Introduction
Osteosarcoma (OS), a malignant tumor with high
recurrence rate, accounts for approximately 60% of all bone cancers
(1,2). Recent advances in the use of
chemotherapy and surgical procedures have resulted in an improved
overall 5-year survival rate of OS patients, which is estimated at
65–75%. However, the progress has not been sufficient to improve
the cure rate of patients. Therefore, it is urgently required to
develop treatment protocols and drugs for OS, equivalent to or
better than the available ones.
Apoptosis, a gene regulated process, is
characterized by a series of changes in cells, including blebbing
of cell membranes, cell shrinkage, DNA fragmentation, nuclear
degradation and the formation of apoptotic bodies (3). As an ancient biological phenomenon,
autophagy forms the basis of the evolution of organisms and widely
occurs in microorganisms and plants. Autophagy play an important
role in eliminating harmful cells in mammals such as tumor cells
(4). Li et al reported that
tumor autophagy and apoptosis could be induced in response to
several chemotherapeutic agents (5). The potential molecular mechanisms
underlying autophagy and apoptosis are quite complex. However,
whether autophagy serves as a mechanism for preventing apoptosis or
activating programmed non-apoptotic cell death remains uncertain.
It has been reported that several drugs may kill tumor cells via
the non-apoptotic pathways, thus these drugs are considered as
promising agents for treating chemoresistant tumors in order to
avoid chemoresistance (6). It has
been well documented that cancerous cells with defects in apoptosis
utilize autophagy, therefore, the inhibition of autophagy may
promote the cell death through alternative pathways (7). Furthermore, autophagy may atenuate
tumor development and progression, and improve the efficacy of
cancer therapy (8).
Several underlying mechanisms have been implicated
the regulation of programmed cell death. Studies have revealed that
the redox status of the tumor is associated with programmed cell
death (9,10). Intracellular hydrogen peroxide
(H2O2), which is produced at the
mitochondrial respiratory chain, is considered as the most
important reactive oxygen species (ROS) involved in the regulation
of the redox status of tumor cells (9). Different levels of
H2O2 have been implicated in the development
or death of tumor cells. Trachootham et al demonstrated that
low H2O2 levels promoted the progression and
development of tumor cells, however, high
H2O2 levels exhibited antitumor properties by
inducing cell apoptosis (11). In
addition, H2O2 acts as an intracellular
signal for the induction of the mitogen-activated protein kinase
(MAPK) pathway [p38, extracellular-regulated kinase (ERK) and c-Jun
N-terminal kinase (JNK)], which in turn promotes tumor cell
apoptosis (12). A previous study
has suggested that the H2O2-mediated
activation of the p38 signaling pathway is involved in the
regulation of Atg7 expression and the fusion of autophagosomes with
lysosomes (13). Emerging evidence
has revealed a close relationship between upregulated
phosphorylated (p)-JNK expression and high intracellular
H2O2 levels (10,14).
Consistent with the previous study, it has been considered that the
H2O2-mediated activation of the JNK signaling
pathway may be a novel mechanism of autophagy (15,16).
In summary, intracellular H2O2 levels mediate
the progression of apoptosis and autophagy via activating the MAPK
pathway.
Polyphyllin VII, a compound extracted from Paris
polyphylla, is a steroidal saponin, which is considered as a
promising antitumor drug (17,18).
It has been well documented that the anticancer activities of
Polyphyllin VII are mediated by inhibiting cell proliferation, cell
cycle arrest, and preventing chemoresistance in several tumor cell
lines (17–20). However, the potential mechanisms
underlying its anticancer activity remain largely unknown. The aim
of the present study was to investigate the effects of Polyphyllin
VII on OS and to identify its potential mechanism underlying the
induction of apoptosis and autophagy in U2OS cells.
Materials and methods
Reagents and antibodies
Polyphyllin VII (purity, 99%; Fig. 1A) was obtained from Beijing Solarbio
Science and Technology Co., Ltd. All cell culture-related reagents
were purchased from Invitrogen (Thermo Fisher Scientific, Inc.).
Propidium iodide (PI), DAPI dye and 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay were obtained from
Sigma-Aldrich (Merck KGaA). In addition, N-acetyl-L cysteine (NAC)
was purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd. The
specific antibodies against Bcl-2, Bax, LC3-I/II, ERK, p-JNK, JNK,
p62, p-ERK, cleaved caspase-3 and −9, cleaved poly (ADP-ribose)
polymerase (PARP), p-p38, p38 and GAPDH were purchased from Cell
Signaling Technology, Inc. Finally, the class III PI 3-kinase
inhibitor (3-MA) was obtained from Tocris Bioscience.
Cell culture
All in vitro experiments were performed on
U2OS human OS cells. Cells were cultured in Dulbecco's modified
Eagles medium (DMEM) supplemented with 10% fetal bovine serum (FBS)
at 37°C in a humidified atmosphere with 5% CO2. Cells
were tested for mycoplasma contamination using immunofluorescence
and DAPI staining according to the manufacturer's guidelines.
Cell viability assay
The U2OS cell viability was determined using an MTT
assay. Briefly, cells were cultured at a density of
6×103 cells/well in 96-well plates. Following incubation
overnight at 37°C, U2OS cells were treated with varying
concentrations of Polyphyllin VII (0, 1.25, 2.5, 5.0 and 10.0 µM)
for 24–48 h. Subsequently, MTT solution (20 µl/well) was added to
each well and U2OS cells were incubated for an additional 2 h at
37°C. Finally, the optical density (OD) of each well was evaluated
by measuring the spectrophotometric absorbance at 490 nm using an
ELX800 Absorbance Microplate Reader (BioTek Instruments, Inc.).
Cytotoxicity was observed with 10 µM Polyphyllin VII, and thus,
this concentration was not used in subsequent experiments.
Detection of apoptosis by Annexin
V-APC/7-AAD and DAPI staining assays
U2OS cells were seeded into 6-well plates at a
density of 1×105 cells/ml for 24 h. Subsequently, cells
in the treatment group were treated with various concentrations of
Polyphyllin VII (1, 2.5 and 5.0 µM), whereas those in the vehicle
group were treated with DMSO (1:10,000 v/v). Following treatment
with Polyphyllin VII for 24 h, cells were then double-stained with
an Annexin V-APC/7-AAD apoptosis detection kit or with DMSO,
according to the manufacturer's instructions. Cells were then
harvested, washed twice with pre-cooled PBS, supplemented with 5 µl
of Annexin V-APC and 5 µl of 7-AAD and incubated at room
temperature in a dark room for 15 min. The early apoptotic (stained
with Annexin V-APC only) and late apoptotic (double-stained with
Annexin V-APC and 7-AAD) cells were assessed using flow cytometry
(Accuri C6; BD Biosciences) and the results were further analyzed
with FCS Express version 3 software (DeNovo Software). For the DAPI
staining, U2OS cells were treated with various concentrations of
Polyphyllin VII (0, 1.25, 2.5, 5.0 µM) for 24 h, washed three times
with PBS and then fixed with 100% ice-cold methyl alcohol for an
additional 10 min at room temperature. Cells were then washed again
thrice with PBS and stained with 10 µg/ml DAPI for 30 min at room
temperature (21,22). Finally, the cells were observed at a
magnification of ×100 under a fluorescence microscope.
