Cancer is a complex disease involving multiple
aspects at the genetic, molecular and cellular levels, which is
characterized by unlimited cell proliferation, invasion and
metastasis (1–3). With global population aging and
lifestyle changes, the incidence and mortality rates of cancer are
continuously rising, posing significant challenges to the global
public health system (4–7). The complexity of cancer treatment lies
in its high heterogeneity and adaptability to therapies, and even
the most advanced treatments face issues of resistance and relapse
(8–10). Therefore, understanding and studying
the fundamental biological characteristics and pathogenesis of
cancer is crucial for developing new therapeutic strategies.
mRNA is a single-stranded ribonucleic acid
transcribed from DNA. Its function is to transfer genetic
information from DNA in the nucleus to ribosomes in the cytoplasm,
directing the synthesis of proteins to execute various functions.
This process is influenced by numerous factors, and the stability
of mRNA can ultimately affect its protein expression levels,
thereby impacting its functional performance (11). The stability of mRNA is related to
the integrity of its structure, which includes the 5′ cap, the 5′
untranslated region (UTR), the open reading frame, the 3′ UTR and
the polyadenylated tail [Poly(A) tail] at the 3′ end (12,13).
The 5′ cap and Poly(A) tail are crucial for maintaining mRNA
stability. When the 5′ cap and Poly(A) tail are present, mRNA is
protected from degradation by exonucleases and can continue to be
translated into proteins. However, when they are removed, mRNA is
degraded by exonucleases, leading to a reduction in the synthesis
of its encoded protein products (14,15).
Some proteins bind to the 5′ UTR or 3′ UTR of mRNA and can maintain
or remove the 5′ cap and Poly(A) tail, thereby affecting whether
the mRNA is degraded; this is a key mechanism for regulating mRNA
stability.
RNA-binding proteins (RBPs) are important regulatory
molecules within cells that bind to RNA to regulate its splicing,
transport, translation and degradation, thereby maintaining normal
cellular physiological functions (16–18).
Studies have found that RBPs can influence mRNA stability through
post-transcriptional regulation, playing a regulatory role in the
occurrence, development and therapeutic resistance of malignant
tumors (19,20). In addition to binding to mRNA, RBPs
can bind to long non-coding RNA (lncRNA). lncRNAs are RNA molecules
longer than 200 nucleotides that do not encode proteins; however,
they maintain normal cellular functions and homeostasis by
regulating gene expression, participating in chromatin remodeling,
and modulating RNA splicing and translation (21–23).
Abnormal expression of lncRNAs and RBPs can significantly affect
the biological behavior of cancer (24,25).
Studies have found that lncRNAs regulate mRNA stability through
interactions with RBPs, which is an important mechanism in cancer
progression and drug resistance (26–28).
For the present review, a systematic literature
search was conducted using the PubMed platform (https://pubmed.ncbi.nlm.nih.gov/) with key words
including ‘lncRNA’, ‘RBP’, ‘mRNA’, ‘RNA stability’, ‘RNA
degradation’ and ‘cancer’. Subsequently, articles on how lncRNAs
regulate mRNA stability in cancer through interactions with RBPs
were screened and analyzed. The present study focused on reviewing
the impact of lncRNA-RBP interactions on mRNA stability in cancer,
and their roles in cancer occurrence, development and drug
resistance, exploring the potential of targeting this mechanism as
a new therapeutic strategy for cancer.
Molecular scaffolds refer to RNA, proteins or other
macromolecules that, during normal cellular physiological processes
and signal transduction, provide binding sites and interact with
multiple molecules. This promotes interactions among these
molecules, ensuring appropriate transmission of signals by kinases,
receptors and other signaling molecules, thus fulfilling biological
functions (29–31). Molecular scaffolds not only speed up
the transmission and efficiency of signals but also ensure that
signals are accurately transmitted to specific cellular locations,
finely regulating cell proliferation, differentiation, metabolism
and other important functions (32,33).
