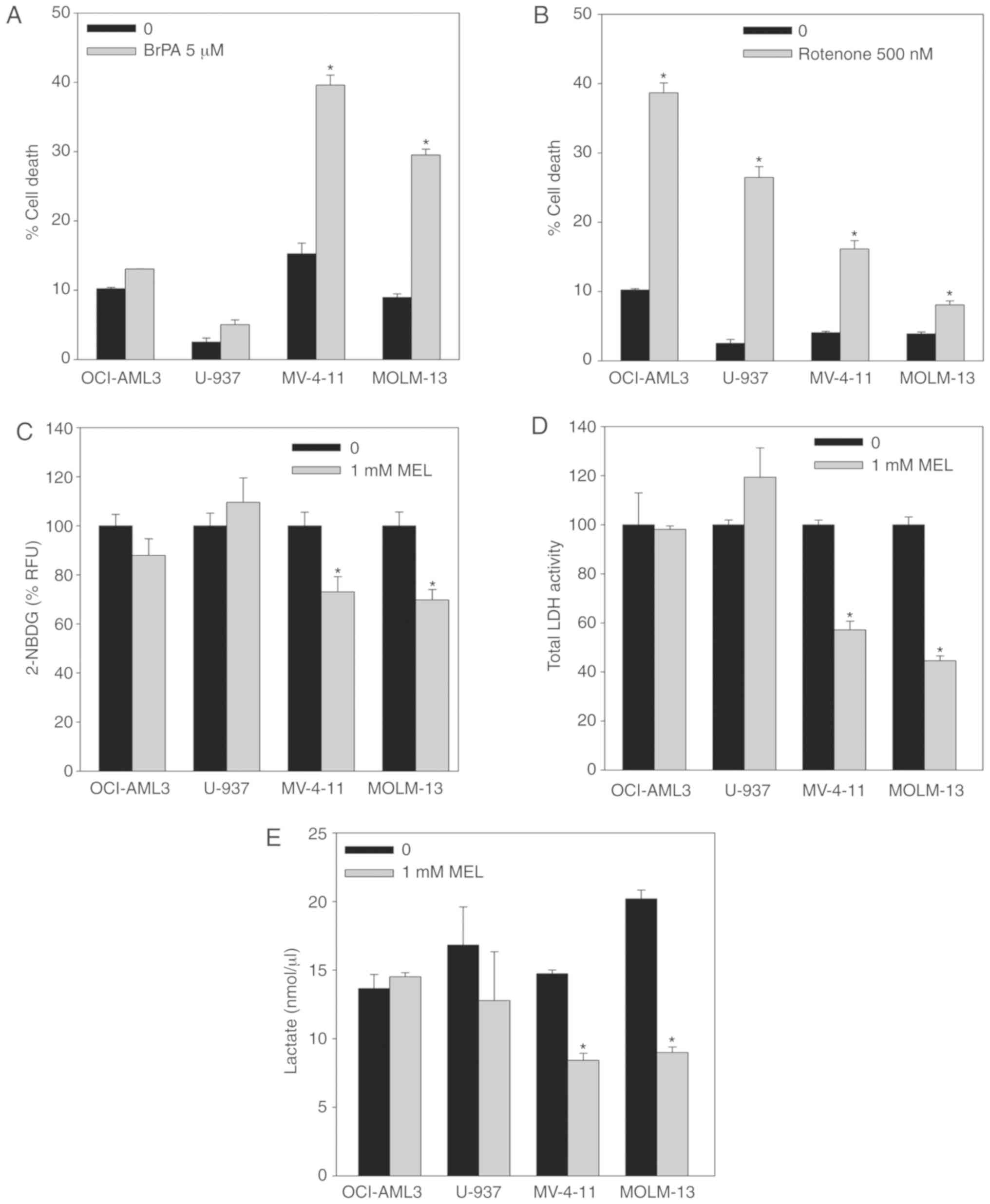
|
1
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel JP, Gönen M, Figueroa ME, Fernandez
H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S,
Aminova O, et al: Prognostic relevance of integrated genetic
profiling in acute myeloid leukemia. N Engl J Med. 366:1079–1089.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leung AY, Man CH and Kwong YL: FLT3
inhibition: A moving and evolving target in acute myeloid
leukaemia. Leukemia. 27:260–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang A, Ju HQ, Liu K, Zhan G, Liu D, Wen
S, Garcia-Manero G, Huang P and Hu Y: Metabolic alterations and
drug sensitivity of tyrosine kinase inhibitor resistant leukemia
cells with a FLT3/ITD mutation. Cancer Lett. 377:149–157. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ju HQ, Zhan G, Huang A, Sun Y, Wen S, Yang
J, Lu WH, Xu RH, Li J, Li Y, et al: ITD mutation in FLT3 tyrosine
kinase promotes Warburg effect and renders therapeutic sensitivity
to glycolytic inhibition. Leukemia. 31:2143–2150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen WL, Wang JH, Zhao AH, Xu X, Wang YH,
Chen TL, Li JM, Mi JQ, Zhu YM, Liu YF, et al: A distinct glucose
metabolism signature of acute myeloid leukemia with prognostic
value. Blood. 124:1645–1654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Favero G, Moretti E, Bonomini F, Reiter
RJ, Rodella LF and Rezzani R: Promising. Antineoplastic Actions of
