|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghaznavi H, Shirvaliloo M, Zarebkohan A,
Shams Z, Radnia F, Bahmanpour Z, Sargazi S, Saravani R, Shirvalilou
S, Shahraki O, et al: An updated review on implications of
autophagy and apoptosis in tumorigenesis: Possible alterations in
autophagy through engineered nanomaterials and their importance in
cancer therapy. Mol Pharmacol. 100:119–143. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown JM and Attardi LD: The role of
apoptosis in cancer development and treatment response. Nat Rev
Cancer. 5:231–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang C, Hu Y, Xiao W and Tian Z: Chimeric
antigen receptor- and natural killer cell receptor-engineered
innate killer cells in cancer immunotherapy. Cell Mol Immunol.
18:2083–2100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling CQ, Yue XQ and Ling C: Three
advantages of using traditional Chinese medicine to prevent and
treat tumor. J Integr Med. 12:331–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
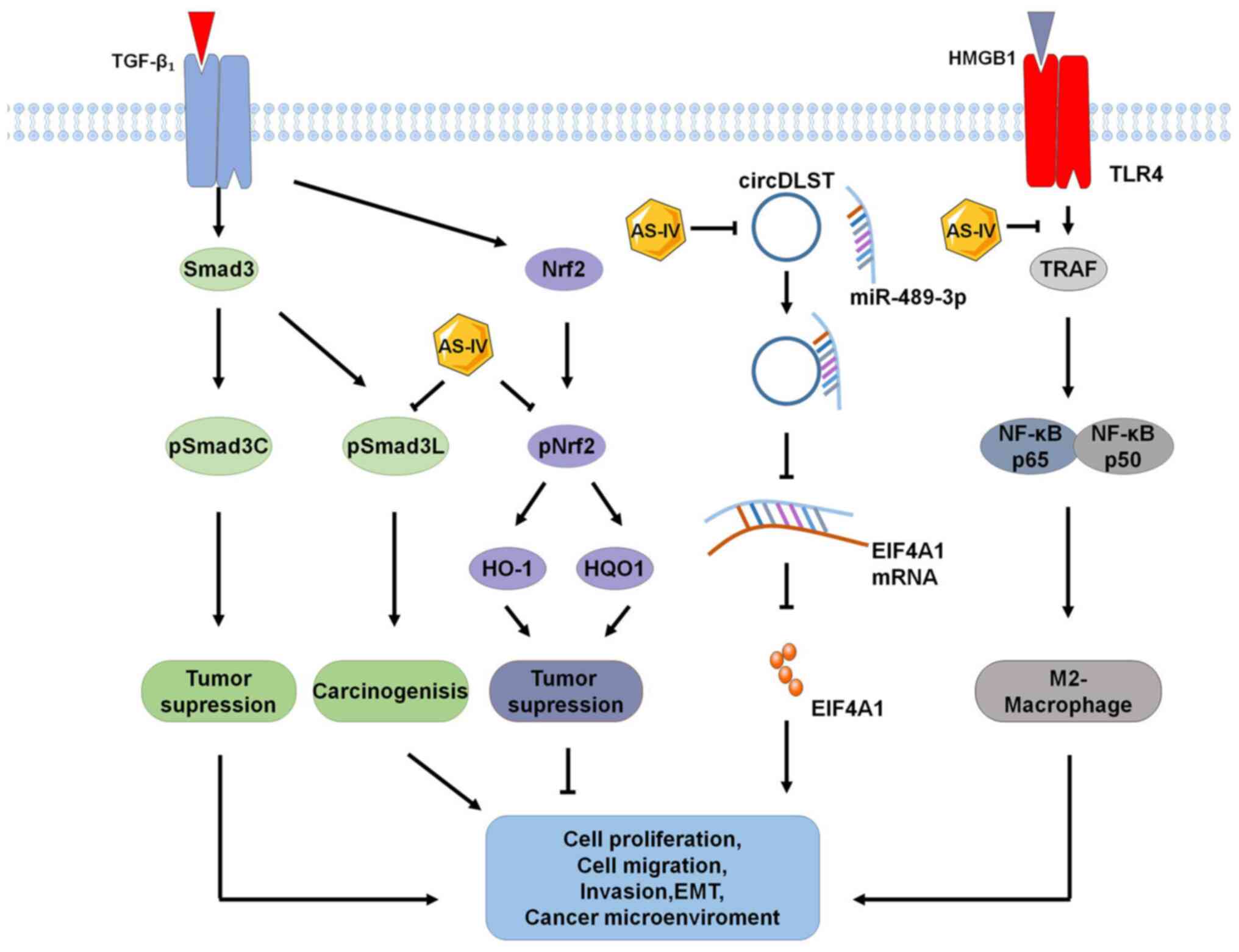
|
7
|
Zhang Y, Lou Y, Wang J, Yu C and Shen W:
Research status and molecular mechanism of the traditional Chinese
medicine and antitumor therapy combined strategy based on tumor
microenvironment. Front Immunol. 11:6097052021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang K, Chen Q, Shao Y, Yin S, Liu C, Liu
Y, Wang R, Wang T, Qiu Y and Yu H: Anticancer activities of TCM and
their active components against tumor metastasis. Biomed
Pharmacother. 133:1110442021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen F, Zhong Z, Tan HY, Guo W, Zhang C,
Tan CW, Li S, Wang N and Feng Y: Uncovering the anticancer
mechanisms of Chinese herbal medicine formulas: Therapeutic
alternatives for liver cancer. Front Pharmacol. 11:2932020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen T, Yang P and Jia Y: Molecular
mechanisms of astragaloside-IV in cancer therapy (review). Int J
Mol Med. 47:132021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu
L, Zhao M, Liu Q, Cheng Z, Zou J, et al: Naturally occurring
anti-cancer compounds: Shining from Chinese herbal medicine. Chin
Med. 14:482019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Zhang Q, Chen Y, Liang CL, Liu H,
Qiu F and Dai Z: Antitumor effects of immunity-enhancing
traditional Chinese medicine. Biomed Pharmacother. 121:1095702020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Royal Botanic Gardens, Kew. https://mpns.science.kew.org/mpns-portal/?_ga=1.111763972.1427522246.1459077346
|
|
14
|
Chang X, Chen X, Guo Y, Gong P, Pei S,
Wang D, Wang P, Wang M and Chen F: Advances in chemical
composition, extraction techniques, analytical methods, and
biological activity of astragali radix. Molecules. 27:10582022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Z, Lou Y, Kong M, Luo Q, Liu Z and Wu