In cancer, some lncRNAs also act as molecular scaffolds, promoting
the binding of RBPs to mRNA, enhancing or weakening mRNA stability,
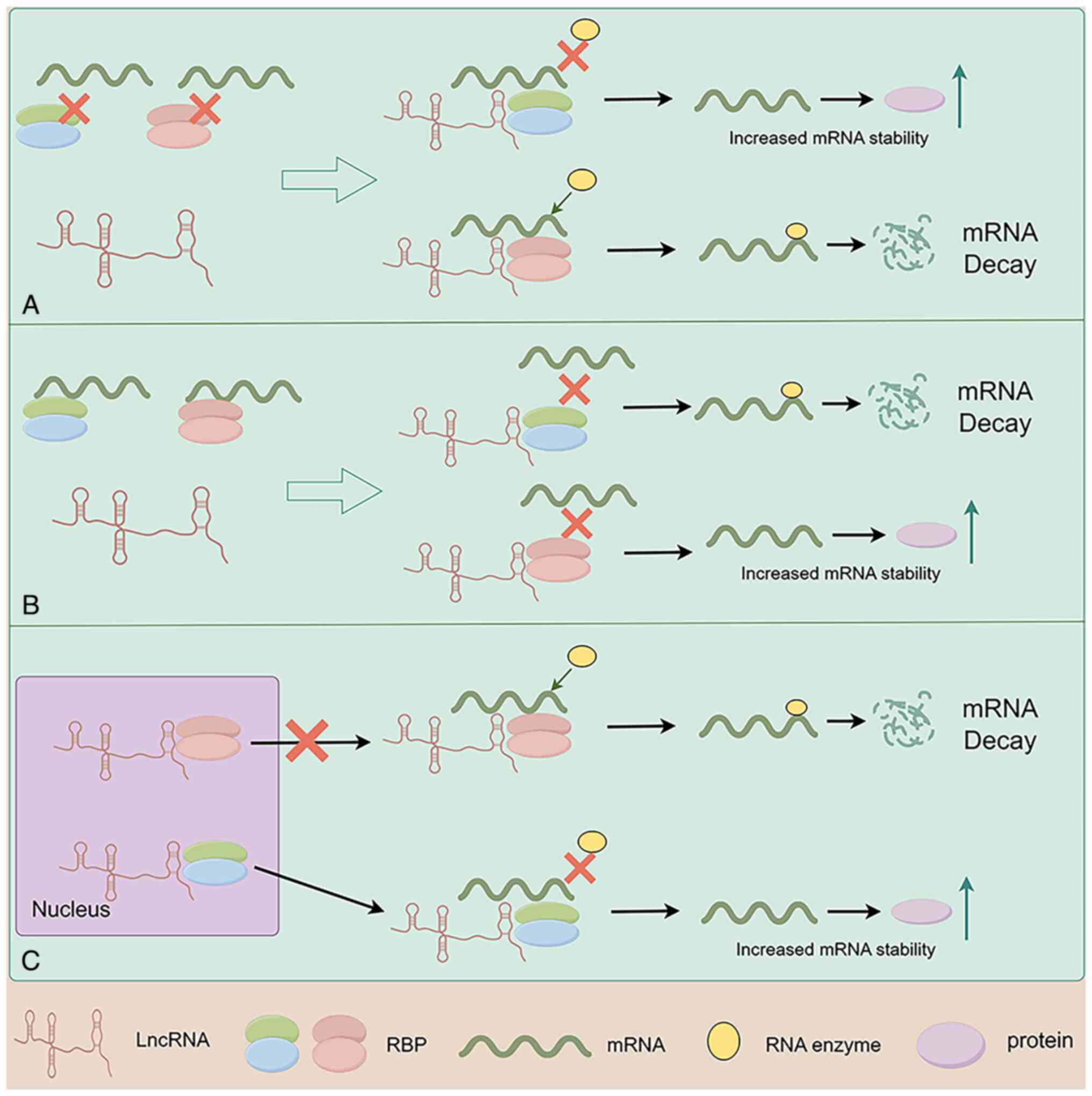
and regulating the synthesis of related proteins (Fig. 1A); this ultimately affects the
malignant biological behavior of tumors and therapeutic resistance
(34–37).
lncRNA acts as a molecular scaffold to promote the
binding of RBPs to the 5′ UTR or 3′ UTR of mRNA, further regulating
mRNA stability (38–40). The regulatory effect depends on the
function of the RBP. When the RBP stabilizes the 5′ cap or Poly(A)
tail of mRNA, it can prevent mRNA from being degraded by
exonucleases, enhancing mRNA stability, increasing the synthesis of
its encoded protein and thus its corresponding biological
functions.
As a molecular scaffold, lncRNA promotes the binding
of RBPs to the mRNA of oncogenes or tumor suppressor genes,
enhancing their stability and exerting oncogenic or
tumor-suppressing effects. Wang et al (41) reported that the lncRNA EGFR-AS1 was
highly expressed in renal cancer. After EGFR-AS1 binds with HuR, it
promotes the binding of human antigen R (HuR) to the ARE sequence
in the 3′ UTR of EGFR mRNA, forming a stable complex that maintains
the stability of the Poly(A) tail at the 3′ end of EGFR mRNA,
preventing its degradation by exonucleases, thereby increasing its
stability. This, in turn, increases EGFR protein synthesis,
promoting renal cancer cell proliferation (41). In a study by Wu et al
(42), the lncRNA small nucleolar
RNA host gene 12 was shown to recruit insulin-like growth factor 2
mRNA binding protein 2 (IGF2BP2) and promote its binding to the 3′
UTR of catenin beta 1 (CTNNB1) mRNA, forming a complex that
prevents CTNNB1 mRNA degradation and increases CTNNB1 protein
synthesis. The CTNNB1 protein further activates the Wnt/β-catenin
signaling pathway, promoting the invasion and metastasis of
esophageal cancer (42). Chen et
al (43) demonstrated that
LINC00659 was lowly expressed in hepatocellular carcinoma (HCC),
and its overexpression significantly inhibited HCC proliferation
and metastasis. Mechanistic studies have indicated that LINC00659
acts as a molecular scaffold to promote the binding of FUS to
SLC10A1 mRNA, preventing its degradation and increasing SLC10A1
protein synthesis. The SLC10A1 protein can block aerobic glycolysis
in liver cancer cells, thereby significantly inhibiting HCC
proliferation and metastasis (43).
Similar mechanisms have also been observed in numerous other types
of cancer (Table I) (44–55).
The regulation of mRNA stability by lncRNA as a
molecular scaffold promoting RBP binding to mRNA not only
influences the malignant biological behavior of cancer, but also
plays a role in regulating cancer treatment resistance (56). Ferroptosis is closely related to
cancer drug resistance. Unlike classical forms of cell death, such
as apoptosis, necrosis or autophagy, ferroptosis is a form of cell
death induced by iron-catalyzed lipid peroxidation, primarily
involving the peroxidative damage of polyunsaturated fatty acids in
cell membranes. Excess iron ions promote the generation of reactive
oxygen species (ROS), disrupt cell membrane integrity, lead to
cellular dysfunction and ultimately trigger cell death (57–59).