H2O2
measurement
To investigate the effects of Polyphyllin VII on the
intracellular levels of H2O2, U2OS cells were
treated with varying concentrations of Polyphyllin VII (0, 1.25,
2.5 and 5.0 µM) for 24 h. Subsequently, cells were supplemented
with 3 µM oxidation-sensitive fluorescent probe
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (Beyotime
Institute of Biotechnology) and incubated at 37°C for 20 min in the
dark. Finally, the relative intracellular
H2O2 levels in U2OS cells were quantitatively
determined using flow cytometry (Accuri C6) and the results were
further analyzed with FCS Express version 3 software.
Detection of acidic vesicular
organelles (AVOs)
To quantitatively detect the effect of Polyphyllin
VII on lysosomes, U2OS cells were cultured in 6-well plates at a
density of 1.5×105 cells/well. Following incubation for
12 h, cells were then treated with various concentrations of
Polyphyllin VII (0, 1.25, 2.5 and 5 µM) and incubated for an
additional 24 h 37°C. Subsequently, the cells were stained with 1
µg/ml of acridine orange solution (Amresco, LLC) at 37°C for 30 min
and washed with PBS. Finally, the lysosomal activity in U2OS cells
was measured by flow cytometry (Accuri C6) and the results were
further analyzed with FCS Express version 3 software.
Mitochondrial membrane potential (Δψm)
measurement
Δψm was determined using the Mitochondrial membrane
potential assay kit and JC-1 (Beyotime Institute of Biotechnology).
Briefly, a total of 1×105 cells were treated for 24 h
with various concentrations of Polyphyllin VII (0, 1.25, 2.5 and 5
µM). Following washing with PBS, cells were cultured in the
presence of the JC-1 staining working solution (1:1,000) at 37°C
for 20 min. U2OS cells were then treated with JC-1 staining buffer
(1X), centrifuged thrice at 600 × g (4°C) for 5 min and Δψm was
determined using flow cytometry (Accuri C6) and the results were
further analyzed with FCS Express version 3 software.
Western blot analysis
For the western blot analysis, U2OS cells were
treated with various concentrations of Polyphyllin VII and then
lysed with RIPA buffer (Solarbio Science & Technology Co.,
Ltd.) supplemented with protease and phosphatase inhibitors (150
µl/well) for 25 min. Protein concentration was determined using the
BCA method. Subsequently, a total of 12 µl of protein extracts (100
µg) from each concentration group were separated by 8–15% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and proteins
were then electrotransferred onto nitrocellulose membranes (Bio-Rad
Laboratories, Inc.). Following blocking with 5% non-fat milk in
Tris-buffered saline (TBS) buffer at 4°C for 8 h, membranes were
then incubated with primary antibodies all diluted 1:1,000 against
p62 (product no. 5114), LC3I/II (product no. 4108), Atg3 (product
no. 3415), Atg5 (product no. 2630), Atg7 (product no. 2631), Atg12
(product no. 2010), Bax (product no. 2774), Bcl-2 (product no.
2875), PARP (product no. 9542), cleaved caspase-3 (product no.
9661) and cleaved caspase-9 (product no. 9505), p-JNK (product no.
9251), JNK (product no. 9252), p-ERK (product no. 9101), ERK
(product no. 9102), p-p38 (product no. 9211), p38 (product no.
9212) and GAPDH (product no. 2118) for 24 h at 4°C. Following 24 h,
the membranes were incubated with corresponding secondary antibody
goat anti-rabbit IgG (HRP) (1:10,000; product code ab205718; Abcam)
at 37°C for 1.5 h. Finally, the Enhanced chemiluminescence (ECL)
system (Thermo Fisher Scientific, Inc.) was applied to detect the
fluorescence intensity of the formed immuno-complexes. The blots
were visualized using Odyssey Sa Infrared Imaging System (LI-COR
Biosciences) and analyzed using ImageJ version 1.51 (National
Institutes of Health).
Statistical analysis
All data were expressed as the mean ± standard
deviation (SD) and all experiments were independently carried out
in triplicate. Differences between groups were compared using
unpaired Student's t-test, one-way ANOVA and Fisher's exact test.
Multiple comparisons between the groups were performed using the
S-N-K method. All statistical analyses were performed using the
SPSS 21.0 statistical software (IBM Corp.) P<0.05 was considered
to indicate a statistically significant difference.
Results
Polyphyllin VII inhibits human U2OS
cell viability
To investigate the potential effect of Polyphyllin
VII on U2OS cell viability, an MTT assay was performed. The results
demonstrated that the IC50 values of Polyphyllin VII in
U2OS cells at 24 and 48 h were 3.48 and 2.58 µM, respectively
(Fig. 1B and C). A previous study
has revealed that Polyphyllin VII exhibited cytotoxic effects on
control cells following treatment for 48 h (19). However, the results of the present
study demonstrated that Polyphyllin VII had less cytotoxic effects
on U2OS cells at 24 h compared with 48 h following treatment. This
finding indicated that treatment with Polyphyllin VII for 24 h
could be considered beneficial for cancer studies. The optimal
concentrations of Polyphyllin VII were 1.25, 2.5 and 5 µM and the
optimal treatment-time was set to 24 h (19,20).
Polyphyllin VII induces apoptosis in
U2OS cells
To investigate whether Polyphyllin VII induced
apoptotic cell death, the changes in the number of apoptotic cells
and expression of apoptosis-related proteins were recorded. The
results of the Annexin V-APC/7-AAD staining indicated that
Polyphyllin VII effectively induced U2OS cell apoptosis in a
dose-dependent manner (Fig. 2A).
This finding was confirmed following DAPI staining. Therefore, the
number of apoptotic U2OS cells was increased after cell treatment
with Polyphyllin VII (Fig. 2B).
Apoptosis is characterized by the activation of executioner
caspases and induction of the caspase-dependent cell death
(23,24). Therefore, the changes in the
expression levels of the apoptosis-related proteins were used to
determine their involvement in the apoptotic process. The western
blot results revealed that Polyphyllin VII upregulated the
expression levels of the apoptotic-promoting proteins (Bax, PARP,
cleaved caspase-3 and cleaved caspase-9) and downregulated those of
the anti-apoptotic protein Bcl-2 (Fig.
2C-F). The aforementioned results suggested that Polyphyllin
VII could effectively induce U2OS cell apoptosis.
