Introduction
Neuroblastoma (NB) is the most frequently diagnosed
cancer during the first year of life and has genetic, morphological
and clinical heterogeneity and can spontaneously regress or
progress aggressively, with many patients succumbing to
recurrent/refractory metastatic disease (1–6). For
children with low- and intermediate-risk disease, survival is
>90%, while for those with high-risk disease, 5-year survival is
<50% despite multimodal application of chemotherapy, stem cell
transplantation, operative treatment, irradiation, retinoid therapy
and different types of immunotherapy (1,6). NB
does not respond well to conventional chemotherapy and irradiation,
and current treatments accepted for NB have narrow targeted
specificity (1,7,8). To
improve individualized therapy for NB, analysis of the molecular
mechanisms underlying the etiology and pathogenesis of NB is
essential and new therapeutic targets have been disclosed (9–13).
The PI3K/Akt signaling pathway has been shown to be
involved in the control of many key cellular responses, such as
proliferation, survival, metabolism and migration (9,14,15).
Moreover, PI3K/Akt is activated in several tumor types, including
NB (16,17). Clinical trials with PI3K inhibitor
monotherapy have been performed for NB; for example, a phase I
clinical trial with SF1126 (PI3K/mTOR inhibitor) for
relapsed/refractory NB (trial no. NCT02337309) (18) has been conducted by the New
Approaches to Neuroblastoma Therapy Consortium.
Furthermore, our previous studies assessed in
vitro effects of the United States Food and Drug Administration
(FDA)-approved PI3K and fibroblast growth factor receptor (FGFR)
inhibitors BYL719 and JNJ- 42756493, respectively, as single or
combined treatment, with or without chemotherapy in NB and
medulloblastoma (MB) cell lines (10–13).
Combined use of PI3K and FGFR inhibitors resulted in synergistic
effects, suggesting possible clinical utility for NB and MB, while
their joint effects with chemotherapy drugs were less clear and
need further investigation. The present study aimed to assess
combinations of targeted therapies.
The contribution of regulators of the cell cycle
such as cyclin-dependent kinase (CDK) 4/6 and WEE1 G2 checkpoint
kinase (WEE1) in NB implies that deregulation of the normal cell
cycle could be important in promoting NB tumorigenesis (19,20).
CDK4 and 6 are enzymes that act with D-type cyclins to promote
progression of the cell cycle from G1 to S phase (21–23).
When the cyclin D/CDK4/6/inhibitor of CDK4/retinoblastoma pathway
is dysregulated, proliferation is increased in many types of cancer
such as glioblastoma, melanoma, liposarcoma and NB (24–26).
Therefore, CDK4/6 inhibitors may serve as therapeutic candidates in
malignant cells. Currently there are three FDA-approved CDK4/6
inhibitors, palbociclib, ribociclib and abemaciclib, which are
primarily used for breast cancer (27). Moreover, several studies have shown
that PI3K/Akt inhibitors combined with CDK4/6 inhibitors are
effective in preclinical cancer models and exhibit synergism
(28–31).
Other FDA approved inhibitors include poly
ADP-ribose polymerase (PARP) inhibitor olaparib and the WEE1
inhibitor IMP7068 (32–37). Olaparib is used in patients with
recurrent ovarian, early breast, metastatic castration-resistant
prostate and metastatic pancreatic cancer, while IMP7068 indirectly
targets non-functional p53 (present in some cases of high-risk NB)
and has potential synergy with PARP inhibitors (32–37).
To explore options for NB therapy, the present study
investigated PI3K, CDK4/6, PARP and WEE1 inhibitors in different
combinations to identify potential synergistic effects in NB cell
lines with different mutational profiles. NB cell lines, SK-N-AS,
SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH, were assessed for their
sensitivity to single and combined treatments with FDA-approved
PI3K, CDK4/6, PARP inhibitors BYL719, PD-0332991 and BMN673,
respectively, as well as the non-FDA-approved WEE1 inhibitor
MK-1775.
Materials and methods
Cell lines and culture
Non-MYCN proto-oncogene, bHLH transcription factor
(MYCN)-amplified SK-N-AS, SK-N-FI (both mutant p53) and SK-N-SH
(wild-type p53) as well as MYCN-amplified SK-N-DZ (deletion of PIK3
catalytic subunit type 2γ) and SK-N-BE(2)-C (both mutant p53) were
a kind gift from Professor Per Kogner, Karolinska Institute
(Stockholm, Sweden) and their identities were verified previously
(10). Cells were all grown in
RPMI)1640 medium including 10% fetal bovine serum (both Gibco;
Thermo Fisher Scientific, Inc.), 1% L-glutamine, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in a humidified
incubator with 5% CO2.
Inhibitors
Stock solutions of the FDA-approved PI3K, CDK4/6 and
PARP inhibitors BYL719, PD-0332991 and BMN673 and non-FDA-approved
WEE1 inhibitor MK-1775 were all purchased from Selleck Chemicals,
prepared in DMSO and then diluted in PBS. Titration experiments
were performed using 0.01–50.0 MK-1775, 0.001–50.0 BMN673, 1.0–20.0
PD-0332991 and 0.25–20.0 µM BYL719) in all cell lines (data not
shown). Subsequently, 1.0–10.0 µM for BYL719 and PD-0332991 and
0.5–1.0 µM for BMN673 and MK-1775 were tested to identify additive
or synergistic effects.
WST-1 viability assay
To assess viability, SK-N-AS, SK-N-BE(2)-C, SK-N-DZ,
SK-N-FI and SK-N-SH cells (5,000 cells/well were cultured in 90 µl
RPMI-1640 medium in 96-well plates at 37°C, 5% CO2 for
24 h followed by treatment with inhibitors. Viability was assessed
after 24, 48 and 72 h exposure to inhibitors by adding 10 µl cell
proliferation WST-1 reagent (Roche Diagnostics GmbH, Solna, Sweden)
per each well as previously described (10,11).
The plates were incubated at 37°C, 5% CO2 for 1 h and
the absorbance was measured at 450 nm using the VersaMax microplate
reader (Molecular Devices, LLC). Moreover, half-maximal inhibitory
concentration (IC50) was estimated from log
concentration-effect curves with non-linear regression analysis in
GraphPad Prism Software version 8 (GraphPad Software, Inc.). All
graphs represent at least three experiments and absorbance values
after treatment were compared with PBS controls.
Confluence, cytotoxicity and apoptosis
assay
To estimate confluence, cytotoxicity and apoptosis,
SK-N-AS, SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH cells (5,000
cells/well) were cultured in 200 µl RPMI-1640 medium in 96-well
plates in an IncuCyte S3 Live® Cell Analysis System
(Essen Bioscience, Welwyn Garden City, UK) at 37°C. After 24 h, the
medium was replaced with fresh RPMI-1640 medium that contained
Incucyte Cytotox Red Reagent to assess cytotoxicity or IncuCyte
Caspase-3/7 Green Apoptosis Assay Reagent [both (Essen Bioscience,
Welwyn Garden City, UK)] to assess apoptosis for 72 h at 37°C. Cell
confluence was assessed by image-based measurements of cell growth
based on area using IncuCyte Analysis Software version 2021A,
Sartorius. Apoptosis was determined when IncuCyte Caspase-3/7 Green
Apoptosis Assay Reagent crossed cell membrane and was cleaved by
active caspase-3/7. DNA intercalating dye was released leading to
the fluorescent staining of nuclear DNA. By using IncuCyte Analysis
Software (Sartorius), fluorescent objects were quantified. Images
were captured with IncuCyte S3 Live® Cell Analysis
System (Essen Bioscience) every 2 h with PBS used as negative
control.