Studies have shown that cancer cells develop drug resistance by
evading ferroptosis (60–62), while inducing ferroptosis in cancer
cells can reverse their drug resistance (63,64).
Temozolomide (TMZ) is a primary drug for the treatment of glioma;
however, drug resistance is a major challenge in its treatment. Luo
et al (65) found that the
lncRNA ataxin-8 opposite strand (ATXN8OS) was lowly expressed in
glioma, and overexpression of ATXN8OS could inhibit glioma
resistance to TMZ. Mechanistic studies indicated that ATXN8OS
recruits adenosine deaminase acting on RNA and promotes its binding
to glutaminase 2 (GLS2) mRNA, enhancing GLS2 mRNA stability and
increasing intracellular GLS2 protein synthesis. GLS2 increases
iron ion and ROS levels, promoting ferroptosis in glioma,
ultimately enhancing the sensitivity of glioma to TMZ (65).
When lncRNA functions as a molecular scaffold to
bind RBPs that remove the 5′ cap or Poly(A) tail of mRNA, it leads
to mRNA degradation, reducing its stability and the synthesis of
its encoded protein, thereby exerting oncogenic or
tumor-suppressive effects.
Staufen (STAU)-mediated mRNA decay (SMD) is a
post-transcriptional regulatory mechanism of gene expression, which
involves the role of STAU in recognizing and promoting the
degradation of specific mRNA molecules (66,67).
Lu et al (68) found that in
breast cancer, the lncRNA TINCR could act as a molecular scaffold
to bind STAU1, a member of the Staufen protein family, thus
promoting the degradation of oligoadenylate synthetase 1 (OAS1)
mRNA through the SMD pathway. Mechanistically, STAU1 binds to the
3′ UTR region of OAS1 mRNA and removes its Poly(A) tail at the 3′
end, ultimately leading to degradation by exonucleases and reducing
OAS1 mRNA stability. OAS1 is a tumor suppressor gene, and reduced
protein synthesis of OAS1 can promote breast cancer cell
proliferation and metastasis (68).
Similarly, in ovarian cancer, the lncRNA SPRY4-IT1 (69) has been revealed to promote mRNA
degradation through a similar mechanism, reducing the synthesis of
tumor suppressor gene-encoded proteins, thereby promoting tumor
proliferation and metastasis.
Additionally, as a molecular scaffold, lncRNA
promotes the binding of RBPs to mRNA encoding
ubiquitination-related proteins, regulating the synthesis of
ubiquitin ligases, and thus the ubiquitin-mediated degradation of
cancer-related proteins, which is another mechanism influencing the
malignant biological behavior of cancer. The BCR protein complex is
a major signaling molecule on the surface of B cells, which is
involved in regulating B-cell activation, proliferation and
differentiation. Aberrant activation of BCR signaling is a key
factor in the proliferation of diffuse large B-cell lymphoma
(DLBCL) cells (70,71). Polypyrimidine tract-binding protein
1 (PTBP1) is a multifunctional RBP that can promote the degradation
of certain mRNAs by recruiting degradation complexes, or can
prevent mRNA degradation by stabilizing its structure; the specific
function depends on the binding site and other regulatory factors
within the cell. Liu et al (72) identified that the lncRNA functional
intergenic repeating RNA element (FIRRE) was highly expressed in
DLBCL. After FIRRE binds to PTBP1, it promotes the binding of PTBP1
to the 3′ UTR of SMAD specific E3 ubiquitin protein ligase 2
(Smurf2) mRNA, possibly leading to Smurf2 mRNA degradation by
recruiting degradation complexes. Smurf2 is a ubiquitin ligase that
promotes the ubiquitin-mediated degradation of BCR proteins. After
Smurf2 mRNA is degraded, the synthesis of Smurf2 decreases,
reducing the ubiquitin-mediated degradation of BCR proteins. This
further maintains the abnormal activation of BCR signaling,
ultimately promoting DLBCL proliferation (72).
lncRNA acting as a molecular scaffold to promote the
binding of RBPs to mRNA is the most frequently reported mechanism
of lncRNA-RBP interactions regulating mRNA stability in cancer
research (Table I). In this
mechanism, lncRNAs exert oncogenic or tumor-suppressive effects, or
regulate chemotherapy sensitivity, by directly modulating the
stability of mRNAs that have cancer-promoting, cancer-suppressing
and chemotherapy sensitivity-influencing roles. On the other hand,
it can regulate the stability of ubiquitin ligase genes,
influencing the expression of tumor-suppressive or oncogenic genes,
thus controlling the malignant biological behavior of tumors.
Competitive binding refers to the phenomenon where
different molecules compete for the same binding site in biological
systems, playing a key role in various biological processes,
including enzyme activity regulation, receptor-ligand interactions,
transcription factor-DNA binding and RBP-RNA interactions (73,74).
In cancer, some lncRNAs bind to RBPs with different functions,
blocking the binding of RBPs to mRNA. This competitive action is
one of the mechanisms that regulates mRNA stability (Fig. 1B).