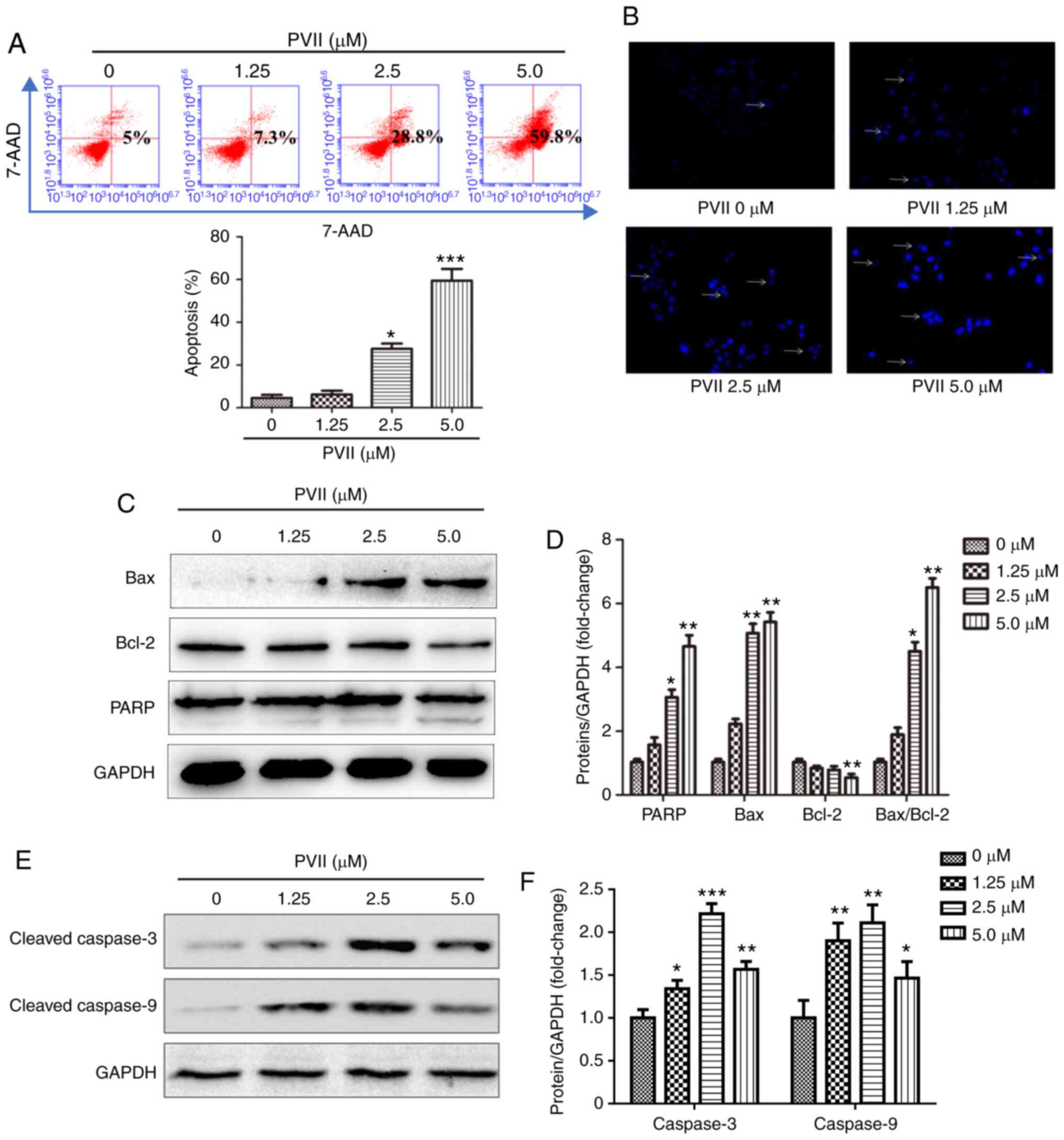 | Figure 2.Polyphyllin VII induces apoptosis in
U2OS cells. (A) Flow cytometric assay revealing the proportion of
apoptotic U2OS cells following treatment with Polyphyllin VII (0,
1.25, 2.5 and 5 µM). (B) DAPI staining of the U2OS cells following
treatment with various concentrations of Polyphyllin VII (0, 1.25,
2.5 and 5 µM) for 24 h. As indicated by the arrows, fragmented or
condensed nuclei could be observed at a magnification of ×100 with
a fluorescence microscope. (C-F) The western blot analysis
revealing the expression levels of cleaved caspase-3, caspase-9,
Bax, Bcl-2 and PARP following the treatment of the U2OS cells with
various concentrations of Polyphyllin VII (0, 1.25, 2.5 and 5 µM)
for 24 h. Data are expressed as the mean ± SD from three
independent experiments. *P<0.05, **P<0.01 and ***P<0.001
compared to the control. DAPI, 4′,6-diamidino-2-phenylindole; PARP,
poly (ADP-ribose) polymerase. |
Polyphyllin VII induces autophagy in
U2OS cells
Since autophagy is considered as an alternative cell
death mechanism, the possible effects of Polyphyllin VII on
autophagy were further investigated. AVO formation is a
characteristic feature of autophagy (25,26).
Therefore, to determine the possible effects on Polyphyllin VII on
AVO formation, U2OS cells were treated with or without Polyphyllin
VII and stained with acridine orange. The results demonstrated that
Polyphyllin VII increased the number of AVOs in a dose-dependent
manner (Fig. 3A and B). It is
widely accepted that LC3, an important regulator of autophagy,
promotes the formation of autophagosomes and activation of the
Atg5-Atg12 complex, which in turn promotes the conjugation of LC3I.
LC3I is further transformed to form LC3II, which interacts with the
membranes of the autophagosome (27,28).
Herein, Polyphyllin VII upregulated the expression of LC3II, Atg5,
Atg7 and the Atg12-Atg5 complex (Fig.
3C-F). However, the expression levels of Atg12 and p62 were
decreased. These results indicated that Polyphyllin VII could
induce autophagy.
 | Figure 3.Polyphyllin VII promotes autophagy in
U2OS cells. (A and B) Polyphyllin VII-treated (0, 1.25, 2.5 and 5
µM) cells were labeled with AO and subjected to flow cytometry. AO
expression increased with the increase of the concentration of
Polyphyllin VII. (C-F) After the U2OS were treated with Polyphyllin
VII (0, 1.25, 2.5 and 5 µM), the expression levels of Atg3, Atg5,
Atg12-Atg5 complex, Atg12 free, Atg7 and GAPDH were determined via
western blotting. Data are expressed as mean ± SD from three
independent experiments. *P<0.05 and **P<0.01 compared to the
control. AO, acridine orange; SD, standard deviation; PVII,
Polyphyllin VII. |
Autophagy inhibitor 3-MA rescues
Polyphyllin VII-induced apoptosis in U2OS cells
To reveal the association between apoptosis and
autophagy, U2OS cells were pre-treated with 3-MA, an autophagy
inhibitor, for 2 h. Subsequently, the cells were treated with or
without Polyphyllin VII for an additional 24 h. The results
demonstrated that Polyphyllin VII (5 µM) increased the expression
levels of the apoptotic proteins caspase-3 and caspase-9 (Fig. 4). However, following pre-treatment
with 3-MA, the apoptosis rate was attenuated, thus suggesting that
inhibition of autophagy decreased apoptosis in U2OS cells treated
with high concentrations of Polyphyllin VII.