FACS analysis
To follow the effects of the drugs on the cell cycle
a FACS analysis was performed. All procedures were performed
according to the protocol of the manufacturer (Invitrogen; Thermo
Fisher Scientific, Inc.), SK-N-AS, SK-N-BE(2)-C, SK-N-DZ, SK-N-FI
and SK-N-SH cells were collected 48 h after treatment with 0.5 and
1.0 MK-1775, 1.0 BMN673, 5.0 and 10.0 PD-0332991 and 5.0 and 10.0
µM BYL719, resuspended in cold PBS in 15-ml tubes and cold 70%
ethanol was included dropwise while vortexing at a speed of 1,800
rpm for 10 sec at room temperature. Controls were made with PBS and
DMSO at the tested concentrations. Subsequently, tubes were stored
at 4°C until they were utilized. A total of 1×106 cells
was analyzed per sample. Following centrifugation at 204 g for 10
min at 4°C, the pellet was dissolved in 0.5 ml FxCyclePI/RNAse
solution according to the manufacturer's instructions (Invitrogen;
Thermo Fisher Scientific, Inc. Stockholm, Sweden) at room
temperature (without light) for 30 min before analysis using FACS
Novocyte, Agilent and Flowjo_v10.8.1 software (Sapio Sciences,
London, UK).
Statistical analysis
The efficacy of the joint therapies was analyzed by
highest single agent effect-based approach and median effect
dose-effect-based approach (based on Loewe additivity) (38,39).
To validate the effect of single drugs or using them in combination
in comparison to a negative control, multiple unpaired t tests
followed by post hoc Holm-Sidak correction was performed (11). All the experiments were performed at
least three times and are presented as mean and SD. Data were
analyzed using Graphpad Prism 9.4.1. Median-effect equation to
calculate a median-effect value D (equivalent to IC50)
and slope, as described previously (10). Goodness-of-fit was analyzed with
linear correlation coefficient; r>0.85 was set as a threshold
for subsequent analysis. The interaction degree of the drugs was
assessed using the combination index (CI) for mutually exclusive
drugs as follows: CI=d1/D1 +
d2/D2, where D1 and D2
represent the doses of drug 1 and 2 alone needed to obtain a
certain effect and d1 and d2 represent the
doses of drug 1 and 2 needed to obtain the same effect in
combination. CI<0.75 was considered to indicate synergy and
CI<1.45; values between the two were considered assessed to
indicate additive effects, in concordance with the recommendations
of the ComboSyn (combosyn.com/). One-way ANOVA with Bonferroni's
post hoc test was used to calculate the difference in mean between
two single drugs and their combined treatment. P<0.05 was
considered to indicate a statistically significant difference.
Results
Viability of the SK-N-AS,
SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH NB cell lines treated
with BYL719, PD-0332991, BMN673 and MK-1775 is affected in a
dose-dependent manner
The effects of BYL719, PD-0332991, BMN673 and
MK-1775 on SK-N-AS, SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH
viability were assessed by WST-1 assay for 72 h following
treatment. When combining PD-0332991 with BYL719 or MK-1775 with
BMN673 only low doses of the drugs exposing possible synergistic
effects were utilized.
All NB cell lines presented dose-dependent responses
following treatment with 1–10 µM PD-0332991. The highest PD-0332991
dose (10 µM) caused a notable decrease in absorbance at 48 and 72 h
after treatment compared with PBS in all lines except for SK-N-AS
(Fig. 1A, D, G, J, M). A similar
response was observed with 5 µM for all cell lines except SK-N-FI
and 1 µM PD-0332991 at 72 h for SK-N-BE(2)-C and SK-N-AS.
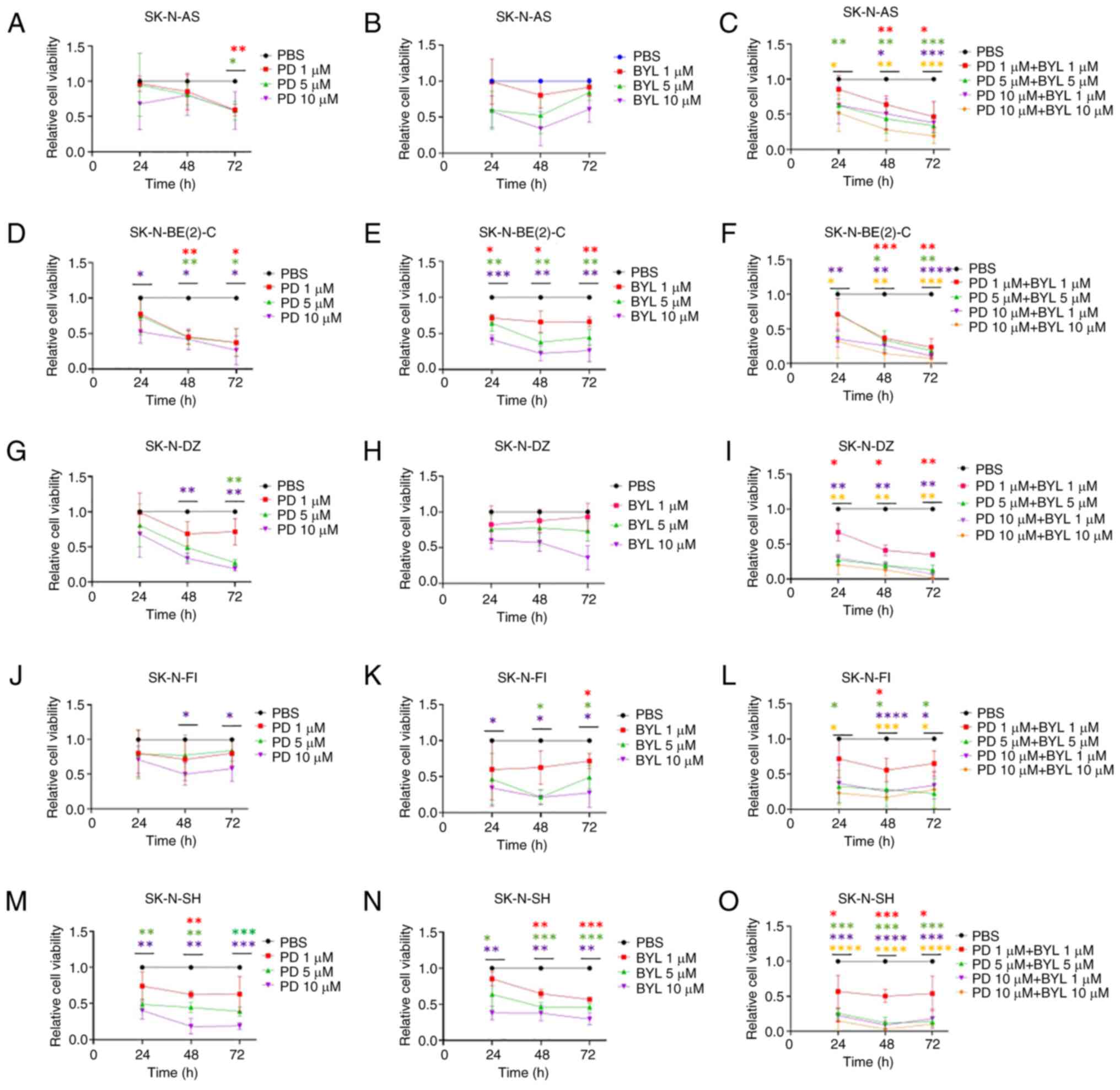 | Figure 1.WST-1 viability assay in NB cell
lines following treatment with CDK4/6 inhibitor PD and PI3K
inhibitor BYL. Viability of SK-N-AS treated with (A) PD, BYL (B)
and combination of PD and BYL (C), viability of SK-N-BE(2)-C
treated with PD (D), BYL (E) and combination of PD and BYL (F),
viability of SK-N-DZ treated with PD (G), BYL (H) and combination
of PD and BYL (I), viability of SK-N-FI treated with PD (J), BYL
(K) and combination of PD and BYL (L) and viability of SK-N-SH
treated with PD (M), BYL (N) and combination of PD and BYL (O) was
measured by absorbance. *P<0.05, **P<0.01, ***P<0.001,
****P<0.0001 treatments compared with PBS each time point. NB,
neuroblastoma; PD, PD-0332991; BYL, BYL719. |
All NB lines exhibited dose-dependent responses to
1–10 µM BYL719 (Fig. 1B, E, H, K,
N). The highest dose (10 µM) gave a notable decrease in
absorbance at 48 and 72 h after treatment compared with PBS in all
lines except for SK-N-AS and SK-N-DZI. A similar response with 1
and 5 µM BYL719 was observed at 72 h for all NB lines except
SK-N-AS and SK-N-DZ.