In cancer, after lncRNA binds to RBPs that promote
mRNA degradation, it blocks the binding of RBPs to mRNA, preventing
mRNA degradation, increasing mRNA stability and the synthesis of
its encoded protein, thus affecting the malignant biological
behavior of cancer. Zhou et al (75) found that the lncRNA
six-transmembrane epithelial antigen of prostate 3 antisense RNA 1
(STEAP3-AS1) was highly expressed in colorectal cancer (CRC), and
promoted CRC proliferation and metastasis. Mechanistically,
STEAP3-AS1 competitively binds to YTHDF2, blocking its binding to
STEAP3 mRNA, further inhibiting the YTHDF2-mediated degradation of
STEAP3 mRNA (75); this increases
STEAP3 mRNA stability, enhances STEAP3 protein synthesis, activates
the Wnt/β-catenin signaling pathway, and promotes CRC cell
proliferation, migration and invasion. In breast cancer studies,
Tao et al (76) demonstrated
that the lncRNA SCAMP1-TV2 could also promote tumor proliferation
and metastasis through a similar mechanism (76).
On the other hand, certain lncRNAs bind to RBPs that
prevent mRNA degradation, blocking the binding of RBPs to mRNA,
leading to mRNA degradation and reduced stability, playing an
important role in regulating the malignant biological behavior of
cancer. Heterogeneous nuclear ribonucleoprotein L (HNRNPL) is an
RBP belonging to the heterogeneous nuclear ribonucleoprotein
family, which can bind to the 3′ UTR of mRNA, forming a stable
complex and preventing its degradation. In HCC studies, Wang et
al (77) found that the lncRNA
small nucleolar RNA host gene 6 could competitively bind to HNRNPL,
blocking its binding to SET domain containing 7 (SETD7) mRNA,
inhibiting the protective effect of HNRNPL on SETD7 mRNA, and
promoting SETD7 mRNA degradation. The SETD7 protein has a
tumor-suppressive role in HCC, and its reduced synthesis can
enhance HCC migration and invasion, playing an important role in
HCC progression (77). He et
al (78) reported that the
lncRNA LINC01093 was significantly downregulated in HCC, and its
overexpression could significantly inhibit HCC progression.
Mechanistically, LINC01093 competitively binds to IGF2BP1, blocking
its binding to GLI1 mRNA, leading to the degradation of GLI1 mRNA
without the protective effect of IGF2BP1. The GLI1 protein can
upregulate cell cycle-related genes, promote cell transition from
the G1 phase to the S phase, and enhance tumor cell
proliferation; therefore, its reduced synthesis can significantly
inhibit HCC proliferation and metastasis (78).
These studies revealed that lncRNAs, through
competitive binding with different functional RBPs, can inhibit the
binding of RBPs to mRNA, regulating mRNA stability and the
synthesis of its encoded protein, thereby exerting oncogenic or
tumor-suppressive effects in cancer (Table II).
The normal distribution of proteins, RNA and other
biological macromolecules between the nucleus and cytoplasm plays
an important regulatory role in biological processes. When the
nuclear-cytoplasmic distribution of these macromolecules is
abnormal, it can lead to cellular dysfunction (79,80).
In cancer, the abnormal nuclear-cytoplasmic distribution of lncRNAs
and RBPs can affect mRNA stability, thereby influencing tumor
progression. Chen et al (81) found that the lncRNA ASNR may have
anti-apoptotic functions. The RBP AUF1 binds to the 3′ UTR region
of Bcl-2 mRNA, promoting Bcl-2 mRNA degradation. Bcl-2 is an
anti-apoptotic protein that serves a key role in apoptosis;
however, when ASNR binds to AUF1 in the nucleus to form a complex,
it prevents AUF1 from entering the cytoplasm, significantly
altering its nuclear-cytoplasmic distribution, reducing Bcl-2 mRNA
degradation and increasing Bcl-2 protein synthesis, thereby
inhibiting tumor cell apoptosis (81). Conversely, Wang et al
(82) demonstrated that after the
lncRNA FIRRE binds to PTBP1 in the nucleus, it can promote the
transfer of PTBP1 from the nucleus to the cytoplasm, forming a
stable complex with Beclin 1 (BECN1) mRNA in the cytoplasm,
preventing BECN1 mRNA degradation by enzymes, increasing its
stability and enhancing BECN1 protein synthesis, thereby further
promoting CRC proliferation and metastasis. This finding contrasts
with that of Liu et al (72), but confirms that PTBP1, as a
multifunctional RBP, affects mRNA stability differently depending
on the binding site and intracellular regulatory factors. Through
the aforementioned mechanisms, lncRNA alters the
nuclear-cytoplasmic distribution of RBPs, thereby increasing or
decreasing the binding of RBPs to mRNA, further regulating mRNA
stability and affecting the malignant biological behavior of cancer
(Fig. 1C; Table II).