J: A systematic review of phytochemistry, pharmacology and
pharmacokinetics on astragali radix: Implications for astragali
radix as a personalized medicine. Int J Mol Sci. 20:14632019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang CH, Yang X, Wei JR, Chen NM, Xu JP,
Bi YQ, Yang M, Gong X, Li ZY, Ren K, et al: Ethnopharmacology,
phytochemistry, pharmacology, toxicology and clinical applications
of radix astragali. Chin J Integr Med. 27:229–240. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Qu L, Dong Y, Han L, Liu E, Fang S,
Zhang Y and Wang T: A review of recent research progress on the
astragalus genus. Molecules. 19:18850–18880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong S, Ou S, Liu Y, Xie M, Mei T, Zhang
Y, Zhang J, Wang Q and Yang B: Surface-enhanced raman spectroscopy
analysis of astragalus saponins and identification of metabolites
after oral administration in rats by ultrahigh-performance liquid
chromatography/quadrupole time-of-flight mass spectrometry
analysis. Front Pharmacol. 13:8284492022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Tang D, Zang W, Yin G, Dai J, Sun
YU, Yang Z, Hoffman RM and Guo X: Synergistic inhibitory effect of
traditional Chinese medicine astragaloside IV and curcumin on tumor
growth and angiogenesis in an orthotopic nude-mouse model of human
hepatocellular carcinoma. Anticancer Res. 37:465–473. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Wu C, Gao L, Du G and Qin X:
Astragaloside IV derived from Astragalus membranaceus: A
research review on the pharmacological effects. Adv Pharmacol.
87:89–112. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong AGW, Duan R, Wang HY, Kong XP, Dong
TTX, Tsim KWK and Chan K: Evaluation of the pharmaceutical
properties and value of astragali radix. Medicines (Basel).
5:462018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Zhou J, Qin X, Huang H and Nie C:
Astragaloside IV inhibits the invasion and metastasis of SiHa
cervical cancer cells via the TGF-β1-mediated PI3K and MAPK
pathways. Oncol Rep. 41:2975–2986. 2019.PubMed/NCBI
|
|
23
|
Sun P, Liu Y, Wang Q and Zhang B:
Astragaloside IV inhibits human colorectal cancer cell growth.
Front Biosci (Landmark Ed). 24:597–606. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim C and Kim B: Anti-cancer natural
products and their bioactive compounds inducing ER stress-mediated
apoptosis: A review. Nutrients. 10:10212018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hata AN, Engelman JA and Faber AC: The
BCL2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia L, Lv D, Zhang S, Wang Z and Zhou B:
Astragaloside IV inhibits the progression of non-small cell lung
cancer through the Akt/GSK-3β/β-catenin pathway. Oncol Res.
