Introduction
Cancer remains a challenging public health issue due
to its high mortality rate. Statistics show that in 2018 over 3
million new cancer cases and more than one million cancer-related
deaths were recorded in Europe (1).
Research in the field currently focuses on finding new anti-tumor
therapies with minimal adverse effects, one of the main research
directions consisting of exploitation of natural occurring
compounds. While conventional cancer treatment is associated with
immunosuppression and the occurrence of infections, new targeted
antitumor therapies induce other severe side effects such as
inflammation and autoimmunity (2).
Therefore, the struggle to find new therapeutic approaches with
high selectivity and targeted action is imperative in order to
reduce the occurrence of systemic toxic effects.
Recently, the scientific interest has focused on the
study of pentacyclic triterpene derivatives, due to their immense
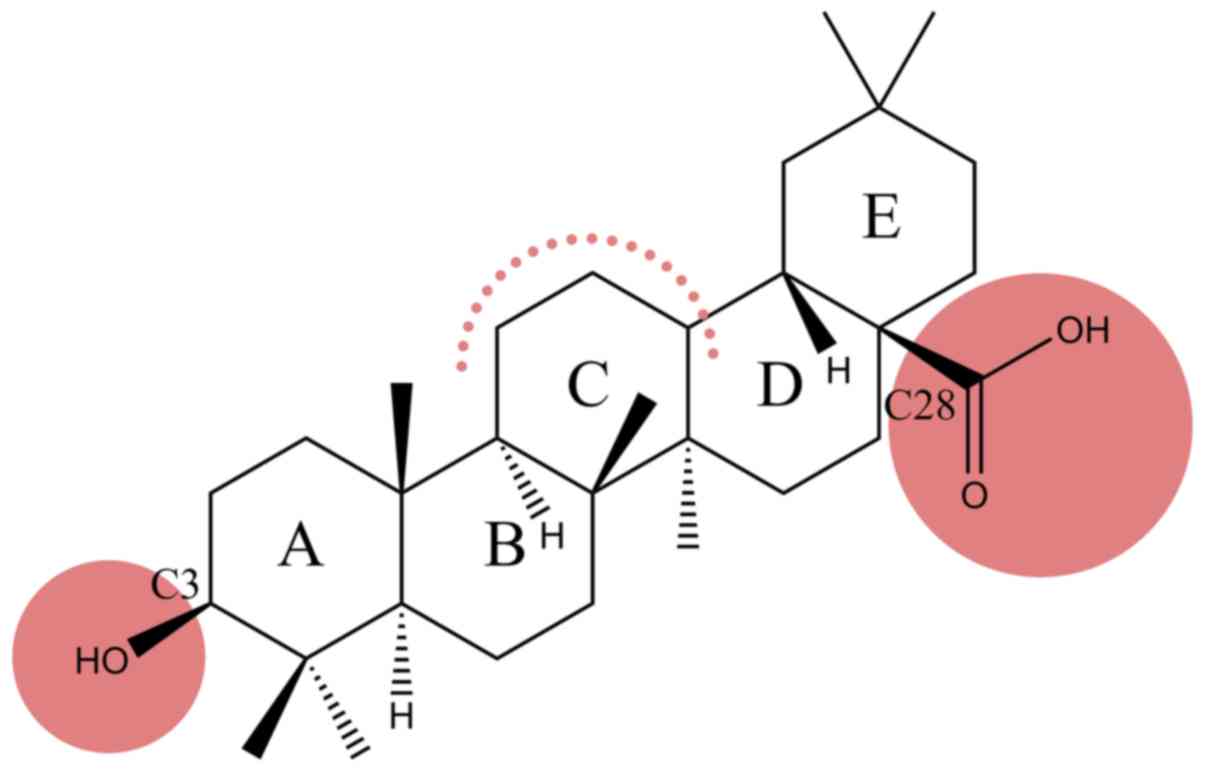
therapeutic potential. Oleanolic acid (OA) (Fig. 1), a pentacyclic triterpenoid widely
found in plants, whose cytotoxic effect has been tested in numerous
tumor cell lines, such as lung cancer (3), breast cancer (4), colon cancer (5) and melanoma cell lines (6), is considered a promising anticancer
drug. Insights have been gained with regard to the complexity of
mechanisms underlying the antitumor effect of OA, as follows: i)
induction of an increased expression of p53 tumor protein,
cytochrome c, caspase-3 apoptosis-promoting protein and Bax;
ii) triggering of mitochondrial mediated apoptosis; iii) inhibition
of Akt, mTOR and S6K protein expression; iv) cell cycle arrest in
different phases in a cancer type-dependent manner by altering the
expression of regulatory cell cycle proteins (7–9); v)
antioxidant activity by exerting scavenging effect against
superoxide anion, hydroxyl radical, nitric oxide and hydrogen
peroxide and also by increasing the ferrous iron chelating activity
(10); and vi) inhibition of
angiogenesis in a dose- dependent manner (11).
Even though OA has proven potent antitumor activity,
the in vivo application is limited due to its very low
solubility in aqueous solutions, inconvenience that led to the
development of novel semisynthetic derivatives with superior
antitumor activity and increased solubility, as:
3-O-acetyl-OA derived carboxamides, and oleanolic
acid-rhodamine B derivatives (12–15).
Rhodamines are xanthene-based derivatives widely
used as fluorescent dyes for cellular and mitochondrial membrane
potential measurement (16).
Moreover, rhodamines were shown to penetrate the mitochondrial
membrane and to accumulate in this organelle, exhibiting high tumor
cell selectivity (17,18). Sommerwerk et al (12) found that the chemical modification
of triterpenoic acid derivatives covalently bonded to rhodamine B,
including oleanolic acid-rhodamine B derivatives (RhodOA),
significantly improves their cytotoxic effect, becoming effective
on tumor lines starting at nanomolar (nM) concentrations. Moreover,
by staining and double-staining experiments it was reported that a
diacetylated maslinic acid derivative, was able to enter the
mitochondria, thus presenting a ‘MITOCAN’ behavior (i.e., agents
that directly target and alter mitochondrial function of cancer
cells causing cancer cell growth inhibition or apoptosis) (12).
A growing body of literature highlights the
essential role that mitochondria play in cancer formation,
progression, malignant transformation and even response to
treatment (19,20). Due to their involvement in energy
production, macromolecule biosynthesis, redox homeostasis, reactive
oxygen species (ROS) generation and the process of cell death
mitochondria have emerged as promising targets for the anticancer
agents (21,22).
Following the findings stated above, the purpose of
the present study was to assess the in vitro and in
ovo biological activity of RhodOA (Fig. 2) in different human tumor and
healthy cell lines (A375 melanoma cell line, MDA-MB-231 breast
adenocarcinoma, A549 lung adenocarcinoma, and HaCaT-healthy
immortalized keratinocytes) in order to gain a deeper insight
regarding their antiproliferative molecular mechanism.
Materials and methods
Cell culture
A375 human melanoma, A549 human lung adenocarcinoma,
and MDA-MB-231 human breast adenocarcinoma cell lines were
purchased from the American Type Culture Collection (ATCC);
HaCaT-human immortalized keratinocyte cell line was provided by the
University of Debrecen, Hungary as a kind gift.
All the cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA)
high-glucose medium supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/Strep,
10,000 IU/ml (Sigma-Aldrich; Merck KGaA). The cells were incubated
under standard temperature conditions of 37°C and humidity
containing 5% CO2.
MTT assay
To study cell viability, the colorimetric
microculture tetrazolium assay (MTT) was used, as described by
Andor et al (23) and Isaia
et al (24). Cells were
cultured in 96-well plates using a number of 1×104
cells/well. After cell attachment, they were treated with five
different concentrations (20, 40, 60, 80 and 100 nM) of RhodOA
solubilized in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA)
and rhodamine B aqueous solution for 24, 48 and 72 h. The control
cells were represented by the cells treated with DMSO, the solvent
of OA derivative conjugated with rhodamine B and water, the solvent
used for rhodamine B, respectively.
Following the treatment period, it was added 10
µl/well of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) solution (5 mg/ml) and after 3 h of incubation, the
MTT precipitates formed were dissolved in 100 µl of solubilization
buffer provided by the manufacturer. Finally, the reduced MTT was
spectrophotometrically measured at 570 nm, using a microplate
reader (xMark Microplate Spectrophotometer; Bio-Rad Laboratories,
Inc.). All experiments were performed in triplicate.