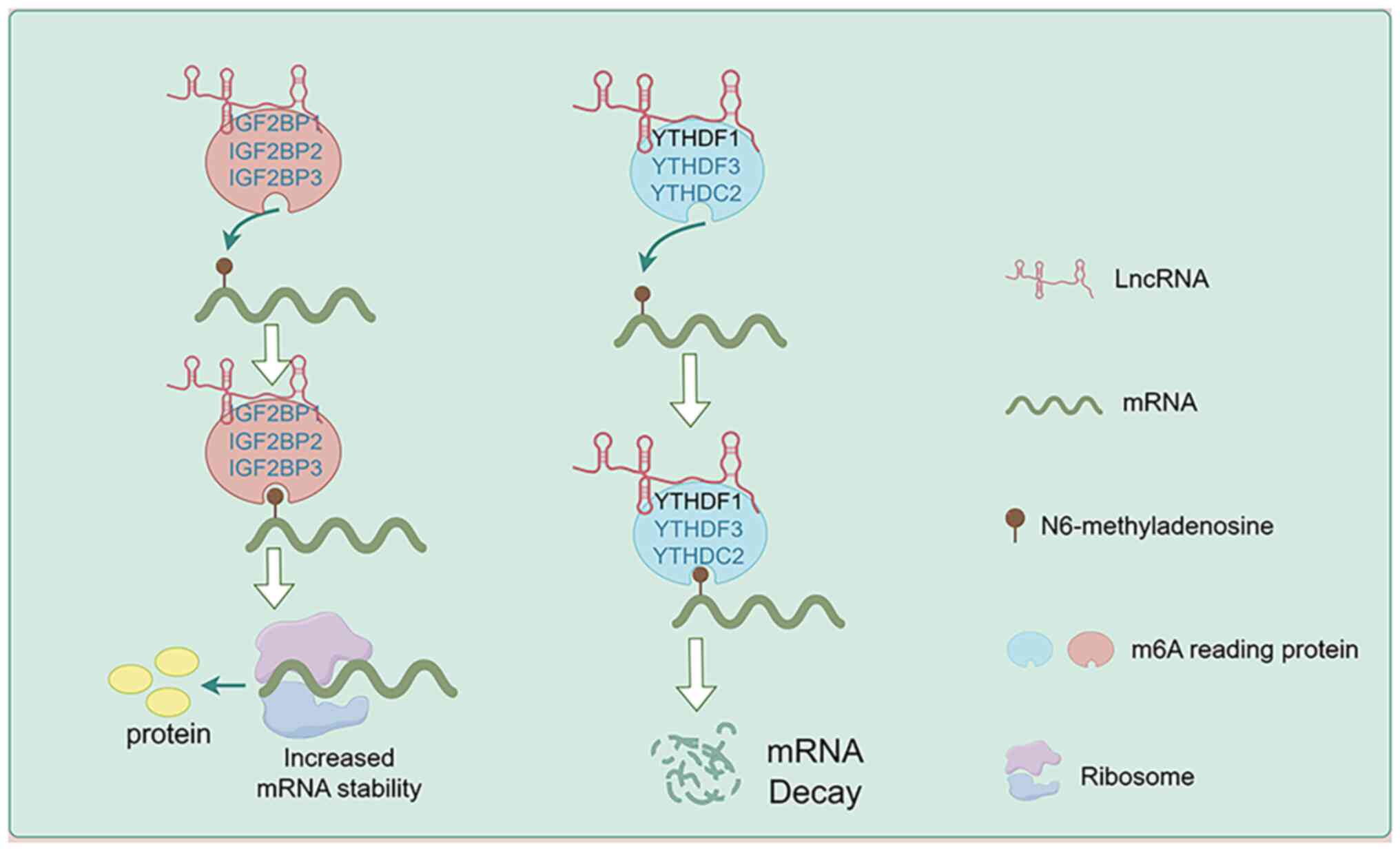
RNA modification is an important mechanism of
post-transcriptional regulation, with m6A modification being the
most common type of RNA modification (83,84).
m6A modification serves a crucial role in regulating mRNA stability
and is an important mechanism for regulating gene expression
(85,86). m6A reader proteins are a special
class of RBPs that can recognize and bind m6A-modified mRNA,
enhancing or reducing mRNA stability, and further influencing
cellular functions (87). The main
m6A reader proteins include the IGF2BP (88) family proteins (including IGF2BP1,
IGF2BP2 and IGF2BP3) and the YTH (89,90)
family proteins (including YTHDF1, YTHDF2 and YTHDF3). In cancer,
lncRNAs bind to these m6A reader proteins, further recognizing and
binding to m6A-modified mRNA, thereby regulating mRNA stability and
influencing the malignant biological behavior and drug resistance
of tumors. This is another mechanism for regulating mRNA stability
(Fig. 2; Table III).
In cancer, lncRNAs and RBPs regulate mRNA stability
through the four aforementioned mechanisms. These findings not only
improve the understanding of cancer progression and drug resistance
mechanisms, but also provide a theoretical basis for developing new
therapeutic strategies. In recent years, small molecule inhibitors,
RNA interference technology and gene editing technology have been
widely applied in molecular biology. By precisely regulating
specific genes and protein functions, these technologies have
advanced biomedical research (95–97).
In addition, these technologies may have potential in cancer
treatment. For example, small molecule inhibitors or RNA
interference technology may be used to target lncRNAs, inhibit
their binding to RBPs, or specifically inhibit RBPs to block their
interactions with lncRNAs and mRNAs. This ultimately reduces the
synthesis of oncogenic proteins or increases the synthesis of
tumor-suppressive proteins, inhibiting tumor growth and metastasis,
thereby playing a role in cancer treatment.