27:503–508. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu T, Fei Z and Wei N: Chemosensitive
effects of astragaloside IV in osteosarcoma cells via induction of
apoptosis and regulation of caspase-dependent Fas/FasL signaling.
Pharmacol Rep. 69:1159–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang S, Mou J, Cui L, Wang X and Zhang Z:
Astragaloside IV inhibits cell proliferation of colorectal cancer
cell lines through down-regulation of B7-H3. Biomed Pharmacother.
102:1037–1044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su CM, Wang HC, Hsu FT, Lu CH, Lai CK,
Chung JG and Kuo YC: Astragaloside IV induces apoptosis,
G1-phase arrest and inhibits anti-apoptotic signaling in
hepatocellular carcinoma. In vivo. 34:631–638. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim MO, Lee HS, Chin YW, Moon DO and Ahn
JS: Gartanin induces autophagy through JNK activation which
extenuates caspase-dependent apoptosis. Oncol Rep. 34:139–146.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amaravadi RK, Kimmelman AC and Debnath J:
Targeting autophagy in cancer: Recent advances and future
directions. Cancer Discov. 9:1167–1181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Russell RC, Yuan HX and Guan KL: Autophagy
regulation by nutrient signaling. Cell Res. 24:42–57. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu
HL, Yang C and Liu HF: p62 links the autophagy pathway and the
ubiqutin-proteasome system upon ubiquitinated protein degradation.
Cell Mol Biol Lett. 21:292016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, Li G, Chen M and Cai R:
Astragaloside IV enhances the sensibility of lung adenocarcinoma
cells to bevacizumab by inhibiting autophagy. Drug Dev Res.
83:461–469. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li QW, Zhang GL, Hao CX, Ma YF, Sun X,
Zhang Y, Cao KX, Li BX, Yang GW and Wang XM: SANT, a novel Chinese
herbal monomer combination, decreasing tumor growth and
angiogenesis via modulating autophagy in heparanase overexpressed
triple-negative breast cancer. J Ethnopharmacol. 266:1134302021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lai ST, Wang Y and Peng F: Astragaloside
IV sensitizes non-small cell lung cancer cells to cisplatin by
suppressing endoplasmic reticulum stress and autophagy. J Thorac
Dis. 12:3715–3724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang B, Yang N, Chen Y, Zhu M, Lian Y,
Xiong Z, Wang B, Feng L and Jia X: An integrated strategy for
effective-component discovery of astragali radix in the treatment
of lung cancer. Front Pharmacol. 11:5809782021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qu X, Gao H, Zhai J, Sun J, Tao L, Zhang
Y, Song Y and Hu T: Astragaloside IV enhances cisplatin
chemosensitivity in hepatocellular carcinoma by suppressing MRP2.
Eur J Pharm Sci. 148:1053252020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia C, He Z and Cai Y: Quantitative
proteomics analysis of differentially expressed proteins induced by
astragaloside IV in cervical cancer cell invasion. Cell Mol Biol
Lett. 25:252020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang X: EMT: New signals from the
invasive front. Oral Oncol. 47:686–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ribatti D: Epithelial-mesenchymal
transition in morphogenesis, cancer progression and angiogenesis.
Exp Cell Res. 353:1–5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kozak J, Forma A, Czeczelewski M, Kozyra
P, Sitarz E, Radzikowska-Büchner E, Sitarz M and Baj J: Inhibition
or reversal of the epithelial-mesenchymal transition in gastric
cancer: Pharmacological approaches. Int J Mol Sci. 22:2772020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu J and Wen K: Astragaloside IV inhibits
TGF-β1-induced epithelial-mesenchymal transition through inhibition
of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother
Res. 32:1289–1296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han J, Shen X, Zhang Y, Wang S and Zhou L:
Astragaloside IV suppresses transforming growth factor-β1-induced
epithelial-mesenchymal transition through inhibition of
Wnt/β-catenin pathway in glioma U251 cells. Biosci Biotechnol
Biochem. 84:1345–1352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Gao S, Song L, Liu M, Sun Z and
Liu J: Astragaloside IV antagonizes M2 phenotype macrophage
polarization-evoked ovarian cancer cell malignant progression by
suppressing the HMGB1-TLR4 axis. Mol Immunol. 130:113–121. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang ZF, Ma DG, Zhu Z, Mu YP, Yang YY,
Feng L, Yang H, Liang JQ, Liu YY, Liu L and Lu HW: Astragaloside IV
inhibits pathological functions of gastric cancer-associated
fibroblasts. World J Gastroenterol. 23:8512–8525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C,
Lin F, Liao H, You Z and Liu L: Prognostic impact of
tumor-associated macrophage infiltration in non-small cell lung
cancer: A systemic review and meta-analysis. Oncotarget.