NB cell lines presented a significant drop in
absorbance following treatment with almost all PD-0332991 + BYL719
combinations at 48 and 72 h (Fig. 1C,
F, I, L, O).
SK-N-BE(2)-C and SK-N-SH were more sensitive to
single treatment with PD-0332991 and BYL719, while the other cell
lines were more resistant, but upon combining the drugs this was
not the case for any of the NB lines.
All NB cell lines presented dose-dependent responses
with 0.5 and 1 µM MK-1775; SK-N-AS and SK-N-DZ were the most and
SK-N-BE(2)-C the least sensitive (Fig.
2A, D, G, J, M). The higher MK-1775 dose (1 µM) resulted in a
notable absorbance decrease at 48 and 72 h compared with PBS in all
NB lines except SK-N-BE(2)-C, while the lower dose (0.5 µM) showed
a significant effect at all timepoints only in SK-N-AS and
SK-N-DZ.
 | Figure 2.WST-1 viability assay in NB cell
lines after treatment with WEE1 inhibitor MK and PARP inhibitor
BMN. Viability of SK-N-AS treated with MK (A), BMN (B) and
combination of MK and BMN (C), viability of SK-N-BE(2)-C treated
with MK (D), BMN (E) and combination of MK and BMN (F), viability
of SK-N-DZ treated with MK (G), BMN (H) and combination of MK and
BMN (I), viability of SK-N-FI treated with MK (J), BMN (K) and
combination of MK and BMN (L) and viability of SK-N-SH treated with
MK (M), BMN (N) and combination of MK and BMN (O) was measured by
absorbance 24, 48 and 72 h. *P<0.05, **P<0.01, ***P<0.001,
****P<0.0001 vs. PBS control at each time point. NB,
neuroblastoma; BMN, BMN673; MK, MK-1775; WEE1, WEE1 G2 checkpoint
kinase; PARP, poly-ADP-ribose-polymerase. |
BMN673 was less potent when administered alone
compared with MK-1775, although dose-dependent responses were
observed in all the NB cell lines (Fig.
2B, E, H, K, N). The higher BMN673 dose (1 µM) significantly
decreased absorbance 72 h after treatment in all cell lines except
SK-N-FI, while the lower dose (0.5 µM) showed a notable effect
after 72 h only in SK-N-SH and SK-N-DZ cells.
MK-1775 + BMN673 (both 0.5–1.0 µM) resulted in a
notable decrease in absorbance for all cell lines at 72 h after
treatment; SK-N-SH and SK-N-DZ showed significant decreases in
absorbance with almost all combinations and timepoints (Fig. 2C, F, I, L, O).
To summarize, all NB lines except SK-N-BE(2)-C were
sensitive to MK-1775, while only SK-N-SH showed pronounced
sensitivity to BMN673; when combining MK-1775 + BMN673, all cell
lines showed a consistent sensitivity especially after 72 h.
IC50 of PD-0332991, BMN673
and MK-1775
IC50 values for PD-0332991, BMN673 and
MK-1775 for SK-N-AS, SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH at
24–72 h after treatment are presented in Table I. BYL719 IC50 values have
been reported previously (12).
PD-0332991 IC50 values were 0.05–1532.00 µM.
SK-N-BE(2)-C was the most sensitive cell line, followed by SK-N-SH
and SK-N-DZ, while SK-N-FI and SK-N-AS were less sensitive.
IC50 values for MK-1775 were 0.14–6.61 µM. SK-N-DZ and
SK-N-AS were the most sensitive lines, while SK-N-BE(2)-C, SK-N-FI
and SK-N-SH were less sensitive. For BMN673, IC50 values
were 0.99–621.20 µM. SK-N-SH was the most and SK-N-FI the least
sensitive NB cell line.
 | Table I.IC50 based on WST-1
viability assay following treatment with CDK4/6 PD-0332991, WEE1 G2
checkpoint kinase MK-1775 and poly-ADP-ribose-polymerase inhibitor
BMN-673. |
Table I.
IC50 based on WST-1
viability assay following treatment with CDK4/6 PD-0332991, WEE1 G2
checkpoint kinase MK-1775 and poly-ADP-ribose-polymerase inhibitor
BMN-673.
| A, PD-0332991 |
|---|
|
|---|
|
| IC50,
µM |
|---|
|
|
|
|---|
| Cell line | 24 h | 48 h | 72 h |
|---|
| SK-N-AS | 12.7a | 24.5a | 11.5a |
| SK-N-BE(2)-C | 19.8a | 0.1a | 0.1a |
| SK-N-DZ | 18.8a | 3.9 | 2.3 |
| SK-N-FI | 1532a | 26.4a | 34.9a |
| SK-N-SH | 5.0 | 2.3 | 2.1 |
|
| B,
MK-1775 |
|
|
| IC50,
µM |
|
|
|
| Cell
line | 24 h | 48 h | 72 h |
|
| SK-N-AS | 1.5 | 0.3 | 0.3 |
| SK-N-BE(2)-C | 1.3 | 1.0 | 1.0 |
| SK-N-DZ | 0.4 | 0.2 | 0.1 |
| SK-N-FI | 6.6 | 1.8 | 0.6 |
| SK-N-SH | 4.9 | 2.5 | 1.0 |
|
| C,
BMN673 |
|
|
| IC50,
µM |
|
|
|
| Cell
line | 24 h | 48 h | 72 h |
|
| SK-N-AS | 109.8a | 174.5a | 63.6 |
| SK-N-BE(2)-C | 152.1a | 20.4 | 7.1 |
| SK-N-DZ | 109.6a | 621.2a | 13.2 |
| SK-N-FI | 195.4a | - | - |
| SK-N-SH | 83.0 | 1.0 | 0.1 |
To summarize, most cell lines were more sensitive to
MK-1775 than BMN673 but response to PD-0332991 varied.
Highest single agent and median effect
following combined inhibitor treatment
CIs of PD-0332991 + BYL719 and MK-1775 + BMN673 were
measured using highest single agent and dose-effect-based median
effect (38,39). To summarize, when using the highest
single agent, CI<1 indicates a positive effect, while a CI>1
suggests a negative effect. When using the dose-effect-based median
effect, CI<0.75 indicates synergism, 0.75≤CI<1.45 indicates
an additive effect, CI=1.45 indicates a neutral effect and
CI>1.45 suggests an antagonistic effect. CI was measured 24
(data not shown), 48 and 72 h (data not shown) following drug
administration for all NB lines; CI at 48 h is presented in
Fig. 3.