Although small molecule inhibitors, RNA interference
technology and gene editing technology show great potential in
cancer treatment and may provide novel options for precision cancer
therapy, most research remains at the cellular and animal model
stage, with only a few methods entering preclinical studies. These
technologies face a series of challenges and limitations in
practical application. Small molecule inhibitors lack selectivity,
and the bioavailability and cellular delivery efficiency of drugs
are key obstacles. The main challenge of RNA interference
technology is to develop delivery systems that ensure siRNA or
short hairpin RNA reaches target cells safely and effectively, and
functions stably. Although gene editing technology can precisely
modify genes, off-target effects, editing efficiency and long-term
genetic impacts remain major challenges, and ethical issues also
limit its clinical application. Therefore, these technologies have
provided potential new areas for cancer treatment, but overcoming
the aforementioned issues is necessary for them to be translated
into safe and effective therapeutic approaches.
The interaction between lncRNAs and RBPs serves an
important role in regulating gene expression, maintaining genome
stability and participating in cell signal transduction, and it has
a key role in cancer progression and drug resistance. In-depth
analysis of lncRNA-RBP interactions is of great significance for
revealing cancer molecular mechanisms, discovering new biomarkers
and developing new therapeutic strategies. The present study
reviewed the various mechanisms by which lncRNA regulates mRNA
stability, including acting as a molecular scaffold to promote RBP
binding to mRNA, competitively binding RBPs, affecting the
nuclear-cytoplasmic localization of RBPs, and binding to m6A reader
RBPs to regulate mRNA stability. These mechanisms affect the
malignant biological behavior of cancer cells and drug resistance,
highlighting their importance in cancer biology. Targeting these
mechanisms via small molecule inhibitors, RNA interference
technology and gene editing technology may provide new therapeutic
options, but these still face challenges and limitations, such as
the precise delivery of interfering molecules within cells,
avoiding non-specific binding and reducing immune responses. This
requires continuous optimization of existing technologies,
development of more efficient delivery systems, and strengthening
of ethical and safety regulations. Additionally, research on the
impact of lncRNA-RBP interactions on mRNA stability in cancer
remains limited in both scope and the range of cancers
investigated, which constitutes a limitation of the present review.
Future research should focus on large-scale screening methods, such
as high-throughput sequencing or single-cell sequencing
technologies, to more comprehensively reveal the network of
lncRNA-RBP interactions and their specific roles in different types
of cancer.
Not applicable.
The present review was supported by the Guizhou Science and
Technology Planning Project [grant nos. Qiankehe
Basics-ZK(2024)general 302 and Qiankehe Basics-ZK(2022)general
631].
Not applicable.
NJZ and KW conceived the study. NJZ conducted the
literature search and data analysis for inclusion in the review,
drafted the manuscript and produced figures. KW edited and revised
the manuscript. Data authentication is not applicable. All authors
read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Erber J and Herndler-Brandstetter D:
Regulation of T cell differentiation and function by long noncoding
RNAs in homeostasis and cancer. Front Immunol. 14:11814992023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Li Y, Jiang X, Deng Y, Ma C, Yu Q
and Gao D: Long non-coding RNA: Multiple effects on the
differentiation, maturity and cell function of dendritic cells.
Clin Immunol. 245:1091672022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mattick JS, Amaral PP, Carninci P,
Carpenter S, Chang HY, Chen LL, Chen R, Dean C, Dinger ME,
Fitzgerald KA, et al: Long non-coding RNAs: Definitions, functions,
challenges and recommendations. Nat Rev Mol Cell Biol. 24:430–447.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan J, Li H, Xie R, Zhang X, Nie X, Shi X,
Zhan J, Yin Z, Zhao Y, Dai B, et al: LncRNA ZNF593-AS alleviates
contractile dysfunction in dilated cardiomyopathy. Circ Res.
128:1708–1723. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang H, Yan J, Lan X, Guo Y, Sun M, Zhao
Y, Zhang F, Sun J and Lu S: LncRNA WDR11-AS1 promotes extracellular
matrix synthesis in osteoarthritis by directly interacting with
RNA-binding protein PABPC1 to stabilize SOX9 expression. Int J Mol
Sci. 24:8172023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu F, Cao Y, Zhang C and Su H: Decreased
DANCR contributes to high glucose-induced extracellular matrix
accumulation in human renal mesangial cell via regulating the
TGF-β/Smad signaling. FASEB J. 37:e229262023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Z, Wan J, Ma L, Li Z, Yang R, Yang H,
Li J, Zhou F and Ming L: Long non-coding RNA HOXC-AS1 exerts its
oncogenic effects in esophageal squamous cell carcinoma by
interaction with IGF2BP2 to stabilize SIRT1 expression. J Clin Lab
Anal. 37:e248012023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai Z, Shi Q, Li Y, Jin L, Li S, Wong LL,
Wang J, Jiang X, Zhu M, Lin J, et al: LncRNA EILA promotes CDK4/6
inhibitor resistance in breast cancer by stabilizing cyclin E1
protein. Sci Adv. 9:eadi38212023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu M, Li H, Li X, Pan B, Zhang J, Pan Y,
Shen M and Liu L: A novel lncRNA FUAT1/TNS4 axis confers
chemoresistance by suppressing reactive oxygen species-mediated
apoptosis in gastric cancer. Antioxid Redox Signal. 41:24–41. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng Z, Wu M, Li H, Xu W, Yang M, Pan K,
Ni Y, Jiang T, Zheng H, Jin X, et al: Downregulation of AC092894.1
promotes oxaliplatin resistance in colorectal cancer via the
USP3/AR/RASGRP3 axis. BMC Med. 21:1322023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bae H and Coller J: Codon
optimality-mediated mRNA degradation: Linking translational
elongation to mRNA stability. Mol Cell. 82:1467–1476. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schuster SL, Arora S, Wladyka CL, Itagi P,
Corey L, Young D, Stackhouse BL, Kollath L, Wu QV, Corey E, et al:
Multi-level functional genomics reveals molecular and cellular
oncogenicity of patient-based 3′ untranslated region mutations.