Immunofluorescence assay
The immunofluorescence assay was performed only on
A375-human melanoma cells, as tumor cells, the selection was based
on cell viability and cell respiration results; and also, on
HaCaT-human keratinocytes as the healthy cells. Cells were cultured
in 6-well plates on slides, 1×106 cells/well and were
stimulated with four different concentrations: 10, 20, 30 nM (the
concentrations tested on cell respiration) and 100 nM (highest
concentration tested in MTT assay).
The protocol applied for immunofluorescence
staining, was based on a protocol described by Gheorgheosu et
al (25) and adapted to our
laboratory conditions. After 24 h of stimulation, the cells were
fixed with 4% paraformaldehyde for one hour at room temperature.
After fixation, cells were permeabilized using 2% Triton X-100
solution in phosphate-buffered saline (PBS) for 30 min at room
temperature. To block permeabilization, 30% FBS solution in 0.01%
Triton X-100 was used for 1 h. Cells were incubated overnight at
4°C with primary antibodies Alexa Fluor® 555 Phalloidin
antibody (Cell Signaling Technology, Inc.) in a 1:20 dilution for
visualization of actin fibers and Anti-COX IV antibody
Mitochondrial marker (ab33985) in a dilution of 1:500. The
following day, the primary antibodies were washed with 0.01% Triton
X-100 solution in PBS. The secondary antibody specific for COX IV
mitochondrial marker-Donkey anti-goat IgG H&L (Alexa
Fluor® 488-ab150129) was added for 2 h at room
temperature in the dark. The 4′,6-diamidino-2-phenylindole (DAPI)
staining was used to visualize the nuclei. The images were captured
using an Olympus IX73 inverted microscope provided with DP74 camera
and analyzed with CellSens V1.15 software (Olympus Corporation) and
Image J software.
Scratch assay
To determine the ability of RhodOA to inhibit the
migration of human melanoma A375 cells, scratch assay was
performed. The protocol applied was previously described in the
literature (26). Briefly,
2×105 A375 cells/well were cultured in 12-well plates
until it reached 80–90% confluence. Using a sterile pipette tip, a
line was drawn in the middle of the well through the single cell
layer. The detached cells were removed by washing with PBS before
stimulation. Then, the cells were stimulated with four different
concentrations of the derivative (20, 40, 60 and 80 nM) and with
the solvent used for the preparation of the derivative solutions
(DMSO). The cells were immortalized at the time interval of 0 h and
24 h and were compared with the unstimulated control cells. Images
were taken using the Olympus IX73 inverted microscope provided with
DP74 camera (Olympus Corporation), and cell migration analysis was
performed using cell Sense Dimension software. In order to
calculate the percentage of migration, the formula previously
described by Felice et al (27) was applied:
Scratch closure rate =[(At0-At/At0)] x 100
where, At0, scratch
area at time 0; At, scratch area at 24 h.
High resolution respirometry
Cellular respiratory function was determined by the
means of high resolution respirometry studies (Oxygraph-2k Oroboros
Ltd.) at 37°C. To obtain a comprehensive analysis of respiratory
control, a substrate-uncoupler-inhibitor titration (SUIT) protocol
was followed, designed to allow the measurements of respiratory
rates with both separate and convergent Complex I and II (CI+CII)
electron input, as described by Petruș et al (28). The cells (1×106/ml) were
suspended in a mitochondrial respiration medium (MIRO5:
MgCl2 3 mM, EGTA 0.5 mM, taurine 20 mM,
KH2PO4 10 mM, K-lactobionate 60 mM, D-sucrose
110 mM, HEPES 20 mM, BSA 1 g/l, pH 7.1).
In order to allow the passage of soluble molecules
between external media and cytosol and to analyze the extended
functional oxidative phosphorylation (OXPHOS), plasma membrane was
permeabilized by adding a mild detergent: digitonin (35
µg/lx106 cells). The optimum digitonin concentration for
selective and complete plasma membrane permeabilization was
determined directly in a respirometric protocol, as described by
Pesta and Gnaiger (29).
Routine respiratory rates were measured in the
presence of cells suspended in mitochondrial respiration media for
10–15 min. The SUIT protocol comprises several steps, as follows:
i) addition of digitonin (cells permeabilizer) and the CI
substrates (glutamate-G, 10 mM and malate-M, 5 mM) for State2
(basal respiratory rate) CI assessment; ii) addition of ADP (5 mM)
for CI-dependent active respiration (OXPHOS CI) measurement; iii)
addition of CII substrate succinate (S, 10 mM) allowed the
convergent electron flow from both CI and CII and the measurement
of maximal OXPHOS capacity (OXPHOS CI+CII); iv) addition of
oligomycin (1 µg/ml) (inhibitor of ATP synthesis) resulted in a
non-phosphorylative state and return to basal respiration (State4
CI+CII); v) addition of a decoupling agent, p- (trifluoromethoxy)
phenylhydrazone carbonyl cyanide (FCCP) determined the maximum
respiratory capacity of the electron transport system (ETS CI+CII);
vi) addition of the CI inhibitor rotenone (0.5 µM) was used to
measure the ETS capacity, dependent on CII alone (ETS CII); and
vii) antimycin A (1 µg/ml), a CIII inhibitor, was added to allow
residual oxygen consumption (ROX) to be measured. All respiratory
rates were corrected for ROX. After data acquisition, the
corresponding flux control ratios (FCRs) that express respiratory
control independent of cell size and content in mitochondria was
calculated, as follows: i) P/E ratio (OXPHOS CI+CII/ETS CI+CII)
describes the function of the phosphorylation system; ii) L/E
(LEAK/ETS CI+CII) an indicator of mitochondrial coupling; iii) R/E
(Routine/ETS CI+CII) shows how close the routine respiration is
operating to the maximum ETS capacity, thus reflecting the degree
of mitochondrial dysfunction; and iv) RCR (OXPHOS CI+CII/STATE2
CI)-indicates the OXPHOS coupling efficiency. The oxygen
consumption rates (respiratory rates) were obtained using the
software support DatLab 4 (Oroboros Instruments) and statistically
analyzed using the GraphPad Prism 5 software (GraphPad Software,
Inc.).
Antioxidant activity
The antioxidant capacity of RhodOA and Rhod was
measured by their ability to scavenge free radicals using a
2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma-Aldrich; Merck KGaA)
ethanolic solution 0.1 mM. DMSO (Sigma-Aldrich; Merck KGaA) and
distilled water (Chemical Company SA Iasi) were used as solvents
for the dilution of the first (RhodOA) and second (Rhod),
respectively. An ethanolic solution (ethanol 80% (v/v); Chemical
Company SA Iasi) of ascorbic acid (Lach-Ner Company) 2 mM, was used
as standard.
Experiments were carried out using the slightly
modified method of Manzocco et al (30). Briefly, 2.5 ml of each sample was
mixed with 0.5 ml of DPPH 0.1 mM. The absorbance of each sample was
recorded continuously at 517 nm for 10 min, using a T70 UV/VIS
spectrophotometer (PG Instruments). The antioxidant activity was
calculated using the following formula:
%AOA=(Ainitial-Afinal)/Ainitial x 100
where, Ainitial, the absorbance of
the DPPH 0.1 mM free radical, without the sample;
Afinal, the absorbance of each sample in mixture
with the DPPH 0.1 mM free radical.
The antioxidant activity of each sample was compared
to the antioxidant activity of the standard solution (ascorbic acid
ethanolic solution 2 mM). The graphic data was interpreted using
Origin 8 Lab software.
The IC50DPPH, defined
as the concentration of antioxidant compounds from each sample that
causes 50% loss of the DPPH activity was also determined, by using
GraphPad Prism 8 software (GraphPad Software, Inc.).
Chorioallantoic membrane (CAM)
assay
Fertilized eggs (Gallus gallus domesticus)
obtained from a local farm were disinfected with alcohol 70°, dated
and incubated in a horizontal position under constant humidity
conditions and a temperature of 37°C. On the 3rd day of incubation,
7 ml of albumen was extracted to allow the CAM to detach from the
eggshell so that the blood vessels could be easily observed. On the
fourth day of incubation, a window was cut and covered with
adhesive tape, and the eggs were incubated until the day of the
experiment.