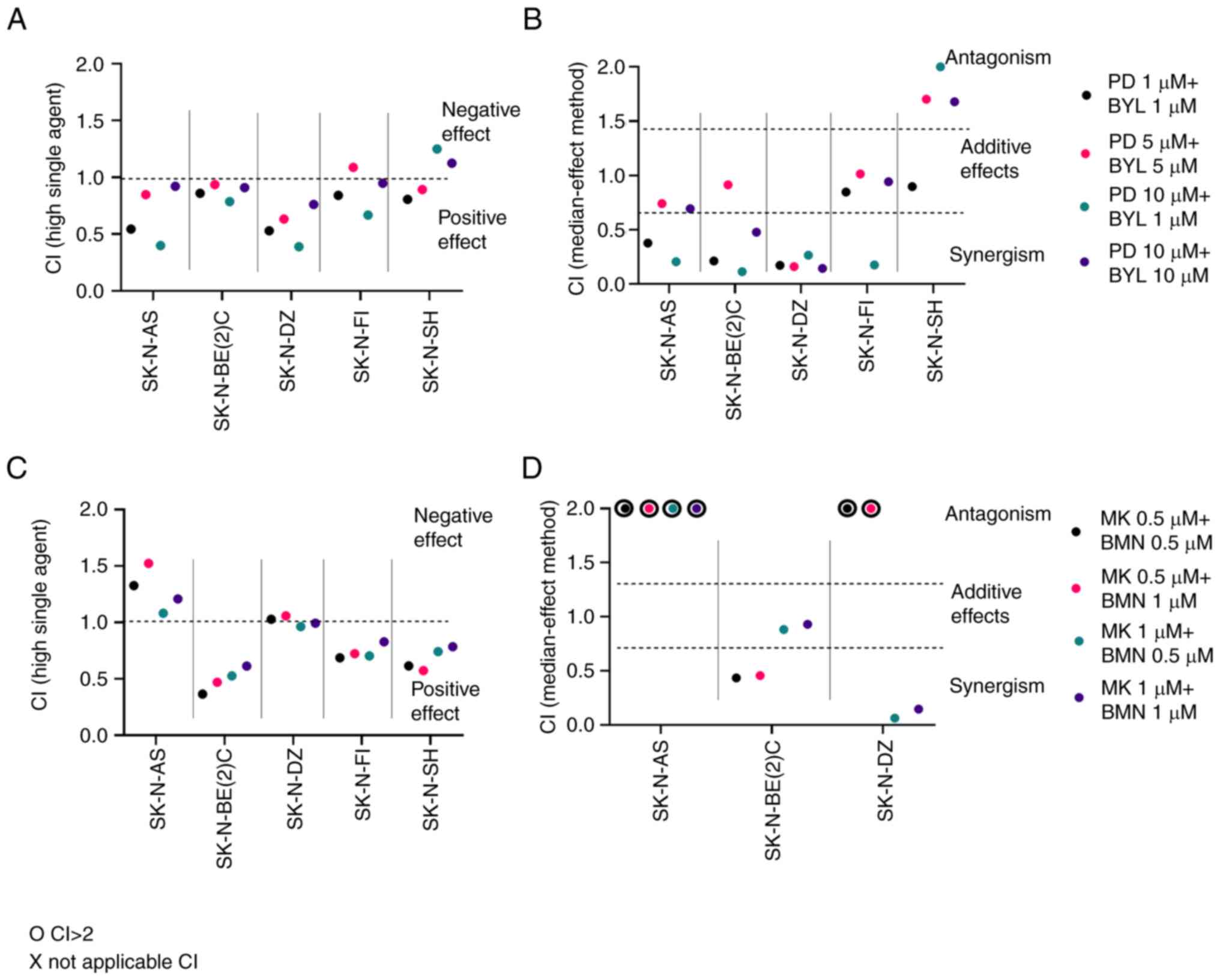 | Figure 3.Effect of combined PI3K and CDK4/6
inhibitors WEE1 G2 checkpoint kinase and PARP inhibitors on
neuroblastoma cell lines. Upon combination of BYL and PD, CIs were
calculated by (A) highest single agent and (B) median effect method
after 48 h. When combining MK together with BMN, CIs were
calculated by (C) highest single agent and (D) median effect method
after 48 h. CI>1.45 suggests antagonism, 0.75<CI>1.45
additive, and CI<0.75 synergistic effects. o indicates CI>2,
which implies a negative combination effect. CI, combination index;
PD, PD-0332991; BYL, BYL719; BMN, BMN673; MK, MK-1775; PARP,
poly-ADP-ribose-polymerase. |
The lowest doses (1 and 5 µM) of PD-0332991 + BYL719
yielded synergistic or neutral combinational effects (CI<1) 48 h
after treatment in all NB lines except SK-N-SH, where a slightly
negative effect was noted (Fig.
3A). In addition, combining 10 PD-0332991 + 1 or 10 µM BYL719
resulted in positive or neutral combinational effects in all NB
lines except SK-N-SH.
PD-0332991 + BYL719 presented synergistic or neutral
combinational effects in all NB lines with the exception of
SK-N-SH, where some antagonistic effects were observed (Fig. 3A and B).
Low doses (0.5 and 1.0 µM) of MK-1775 + BMN673
analyzed according to the highest single agent approach showed
mainly positive or neutral effects (CI<1) 48 h after treatment
for all NB cell lines except SK-N-AS, where negative effects were
observed (Fig. 3C). Using the
dose-effect-based median effect approach, synergistic effects were
observed in SK-N-BE(2)-C and SK-N-DZ, while for SK-N-FI and SK-N-SH
they could not be analyzed (Fig.
3D).
To summarize, MK-1775 + BMN673 exhibited positive
and neutral effects for NB cell lines except SK-N-AS, but it was
not possible to calculate the dose-effect-based median effect in
all cases.
Cell confluence after single and
combined treatments with PD-0332991, BYL719, BMN673 and
MK-1775
Confluence as a measure of proliferation of NB cell
lines following single and combined treatment with PD-0332991,
BYL719, BMN673 and MK-1775 was analyzed using the IncuCyte S3
Live-Cell Analysis System every 2 h for 72 h.
Monotherapy with 5–10 µM PD-0332991 decreased
confluence in all NB cell lines, while 1 µM PD-0332991 decreased
confluence only in SK-N-BE(2)-C and SK-N-SH cells with only
marginal effects on the other cell lines (Fig. 4A, D, G, J, M).
 | Figure 4.Effect of PD and BYL on confluence of
neuroblastoma cell lines. Confluence of SK-N-AS cells treated with
PD (A), BYL (B) and combination of PD and BYL (C), confluence of
SK-N-BE(2)-C treated with PD (D), BYL (E) and combination of PD and
BYL (F), confluence of SK-N-DZ treated with PD (G), BYL (H) and
combination of PD and BYL (I), confluence of SK-N-FI treated with
PD (J), BYL (K) and combination of PD and BYL (L) and confluence of
SK-N-SH treated with PD (M), BYL (N) and combination of PD and BYL
(O) was measured every 2 h up to 72 h after the treatment.
Confluence indicates proliferation. BYL, BYL719; PD, PD-0332991;
CDK4/6, cyclin-dependent kinase-4/6; PI3K, phosphoinositide
3-kinase. |
Monotherapy with 1–10 µM BYL719 showed decreases in
confluence in the NB cell lines, with SK-N-AS and SK-N-DZ
exhibiting the least difference (Fig.
4B, E, H, K, N).
PD-0332991 + BYL719 (both 1–10 µM) decreased
confluence for all NB lines, except SK-N-DZ and SK-N-FI at the
lowest dose combinations (Fig. 4C, F,
I, L, O).
Low doses (0.5 and 1.0 µM) of MK-1775 alone
decreased cell confluence in all NB cell lines 72 h after treatment
compared with PBS control (Fig. 5A, D,
G, J, M).