Polyphyllin VII promotes autophagy and
apoptosis via intracellular levels of H2O2
and the MAPK signaling pathway
The stability of ΔΨm is important for cell survival
(9). When ΔΨm is decreased, the
production of H2O2 is increased (9). Therefore, in the present study,
Polyphyllin VII significantly decreased the ΔΨm in U2OS cells
(Fig. 5A and B). Emerging evidence
has suggested that certain chemotherapeutic drugs may induce tumor
cell apoptosis via promoting H2O2 production
(25). In addition,
H2O2 is tightly associated with
starvation-induced autophagy (29).
Therefore, the possible molecular mechanism underlying Polyphyllin
VII-mediated apoptosis and autophagy was explored. In U2OS cells
treated with Polyphyllin VII, the levels of intracellular
H2O2 were significantly increased (Fig. 5C and D). However, pre-treatment of
cells with ROS scavenger NAC (10 mM) entirely reversed this effect
(Fig. 5E and F). These findings
indicated that Polyphyllin VII increased the levels of
intracellular H2O2 in U2OS cells and this
effect was reversed following pre-treatment with NAC.
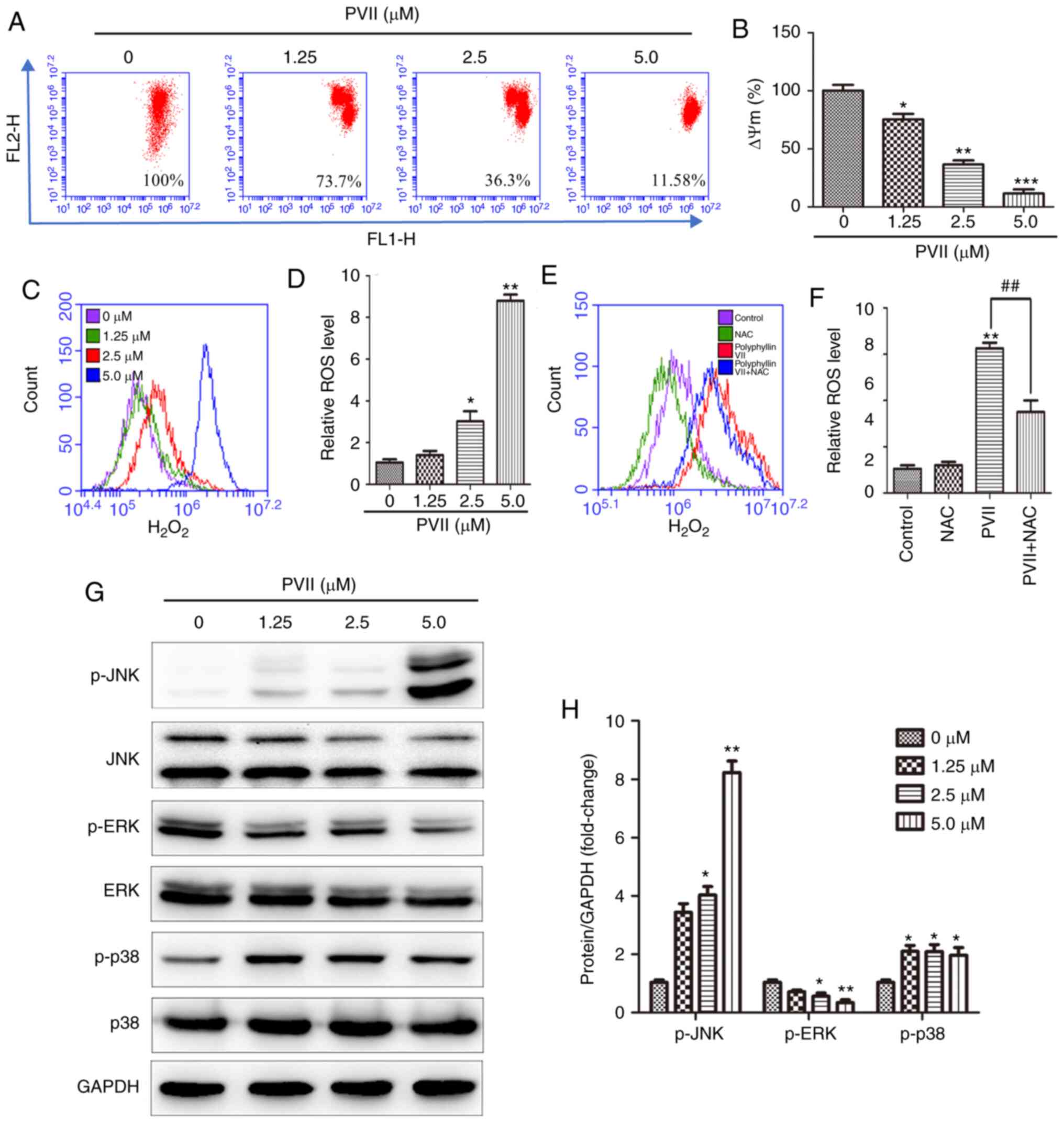 | Figure 5.Polyphyllin VII induces autophagy and
apoptosis via intracellular H2O2 and
activated JNK and p38 MAPK pathways. (A and B) After the U2OS cells
were treated with the indicated concentrations of Polyphyllin VII
(0, 1.25, 2.5 and 5 µM), the mitochondrial membrane potentials were
measured via flow cytometry. (C and D) The relative
H2O2 levels in the U2OS cells treated with
various concentrations of Polyphyllin VII (0, 1.25, 2.5 and 5 µM).
(E and F) Flow cytometric analysis of the relative
H2O2 levels in U2OS cells incubated with 2.5
µM Polyphyllin VII alone or in combination with 10 mM NAC. (G and
H) The western blot analysis revealing the levels of p-JNK, p-ERK,
and p-p38 following the treatment of U2OS cells with varying
concentrations of Polyphyllin VII (0, 1.25, 2.5 and 5 µM) for 24 h.
Data are expressed as mean ± SD from three independent experiments.
*P<0.05, **P<0.01 and ***P<0.001 compared to the control.
##P<0.01 compared to Polyphyllin VII alone. MAPK,
mitogen-activated protein kinase pathway; JNK, c-Jun N-terminal
kinase; NAC, N-acetyl-L cysteine; ERK, extracellular-regulated
kinase; p-, phosphorylated; PVII, Polyphyllin VII. |
Previous studies have revealed that apoptosis and
autophagy occur in different tumor cells via the activation of the
JNK, ERK and p38 pathways (27,30).