The study was conducted starting with the 7th day of
incubation. The effect of the RhodOA on angiogenesis was recorded
for 5 days. During this time, the blood vessels have an increased
rate of development similar to the tumor angiogenesis process
(31). Five concentrations of
RhodOA (20, 40, 60, 80 and 100 nM) and the 0.5% DMSO solvent were
tested in triplicates. A volume of 10 µl was applied inside a 5 mm
diameter ring placed previously on the CAM surface. The microscopic
evaluation was done daily by means of a Discovery 8
Stereomicroscope (Zeiss), while images were captured for analysis
using Axio CAM 105 color (Zeiss) and Image J software.
Hen egg test-CAM (HET-CAM) assay
To evaluate the biocompatibility and toxicity of
RhodOA, the Hen egg test (HET-CAM) was assessed. The eggs were
prepared as described above in the CAM method. Five eggs were used
for each test solution. For each solution, a volume of 500 µl was
tested. The changes observed in CAM were evaluated using a
stereomicroscope (Discovery 8 Stereomicroscope; Zeiss) and the
images were performed (Axio CAM 105 color; Zeiss) before and after
a 5 min application. All images were processed using ImageJ v 1.50e
software (U.S. National Institutes of Health).
Water was used as negative control, and 1% sodium
dodecylsulfate (SDS) in H2O was used as positive
control. The test samples were diluted in DMSO at a concentration
of 80 and 100 nM.
The effects on blood vessels, hemorrhage (H), vessel
lysis (L) and coagulation and extra vascular (C) were monitored for
5 min. The analytical method used to determine the irritant effect
was to calculate the irritation score (IS) using the formula
(32):
IS=5×301-H300+7×301-L300+9×301-C300
where, IS was between 0 and 21.
Determination of anti-irritative
potential
The eggs were prepared as indicated above. The
method was adapted to our laboratory conditions (33). To determine the anti-irritant effect
of RhodOA, the two previously tested concentrations (80 and 100 nM)
were applied to the CAM in a volume of 600 µl. The eggs were
introduced into the incubator for 3 h to allow the solution to be
absorbed into the membrane. After 3 h, the eggs were treated with
300 µl 0.5% SDS in H2O. The membrane was observed for 5
min and the vascular effects of lysis, stasis and hemorrhage were
monitored.
Results
RhodOA induces a dose-dependent
decrease of tumor cell viability
In order to determine the impact of the tested
compound on cells viability, five different concentrations (20, 40,
60, 80 and 100 nM) were tested in lung cancer (A549), breast cancer
(MDA-MB-231) and human melanoma (A375) cell lines as compared to a
healthy human keratinocyte cell line (HaCaT). Cell viability was
determined by MTT assay at 3 time intervals: 24, 48 and 72 h,
respectively. At 24 and 48 h no significant effect was observed in
the tumor cell lines; however, at 72 h a dose-dependent decrease in
cell viability was noted in all the tumor lines, the most affected
being the human melanoma cell line A375 (Fig. 3). In contrast, the HaCaT cells
viability was slightly decreased at 20 nM concentration (up to
92%), while the highest concentration of the derivative, 100 nM,
decreased viability to 83%. In parallel, 5 different concentrations
of rhodamine B (20, 40, 60, 80 and 100 nM) were tested; a slight
dose-dependent decrease of cell viability was recorded but compared
to the RhodOA derivative the decrease was not statistically
significant.
RhodOA induces reorganization of A375
cytoskeleton and nuclei condensation
Changes in the nucleus, actin fibers and
mitochondria morphological aspects were assessed by fluorescence
microscopy in human melanoma A375 cells and human keratinocytes
HaCaT.
An immunofluorescence assay was performed to
determine the RhodOA mechanism of action responsible for
cytotoxicity in human melanoma cells. By DAPI staining assay, it
was observed that RhodOA induced a condensation of the nuclei in
the case of the A375 cells, the most visible effect being recorded
at a concentration of 100 nM (Fig.
4). In the case of the human keratinocytes (Fig. 5), the condensation of the nuclei was
not so visible. Therefore, it can be concluded that RhodOA has a
selective proapoptotic effect on the human melanoma cells.
The morphology and organization of actin fibers were
evaluated in human melanoma cells and human keratinocytes after
their treatment with different concentrations of RhodOA. In the
case of human melanoma cells (Fig.
4), in the control cells, a network of branched actin fibers
that runs along the cells was observed. Following treatment with
RhodOA, a condensation of actin fibers was noticed, the strongest
effect being recorded at a concentration of 100 nM. For human
keratinocytes (Fig. 5) no
significant changes in the structure and organization of actin
fibers were noted.
By using the Anti-COX IV antibody Mitochondrial
marker, we were able to gather information on the localization of
mitochondria in A375 and HaCaT cells (Fig. 4) with and without RhodOA
pretreatment. In A375 cells an increase in mitochondria
concentration near the nucleus upon treatment with increasing
concentrations of RhodOA was noted. In the HaCaT cells no
modifications in terms of mitochondria distribution were detected
(Fig. 5).
RhodOA inhibits A375 cell migration in
a dose-dependent manner
In order to determine the effect of the OA
derivative on cellular migration, the scratch assay was performed.
Human melanoma cells were stimulated with four different
concentrations of the derivative (20, 40, 60 and 80 nM), and were
compared to unstimulated control cells and solvent
(DMSO)-stimulated cells.
Stimulation of the A375 cells with RhodOA produced a
dose-dependent anti-migratory effect (Fig. 6). At the lowest concentration of 20
nM, a closure of 35.6% was recorded, similar to that of the control
group. At the concentration of 40 and 60 nM, the closure rate was
22.97 and 16.10%, respectively. At the highest tested concentration
(80 nM), the derivative strongly inhibited cell migration,
presenting the lowest closure rate (10.76%). In addition, after 24
h of stimulation, a slight modification in cell morphology was
noted and some cells detached from the plate, which suggests that
RhodOA has a cytotoxic effect against the melanoma cells (Fig. 7).
High-resolution respirometry
studies
To study the effect on cellular respiration, three
increasing concentrations of RhodOA (10, 20 and 30 nM) were tested
in HaCaT and A375 cells.
The compound produced a dose-dependent increase in
all respiratory rates in the HaCaT cells, whereas, in the case of
A375 cells, a dose-dependent decrease of all respiratory rates, P/E
and RCR and an increase of FCRs: L/E and R/E were recorded. The
decrease of respiratory rates: State2 CI and State4 CI+CII suggests
that the tested compound decreases oxygen consumption when the
phosphorylation system is in an inactive state due to a decrease in
proton leak/slip across the inner mitochondrial membrane. In
addition, our results show that RhodOA inhibits active respiration
and decreases the maximal respiratory capacity of the electron
transfer system, by lowering the respiratory rates of OXPHOS CI and
OXPHOS CI+CII simultaneously with the decrease of ETS CI+CII and
ETS CII. As displayed in Fig. 8, in
A375 cells, the routine respiration (i.e., when cell respiration is
controlled by the physiological aerobic ATP demand) is decreased in
a dose-dependent manner when RhodOA was applied (Table I).
 | Table I.Effect of acute treatment with RhodOA
(10, 20 and 30 nM) on P/E, L/E, R/E and RCR in HaCaT-human
keratinocytes and A375-human melanoma cell lines. |
Table I.
Effect of acute treatment with RhodOA
(10, 20 and 30 nM) on P/E, L/E, R/E and RCR in HaCaT-human
keratinocytes and A375-human melanoma cell lines.
|
| P/E | L/E | R/E | RCR |
|---|
|
|
|
|
|
|
|---|
|
| HaCaT | A375 | HaCaT | A375 | HaCaT | A375 | HaCaT | A375 |
|---|
| Control | 0.787418 | 0.521129 | 0.133967 | 0.141969 | 0.463907 | 0.729738 | 6.213332 | 5.058102 |
| RhodOA 10 nM | 0.722780 | 0.683028 | 0.125734 | 0.164756 | 0.428187 | 0.841086 |
8.509565a | 6.490921 |
| RhodOA 20 nM | 1.010007 | 0.642803 | 0.131158 | 0.138359 | 0.466503 | 1.003413 |
4.770558a | 4.87191 |
| RhodOA 30 nM | 0.751809 | 0.637909 | 0.126915 | 0.217103 | 0.377931 |
1.218005a | 6.422751 | 6.102949 |
Antioxidant activity
The antioxidant activity (AOA) was assessed by means
of the DPPH method. Two sets of samples [RhodOA and rhodamine B
(Rhod)] of different concentrations (20, 40, 60, 80 and 100 nM)
were tested.
As presented in Fig.