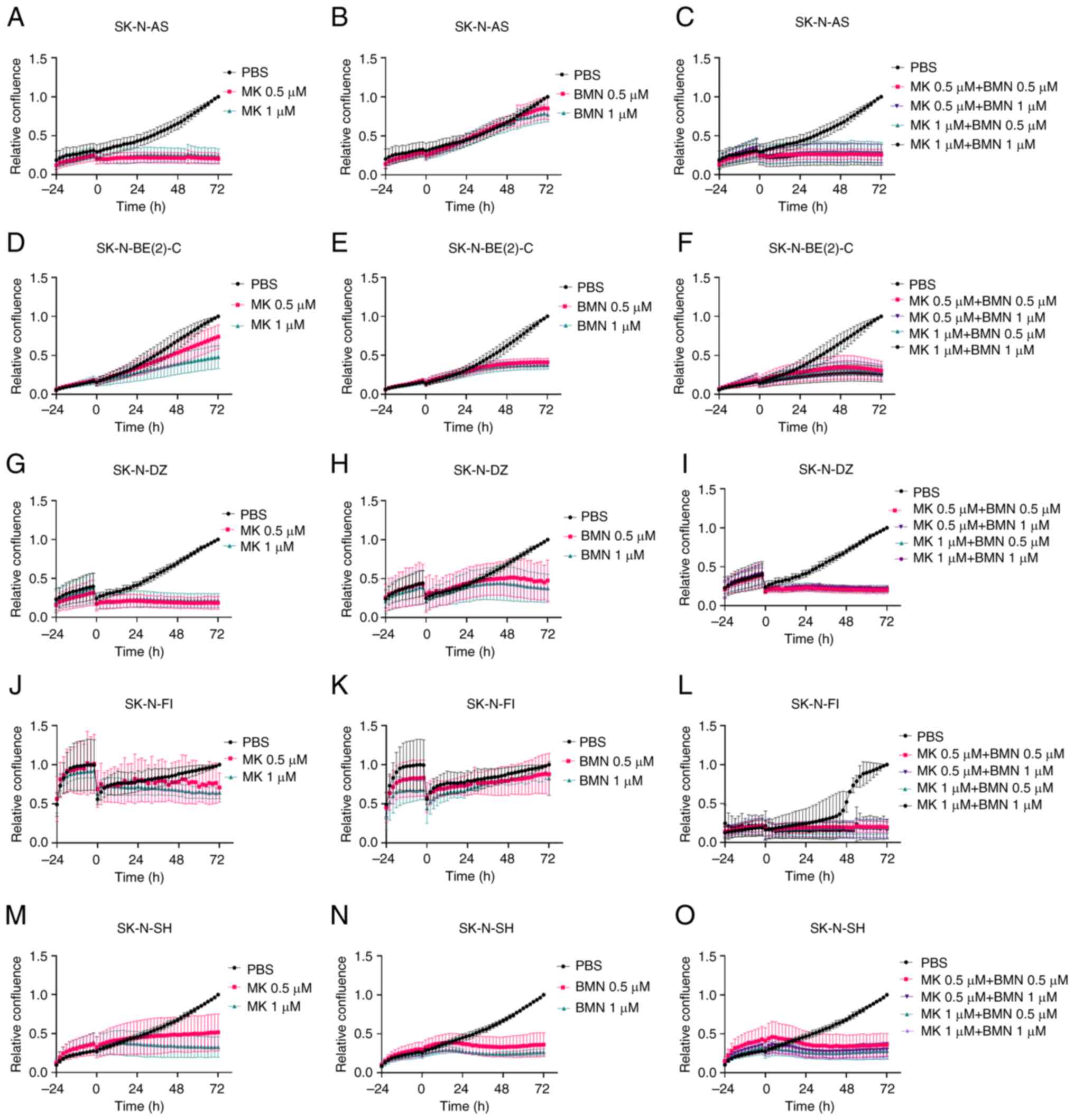 | Figure 5.Confluence of neuroblastoma cell
lines following administration of MK and BMN. Confluence of SK-N-AS
treated with (A) MK (A), BMN (B) and combination of MK and BMN (C),
confluence of SK-N-BE(2)-C treated with (D) MK, BMN (E) and
combination of MK and BMN (F), confluence of SK-N-DZ treated with
MK (G), BMN (H) and combination of MK and BMN (I), confluence of
SK-N-FI treated with MK (J), BMN (K) and combination of MK and BMN
(L) and confluence of SK-N-SH treated with MK (M), BMN (N) and
combination of MK and BMN (O) was measured every 2 h up to 72 h
after the treatment. Confluence denotes proliferation. BMN, BMN673;
MK, MK-1775; PARP, poly-ADP-ribose-polymerase. |
Low doses (0.5 and 1.0 µM) of BMN673 tended to
decrease confluence, especially in SK-N-BE(2)-C and SK-N-SH
compared with PBS control (Fig. 5B, E,
H, K, N).
Combining 0.5 and 1.0 µM MK-1775 + BMN673 decreased
confluence for all NB cell lines compared with PBS control
(Fig. 5C, F, I, L, O).
To summarize, combining PD-0332991 + BYL719 or
MK-1775 + BMN673 tended to decrease cell confluence at lower doses
compared with monotherapy.
Cytotoxic effects of single and
combined treatments of PD-0332991, BYL719, BMN673 and MK-1775
The cytotoxic effects of the drugs on SK-N-AS,
SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH were analyzed utilizing
the Cytotox Red Reagent and IncuCyte S3 Live-Cell Analysis System
(Figs. S1 and S2).
Marked cytotoxicity was not observed in the NB lines
after exposure to 1–10 µM PD-0332991 or BYL719, however, treatment
with 10 µM PD-0332991 induced slight cytotoxic effects in SK-N-FI
and SK-N-SH cells (Fig. S1A, B, D, E,
G, H, J, K, M, N).
When combining 10 µM PD-0332991 + BYL719,
cytotoxicity was observed in almost all NB cell lines. Moreover,
cytotoxicity was observed for SK-N-FI with all combinations of
1.0–10.0 µM PD-0332991 + BYL719 (Fig.
S1C, F, I, L, O).
Cytotoxic effects were observed with 0.5–1.0 µM
MK-1775 in SK-N-AS, S-K-N-FI and SK-N-SH, but not SK-N-BE(2)-C and
SK-N-DZ cells (Fig. S2A, D, G, J,
M).
Cytotoxicity was observed with 0.5–1.0 µM BMN673 in
SK-N-SH but not any of the other cell lines (Fig. S2B, E, H, K, N).
Combined 0.5–1.0 µM MK-1775 + BMN673 tended to
provide similar cytotoxic effects on the NB lines as 0.5–1.0 µM
MK-1775 alone with moderate effects on SK-N-AS, S-K-N-FI and
SK-N-SH (Fig. S2C, F, I, L,
O).
To summarize, marked cytotoxic effects were shown
only when using high doses of PD-0332991 or MK-1775. Moreover,
although combining PD-0332991 with BYL719 gave additive positive
effects while combining MK-1775 + BMN673 did not increase the
cytotoxic effects compared with MK-1775 alone.
Apoptotic effects on SK-N-AS,
SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and SK-N-SH after single and
combined treatments of PD-0332991, BYL719, BMN673 and MK-1775
Apoptotic effects on SK-N-AS, SK-N-BE(2)-C, SK-N-DZ,
SK-N-FI and SK-N-SH was analyzed using the Caspase-3/7 Green
Reagent in the IncuCyte S3 Live-Cell Analysis System (Figs. S3 and S4). Slight apoptosis was observed only
with 10 µM PD-0332991 in SK-N-DZ and SK-N-SH (Fig. S3A, D, G, J, M).
Marked apoptosis was not observed with 1–10 µM
BYL719 in any of the NB lines (Fig.
S3B, E, H, K, N).
When combining 1–10 µM PD-0332991 + BYL719,
apoptosis was observed in SK-N-DZ and 10 µM PD-0332991 + BYL719
caused apoptosis in SK-N-FI and SK-N-BE(2)-C cells (Fig. S3C, F, I, L, O).
Apoptosis was observed in all NB lines at the
highest dose (1 µM) of MK-1775 (Fig. A, D, G, J, M). Apoptosis was
also observed with 0.5–1.0 µM BMN673 in SK-N-BE(2)-C and SK-N-SH
(Fig. S4B, E, H, K, N).
Combining 0.5–1.0 µM MK-1775 + BMN673 did not
enhance the apoptotic effects compared with 0.5–1.0 µM MK-1775 or
BMN673 alone (Fig. S4C, F, I, L,
O).
To summarize, MK-1775 induced apoptosis in almost
all NB lines, as did BMN673 in SK-N-BE(2)-C and SK-N-SH cells.
PD-0332991 induced apoptosis only at high doses, whereas BYL719 did
not induce apoptotic effects. Moreover, combining MK-1775 + BMN673
did not increase the apoptotic effects compared with MK-1775 or
BMN673 alone. However, PD-0332991 + BYL719 increased apoptosis in
SK-N-BE(2)-C, SK-N-DZ and SK-N-FI cells.
Cell cycle distribution after single
and combined treatments with CDK4/6 and PI3K inhibitors PD-0332991
and BYL719 on SK-N-DZ and SK-N-SH and PARP and WEE1 inhibitors
MK-1775 and BMN673 on SK-N-BE(2)-C and SK-N-AS
Effects on cell cycle distribution were examined in
selected samples using FACS Novocyte machine and FlowJo software.