In the present study, the effect of Polyphyllin VII on the
activation of the MAPK signaling pathways was determined in U2OS
cells. Therefore, the phosphorylation levels of JNK, ERK and p38
were assessed using western blot analysis. The results revealed
that Polyphyllin VII attenuated ERK phosphorylation and upregulated
p-JNK and p-p38 expression in a dose-dependent manner (Fig. 5G and H). Therefore, Polyphyllin VII
induced autophagy and apoptosis in U2OS cells via activating the
JNK, p38 and ERK MAPK signaling pathways.
Discussion
Polyphyllin VII exhibits anti-proliferative effects
against various human tumor cell lines (26,31,32).
The present study demonstrated for the first time, to the best of
our knowledge, that Polyphyllin VII induced U2OS cell apoptosis and
autophagy. The results revealed that Polyphyllin VII attenuated
cell growth in a dose-dependent manner following treatment of U2OS
cells with low IC50 values of Polyphyllin VII. In
addition, Polyphyllin VII upregulated the expression of the
apoptosis-related proteins (Bax, Bcl-2, PARP, cleaved caspase-3 and
cleaved caspase-9) and autophagy-related proteins (p62, LC3I/II,
Atg3, Atg5, Atg7 and Atg12) in U2OS cells. The aforementioned
findings indicated that Polyphyllin VII exhibited a powerful
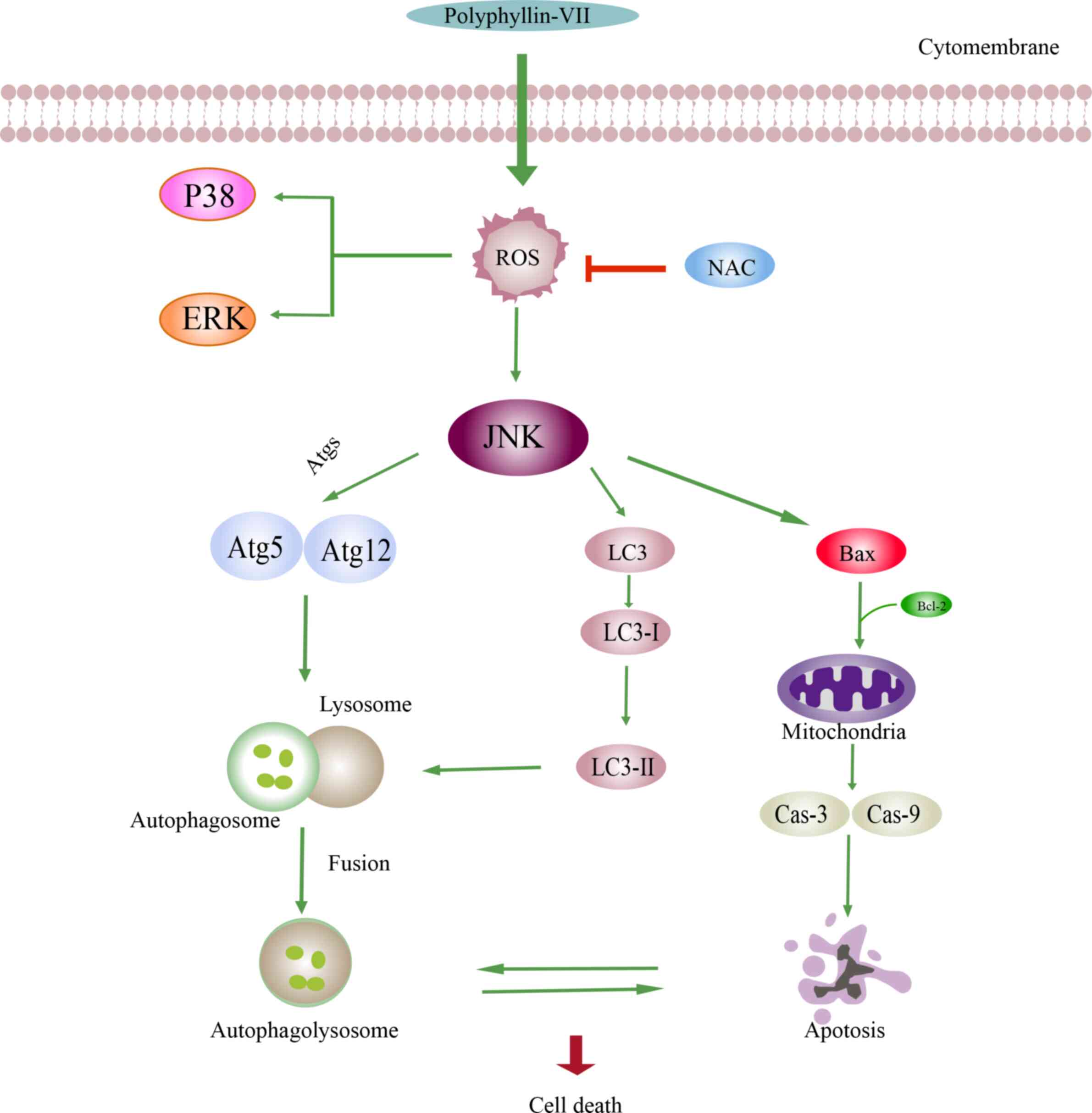
antitumor effect. As revealed in Fig.
6, the possible mechanism underlying the effects of Polyphyllin
VII could be associated with the H2O2 levels
and the MAPK pathways. Polyphyllin VII -induced cell death was
associated with both apoptosis and autophagy. As indicated in
Fig. 6, in the apoptosis pathway,
decreased levels of Bcl-2 and increased levels of Bax can lead to
mitochondrial membrane permeabilization. In addition, the
upregulated expression levels of the apoptotic proteins caspase-3
and caspase-9 result in the occurrence of apoptosis. The autophagic
pathway is triggered by the enhanced conversion of LC3B-I to
LC3B-II, accumulation of Atgs and incorporation of the Atg12-Atg5
protein complex. Furthermore, autophagosome formation is dependent
on the covalent attachment of a series of Atg proteins during
protein ubiquitination. Finally, autophagolysosome formation
combines the autophagosome with the upregulated expression of
LC3B-II. The increased expression of Bax and decreased expression
of Bcl-2 indicated that apoptosis is promoted to lead to cell death
when suppression of autophagy is decreased. Conversely, when
apoptosis is blocked, the upregulated expression of LC3B-II and
Atg5-Atg12 indicated that cell death is caused by the autophagic
pathway.
Several natural substances exhibit strong antitumor
activities via activating both apoptosis and autophagy (33–36),
thus indicating a strong association between these processes.
Although the antitumor effects of Polyphyllin VII have been well
documented, its possible effects in the induction of autophagy and
apoptosis, and its underlying mechanisms of action in U2OS cells
have not yet been fully elucidated. In the present study, the
Annexin V-APC/7-AAD staining indicated that Polyphyllin VII
increased the ratio of apoptotic cells in a dose-dependent manner.