Melatonin. Front Pharmacol. 9:10862018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodriguez C, Martín V, Herrera F,
García-Santos G, Rodriguez-Blanco J, Casado-Zapico S,
Sánchez-Sánchez AM, Suárez S, Puente-Moncada N, Anítua MJ and
Antolín I: Mechanisms involved in the pro-apoptotic effect of
melatonin in cancer cells. Int J Mol Sci. 14:6597–6613. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chuffa LG, Lupi Júnior LA, Seiva FR,
Martinez M, Domeniconi RF, Pinheiro PF, Dos Santos LD and Martinez
FE: Quantitative proteomic profiling reveals that diverse metabolic
pathways are influenced by melatonin in an in vivo model of ovarian
carcinoma. J Proteome Res. 15:3872–3882. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mao L, Dauchy RT, Blask DE, Dauchy EM,
Slakey LM, Brimer S, Yuan L, Xiang S, Hauch A, Smith K, et al:
Melatonin suppression of aerobic glycolysis (Warburg effect),
survival signalling and metastasis in human leiomyosarcoma. J
Pineal Res. 60:167–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanchez-Sanchez AM, Antolin I,
Puente-Moncada N, Suarez S, Gomez-Lobo M, Rodriguez C and Martin V:
Melatonin cytotoxicity is associated to Warburg effect inhibition
in Ewing sarcoma cells. PLoS One. 10:e01354202015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gunawardane RN, Nepomuceno RR, Rooks AM,
Hunt JP, Ricono JM, Belli B and Armstrong RC: Transient exposure to
quizartinib mediates sustained inhibition of FLT3 signaling while
specifically inducing apoptosis in FLT3-activated leukemia cells.