Effects of single and combined PD-0332991 and BYL719 were analyzed
for one sensitive and one resistant cell line, SK-N-DZ and SK-N-SH,
respectively. Likewise, effects of single and combined MK-1775 and
BMN673 were analyzed for one sensitive and one resistant cell line,
SK-N-BE(2)-C and SK-N-AS, respectively.
Using either 5 or 10 µM PD-0332991 increased the
number of cells in G1 and decreased the numbers of cells in S phase
for SK-N-DZ and SK-N-SH, while 5 and 10 µM BYL719 had no effect on
the cell cycle of these NB lines (Fig.
6A and B). In addition, combining PD-0332991 + BYL719 did not
result in any notable changes in the cell cycle distribution
compared with PD-0332991 alone.
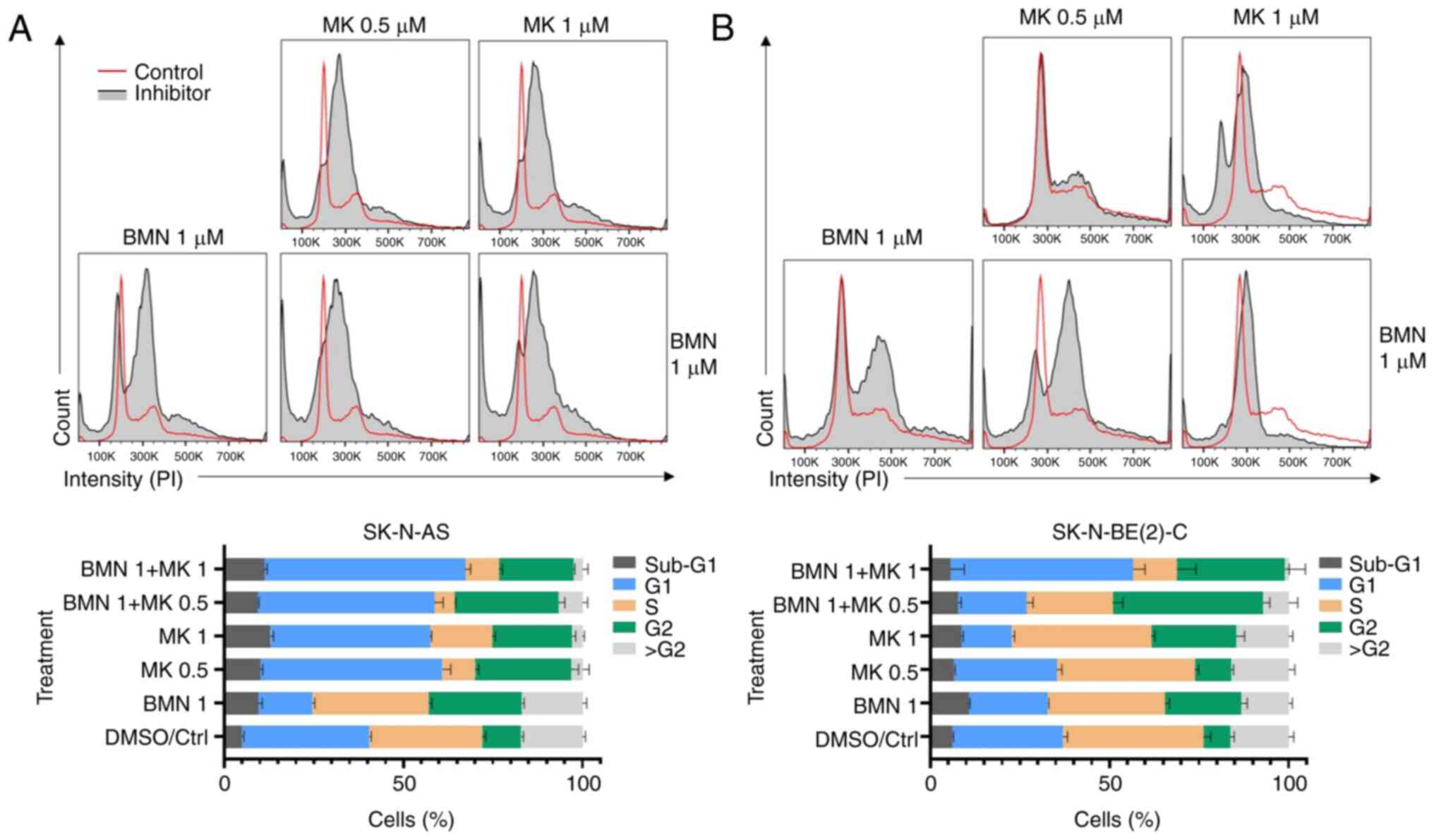
Treatment with 0.5 and 1 µM MK-1775 increased the
number of cells in G1 and G2 and decreased the numbers of cells in
S phase for SK-N-AS (Fig. 7A and
B). For SK-N-BE(2)-C, 1 µM MK-1775 notably decreased number of
cells in G1 and increased number of cells in G2. In addition, 1 µM
BMN673 alone decreased the number of cells in G1 and increased the
number of cells in G2 in both cell lines. Combining 1 µM MK-1775 +
BMN673 induced a notable increase in the number of cells in both G1
and G2 and a notable decrease in number of cells in S phase in both
cell lines.
To summarize, upon combining MK-1775 with BMN673
additive effects were observed for both SK-N-BE(2)-C and SK-N-AS
cells, with large increases in the number of cells in G1 and G2 and
a notable decrease in the numbers of cells in S phase. By contrast,
following treatment with PD-0332991 + BYL719, the effect was
similar to using PD-0332991 alone with a rise in the number of
cells in G1 and a decrease in the number of cells in S phase.
Discussion
Here, the effects of CDK4/6, PI3K, WEE1 and PARP
inhibitors PD-0332991, BYL719, MK-1775 and BMN673 alone or combined
on NB cell lines SK-N-AS, SK-N-BE(2)-C, SK-N-DZ, SK-N-FI and
SK-N-SH were assessed. Briefly, all single inhibitor applications
induced dose-dependent decreases in viability and confluence in all
NB lines and combining PD-0332991 + BYL719 or BMN673 + MK-1775
resulted in additive and synergistic effects in most NB lines. A
slight cytotoxic effect was induced by high doses of PD-0332991 or
MK-1775 and apoptosis was induced in most NB cell lines by MK-1775;
apoptosis induced by PD-0332991, BYL719 and BMN673 was cell
line-specific. In addition, effects on the cell cycle were examined
in SK-N-DZ and SK-N-SH after single and combined PD-0332991 and
BYL719 applications and SK-N-BE(2)-C and SK-N-AS after single and
combined MK-1775 and BMN673 treatment. Notably, while combining
PD-0332991 + BYL719 did not result in any notable effects on cell
cycle, combining MK-1775 + BMN673 showed a notable decrease in the
number of cells in S phase for both cell lines.
As expected, single inhibitor applications induced
dose-dependent responses (12,13).
Similarly, when combining CDK4/6 inhibitor PD-0332991 + PI3K
inhibitor BYL719, positive and synergistic effects on viability and
confluence were observed in almost all NB lines, consistent with
pre-clinical models using other types of tumor (28,29,40,41).
However, SK-N-SH cell line showed mainly antagonistic, despite
decreases in viability and cell confluence. This may be because
SK-N-SH cell line was sensitive and lacked mutated p53 and it was
difficult to observe further decreases in viability upon
combination treatment, especially with doses that induced effects
in more resistant cell lines. Our previous study showed that when
combining PI3K and FGFR inhibitors in NB, MB and head and neck
cancer cell lines, effects in cell lines that were particularly
sensitive to one of the drugs were not observed (10–13,40,42).
It is unknown why combining PD-0332991 + BYL719 resulted in
enhanced inhibition in SK-N-AS, SK-N-BE(2)-C, SK-N-DZ and SK-N-FI,
however all four cell lines have mutated p53. Moreover,
SK-N-BE(2)-C and SK-N-DZ have amplified MYCN. On the other hand,
SK-N-SH with wild-type p53 was also sensitive to treatments with
both PD-0332991 and BYL719 alone.