It has been demonstrated that Polyphyllin VII upregulates Bax,
cleaved caspase-3 and cleaved caspase-9 expression levels in HeLa
cells (17). In addition, a
previous study has revealed that Polyphyllin VII regulates the
protein expression levels of Bax, Bcl-2 and Bcl-xL, and enhances
those of caspase-3, −8, and −9 in NPC-039 and HONE-1 cells
(20). Zhang et al also
demonstrated that Polyphyllin VII downregulated Bcl-2 expression in
HepG2 cells (31). Consistent with
the aforementioned studies, the results of the present study
demonstrated that Polyphyllin VII induced apoptosis via triggering
caspase-3 and caspase-9 expression, and altering Bax, Bcl-2 and
PARP expression levels. The present results revealed that cleaved
caspase-3 and −9 were increased with the increase of Polyphyllin
VII concentration, however the effects were decreased at 5.0 µM. It
was revealed that the U2OS cell viability was significantly
inhibited at 5.0 µM. In addition, the protein expression levels of
caspase-3 and −9 for each group were statistically significant when
compared with the control group. Therefore, it was theorized that
the reason for this phenomenon may be that there was no strict
concentration dependence for cleaved caspase-3 and −9. Following
treatment of U2OS cells with Polyphyllin VII, Bcl-2 was gradually
downregulated as the expression of Bax was increased. The
mitochondrial membrane permeability may be increased through Bax
activation. Therefore, the results revealed that Polyphyllin VII
triggered the apoptotic cascade through the mitochondrial pathway.
The findings of the present study were consistent with previous
studies (17,19) that suggested that Polyphyllin VII
disrupted the integrity of the mitochondrial membrane in U2OS
cells. PARP, a nuclear DNA-binding protein, serves a key role in
DNA repair. Cleavage of PARP by caspase-3, a sign of apoptosis, is
inhibited (37). Herein, the
Polyphyllin VII-mediated apoptosis was determined in U2OS cells via
investigating the PARP cleavage status. The results were consistent
with those obtained using flow cytometric analysis, where treatment
of U2OS cells with Polyphyllin VII increased the cell apoptosis
rate. In addition, cell apoptosis was induced in a dose-dependent
manner following treatment of U2OS cells with Polyphyllin VII for
24 h.
Autophagy is an intracellular degradation mechanism
that improves survival under nutrient starvation or induces type II
programmed cell death under certain conditions (19,20).
During autophagy, a part of the cytoplasm is engulfed by a
crescent-shaped phagophore that elongates to form an autophagosome,
which in turn fuses with a lysosome, thus leading to the
decomposition of cellular content by the lysosomal hydrolases
(7,8). In the present study, following
treatment with Polyphyllin VII, the formation of AVOs, a
characteristic feature of autophagy, was increased in a
concentration-dependent manner. The ability of Polyphyllin VII to
induce autophagy was demonstrated by the transformation of LC3B-I
to LC3B-II and the upregulation of Atg3, Atg5, Atg7 and the
Atg12-Atg5 complex. However, Polyphyllin VII attenuated the
expression levels of Atg12 and p62. These findings indicated that
Polyphyllin VII effectively promoted autophagy in U2OS cells.
However, these apoptosis-related changes were observed following
pre-treatment of U2OS cells with the 3-MA autophagy inhibitor. The
results revealed that the expression of the apoptotic proteins
caspase-3 and caspase-9 was reduced in the presence of 3-MA
compared with that noted in the groups treated with Polyphyllin VII
alone. Therefore, the inhibition of Polyphyllin VII induced
autophagy in U2OS cells, whereas this effect could be reversed by
inhibiting apoptosis.
The equilibrium between the formation and
degradation rates of H2O2 maintains cellular
redox status (9). During the
induction of apoptosis, H2O2 generation is
accompanied by the reduction of ΔΨm and the uncoupling of the
electron transport chain in mitochondria (9). Previous studies have reported that the
induction of autophagy is characterized by the accumulation of
intracellular H2O2, which in the presence of
reversibly oxidizing essential signaling components may act as a
signaling molecule in the autophagic pathway (38,39).
H2O2 has been revealed to be associated with
the antitumor effects of several chemotherapeutic agents, when the
antioxidant defenses of tumor cells become weaker compared with
normal ones (40). A previous study
has provided further evidence about the involvement of
intracellular H2O2 accumulation in cellular
autophagy, which may be induced in response to several factors,
including chemotherapeutic agents, starvation and mitochondrial
injury (41). Herein, Polyphyllin
VII reduced ΔΨm in a dose-dependent manner. Furthermore, the
increased production of H2O2 in the
Polyphyllin VII-treated U2OS cells was effectively inhibited
following treatment with NAC (H2O2
scavenger). Therefore, these findings suggested that Polyphyllin
VII increased the intracellular H2O2 levels
via attenuating ΔΨm. Overall, the aforementioned results indicated
that the promotion of autophagy and apoptosis could be mediated by
H2O2 overproduction via the effects of
Polyphyllin VII on U2OS cells.
It is generally acknowledged that
H2O2 plays an important role in autophagy and
apoptosis via regulating the MAPK family members (42,43).
The MAPK pathway is closely associated with autophagy and apoptosis
(20). In the present study,
Polyphyllin VII treatment significantly induced the expression of
p-JNK in U2OS cells, thus suggesting that Polyphyllin VII only
affected the JNK signaling pathway. The expression levels of p-p38
and p-ERK were not strongly affected by Polyphyllin VII. JNK plays
a key role in inducing autophagy and apoptosis in response to
various stress signals (44). The
JNK signaling pathway affected the ΔΨm to inhibit mitochondrial
dysfunction via altering the protein expression levels of Bax and
Bcl-2, thus resulting in apoptotic response. The increased JNK
signaling was also associated with the induction of autophagy via
upregulating LC3 expression. A previous study has revealed that the
activation of the JNK signaling pathway promotes apoptosis and
autophagy in human non-small cell lung cancer NCI-H460 cells. This
effect was accompanied by increased H2O2
levels (14). Therefore, we
hypothesized that Polyphyllin VII induced apoptosis and autophagy
via mediating the activation of the JNK pathway and overproduction
of H2O2.
However, the present study has certain limitations.
Firstly, this study demonstrated that Polyphyllin VII increased the
p-JNK expression and H2O2 levels in a
dose-dependent manner. However, whether the
H2O2 scavenger, NAC, downregulates p-JNK
expression requires further investigation. Secondly, 3-MA
downregulated caspase-3 and caspase-9, however, the association
between autophagy and the apoptosis-related MAPK pathway requires
further elucidation. Thirdly, only one cell line was used for the
confirmation of the effect of Polyphyllin VII on apoptosis and
autophagy. Fourthly, in the present study only in vitro
experiments were performed. Therefore, the inhibitory effects of
Polyphyllin VII should be explored on an OS xenograft in
vivo model. The aforementioned limitations will be the focus of
our upcoming studies on the effects of Polyphyllin VII in OS. In
addition, mitochondrial marker immunofluorescence assays for
autophagy should also be included in future studies.