Cell Rep. 42:1128402023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia L, Mao Y, Ji Q, Dersh D, Yewdell JW
and Qian SB: Decoding mRNA translatability and stability from the
5′ UTR. Nat Struct Mol Biol. 27:814–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Passmore LA and Coller J: Roles of mRNA
poly(A) tails in regulation of eukaryotic gene expression. Nat Rev
Mol Cell Biol. 23:93–106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bednarek S, Madan V, Sikorski PJ,
Bartenschlager R, Kowalska J and Jemielity J: mRNAs biotinylated
within the 5′ cap and protected against decapping: New tools to
capture RNA-protein complexes. Philos Trans R Soc Lond B Biol Sci.
373:201801672018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Korn SM, Ulshöfer CJ, Schneider T and
Schlundt A: Structures and target RNA preferences of the
RNA-binding protein family of IGF2BPs: An overview. Structure.
29:787–803. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mushtaq A, Mir US and Altaf M:
Multifaceted functions of RNA-binding protein vigilin in gene
silencing, genome stability, and autism-related disorders. J Biol
Chem. 299:1029882023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao ZT, Yang YM, Sun MM, He Y, Liao L,
Chen KS and Li B: New insights into the interplay between long
non-coding RNAs and RNA-binding proteins in cancer. Cancer Commun
(Lond). 42:117–140. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Deng X and Chen J: RNA-binding
proteins in regulating mRNA stability and translation: Roles and
mechanisms in cancer. Semin Cancer Biol. 86:664–677. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin H, Ni H, Liu Y, Yuan Y, Xi T, Li X and
Zheng L: RNA-binding proteins in tumor progression. J Hematol
Oncol. 13:902020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ouyang J, Zhong Y, Zhang Y, Yang L, Wu P,
Hou X, Xiong F, Li X, Zhang S, Gong Z, et al: Long non-coding RNAs
are involved in alternative splicing and promote cancer
progression. Br J Cancer. 126:1113–1124. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaath H, Vishnubalaji R, Elango R,
Kardousha A, Islam Z, Qureshi R, Alam T, Kolatkar PR and Alajez NM:
Long non-coding RNA and RNA-binding protein interactions in cancer:
Experimental and machine learning approaches. Semin Cancer Biol.
86:325–345. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramesh-Kumar D and Guil S: The IGF2BP
family of RNA binding proteins links epitranscriptomics to cancer.
Semin Cancer Biol. 86:18–31. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miao W, Porter DF, Lopez-Pajares V,
Siprashvili Z, Meyers RM, Bai Y, Nguyen DT, Ko LA, Zarnegar BJ,
Ferguson ID, et al: Glucose dissociates DDX21 dimers to regulate
mRNA splicing and tissue differentiation. Cell. 186:80–97.e26.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gebauer F, Schwarzl T, Valcárcel J and
Hentze MW: RNA-binding proteins in human genetic disease. Nat Rev
Genet. 22:185–198. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Herman AB, Tsitsipatis D and Gorospe M:
Integrated lncRNA function upon genomic and epigenomic regulation.
Mol Cell. 82:2252–2266. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nojima T and Proudfoot NJ: Mechanisms of
lncRNA biogenesis as revealed by nascent transcriptomics. Nat Rev
Mol Cell Biol. 23:389–406. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Knott GJ, Bond CS and Fox AH: The DBHS
proteins SFPQ, NONO and PSPC1: A multipurpose molecular scaffold.
Nucleic Acids Res. 44:3989–4004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Y, Lu Y, Yang L, Ma W, Dong Y, Zhou
S, Liu N, Gan W and Li D: LncRNA like NMRK2 mRNA functions as a key
molecular scaffold to enhance mitochondrial respiration of
NONO-TFE3 rearranged renal cell carcinoma in an NAD+
kinase-independent manner kinase-independent manner. J Exp Clin
Cancer Res. 42:2522023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao P, Ji MM, Fang Y, Li X, Yi HM, Yan
ZX, Cheng S, Xu PP, Janin A, Wang CF, et al: A novel lncRNA TCLlnc1
promotes peripheral T cell lymphoma progression through acting as a
modular scaffold of HNRNPD and YBX1 complexes. Cell Death Dis.
12:3212021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng Y, Zhang T, Zhang Z, Liang Y, Wang H,
Chen Y, Yu X, Song X, Mao Q, Xia W, et al: The
super-enhancer-driven lncRNA LINC00880 acts as a scaffold between
CDK1 and PRDX1 to sustain the malignance of lung adenocarcinoma.
Cell Death Dis. 14:5512023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang W, Zhao J, Deng L, Ishimwe N, Pauli
J, Wu W, Shan S, Kempf W, Ballantyne MD, Kim D, et al: INKILN is a
novel long noncoding RNA promoting vascular smooth muscle
inflammation via scaffolding MKL1 and USP10. Circulation.
148:47–67. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Zhang H, Zhang X and Liu B:
CBR3-AS1 accelerates the malignant proliferation of gestational
choriocarcinoma cells by stabilizing SETD4. Dis Markers.