WEE1 inhibitor MK-1775 + PARP inhibitor BMN673
induced some positive and synergistic effects on viability and
confluence in SK-N-BE(2)-C, SK-N-DZ, SK-N-FI, SK-N-SH cells,
similar to what has been shown for advanced prostate and ovarian
cancer (36,37). SK-N-AS and SK-N-DZ cells exhibited
antagonistic effects; this could be because they were both
sensitive to MK-1775 but resistant to BMN673.
Cytotoxicity and apoptosis were assessed. For
PD-0332991, only high dose treatment induced cytotoxicity and
apoptosis in NB cell lines, while for BYL719 cytotoxic effects were
not observed and combining the two did not markedly improve
cytotoxic or apoptotic effects compared with PD-0332991 alone.
However, when combining PD-0332991 + BYL719 in SK-N-BE(2)-C,
SK-N-DZ and SK-N-FI cells, enhanced apoptosis was observed. These
data are similar to our previous study on MB cell lines DAOY and
UW228-3, where PD-0332991 exhibited a greater effect on both
cytotoxicity and apoptosis (13).
Similarly, while MK-1775 induced both cytotoxicity
and apoptosis in some NB cell lines, BMN673 did not show cytotoxic
and apoptotic effects and when combining them their effects were
not enhanced. MK-1775 induced cytotoxic effects in SK-N-AS, SK-N-FI
and SK-N-SH, but these effects were not enhanced by the addition of
BMN673 in any cell lines. Likewise, when analyzing apoptosis, no
additive effect was observed upon combining MK-1775 + BMN673
compared with single MK-1775 treatment. To conclude, there were no
additive effects on either cytotoxicity or apoptosis when combining
MK-1775 + BMN673.
PD-0332991 increased the proportion of cells in G1
and limited the proportion of cells in S phase for SK-N-DZ and
SK-N-SH, while using BYL719 alone had no effect on the cell cycle
distribution of either NB line. MK-1775 increased the proportion of
cells in G1 and G2 and decreased the proportion of cells in S phase
for SK-N-AS, effects on SK-N-BE(2)-C were less pronounced. All the
effects of using BMN673 alone were limited in comparison to
MK-1775, although an increase in numbers of cells in G2 was
observed following treatment with BMN673 in both SK-N-AS and
SK-N-BE(2)-C.
The observation that both PD-0332991 and MK-1775
increased the proportion of cells in G1 and limited the number of
cells in S phase and that MK-1775 can have effects on the cell
cycle and mitosis by increasing the number of cells arresting in G2
is in line with previous data (43,44).
Likewise, BMN673 has also been shown to induce cell cycle arrest
(42).
Notably, combining MK-1775 + BMN673 in SK-N-BE(2)-C
and SK-N-AS cells resulted in a marked decrease in the number of
cells in the S phase; higher doses resulted in a marked increase in
the proportion of cells in G1 and G2. This may be due to the
effects of BMN673, as previously described (45).
The present study had limitations. Lower doses in
combination were not used when the NB lines were sensitive to one
drug alone. However, if a cell line is sensitive to low doses of
one inhibitor, combination therapies may not be needed.
Furthermore, the present study did not investigate changes in
pathways in relation to the molecular profile of each cell line.
Another limitation of the present study was that all treatments
were performed in vitro; studies on cells grown as spheroids
and treatment in in vivo in mouse models will be performed
in future.
To summarize, PD-0332991, BYL719, MK-1775 and BMN673
gave dose dependent effects on viability the NB cell lines. Notably
however, using combinations of PD-0332991 with BYL719 and
combinations of MK-1775 and BMN763 gave additive and synergistic
effects on the inhibition of viability compared with each drug
alone. Inhibition of cell confluence tended to be more prominent
when combining two drugs especially for some of the cell lines
which tended to be relatively resistant to one drug alone. Enhanced
cytotoxicity or apoptosis was not observed when combining
PD-0332991 + BYL719 or MK-1775 + BMN673. BYL719 did not affect
PD-0332991-induced changes in the cell cycle distribution. Notably,
MK-1775 + BMN673 resulted in a notable increase of cells in G1 and
G2 and a marked decrease of cells in S phase.
Altogether, the aforementioned data indicated that
NB, similar to other tumor types, may be sensitive to single and
combined treatment with CDK4/6, PI3K, PARP and WEE1 inhibitors
(40,41). It is unknown why some NB cell lines
were more sensitive to some combinations.
To conclude, combined treatment with either CDK4/6
and PI3K inhibitor PD-0332991 + BYL719 or WEE1 and PARP inhibitors
MK-1775 + BMN673 synergistically decreased viability and cell
confluence in most NB cell lines. These combinations did not have
any marked effects on cytotoxicity or apoptosis. MK-1775 + BMN673
resulted in marked effects on the cell cycle distribution compared
with PD-0332991 + BYL719 combination. The present data suggest that
in the future, combinations of MK-1775 + BMN673 or PD-0332991 and
BYL719 could serve as a treatment of resilient or recurrent NB.
Further studies utilizing NB cell lines with specific molecular
characteristics grown as spheroid cultures or in vivo models
are required to determine the best drug combination for each cell
line based on its molecular profile.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Per
Kogner, Karolinska Institute (Stockholm, Sweden) for kindly
providing neuroblastoma cell lines.
Funding
The present study was supported by Swedish Childhood Cancer
Foundation (grant no. TJ2022-0067), Swedish Cancer Foundation
(grant no. 20 0704), the Stockholm Cancer Society (grant no.
201092), Stockholm City Council (grant no. 20180037), AnnaBrita o
Bo Casters Minne Foundation (Lindhés Advokatbyrå) (grant no.
LA2022-0070), Svenska Läkaresällskapet (grant no. SLS-934161), Åke
Wiberg Foundation (grant no. M21-0012), Karolinska Institutet
Sweden (grant no. 2022-01587), Tornspiran Foundation (grant no.
839), Mary Beves Foundation and Magnus Bergvalls Foundation (grant
no. 2022-109).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and ONK performed experiments, interpreted the
data and wrote the manuscript. SH and EP performed experiments. SH
and ML performed experiments, analyzed data and wrote the
manuscript. TM analyzed FACS data and constructed figures. TD and
ONK conceived and designed the study, analyzed and interpreted data
and wrote and revised the manuscript. ML and ONK confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ward E, DeSantis C, Robbins A, Kohler B
and Jemal A: Childhood and adolescent cancer statistics, 2014. CA
Cancer J Clin. 64:83–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Angio GJ, Evans AE and Koop CE: Special
pattern of widespread neuroblastoma with a favourable prognosis.
Lancet. 1:1046–1049. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakagawara A, Li Y, Izumi H, Muramori K,
Inada H and Nishi M: Neuroblastoma. Jpn J Clin Oncol. 1:214–241.
2018. View Article : Google Scholar
|
|
4
|
Bo Q and Matthay KK: Advancing therapy for
neuroblastoma. Nat Rev Clin Oncol. 19:515–533. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hero B, Simon T, Spitz R, Ernestus K,
Gnekow AK, Scheel-Walter HG, Schwabe D, Schilling FH, Benz-Bohm G
and Berthold F: Localized infant neuroblastomas often show
spontaneous regression: Results of the prospective trials NB95-S
and NB97. J Clin Oncol. 26:1504–1510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matthay KK, Maris JM, Schleiermacher G,
Nakagawara A, Mackall CL, Diller L and Weiss WA: Neuroblastoma. Nat
Rev Dis Prim. 2:160782016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
London WB, Bagatell R, Weigel BJ, Fox E,
Guo D, Van Ryn C, Naranjo A and Park JR: Historical time to disease
progression and progression-free survival in patients with
recurrent/refractory neuroblastoma treated in the modern era on
children's oncology group early-phase trials. Cancer.
123:4914–4923. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnsen JI, Dyberg C and Wickström M:
Neuroblastoma-A neural crest derived embryonal malignancy. Front
Mol Neurosci. 12:92019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hemmings BA and Restuccia DF: The
PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol.