In conclusion, the present study demonstrated that
Polyphyllin VII attenuated cell viability, and induced apoptosis
and autophagy in U2OS cells. The mechanisms underlying the effects
of Polyphyllin VII may be associated with the regulation of the
H2O2 levels and the JNK/MAPK pathway. In
addition, the results indicated that Polyphyllin VII could be a
promising candidate compound for cancer therapy. Therefore,
Polyphyllin VII could be considered as an attractive and effective
treatment approach for OS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangxi Province (grant nos.
2017GXNSFBA198098, 2018GXNSFBA281090 and 2019JJA140408), the Open
Project of Guangxi Key Laboratory of Regenerative Medicine (grant
no. 201806), the Innovative Project for Guangxi Graduate Education
(grant no. YCBZ2018035), the Guangxi Young and Middle-aged Teachers
Basic Ability Promoting Project (grant no. 2019KY0111), the Project
of Guangxi Health Department (grant nos. Z2016313 and Z20181011)
and the Science Foundation of the People's Hospital of Guangxi
Zhuang Autonomous Region (grant no. QN2017-11).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
KW and CW conceived the study. XL, YL, SL, CL and WF
performed the experiments. XL, YL, SL, CL, AM and JL analyzed the
data. KW, XL, YL, SL and AM wrote the manuscript. All authors read
and approved the final manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Whelan JS and Davis LE: Osteosarcoma,
chondrosarcoma, and chordoma. J Clin Oncol. 36:188–193. 2018.
View Article : Google Scholar
|
|
2
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.
View Article : Google Scholar
|
|
3
|
Burgess DJ: Apoptosis: Refined and lethal.
Nat Rev Cancer. 13:792013. View
Article : Google Scholar
|
|
4
|
Li Z, Dong H, Li M, Wu Y, Liu Y, Zhao Y,
Chen X and Ma M: Honokiol induces autophagy and apoptosis of
osteosarcoma through PI3K/Akt/mTOR signaling pathway. Mol Med Rep.
17:2719–2723. 2018.
|
|
5
|
Li Y, Zhu H, Zeng X, Fan J, Qian X, Wang
S, Wang Z, Sun Y, Wang X, Wang W and Ju D: Suppression of autophagy
enhanced growth inhibition and apoptosis of interferon-β in human
glioma cells. Mol Neurobiol. 47:1000–1010. 2013. View Article : Google Scholar
|
|
6
|
Wong RW and Rabie AB: Effect of psoralen
on bone formation. J Orthop Res. 29:158–164. 2011. View Article : Google Scholar
|
|
7
|
Zhang XD, Wang Y, Wang Y, Zhang X, Han R,
Wu JC, Liang ZQ, Gu ZL, Han F, Fukunaga K and Qin ZH: p53 mediates
mitochondria dysfunction-triggered autophagy activation and cell
death in rat striatum. Autophagy. 5:339–350. 2009. View Article : Google Scholar
|
|
8
|
Hsieh MJ, Yang SF, Hsieh YS, Chen TY and
Chiou HL: Autophagy inhibition enhances apoptosis induced by
dioscin in huh7 cells. Evid Based Complement Alternat Med.
2012:1345122012. View Article : Google Scholar
|
|
9
|
Orrenius S: Reactive oxygen species in
mitochondria-mediated cell death. Drug Metab Rev. 39:443–455. 2007.
View Article : Google Scholar
|
|
10
|
Zhang C, Yang L, Wang XB, Wang JS, Geng
YD, Yang CS and Kong LY: Calyxin Y induces hydrogen
peroxide-dependent autophagy and apoptosis via JNK activation in
human non-small cell lung cancer NCI-H460 cells. Cancer Lett.
340:51–62. 2013. View Article : Google Scholar
|
|
11
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar
|
|
12
|
Torres M and Forman HJ: Redox signaling
and the MAP kinase pathways. Biofactors. 17:287–296. 2003.
View Article : Google Scholar
|
|
13
|
McClung JM, Judge AR, Powers SK and Yan Z:
p38 MAPK links oxidative stress to autophagy-related gene
expression in cachectic muscle wasting. Am J Physiol Cell Physiol.
298:C542–C549. 2010. View Article : Google Scholar
|
|
14
|
Kamata H, Honda S, Maeda S, Chang L,
Hirata H and Karin M: Reactive oxygen species promote
TNFalpha-induced death and sustained JNK activation by inhibiting
MAP kinase phosphatases. Cell. 120:649–661. 2005. View Article : Google Scholar
|
|
15
|
Trejo-Solis C, Jimenez-Farfan D,
Rodriguez-Enriquez S, Fernandez-Valverde F, Cruz-Salgado A,
Ruiz-Azuara L and Sotelo J: Copper compound induces autophagy and
apoptosis of glioma cells by reactive oxygen species and JNK
activation. BMC Cancer. 12:1562012. View Article : Google Scholar
|
|
16
|
Wong CH, Iskandar KB, Yadav SK, Hirpara
JL, Loh T and Pervaiz S: Simultaneous induction of non-canonical
autophagy and apoptosis in cancer cells by ROS-dependent ERK and
JNK activation. PLoS One. 5:e99962010. View Article : Google Scholar
|
|
17
|
Zhang W, Zhang D, Ma X, Liu Z, Li F and Wu
D: Paris saponin VII suppressed the growth of human cervical cancer
Hela cells. Eur J Med Res. 19:412014. View Article : Google Scholar
|
|
18
|
Li Y, Sun Y, Fan L, Zhang F, Meng J, Han
J, Guo X, Zhang D, Zhang R, Yue Z and Mei Q: Paris saponin VII
inhibits growth of colorectal cancer cells through Ras signaling
pathway. Biochem Pharmacol. 88:150–157. 2014. View Article : Google Scholar
|
|
19
|
Zhang C, Jia X, Bao J, Chen S, Wang K,
Zhang Y, Li P, Wan JB, Su H, Wang Y, et al: Polyphyllin VII induces
apoptosis in HepG2 cells through ROS-mediated mitochondrial
dysfunction and MAPK pathways. BMC Complement Altern Med.
16:582016. View Article : Google Scholar
|
|
20
|
Chen JC, Hsieh MJ, Chen CJ, Lin JT, Lo YS,
Chuang YC, Chien SY and Chen MK: Polyphyllin G induce apoptosis and
autophagy in human nasopharyngeal cancer cells by modulation of AKT
and mitogen-activated protein kinase pathways in vitro and in vivo.