9, when the 0.1 mM ethanolic DPPH solution was used, both
RhodOA and Rhod showed antioxidant activity compared to ascorbic
acid. More precisely, the antioxidant activity of RhodOA and Rhod
samples was above 80%, being slightly increased at the beginning of
the reaction and gently decreasing towards the end of the reaction.
This pattern was similar for almost all samples, except RhodOA 20
nM and RhodOA 60 nM, in which antioxidant activity was slightly
higher at the end of the reaction compared to the initial moment.
The maximum antioxidant activity was reached in the case of RhodOA
100 and 60 nM (85.03%) and Rhod 100 nM (84.71%) samples; the
antioxidant activity of ascorbic acid was 94.19%.
The RhodOA and Rhod samples quenche the DPPH free
radical after the first 50 sec, subsequently the reaction reaches
equilibrium, except RhodOA 40 nM, Rhod 20 and 40 nM, in which
antioxidant activity decreases visibly.
As shown in Fig. 9,
the antioxidant activity of the analyzed samples is not
concentration-dependent, at the initial moment nor after 10 min of
reaction with DPPH free radical, its values oscillating over time
(Table II).
 | Table II.The inhibition percentage of ascorbic
acid 2 mM, RhodOA and Rhod (20, 40, 60 and 80 nM), when reacting
with 0.1 mM DPPH, after 10 min. |
Table II.
The inhibition percentage of ascorbic
acid 2 mM, RhodOA and Rhod (20, 40, 60 and 80 nM), when reacting
with 0.1 mM DPPH, after 10 min.
| Ascorbic acid in
ethanol 80% | RhodOA solution in
DMSO | Rhod solution in
distilled water |
|---|
|
|
|
|---|
| Concentration
(mM) | % inhibition | Concentration
(nM) | % inhibition | Concentration
(nM) | % inhibition |
|---|
| 2 | 94.16±0.0589 | 20 | 83.08±0.0089 | 20 | 83.70±0.0086 |
| 1 | 91.60±0.0075 | 40 | 82.27±0.0093 | 40 | 83.70±0.0086 |
| 0.6 | 49.51±0.0451 | 60 | 83.34±0.0623 | 60 | 84.68±0.0571 |
| 0.3 | 31.72±0.0610 | 80 | 84.61±0.0081 | 80 | 83.60±0.0086 |
| 0.1 |
9.20±0.0811 | 100 | 84.31±0.0083 | 100 | 84.71±0.0080 |
The IC50DPPH values for
ascorbic acid 2 mM (IC50=63.65 nM), RhodOA
(IC50=60.44 nM) and Rhod (IC50=52.82 nM) were
also calculated when reacting with 0.1 mM DPPH ethanolic solution,
using equation (2) described
above.
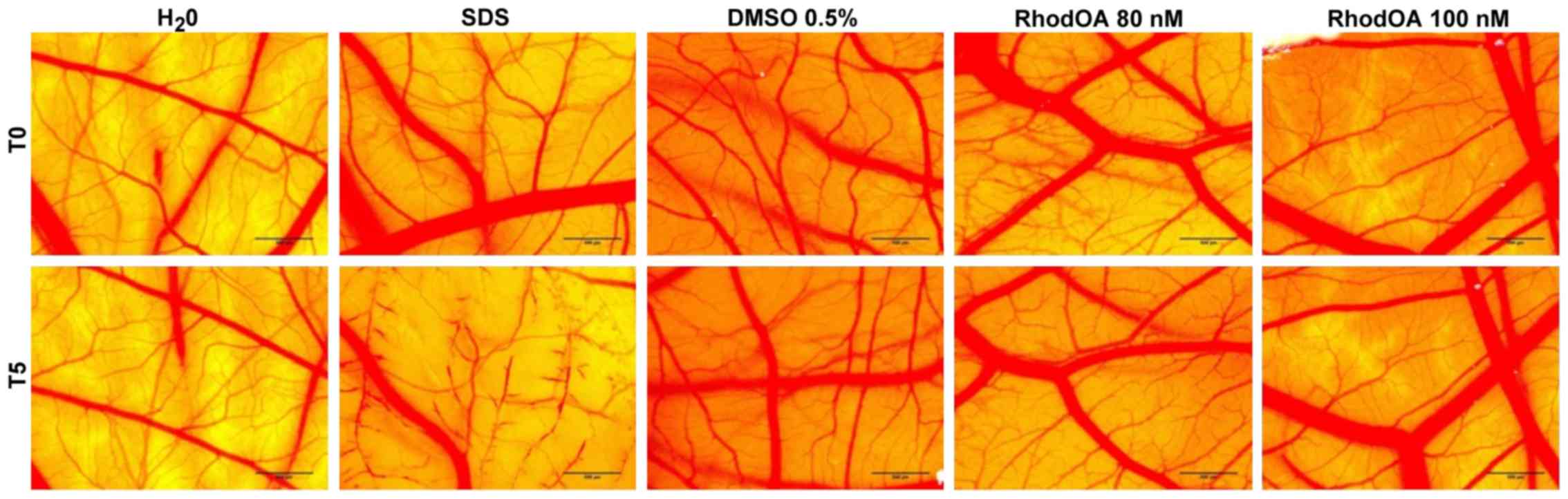
RhodOA has no toxic effect in ovo
The toxic potential of the RhodOA was tested in
ovo using chicken CAM as the biological medium; the protocol
allowed the assessment of its potential irritant effect following
topical application. The effect induced by the compound along with
the effects induced by positive (SDS) and negative controls
(water), respectively, were recorded as photographs before and
after 5 min of contact with the CAM. Hemorrhage, coagulation and
lysis of blood vessels were detected only in SDS treated CAMs.
The highest IS was present only in the SDS-treated
CAM, whereas the negative control and the test compound caused no
irritant effect. The application of 500 µl SDS induces massive
damage to blood vessels, causing micro-hemorrhage, coagulation and
vascular lysis occurring on an extended membrane area. The death of
specimens treated with SDS was noted after 60 min. For
non-irritative samples, a viability of over 24 h was recorded.
Both tested concentrations of RhodOA, 80 and 100 nM
were non-irritative to the vascular plexus of the CAM (Fig. 10; Table III).
 | Table III.IS for SDS, DMSO and RhodOA 80 and
100 nM and the occurrence time of hemorrhage (tH), lysis (tL) and
coagulation (tC). |
Table III.
IS for SDS, DMSO and RhodOA 80 and
100 nM and the occurrence time of hemorrhage (tH), lysis (tL) and
coagulation (tC).
|
| SDS 1% | DMSO 0.5% | RhodOA 80 nM | RhodOA 100 nM |
|---|
| IS | 19.17 | 1.15 | 1.87 | 2.07 |
| tH | 18 sec | 300 | 300 | 180 |
| tL | 23 sec | 300 | 300 | 300 |
| tC | 35 sec | 264 | 240 | 300 |
RhodOA exhibits a moderate
anti-irritant potential
In order to determine the potential anti-irritant
effect of the tested compound, we proceeded with a preventive
application of the test solutions and DMSO as control, prior to the
SDS irritative intervention. The same parameters as for the HET-CAM
assay were registered (H, L and S time in seconds), both with and
without pre-treatment of test samples. Thus, the following
parameters for the anti-irritant effect were calculated:
HAI = H/HSDS: hemorrhage time
after pretreatment with RhodOA and SDS addition 0.5%/hemorrhage
time without pretreatment with RhodOA; LAI =
L/LSDS: vascular lysis time after pretreatment
with RhodOA and SDS addition 0.5%/vascular lysis time without
pretreatment with RhodOA; CAI =
C/CSDS: vascular coagulation time after
pretreatment with RhodOA and SDS addition 0.5%/vascular coagulation
time without pretreatment with RhodOA.
Table IV presents
the irritability score (IS) after treatment with RhodOA 80 nM
(ISRhodOA80) and 100 nM (ISRhodOA100), H, L
and C onset times before treatment; HRhodOA,
LRhodOA and CRhodOA after pretreatment and
administration of 0.5% SDS and HAI, LAI and
CAI parameters.
 | Table IV.Anti-irritant effect of RhodOA 80 and
100 nM and solvent DMSO 0.5% after CAM irritation with SDS
0.5%. |
Table IV.