7:a0266092015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kostopoulou ON, Holzhauser S, Lange BKA,
Ohmayer A, Andonova T, Bersani C, Wickström M and Dalianis T:
Analyses of FGFR3 and PIK3CA mutations in neuroblastomas and the
effects of the corresponding inhibitors on neuroblastoma cell
lines. Int J Oncol. 55:1372–1384. 2019.PubMed/NCBI
|
|
11
|
Holzhauser S, Lukoseviciute M, Andonova T,
Ursu RG, Dalianis T, Wickström M and Kostopoulou ON: Targeting
fibroblast growth factor receptor (FGFR) and Phosphoinositide
3-kinase (PI3K) signaling pathways in medulloblastoma cell lines.
Anticancer Res. 40:53–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holzhauser S, Lukoseviciute M,
Papachristofi C, Vasilopoulou C, Herold N, Wickström M, Kostopoulou
ON and Dalianis T: Effects of PI3K and FGFR inhibitors alone and in
combination, and with/without cytostatics in childhood
neuroblastoma cell lines. Int J Oncol. 58:211–225. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lukoseviciute M, Maier H,
Poulou-Sidiropoulou E, Rosendahl E, Holzhauser S, Dalianis T and
Kostopoulou ON: Targeting PI3K, FGFR, CDK4/6 signaling pathways
together with cytostatics and radiotherapy in two medulloblastoma
cell lines. Front Oncol. 11:7486572021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katso R, Okkenhaug K, Ahmadi K, White S,
Timms J and Waterfield MD: Cellular function of phosphoinositide
3-kinases: Implications for development, homeostasis, and cancer.
Annu Rev Cell Dev Biol. 17:615–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
King D, Yeomanson D and Bryant HE: PI3King
the lock: Targeting the PI3K/Akt/mTOR pathway as a novel
therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol.
37:245–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khezri MR, Jafari R, Yousefi K and
Zolbanin NM: The PI3K/AKT signaling pathway in cancer: Molecular
mechanisms and possible therapeutic interventions. Exp Mol Pathol.
127:1047872022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zage PE: Novel therapies for relapsed and
refractory neuroblastoma. Children (Basel). 5:1482018.PubMed/NCBI
|
|
19
|
Creevey L, Ryan J, Harvey H, Bray IM,
Meehan M, Khan AR and Stallings RL: MicroRNA-497 increases
apoptosis in MYCN amplified neuroblastoma cells by targeting the
key cell cycle regulator WEE1. Mol Cancer. 12:232013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rader J, Russell MR, Hart LS, Nakazawa MS,
Belcastro LT, Martinez D, Li Y, Carpenter EL, Attiyeh EF, Diskin
SJ, et al: Dual CDK4/CDK6 inhibition induces cell-cycle arrest and
senescence in neuroblastoma. Clin Cancer Res. 19:6173–6182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knudsen ES, Pruitt SC, Hershberger PA,
Witkiewicz AK and Goodrich DW: Cell cycle and beyond: Exploiting
new RB1 controlled mechanisms for cancer therapy. Trends Cancer.
5:308–324. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morgan DO: Cyclin-dependent kinases:
Engines, clocks, and microprocessors. Annu Rev Cell Dev Biol.
13:261–291. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sherr CJ and Roberts JM: Living with or
without cyclins and cyclin-dependent kinases. Genes Dev.
18:2699–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamilton E and Infante JR: Targeting
CDK4/6 in patients with cancer. Cancer Treat Rev. 45:129–138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ortega S, Malumbres M and Barbacid M:
Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim
Biophys Acta. 1602:73–87. 2022.PubMed/NCBI
|
|
26
|
Braal CL, Jongbloed EM, Wilting SM,
Mathijssen RHJ, Koolen SLW and Jager A: Inhibiting CDK4/6 in breast
cancer with palbociclib, ribociclib, and abemaciclib: Similarities
and differences. Drugs. 81:317–331. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmidt EE, Ichimura K, Reifenberger G and
Collins VP: CDKN2 (p16/MTS1) gene deletion or CDK4 amplification
occurs in the majority of glioblastomas. Cancer Res. 15:6321–6324.
1994.PubMed/NCBI
|
|
28
|
Muranen T, Meric-Bernstam F and Mills GB:
Promising rationally derived combination therapy with PI3K and
CDK4/6 inhibitors. Cancer Cell. 26:7–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonelli MA, Digiacomo G, Fumarola C,
Alfieri R, Quaini F, Falco A, Madeddu D, Monica SL, Cretella D,
Ravelli A, et al: Combined inhibition of CDK4/6 and PI3K/AKT/mTOR
pathways induces a synergistic anti-tumor effect in malignant
pleural mesothelioma cells. Neoplasia. 19:637–648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Loibl S and Furlanetto J: Integrating
CDK4/6 inhibitors in the treatment of patients with early breast
cancer. Breast. 62:S70–S79. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vora SR, Juric D, Kim N, Mino-Kenudson M,
Huynh T, Costa C, Lockerman EL, Pollack SF, Liu M, Li X, et al: CDK
4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K
inhibitors. Cancer Cell. 26:136–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paik J: Olaparib: A review as first-line
maintenance therapy in advanced ovarian cancer. Target Oncol.
16:847–856. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brown TJ and Reiss KA: PARP inhibitors in
pancreatic cancer. Cancer J. 27:465–475. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Bono J, Mateo J, Fizazi K, Saad F,
Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, et al:
Olaparib for metastatic castration-resistant prostate cancer. N
Engl J Med. 382:2091–2102. 2022. View Article : Google Scholar
|
|
35
|
Banerjee S, Moore KN, Colombo N, Scambia
G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary
A, et al: Maintenance olaparib for patients with newly diagnosed
advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004):
5-year follow-up of a randomised, double-blind, placebo-controlled,
phase 3 trial. Lancet Oncol. 22:1721–1731. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fang Y, McGrail DJ, Sun C, Labrie M, Chen
X, Zhang D, Ju Z, Vellano CP, Lu Y, LI Y, et al: Sequential therapy
with PARP and WEE1 inhibitors minimizes toxicity while maintaining
efficacy. Cancer Cell. 35:851–867.e857. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
di Rorà AG, Cerchione C, Martinelli G and
Simonetti G: A WEE1 family business: Regulation of mitosis, cancer
progression, and therapeutic target. J Hematol Oncol. 13:1262020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Foucquier J and Guedj M: Analysis of drug
combinations: Current methodological landscape. Pharmacol Res
Perspect. 3:e001492015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kostopoulou ON, Zupancic M, Pont M, Papin
E, Lukoseviciute M, Mikelarena BA, Holzhauser S and Dalianis T:
Targeted therapy of HPV positive and negative tonsillar squamous
cell carcinoma cell lines reveals synergy between CDK4/6, PI3K and
sometimes FGFR inhibitors, but rarely between PARP and WEE1
inhibitors. Viruses. 14:13722022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Agostinetto E, Debien V, Marta GN,
Lambertini M, Piccart-Gebhart M and de Azambuja E: CDK4/6 and PI3K
inhibitors: A new promise for patients with HER2-positive breast
cancer. Eur J Clin Invest. 51:e135352021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Holzhauser S, Wild N, Zupancic M, Ursu RG,
Bersani C, Näsman A, Kostopoulou Ourania N and Dalianis T: Targeted
therapy with PI3K and FGFR inhibitors on human papillomavirus
positive and negative tonsillar and base of tongue cancer lines
with and without corresponding mutations. Front Oncol.
11:6404902021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rihani A, Vandesompele J, Speleman F and
Van Maerken T: Inhibition of CDK4/6 as a novel therapeutic option
for neuroblastoma. Cancer Cell Int. 15:762015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fry DW, Harvey PJ, Keller PR, Elliott WL,
Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK and
Toogood PL: Specific inhibition of cyclin-dependent kinase 4/6 by
PD 0332991 and associated antitumor activity in human tumor
xenografts. Mol Cancer Ther. 3:1427–1438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Challa S and Kraus WL: Two birds, one
stone: Non-canonical therapeutic effects of the PARP inhibitor
Talazoparib. Cell Chem Biol. 29:171–173. 2022. View Article : Google Scholar : PubMed/NCBI
|