7:34217–34228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cuccarese MF, Dubach JM, Pfirschke C,
Engblom C, Garris C, Miller MA, Pittet MJ and Weissleder R:
Heterogeneity of macrophage infiltration and therapeutic response
in lung carcinoma revealed by 3D organ imaging. Nat Commun.
8:142932017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sawa-Wejksza K, Dudek A, Lemieszek M,
Kaławaj K and Kandefer-Szerszeń M: Colon cancer-derived conditioned
medium induces differentiation of THP-1 monocytes into a mixed
population of M1/M2 cells. Tumour Biol. 40:10104283187978802018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li N, Qin J, Lan L, Zhang H, Liu F, Wu Z,
Ni H and Wang Y: PTEN inhibits macrophage polarization from M1 to
M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer
Biol Ther. 16:297–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL,
Gong WY, Dong JC and Liu BJ: Astragaloside IV inhibits lung cancer
progression and metastasis by modulating macrophage polarization
through AMPK signaling. J Exp Clin Cancer Res. 37:2072018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu F, Ran F, He H and Chen L:
Astragaloside IV exerts anti-tumor effect on murine colorectal
cancer by re-educating tumor-associated macrophage. Arch Immunol
Ther Exp (Warsz). 68:332020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen J, Ye X, Pitmon E, Lu M, Wan J,
Jellison ER, Adler AJ, Vella AT and Wang K: IL-17 inhibits
CXCL9/10-mediated recruitment of CD8+ cytotoxic T cells
and regulatory T cells to colorectal tumors. J Immunother Cancer.
7:3242019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang LF, Yao YM, Li JF, Zhang SW, Li WX,
Dong N, Yu Y and Sheng ZY: The effect of astragaloside IV on immune
function of regulatory T cell mediated by high mobility group box 1
protein in vitro. Fitoterapia. 83:1514–1522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang A, Zheng Y, Que Z, Zhang L, Lin S,
Le V, Liu J and Tian J: Astragaloside IV inhibits progression of
lung cancer by mediating immune function of Tregs and CTLs by
interfering with IDO. J Cancer Res Clin Oncol. 140:1883–1890. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu W, Chen H and Wang D: Protective role
of astragaloside IV in gastric cancer through regulation of
microRNA-195-5p-mediated PD-L1. Immunopharmacol Immunotoxicol.
43:443–451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang PP, Luan JJ, Xu WK, Wang L, Xu DJ,
Yang CY, Zhu YH and Wang YQ: Astragaloside IV downregulates the
expression of MDR1 in Bel-7402/FU human hepatic cancer cells by
inhibiting the JNK/c-Jun/AP-1 signaling pathway. Mol Med Rep.
16:2761–2766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xie T, Li Y, Li SL and Luo HF:
Astragaloside IV enhances cisplatin chemosensitivity in human
colorectal cancer via regulating NOTCH3. Oncol Res. 24:447–453.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dulloo I, Phang BH, Othman R, Tan SY,
Vijayaraghavan A, Goh LK, Martin-Lopez M, Marques MM, Li CW, Wang
de Y, et al: Hypoxia-inducible TAp73 supports tumorigenesis by
regulating the angiogenic transcriptome. Nat Cell Biol. 17:511–523.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu L, Zhou XM, Yang FF, Miao Y, Yin Y, Hu
XJ, Hou G, Wang QY and Kang J: TRIM22 confers poor prognosis and
promotes epithelial-mesenchymal transition through regulation of
AKT/GSK3β/β-catenin signaling in non-small cell lung cancer.
Oncotarget. 8:62069–62080. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qin CD, Ma DN, Ren ZG, Zhu XD, Wang CH,
Wang YC, Ye BG, Cao MQ, Gao DM and Tang ZY: Astragaloside IV
inhibits metastasis in hepatoma cells through the suppression of
epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin
pathway. Oncol Rep. 37:1725–1735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Y, Liu C, Xie Z and Lu H: Knockdown
of TRIM47 inhibits breast cancer tumorigenesis and progression
through the inactivation of PI3K/Akt pathway. Chem Biol Interact.