Anti-irritant effect of RhodOA 80 and
100 nM and solvent DMSO 0.5% after CAM irritation with SDS
0.5%.
|
| SDS 0.5% | DMSO 0.5% +
SDS | RhodOA 80nM +
SDS | RhodOA 100 nM +
SDS |
|---|
| IS | 18.68 | 18.43 | 17.03 | 17 |
| tH | 55 sec | 24 sec | 90 sec | 90 sec |
| tL | 44 sec | 90 sec | 70 sec | 80 sec |
| tC | 14 sec | 4
sec | 30 sec | 23 sec |
| HAI |
| 0.43 | 1.63 | 1.63 |
| LAI |
| 2.04 | 1.59 | 1.81 |
| CAI |
| 0.28 | 2.14 | 1.64 |
The lack of anti-irritative effect can clearly be
seen for DMSO, while RhodOA induces a reduction of the IS value of
SDS with no pre-treatment. Both concentrations of RhodOA induce a
similar moderate anti-irritative effect (Fig. 11).
RhodOA presents a dose-dependent
anti-angiogenic effect
The anti-angiogenic effect of the OA-rhodamine B
derivative was tested in vivo using the CAM assay. Chicken
embryo specimens showed good viability and survival rate, allowing
compound testing up to approximately ED11. After 3 days of
treatment, a change in the vascular network was observed. Fig. 12 shows a decrease in vascular
density, with numerous areas with a low number of capillaries
inside the ring. This decrease in the number of newly formed
vessels is directly proportional to the concentration tested,
significant results were obtained in the case of the highest tested
concentration (100 nM).
Discussion
Old strategies, which aimed to treat cancer just by
the induction of cell death, are no longer relevant. New
therapeutic approaches that also target the tumor microenvironment
treatment, immune/inflammatory response modulation, stopping
angiogenesis and resistance of cancer cell death by using highly
selective compounds with a specific action and with reduced
systemic toxic effects, represent the focus of the current trends
in cancer research. Therefore, an increasing number of preclinical
and epidemiological studies support the beneficial effect of
phytochemicals in cancer (34,35).
Due to their selectivity against cancer cells and the plethora of
biological activities (anticancer, anti-inflammatory,
anti-angiogenic, antioxidant, antibacterial, antiviral and
antifungal), the use of triterpenes have emerged as a multifaceted
potential alternative in cancer prevention and treatment (36). Among triterpenes, the
antiproliferative effect of OA has been described in various types
of cancer, such as: breast cancer (4), lung cancer (3), liver cancer (37), skin cancer (38,39),
gastric cancer (40), pancreatic
cancer (41), prostate cancer
(42) and in leukemia (43).
In order to increase its therapeutic potential in
cancer treatment, numerous derivatizations of OA have been
performed. A study by Wiemann et al (44) evaluated the cytotoxic potential of
hydroxiaminated derivatives of OA and revealed that the obtained
derivatives exhibit higher cytotoxic effect on certain tumor cell
lines (breast cancer, MCF-7, lung cancer, A549) and a lower
cytotoxic activity against healthy cell lines (nonmalignant mouse
fibroblasts, NIH 3T3). Another study demonstrated that by
introducing a double bond at C ring, a derivative with superior
antitumor effect is obtained by increasing ROS production,
caspase-9 activity and inducing mitochondrial mediated apoptosis in
MCF-7, breast cancer cell line (14). Heller et al (15) showed that 3-O-acetyl-OA
derived carboxamides also have a superior antitumor effect compared
to OA when applied on several human tumor cell lines, by inducing
cell cycle arrest and cell death via autophagy or apoptosis. In
line with the previous stated findings, Xie et al (45), reported the synthesis of a
rhodamine-B-docetaxel conjugate, in order to obtain improved
mitochondrial delivery and increased cytotoxicity; results showed
the specific delivery of the compound to mitochondria and improved
antitumor effect.
Similarly, pentacyclic triterpenoic acid rhodamine B
esters or piperazine-spacered rhodamine B amides were shown to be
more cytotoxic on various cancer cell lines compared with their
parent compound (12,46). The present work aimed to further
investigate oleanolic acid-rhodamine B (RhodOA) mechanism of action
by a series of in vitro and in ovo biological
studies. Thus, the effect of RhodOA on cellular viability was first
assessed in three tumor cell lines: lung cancer (A549), breast
cancer (MDA-MB-231) and human melanoma (A375) cell lines and in a
healthy human keratinocyte cell line (HaCaT). We found that after
72 h exposure, RhodOA significantly decreased cell viability, in a
dose-dependent manner, in all three different types of cancer cell
lines (the most significant effect was observed in A375 cells, cell
line selected for further investigations), whereas healthy human
keratinocyte cells were not affected. Moreover, RhodOA induced a
dose-dependent anti-migratory effect in A375 cell line (Fig. 6). As suggested by the obtained data,
RhodOA presents selective antitumor activity as well as reduced
toxicity on healthy cells.
One of the hallmarks of cancer is the immense
ability of tumor cells to evade apoptosis. A large number of
literature studies revealed that defects in the apoptotic pathways
have an important role in carcinogenesis and thus, treatment
strategies that target apoptosis can be successfully used in cancer
therapy (47,48). The morphological changes associated
with apoptosis include: formation of apoptotic bodies, nuclear
condensation, DNA fragmentation, cell shrinkage and membrane
alterations (49). Several recent
studies indicated that OA can induce the above-mentioned
morphological changes in HepG2-human hepatocellular carcinoma cell
line (50), in HeLa-human cervical
cancer cell line (51) and in
various human astrocytoma cell lines (52). Similar morphological changes of
apoptosis were reported when OA derivatives were used against
SMMC-7721 human hepatocellular carcinoma cell line (53), K562 human leukemia cell line
(54) and B16-F10 melanoma cell
line, respectively (55). Moreover,
another cytotoxic triterpenoid, piperazine, of rhodamine B
derivatives were reported to accumulate in mitochondria and to
trigger apoptosis in A2780 ovarian carcinoma cells (46). Our results are in line with all
these findings, showing that RhodOA produced a condensation of the
nuclei, a hallmark of apoptosis, with the highest effect being
detected when the highest concentration was used (100 nM, Fig. 4), visible only in the case of A375
cells and not in the HaCaT cells; therefore, a high degree of
selectivity can be assumed. However, a deeper analysis is required
to evaluate all the morphological changes associated with apoptosis
and to conclude on the pro-apoptotic effect of the new OA-rhodamine
B derivative.
The tumor invasion and metastasizing capacity
depends on the cancer cell ability to move, and hence by the actin
content. Our findings regarding the evaluation of actin fiber
organization, in A375 human melanoma cells, revealed that treatment
with RhodOA causes significant changes in the F-actin pattern,
increasing the condensation of F-actin, the strongest effect being
observed at 100 nM, while untreated A375 human melanoma cells
presented a normal morphology and actin spread (Fig. 4). For HaCaT human keratinocytes, no
significant changes in the structure and organization of actin
fibers were observed (Fig. 5).
These results suggest that the RhodOA influences the cytoskeleton
arrangement of human melanoma cells; similar results were obtained
in astrocytoma cell lines treated with OA (52).
As reviewed by Kim et al (56), due to the essential role that
mitochondria play in energy metabolism, signaling pathways and cell
death, they are implicitly involved in tumor initiation, growth,
metastasis and even in drug resistance. Numerous studies have tried
to identify the antitumor mechanisms of action of OA. Zhu et
al (50) found that OA induces
apoptosis in liver cancer cells by affecting mitochondria in a
dose-dependent manner (50).
Another study reported that OA induced apoptosis in human
hepatocellular carcinoma (HuH7) cell lines through
mitochondria-mediated pathway (57). Similarly, when tested on A549 lung
cancer cells, OA produces ultrastructural alterations of the
mitochondria, increases ROS production and causes mitochondria
autophagy, a process known as mitophagy (58).
In the present study, we evaluated the effects of a
new RhodOA conjugate on mitochondrial respiration in healthy human
keratinocytes (HaCaT) and on human melanoma tumor cells (A375).