Oncotarget. 7:70276–70289. 2016. View Article : Google Scholar
|
|
21
|
Chen S, Jin Z, Dai L, Wu H, Wang J, Wang
L, Zhou Z, Yang L and Gao W: Aloperine induces apoptosis and
inhibits invasion in MG-63 and U2OS human osteosarcoma cells.
Biomed Pharmacother. 97:45–52. 2018. View Article : Google Scholar
|
|
22
|
Li AX, Sun M and Li X: Withaferin-A
induces apoptosis in osteosarcoma U2OS cell line via generation of
ROS and disruption of mitochondrial membrane potential. Eur Rev Med
Pharmacol Sci. 21:1368–1374. 2017.
|
|
23
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar
|
|
24
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar
|
|
25
|
Liu C, Gong K, Mao X and Li W: Tetrandrine
induces apoptosis by activating reactive oxygen species and
repressing Akt activity in human hepatocellular carcinoma. Int J
Cancer. 129:1519–1531. 2011. View Article : Google Scholar
|
|
26
|
He DX, Li GH, Gu XT, Zhang L, Mao AQ, Wei
J, Liu DQ, Shi GY and Ma X: A new agent developed by
biotransformation of Polyphyllin VII inhibits chemoresistance in
breast cancer. Oncotarget. 7:31814–31824. 2016. View Article : Google Scholar
|
|
27
|
Wang X, Qi H, Wang Q, Zhu Y, Wang X, Jin
M, Tan Q, Huang Q, Xu W, Li X, et al: FGFR3/fibroblast growth
factor receptor 3 inhibits autophagy through decreasing the
ATG12-ATG5 conjugate, leading to the delay of cartilage development
in achondroplasia. Autophagy. 11:1998–2013. 2015. View Article : Google Scholar
|
|
28
|
Fan X, Han S, Yan D, Gao Y, Wei Y, Liu X,
Liao Y, Guo H and Sun S: Foot-and-mouth disease virus infection
suppresses autophagy and NF-κB antiviral responses via degradation
of ATG5-ATG12 by 3Cpro. Cell Death Dis. 8:e25612017.
View Article : Google Scholar
|
|
29
|
Scherz-Shouval R, Shvets E, Fass E, Shorer
H, Gil L and Elazar Z: Reactive oxygen species are essential for
autophagy and specifically regulate the activity of Atg4. EMBO J.
26:1749–1760. 2019. View Article : Google Scholar
|
|
30
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar
|
|
31
|
Zhang C, Jia X, Wang K, Bao J, Li P, Chen
M, Wan JB, Su H, Mei Z and He C: Polyphyllin VII induces an
autophagic cell death by activation of the JNK pathway and
inhibition of PI3K/AKT/mTOR pathway in HepG2 cells. PLoS One.
11:e01474052016. View Article : Google Scholar
|
|
32
|
Lin Z, Liu Y, Li F, Wu J, Zhang G, Wang Y,
Lu L and Liu Z: Anti-lung cancer effects of polyphyllin VI and VII
potentially correlate with apoptosis in vitro and in vivo.
Phytother Res. 29:1568–1576. 2015. View Article : Google Scholar
|
|
33
|
Kim AD, Kang KA, Kim HS, Kim DH, Choi YH,
Lee SJ, Kim HS and Hyun JW: A ginseng metabolite, compound K,
induces autophagy and apoptosis via generation of reactive oxygen
species and activation of JNK in human colon cancer cells. Cell
Death Dis. 4:e7502013. View Article : Google Scholar
|
|
34
|
Hsieh MJ, Tsai TL, Hsieh YS, Wang CJ and
Chiou HL: Dioscin-induced autophagy mitigates cell apoptosis
through modulation of PI3K/Akt and ERK and JNK signaling pathways
in human lung cancer cell lines. Arch Toxicol. 87:1927–1937. 2013.
View Article : Google Scholar
|
|
35
|
Ko CP, Lin CW, Chen MK, Yang SF, Chiou HL
and Hsieh MJ: Pterostilbene induce autophagy on human oral cancer
cells through modulation of Akt and mitogen-activated protein
kinase pathway. Oral Oncol. 51:593–601. 2015. View Article : Google Scholar
|
|
36
|
Kim A, Yim NH and Ma JY: Samsoeum, a
traditional herbal medicine, elicits apoptotic and autophagic cell
death by inhibiting Akt/mTOR and activating the JNK pathway in
cancer cells. BMC Complement Altern Med. 13:2332013. View Article : Google Scholar
|
|
37
|
Morales J, Li L, Fattah FJ, Dong Y, Bey
EA, Patel M, Gao J and Boothman DA: Review of poly (ADP-ribose)
polymerase (PARP) mechanisms of action and rationale for targeting
in cancer and other diseases. Crit Rev Eukaryot Gene Expr.
24:15–28. 2014. View Article : Google Scholar
|
|
38
|
Li Y, Luo Q, Yuan L, Miao C, Mu X, Xiao W,
Li J, Sun T and Ma E: JNK-dependent Atg4 upregulation mediates
asperphenamate derivative BBP-induced autophagy in MCF-7 cells.
Toxicol Appl Pharmacol. 263:21–31. 2012. View Article : Google Scholar
|
|
39
|
Gong K, Chen C, Zhan Y, Chen Y, Huang Z
and Li W: Autophagy-related gene 7 (ATG7) and reactive oxygen
species/extracellular signal-regulated kinase regulate
tetrandrine-induced autophagy in human hepatocellular carcinoma. J
Biol Chem. 287:35576–35588. 2012. View Article : Google Scholar
|
|
40
|
Hileman EO, Liu J, Albitar M, Keating MJ
and Huang P: Intrinsic oxidative stress in cancer cells: A
biochemical basis for therapeutic selectivity. Cancer Chemother
Pharmacol. 53:209–219. 2004. View Article : Google Scholar
|
|
41
|
Donadelli M, Dando I, Zaniboni T, Costanzo
C, Dalla Pozza E, Scupoli MT, Scarpa A, Zappavigna S, Marra M,
Abbruzzese A, et al: Gemcitabine/cannabinoid combination triggers
autophagy in pancreatic cancer cells through a ROS-mediated
mechanism. Cell Death Dis. 2:e1522011. View Article : Google Scholar
|
|
42
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar
|
|
43
|
Sato A, Okada M, Shibuya K, Watanabe E,
Seino S, Narita Y, Shibui S, Kayama T and Kitanaka C: Pivotal role
for ROS activation of p38 MAPK in the control of differentiation
and tumor-initiating capacity of glioma-initiating cells. Stem Cell
Res. 12:119–131. 2014. View Article : Google Scholar
|
|
44
|
Xu J, Qin X, Cai X, Yang L, Xing Y, Li J,
Zhang L, Tang Y, Liu J, Zhang X and Gao F: Mitochondrial JNK
activation triggers autophagy and apoptosis and aggravates
myocardial injury following ischemia/reperfusion. Biochim Biophys
Acta. 1852:262–270. 2015. View Article : Google Scholar
|