317:1089602020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li R, Song Y, Zhou L, Li W and Zhu X:
Downregulation of RAGE inhibits cell proliferation and induces
apoptosis via regulation of PI3K/AKT pathway in cervical squamous
cell carcinoma. Onco Targets Ther. 13:2385–2397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao Y, Wang L, Wang Y, Dong S, Yang S,
Guan Y and Wu X: Astragaloside IV inhibits cell proliferation in
vulvar squamous cell carcinoma through the TGF-β/Smad signaling
pathway. Dermatol Ther. 32:e128022019.PubMed/NCBI
|
|
70
|
Zhang XQ, Yao C, Bian WH, Chen X, Xue JX,
Zhu ZY, Ying Y, Xu YL and Wang C: Effects of astragaloside IV on
treatment of breast cancer cells execute possibly through
regulation of Nrf2 via PI3K/AKT/mTOR signaling pathway. Food Sci
Nutr. 7:3403–3413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
He Y, Zhang Q, Chen H, Guo Q, Zhang L,
Zhang Z and Li Y: Astragaloside IV enhanced carboplatin sensitivity
in prostate cancer by suppressing AKT/NF-κB signaling pathway.
Biochem Cell Biol. 99:214–222. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tanaka Y, Kobayashi H, Suzuki M, Kanayama
N and Terao T: Transforming growth factor-beta1-dependent urokinase
up-regulation and promotion of invasion are involved in
Src-MAPK-dependent signaling in human ovarian cancer cells. J Biol
Chem. 279:8567–8576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Anfuso CD, Motta C, Giurdanella G, Arena
V, Alberghina M and Lupo G: Endothelial PKCα-MAPK/ERK-phospholipase
A2 pathway activation as a response of glioma in a triple culture
model. A new role for pericytes? Biochimie. 99:77–87.
2014.PubMed/NCBI
|
|
74
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI
|
|
75
|
Li B, Wang F, Liu N, Shen W and Huang T:
Astragaloside IV inhibits progression of glioma via blocking
MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 491:98–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jiang K, Lu Q, Li Q, Ji Y, Chen W and Xue
X: Astragaloside IV inhibits breast cancer cell invasion by
suppressing Vav3 mediated Rac1/MAPK signaling. Int Immunopharmacol.
42:195–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB
pathway. Int Immunopharmacol. 23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Komohara Y, Fujiwara Y, Ohnishi K and
Takeya M: Tumor-associated macrophages: Potential therapeutic
targets for anti-cancer therapy. Adv Drug Deliv Rev. 99:180–185.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xie C, Liu D, Chen Q, Yang C, Wang B and
Wu H: Soluble B7-H3 promotes the invasion and metastasis of
pancreatic carcinoma cells through the TLR4/NF-κB pathway. Sci Rep.
6:275282016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hong F, Xiao W, Ragupathi G, Lau CB, Leung
PC, Yeung KS, George C, Cassileth B, Kennelly E and Livingston PO:
The known immunologically active components of astragalus account
for only a small proportion of the immunological adjuvant activity
when combined with conjugate vaccines. Planta Med. 77:817–824.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Min L, Wang H and Qi H: Astragaloside IV
inhibits the progression of liver cancer by modulating macrophage
polarization through the TLR4/NF-κB/STAT3 signaling pathway. Am J
Transl Res. 14:1551–1566. 2022.PubMed/NCBI
|
|
82
|
Zhang C, Li L, Hou S, Shi Z, Xu W, Wang Q,
He Y, Gong Y, Fang Z and Yang Y: Astragaloside IV inhibits
hepatocellular carcinoma by continually suppressing the development
of fibrosis and regulating pSmad3C/3L and Nrf2/HO-1 pathways. J
Ethnopharmacol. 279:1143502021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li F, Cao K, Wang M, Liu Y and Zhang Y:
Astragaloside IV exhibits anti-tumor function in gastric cancer via
targeting circRNA dihydrolipoamide S-succinyltransferase
(circDLST)/miR-489-3p/eukaryotic translation initiation factor
4A1(EIF4A1) pathway. Bioengineered. 13:10111–10122. 2022.PubMed/NCBI
|
|
84
|
Zhang J, Hou L, Liang R, Chen X, Zhang R,
Chen W and Zhu J: CircDLST promotes the tumorigenesis and
metastasis of gastric cancer by sponging miR-502-5p and activating
the NRAS/MEK1/ERK1/2 signaling. Mol Cancer. 18:802019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cui X, Jiang X, Wei C, Xing Y and Tong G:
Astragaloside IV suppresses development of hepatocellular carcinoma
by regulating miR-150-5p/β-catenin axis. Environ Toxicol Pharmacol.
78:1033972020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang L, Botchway BOA and Liu X: The
repression of the HMGB1-TLR4-NF-κB signaling pathway by safflower
yellow may improve spinal cord injury. Front Neurosci.
15:8038852021. View Article : Google Scholar : PubMed/NCBI
|