When applied to the A375 cells, RhodOA decreased routine
respiration in a dose-dependent manner (Fig. 8). Mitochondrial respiration and
phosphorylation in the routine state are controlled by energy
demand and turnover and the degree of mitochondrial coupling
(29). The results also revealed a
dose-dependent inhibition of the active respiration (OXPHOS) in the
A375 cells as well as an increase of the active respiration in
HaCaT cells (Fig. 8). However,
mitochondrial uncoupling/toxicological dyscoupling can contribute
to flux control in the OXPHOS state. In order to see if the
decrease of OXPHOS observed in the A375 cells treated with RhodOA
is a result of RhodOA acting as a mitochondrial uncoupler (i.e., by
increasing the proton leak across the inner mitochondrial membrane,
thus uncoupling the link between ADP phosphorylation in ATP and
substrate oxidation) the values of State4 CI+CII were analyzed
(Fig. 8). As presented by Terada
(59) uncoupling agents increase
State4 respiratory rate. Interestingly, our results showed a
decrease of the State4 CI+CII respiratory rate, suggesting that
RhodOA decreases proton leak/slip across the inner mitochondrial
membrane and does not act as mitochondrial uncoupler. Hence, we can
assume that the detected decrease of OXPHOS is a direct consequence
of low ADP phosphorylation in ATP. Taken together these results
suggests that treatment with RhodOA impaired mitochondrial function
and the capacity to produce sufficient levels of ATP in A375
melanoma cells without inducing toxicity in the healthy human
keratinocyte cells HaCaT. As previously demonstrated, ATP loss in
cancer cells leads to energy depletion, cellular stress, and
ultimately may induce cell death through different pathways
(60,61). Contrary to the traditional belief
that cancer cells present mitochondrial damage and consecutive
metabolic inability, current studies have demonstrated that
mitochondrial respiration is not impaired in many cancers (62). By calculating FCRs (Table I), we obtained valuable information
regarding mitochondrial integrity, efficiency and more importantly,
how the tested compound influenced mitochondrial functions. In A375
cells treated with RhodOA the results revealed a decrease of RCR
and P/E parallel with an increase of L/E and R/E vs. control, thus
suggesting that RhodOA decreases the capacity of the
phosphorylation system. In addition, the L/E and R/E increase
observed in the A375 cell tests (Table
I) suggests an increased cellular ATP demand or the existence
of a limitation in the mitochondrial respiratory capacity produced
by defects in substrate oxidation or in ETS complexes after
treatment with RhodOA (29). By
correlating the high antitumor effect with the relevant
intervention on mitochondrial respiration, we can presume that the
new OA-rhodamine B derivative exhibits a ‘MITOCAN’ behavior.
As presented in a recent review by Yang et
al (63), ROS role in cancer is
like a double-edged sword, being a tumor-suppressing or a
tumor-promoting agent, with vast evidence that supports both cases.
However, this dichotomy of ROS in cancer cells depends on the stage
of cancer progression; in early stages moderate ROS induce tumor
growth and metastasis, whilst in late stages, with tumor
progression, increasing ROS levels can lead to apoptosis, cell
death and senescence (64). In the
case of human melanoma cells, studies concluded that ROS play an
important role in increasing the metastasis potential, by inducing
DNA mutations and cell proliferation and by activating molecules
involved in metastasis, such as: urokinase plasminogen receptor
activator, interleukin-8, epidermal growth factor receptor, and
vascular endothelial growth factor (65). In lung cancer, increasing ROS
produce changes at the DNA level and in the structure of lipids,
carbohydrates and proteins, thus leading to proliferation and
metastasis of cancer cells (66). A
study performed on MDA-MB-231 breast cancer cell lines concluded
that ROS stimulate angiogenesis and favor the metastasis process
(67). In terms of the effect
phytochemicals exhibit in cancer, a study revealed that OA had an
anti-proliferative effect while increasing the oxidative stress in
various breast cancer cell lines, thus being a pro-oxidant agent
(68). Controversially, on HCT 116
colon cancer cell line, OA exhibited anti-proliferative and
antioxidant activity (69). The
free radical scavenging activity of OA, analyzed through the same
DPPH method used in the current work, showed that OA has a
significant free radical inhibitory activity compared to the
standard antioxidant ascorbic acid (IC50=61.5 µg/ml; 132
µM) (69). This is in line with our
findings, according to which RhodOA maintains the free radical
scavenging activity exhibited by OA; moreover, RhodOA has a
stronger antioxidant effect at 100 nM, comparable to that of the
ascorbic acid control solution (RhodOA IC50=60.44
nM).
Tumor development and progression represents a
cumulative molecular and phenotypic modification of epithelial
cells (70). Angiogenesis is one of
the processes that underlies tumor development and progression. New
blood vessel development is stimulated when tumor cells require
increasing amounts of nutrients and oxygen due to a high and rapid
proliferation rate (71,72). Therefore, inhibition of angiogenesis
can result in tumor growth inhibition. Regarding the effect of OA
on angiogenesis, an important effect in inhibiting the formation of
blood vessels has been observed in the case of Sk-Mel-2 melanoma
cells (73). The anti-angiogenic
effect of OA has been previously tested using the CAM method and it
was proven to have the potential to reduce vascular density and
reduce capillary count in a concentration of 30 µM (73).
In this study, the effects of a novel OA-rhodamine
B derivative were evaluated on angiogenesis and a decrease was
detected in the vascular density and a lower number of capillaries
after 3 days of treatment (Fig.
12). The best recorded results in terms of the number of newly
formed vessels were recorded for the highest tested concentration
(100 nM), a lower dose compared to that previously used for the
parent compound (74). The
literature contains data regarding optimized anti-angiogenic effect
of certain OA derivatives; CDDO-Me (methyl
2-cyano-3,12-dioxoolean-1,9-dien-28-oate) and CDDO-Imm
(2-cyano-3,12-dioxoolean-1,9-dien-28-oic imidazolide), exhibited
increased anti-angiogenic effect, by preventing endothelial cell
tubulogenesis, at low doses in the Matrigel sponge assay, being
also able to inhibit tumor growth in an immortalized Kaposi's
sarcoma cell line (74).
In conclusion, this study brings valuable evidence
that an oleanolic acid-rhodamine B derivative, RhodOA, display
antitumor properties against lung cancer, breast cancer and human
melanoma cell lines without inducing toxic effects on human healthy
keratinocytes. Moreover, when tested in human melanoma cells,
RhodOA was able to produce a condensation of the nuclei and of
F-actin. Despite the limitations of this study, the above-mentioned
results suggest that RhodOA is involved in the apoptotic process of
cancer cells as well as in suppressing tumor invasion and
metastasis. A significant improvement of the anti-angiogenic
potential compared to the parent compound OA was observed for the
novel rhodamine B derivative. Furthermore, the novel formulation
RhodOA showed an increased free radical scavenging activity
compared to OA alone. In melanoma cells, RhodOA impaired
mitochondrial function and the capacity to produce sufficient
levels of ATP, whilst the mitochondrial function of healthy human
keratinocytes was left intact. The observed antitumor effect can be
correlated with the intervention that RhodOA has on mitochondrial
respiration, therefore the new OA-rhodamine B derivative can be
classified as a ‘MITOCAN’. Even though further studies are still
needed to unravel its full mechanism of action, this study sheds
light on the immense therapeutic anticancer potential of this novel
OA derivative.
Acknowledgments
Not applicable.
Funding
This study was supported by an internal grant at
‘Victor Babes’ University of Medicine and Pharmacy (grant no.
2DOC/1299/31.01.2020.
Availability of data and materials
Not applicable.
Authors' contributions
VD, CD, CȘ: designed and directed the project; CD,
AT: provided critical feedback and helped shape the research,
analysis and manuscript; IM, AM, DC, VLD: contributed to the
interpretation of the results; IM, ȘA, AM: carried out the
experiments; IM, DAS, AM, CȘ, DC: wrote the manuscript with input
from all authors. All the authors discussed the results and
contributed to the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer
incidence and mortality patterns in Europe: Estimates for 40
countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kroschinsky F, Stölzel F, von Bonin S,
Beutel G, Kochanek M, Kiehl M and Schellongowski P; Intensive Care
in Hematological and Oncological Patients (iCHOP) Collaborative
Group, : New drugs, new toxicities: Severe side effects of modern
targeted and immunotherapy of cancer and their management. Crit
Care. 21:892017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao X, Liu M and Li D: Oleanolic acid
suppresses the proliferation of lung carcinoma cells by
miR-122/Cyclin G1/MEF2D axis. Mol Cell Biochem. 400:1–7. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu J, Yang C, Guo C, Li X, Yang N, Zhao L,
Hang H, Liu S, Chu P, Sun Z, et al: SZC015, a synthetic oleanolic
acid derivative, induces both apoptosis and autophagy in MCF-7
breast cancer cells. Chem Biol Interact. 244:94–104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Wei L, Shen A, Chu J, Lin J and Peng
J: Oleanolic acid modulates multiple intracellular targets to
inhibit colorectal cancer growth. Int J Oncol. 47:2247–2254. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oprean C, Ivan A, Bojin F, Cristea M,
Soica C, Drăghia L, Caunii A, Paunescu V and Tatu C: Selective in
vitro anti-melanoma activity of ursolic and oleanolic acids.