Mol Cancer Ther. 12:438–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
León J, Casado J, Jiménez Ruiz SM, Zurita
MS, González-Puga C, Rejón JD, Gila A, Muñoz de Rueda P, Pavón EJ,
Reiter RJ, et al: Melatonin reduces endothelin-1 expression and
secretion in colon cancer cells through the inactivation of FoxO-1
and NF-κβ. J Pineal Res. 56:415–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin V, Herrra F, Carrera-Gonzalez P,
García-Santos G, Antolín I, Rodriguez-Blanco J and Rodriguez C:
Intracellular signaling pathways involved in the cell growth
inhibition of glioma cells by melatonin. Cancer Res. 66:1081–1088.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Puente-Moncada N, Costales P, Antolín I,
Núñez LE, Oro P, Hermosilla MA, Pérez-Escuredo J, Ríos-Lombardía N,
Sanchez-Sanchez AM, Luño E, et al: Inhibition of FLT3 and PIM
kinases by EC-70124 exerts potent activity in preclinical models of
acute myeloid leukemia. Mol Cancer Ther. 17:614–624. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stubbs M and Griffiths JR: The altered
metabolism of tumors: HIF-1 and its role in the Warburg effect. Adv
Enzyme Regul. 50:44–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marini C, Ravera S, Buschiazzo A, Bianchi
G, Orengo AM, Bruno S, Bottoni G, Emionite L, Pastorino F,
Monteverde E, et al: Discovery of a novel glucose metabolism in
cancer: The role of endoplasmic reticulum beyond glycolysis and
pentose phosphate shunt. Sci Rep. 6:250922016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rogoff D, Black K, McMillan DR and White
PC: Contribution of hexose-6-phosphate dehydrogenase to NADPH
content and redox environment in the endoplasmic reticulum. Redox
Rep. 15:64–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sánchez-Sánchez AM, Martín V,
García-Santos G, Rodríguez-Blanco J, Casado-Zapico S,
Suarez-Garnacho S, Antolín I and Rodriguez C: Intracellular redox
state as determinant for melatonin antiproliferative vs. cytotoxic
effects in cancer cells. Free Radic Res. 45:1333–1341. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ambrus A: An updated view on the molecular
pathomechanisms of human dihydrolipoamide dehydrogenase deficiency
in light of novel crystallographic evidence. Neurochem Res.
44:2307–2313. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Owen OE, Kalhan SC and Hanson RW: The key
role of anaplerosis and cataplerosis for citric acid cycle
function. J Biol Chem. 277:30409–30412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Montal ED, Dewi R, Bhalla K, Ou L, Hwang
BJ, Ropell AE, Gordon C, Liu WJ, DeBerardinis RJ, Sudderth J, et
al: PEPCK coordinates the regulation of central carbon metabolism
to promote cancer cell growth. Mol Cell. 60:571–583. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grasmann G, Smolle E, Olschewski H and
Leithner K: Gluconeogenesis in cancer cells-repurposing of a
starvation-induced metabolic pathway? Biochim Biophys Acta Rev
Cancer. 1872:24–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu MX, Jin L, Sun SJ, Liu P, Feng X,
Cheng ZL, Liu WR, Guan KL, Shi YH, Yuan HX and Xiong Y: Metabolic
reprogramming by PCK1 promotes TCA cataplerosis, oxidative stress
and apoptosis in liver cancer cells and suppresses hepatocellular
carcinoma. Oncogene. 37:1637–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma R, Zhang W, Tang K, Zhang H, Zhang Y,
Li D, Li Y, Xu P, Luo S, Cai W, et al: Switch of glycolysis to
gluconeogenesis by dexamethasone for treatment of hepatocarcinoma.
Nat Commun. 4:25082013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park EJ, Woo SM, Min KJ and Kwon TK:
Transcriptional and post-translational regulation of Bim controls
apoptosis in melatonin-treated human renal cancer Caki cells. J
Pineal Res. 56:97–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang TH, Wu CH, Yeh CT, Su SC, Hsia SM,
Liang KH, Chen CC, Hsueh C and Chen CY: Melatonin suppresses
hepatocellular carcinoma progression via lncRNA-CPS1-IT-mediated
HIF-1α inactivation. Oncotarget. 8:82280–82293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cardaci S, Desideri E and Ciriolo MR:
Targeting aerobic glycolysis: 3-bromopyruvate as a promising
anticancer drug. J Bioenerg Biomembr. 44:17–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akers LJ, Fang W, Levy AG, Franklin AR,
Huang P and Zweidler-McKay PA: Targeting glycolysis in leukemia: A
novel inhibitor 3-BrOP in combination with rapamycin. Leuk Res.
35:814–820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hevia D, Gonzalez-Menendez P,
Fernandez-Fernandez M, Cueto S, Rodriguez-Gonzalez P, Garcia-Alonso
JI, Mayo JC and Sainz RM: Melatonin decreases glucose metabolism in
prostate cancer cells: A 13C stable isotope-resolved
metabolomic study. Int J Mol Sci. 18:E16202017. View Article : Google Scholar : PubMed/NCBI
|