Toxicol Mech Methods. 28:148–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Bai H, Zhang X, Liu J, Cao P, Liao
N, Zhang W, Wang Z and Hai C: Inhibitory effect of oleanolic acid
on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest
and mitochondrial-dependent apoptosis. Carcinogenesis.
34:1323–1330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim GJ, Jo HJ, Lee KJ, Choi JW and An JH:
Oleanolic acid induces p53-dependent apoptosis via the ERK/JNK/AKT
pathway in cancer cell lines in prostatic cancer xenografts in
mice. Oncotarget. 9:26370–26386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mu DW, Guo HQ, Zhou GB, Li JY and Su B:
Oleanolic acid suppresses the proliferation of human bladder cancer
by Akt/mTOR/S6K and ERK1/2 signaling. Int J Clin Exp Pathol.
8:13864–13870. 2015.PubMed/NCBI
|
|
10
|
Senthilkumar PK, Kandhavelu M and Reetha
D: Antioxidant properties of the oleanolic acid isolated from
Cassia auriculata (Linn). J Pharm Res Clin Pract. 4:30–36.
2014.
|
|
11
|
Sohn KH, Lee HY, Chung HY, Young HS, Yi SY
and Kim KW: Anti-angiogenic activity of triterpene acids. Cancer
Lett. 94:213–218. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sommerwerk S, Heller L, Kerzig C, Kramell
AE and Csuk R: Rhodamine B conjugates of triterpenoic acids are
cytotoxic mitocans even at nanomolar concentrations. Eur J Med
Chem. 127:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salvador JAR, Leal AS, Valdeira AS,
Gonçalves BMF, Alho DPS, Figueiredo SAC, Silvestre SM and Mendes
VIS: Oleanane-, ursane-, and quinone methide friedelane-type
triterpenoid derivatives: Recent advances in cancer treatment. Eur
J Med Chem. 142:95–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pattnaik B, Lakshma Nayak V, Ramakrishna S
and Venkata Mallavadhani U: Synthesis of ring-C modified oleanolic
acid derivatives and their cytotoxic evaluation. Bioorg Chem.
68:152–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heller L, Knorrscheidt A, Flemming F,
Wiemann J, Sommerwerk S, Pavel IZ, Al-Harrasi A and Csuk R:
Synthesis and proapoptotic activity of oleanolic acid derived
amides. Bioorg Chem. 68:137–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan X, Zhou Y and Liu S: Optical imaging
of tumors with copper-labeled rhodamine derivatives by targeting
mitochondria. Theranostics. 2:988–998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lampidis TJ, Hasin Y, Weiss MJ and Chen
LB: Selective killing of carcinoma cells ‘in vitro’ by
lipophilic-cationic compounds: A cellular basis. Biomed
Pharmacother. 39:220–226. 1985.PubMed/NCBI
|
|
18
|
Johnson LV, Walsh ML and Chen LB:
Localization of mitochondria in living cells with rhodamine 123.
Proc Natl Acad Sci USA. 77:990–994. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vyas S, Zaganjor E and Haigis MC:
Mitochondria and Cancer. Cell. 166:555–566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Porporato PE, Filigheddu N, Pedro JMB,
Kroemer G and Galluzzi L: Mitochondrial metabolism and cancer. Cell
Res. 28:265–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weinberg SE and Chandel NS: Targeting
mitochondria metabolism for cancer therapy. Nat Chem Biol. 11:9–15.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, Karakhanova S, Hartwig W, D'Haese
JG, Philippov PP, Werner J and Bazhin AV: Mitochondria and
mitochondrial ROS in cancer: novel targets for anticancer therapy.
J Cell Physiol. 231:2570–2581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andor B, Tischer AA (Tucuina),
Berceanu-Vaduva D, Lazureanu V, Cheveresan A and Poenaru M:
Antimicrobial activity and cytotoxic effect on gingival cells of
silver nanoparticles obtained by biosynthesis. Rev Chim.
70:781–783. 2019. View Article : Google Scholar
|
|
24
|
Isaia AI (Oarcea), Ienascu IMC, Andrica
FM, Georgescu D, Bratosin D and Pinzaru IA: Preliminary in vitro
evaluation of seven different plant extracts on A375, B164A5 and
HaCat cell lines. Rev Chim. 68:1633–1636. 2016.
|
|
25
|
Gheorgheosu D, Jung M, Ören B, Schmid T,
Dehelean C, Muntean D and Brüne B: Betulinic acid suppresses
NGAL-induced epithelial-to-mesenchymal transition in melanoma. Biol
Chem. 394:773–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghițu A, Schwiebs A, Radeke HH, Avram S,
Zupko I, Bor A, Pavel IZ, Dehelean CA, Oprean C, Bojin F, et al: A
comprehensive assessment of apigenin as an antiproliferative,
proapoptotic, antiangiogenic and immunomodulatory phytocompound.
Nutrients. 11:8582019. View Article : Google Scholar
|
|
27
|
Felice F, Zambito Y, Belardinelli E,
Fabiano A, Santoni T and Di Stefano R: Effect of different chitosan
derivatives on in vitro scratch wound assay: A comparative study.
Int J Biol Macromol. 76:236–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Petruș A, Rațiu C, Noveanu L, Lighezan R,
Roșca M and Muntean DO: Assessment of mitochondrial respiration in
human platelets. Revista De Chimie. 68:768–771. 2017. View Article : Google Scholar
|
|
29
|
Pesta D and Gnaiger E: High-resolution
respirometry: OXPHOS protocols for human cells and permeabilized
fibers from small biopsies of human muscle. Methods Mol Biol.
810:25–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Manzocco L, Anese M and Nicoli MC:
Antioxidant properties of tea extracts as affected by processing.
LWT - Food Sci Technol. 31:694–698. 1998. View Article : Google Scholar
|
|
31
|
Nowak-sliwinska P, Segura T and
Iruela-Arispe ML: The chicken chorioallantoic membrane model in
biology, medicine and bioengineering. Angiogenesis. 17:779–804.
2015. View Article : Google Scholar
|
|
32
|
Batista-Duharte A, Jorge Murillo G, Pérez
UM, Tur EN, Portuondo DF, Martínez BT, Téllez-Martínez D,
Betancourt JE and Pérez O: The hen's egg test on chorioallantoic
membrane: An alternative assay for the assessment of the irritating
effect of vaccine adjuvants. Int J Toxicol. 35:627–633. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moacă EA, Farcaş C, Coricovac D, Avram S,
Mihali CV, Drâghici GA, Loghin F, Păcurariu C and Dehelean C: Oleic
acid double coated Fe3O4 nanoparticles as
anti-melanoma compounds with a complex mechanism of activity - in
vitro and in ovo assessment. J Biomed Nanotechnol. 15:893–909.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Orlikova B, Legrand N, Panning J, Dicato M
and Diederich M: Anti-inflammatory and anticancer drugs from
nature. Cancer Treat Res. 159:123–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tuorkey MJ: Cancer therapy with
phytochemicals: Present and future perspectives. Biomed Environ
Sci. 28:808–819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chudzik M, Korzonek-Szlacheta I and Król
W: Triterpenes as potentially cytotoxic compounds. Molecules.
20:1610–1625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi Y, Song Q, Hu D, Zhuang X, Yu S and
Teng D: Oleanolic acid induced autophagic cell death in
hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent
pathway. Korean J Physiol Pharmacol. 20:237–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lúcio KA, Rocha G da G, Monção-Ribeiro LC,
Fernandes J, Takiya CM and Gattass CR: Oleanolic acid initiates
apoptosis in non-small cell lung cancer cell lines and reduces
metastasis of a B16F10 melanoma model in vivo. PLoS One.
6:e285962011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tokuda H, Ohigashi H, Koshimizu K and Ito
Y: Inhibitory effects of ursolic and oleanolic acid on skin tumor
promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer
Lett. 33:279–285. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao L, Xu Z, Wang Y, Sun B, Song Z, Yang
B, Liu X, Lin Y, Peng J, Han G, et al: Anticancer effect of SZC017,
a novel derivative of oleanolic acid, on human gastric cancer
cells. Oncol Rep. 35:1101–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei J, Liu M, Liu H, Wang H, Wang F, Zhang
Y, Han L and Lin X: Oleanolic acid arrests cell cycle and induces
apoptosis via ROS-mediated mitochondrial depolarization and
lysosomal membrane permeabilization in human pancreatic cancer
cells. J Appl Toxicol. 33:756–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Song Y, Zhang P, Zhu H, Chen L, Xiao
Y and Xing Y: Oleanolic acid inhibits cell survival and
proliferation of prostate cancer cells in vitro and in vivo through
the PI3K/Akt pathway. Tumour Biol. 37:7599–7613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang P, Li H, Chen D, Ni J, Kang Y and
Wang S: Oleanolic acid induces apoptosis in human leukemia cells
through caspase activation and poly(ADP-ribose) polymerase
cleavage. Acta Biochim Biophys Sin (Shanghai). 39:803–809. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wiemann J, Heller L and Csuk R: Targeting
cancer cells with oleanolic and ursolic acid derived hydroxamates.
Bioorg Med Chem Lett. 26:907–909. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie C, Chang J, Hao X-D, Yu J-M, Liu H-R
and Sun X: Mitochondrial-targeted prodrug cancer therapy using a
rhodamine B labeled fluorinated docetaxel. Eur J Pharm Biopharm.
85:(3 Pt A). 541–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wolfram RK, Heller L and Csuk R: Targeting
mitochondria: Esters of rhodamine B with triterpenoids are
mitocanic triggers of apoptosis. Eur J Med Chem. 152:21–30. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ferreira CG, Epping M, Kruyt FAE and
Giaccone G: Apoptosis: Target of cancer therapy. Clin cancer Res.
8:2024–2034. 2002.PubMed/NCBI
|
|
49
|
Balba A and Catoi C: Tumor cell
morphology. Comparative Oncology. The Publishing House of the
Romanian Academy. (Bucharest). 2007.
|
|
50
|
Zhu YY, Huang HY and Wu YL: Anticancer and
apoptotic activities of oleanolic acid are mediated through cell
cycle arrest and disruption of mitochondrial membrane potential in
HepG2 human hepatocellular carcinoma cells. Mol Med Rep.
12:5012–5018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Song X, Liu CC, Hong YR and Zhu XC:
Anticancer activity of novel oleanolic acid methyl ester derivative
in HeLa cervical cancer cells is mediated through apoptosis
induction and reactive oxygen species production. Bangladesh J
Pharmacol. 10:8962015. View Article : Google Scholar
|
|
52
|
Martín R, Carvalho-Tavares J, Ibeas E,
Hernández M, Ruiz-Gutierrez V and Nieto ML: Acidic triterpenes
compromise growth and survival of astrocytoma cell lines by
regulating reactive oxygen species accumulation. Cancer Res.
67:3741–3751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fan X, Wang P, Sun Y, Jiang J, Du H, Wang
Z, Duan Z, Lei H and Li H: Induction of apoptosis by an oleanolic
acid derivative in SMMC-7721 human hepatocellular carcinoma cells
is associated with mitochondrial dysfunction. Oncol Lett.
15:2821–2828. 2017.PubMed/NCBI
|
|
54
|
Pan S, Hu J, Zheng T, Liu X, Ju Y and Xu
C: Oleanolic acid derivatives induce apoptosis in human leukemia
K562 cell involved in inhibition of both Akt1 translocation and
pAkt1 expression. Cytotechnology. 67:821–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Reyes-Zurita FJ, Medina-O'Donnell M,
Ferrer-Martin RM, Rufino-Palomares EE, Martin-Fonseca S, Rivas F,
Martínez A, García-Granados A, Pérez-Jiménez A, García-Salguero L,
et al: The oleanolic acid derivative,
3-O-succinyl-28-O-benzyl oleanolate, induces
apoptosis in B16-F10 melanoma cells via the mitochondrial apoptotic
pathway. RSC Advances. 6:93590–93601. 2016. View Article : Google Scholar
|
|
56
|
Kim HK, Noh YH, Nilius B, Ko KS, Rhee BD,
Kim N and Han J: Current and upcoming mitochondrial targets for
cancer therapy. Semin Cancer Biol. 47:154–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shyu MH, Kao TC and Yen GC: Oleanolic acid
and ursolic acid induce apoptosis in HuH7 human hepatocellular
carcinoma cells through a mitochondrial-dependent pathway and
downregulation of XIAP. J Agric Food Chem. 58:6110–6118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Castrejón-Jiménez NS, Leyva-Paredes K,
Baltierra-Uribe SL, Castillo-Cruz J, Campillo-Navarro M,
Hernández-Pérez AD, Luna-Angulo AB, Chacón-Salinas R, Coral-Vázquez
RM, Estrada-García I, et al: Ursolic and oleanolic acids induce
mitophagy in A549 human lung cancer cells. Molecules. 24:34442019.
View Article : Google Scholar
|
|
59
|
Terada H: Uncouplers of oxidative
phosphorylation. Environ Health Perspect. 87:213–218. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Iglesias-Figueroa BF, Siqueiros-Cendón TS,
Gutierrez DA, Aguilera RJ, Espinoza-Sánchez EA, Arévalo-Gallegos S,
Varela-Ramirez A and Rascón-Cruz Q: Recombinant human lactoferrin
induces apoptosis, disruption of F-actin structure and cell cycle
arrest with selective cytotoxicity on human triple negative breast
cancer cells. Apoptosis. 24:562–577. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Izyumov DS, Avetisyan AV, Pletjushkina OY,
Sakharov DV, Wirtz KW, Chernyak BV and Skulachev VP: ‘Wages of
fear’: Transient threefold decrease in intracellular ATP level
imposes apoptosis. Biochim Biophys Acta. 1658:141–147. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Potter M, Newport E and Morten KJ: The
Warburg effect: 80 years on. Biochem Soc Trans. 44:1499–1505. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang H, Villani RM, Wang H, Simpson MJ,
Roberts MS, Tang M and Liang X: The role of cellular reactive
oxygen species in cancer chemotherapy. J Exp Clin Cancer Res.
37:2662018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Aggarwal V, Tuli HS, Varol A, Thakral F,
Yerer MB, Sak K, Varol M, Jain A, Khan MA and Sethi G: Role of
reactive oxygen species in cancer progression: molecular mechanisms
and recent advancements. Biomolecules. 9:92019. View Article : Google Scholar
|
|
65
|
Mishra R, Patel H, Yuan L and Garrett JT:
Role of reactive oxygen species target metastatic melanoma. Cancer
Res Front. 4:101–130. 2018. View Article : Google Scholar
|
|
66
|
Azad N, Rojanasakul Y and Vallyathan V:
Inflammation and Lung cancer: roles of reactive oxygen/nitrogen
species. J Toxicol Environ Health B Crit Rev. 11:1–15. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu Y, Cui Y, Shi M, Zhang Q, Wang Q and
Chen X: Deferoxamine promotes MDA-MB-231 cell migration and
invasion through increased ROS-dependent HIF-1α accumulation. Cell
Physiol Biochem. 33:1036–1046. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sánchez-Quesada C, López-Biedma A and
Gaforio JJ: Oleanolic acid, a compound present in grapes and
olives, protects against genotoxicity in human mammary epithelial
cells. Molecules. 20:13670–13688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sasikumar K, Dubey V and Ghosh AR:
Oleanolic acid from black raisins, Vitis vinifera with antioxidant
and antiproliferative potentials on HCT 116 colon cancer cell line.
Braz J Pharm Sci. 56:562020. View Article : Google Scholar
|
|
70
|
Hu M and Polyak K: Microenvironmental
regulation of cancer development. Curr Opin Genet Dev. 18:27–34.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nishida N, Yano H, Nishida T, Kamura T and
Kojiro M: Angiogenesis in cancer. Vasc Health Risk Manag.
2:213–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rajabi M and Mousa SA: The role of
angiogenesis in cancer treatment. Biomedicines. 5:342017.
View Article : Google Scholar
|
|
73
|
Caunii A, Oprean C, Cristea M, Ivan A,
Danciu C, Tatu C, Paunescu V, Marti D, Tzanakakis G, Spandidos DA,
et al: Effects of ursolic and oleanolic on SK-MEL-2 melanoma cells:
In vitro and in vivo assays. Int J Oncol.
51:1651–1660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sogno I, Vannini N, Lorusso G, Cammarota
R, Noonan DM, Generoso L, Sporn MB and Albini A: Anti-angiogenic
activity of a novel class of chemopreventive compounds: oleanic
acid terpenoids. Recent Results Cancer Res. 181:209–212. 2009.
View Article : Google Scholar : PubMed/NCBI
|