2022:71555252022.PubMed/NCBI
|
|
35
|
Xie M, Ma T, Xue J, Ma H, Sun M, Zhang Z,
Liu M, Liu Y, Ju S, Wang Z and De W: The long intergenic
non-protein coding RNA 707 promotes proliferation and metastasis of
gastric cancer by interacting with mRNA stabilizing protein HuR.
Cancer Lett. 443:67–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ni T, Guo D, Tan L, Xiao Z and Shi Y:
NPSR1-AS1 activates the MAPK pathway to facilitate thyroid cancer
cell malignant behaviors via recruiting ELAVL1 to stabilize NPSR1
mRNA. Cell Cycle. 21:439–449. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jalali S, Gandhi S and Scaria V: Distinct
and modular organization of protein interacting sites in long
non-coding RNAs. Front Mol Biosci. 5:272018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang C, Yang Y, Zhang G, Li J, Wu X, Ma X,
Shan G and Mei Y: Long noncoding RNA EMS connects c-Myc to cell
cycle control and tumorigenesis. Proc Natl Acad Sci USA.
116:14620–14629. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pan L, Li Y, Jin L, Li J and Xu A:
TRPM2-AS promotes cancer cell proliferation through control of
TAF15. Int J Biochem Cell Biol. 120:1056832020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang L, Ye S, Wang J, Gu Z, Zhang Y, Zhang
C and Ma X: HuR stabilizes lnc-Sox5 mRNA to promote tongue
carcinogenesis. Biochemistry (Mosc). 82:438–445. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang A, Bao Y, Wu Z, Zhao T, Wang D, Shi
J, Liu B, Sun S, Yang F, Wang L and Qu L: Long noncoding RNA
EGFR-AS1 promotes cell growth and metastasis via affecting HuR
mediated mRNA stability of EGFR in renal cancer. Cell Death Dis.
10:1542019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu D, He X, Wang W, Hu X, Wang K and Wang
M: Long noncoding RNA SNHG12 induces proliferation, migration,
epithelial-mesenchymal transition, and stemness of esophageal
squamous cell carcinoma cells via post-transcriptional regulation
of BMI1 and CTNNB1. Mol Oncol. 14:2332–2351. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen B, Xu X, Wu W, Zheng K and Yu Y:
LINC00659 inhibits hepatocellular carcinoma malignant progression
by blocking aerobic glycolysis through FUS recruitment and SLC10A1
modulation. Anal Cell Pathol (Amst). 2023:58529632023.PubMed/NCBI
|
|
44
|
Tuo H, Liu R, Wang Y, Yang W and Liu Q:
Hypoxia-induced lncRNA MRVI1-AS1 accelerates hepatocellular
carcinoma progression by recruiting RNA-binding protein CELF2 to
stabilize SKA1 mRNA. World J Surg Oncol. 21:1112023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tian C, Abudoureyimu M, Lin X, Chu X and
Wang R: Linc-ROR facilitates progression and angiogenesis of
hepatocellular carcinoma by modulating DEPDC1 expression. Cell
Death Dis. 12:10472021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ren L, Fang X, Shrestha SM, Ji Q, Ye H,
Liang Y, Liu Y, Feng Y, Dong J and Shi R: LncRNA SNHG16 promotes
development of oesophageal squamous cell carcinoma by interacting
with EIF4A3 and modulating RhoU mRNA stability. Cell Mol Biol Lett.
27:892022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang Z and Hu T: Long noncoding RNA
HOXC-AS3 facilitates the progression of invasive mucinous
adenocarcinomas of the lung via modulating FUS/FOXM1. In Vitro Cell
Dev Biol Anim. 56:15–23. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu L, Liu Y, Tang H and Wang P: FOXP3
activated-LINC01232 accelerates the stemness of non-small cell lung
carcinoma by activating TGF-β signaling pathway and recruiting
IGF2BP2 to stabilize TGFBR1. Exp Cell Res. 413:1130242022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang F, Xue X, Zheng L, Bi J, Zhou Y, Zhi
K, Gu Y and Fang G: Long non-coding RNA GHET1 promotes gastric
carcinoma cell proliferation by increasing c-Myc mRNA stability.
FEBS J. 281:802–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Luo N, Zhang K, Li X and Hu Y: ZEB1
induced-upregulation of long noncoding RNA ZEB1-AS1 facilitates the
progression of triple negative breast cancer by binding with ELAVL1
to maintain the stability of ZEB1 mRNA. J Cell Biochem.
121:4176–4187. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ning X, Zhao J, He F, Yuan Y, Li B and
Ruan J: lncRNA NUTM2A-AS1 targets the SRSF1/Trim37 signaling
pathway to promote the proliferation and invasion of breast cancer.
Comput Math Methods Med. 2022:32993362022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wen D, Huang Z, Li Z, Tang X, Wen X, Liu J
and Li M: LINC02535 co-functions with PCBP2 to regulate DNA damage
repair in cervical cancer by stabilizing RRM1 mRNA. J Cell Physiol.
235:7592–7603. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang L, Ruan Y, Wu X and Zhou X: lncRNA
ZFAS1 promotes HMGCR mRNA stabilization via binding U2AF2 to
modulate pancreatic carcinoma lipometabolism. J Immunol Res.
2022:41631982022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen R, Zhang X and Wang C: LncRNA
HOXB-AS1 promotes cell growth in multiple myeloma via FUT4 mRNA
stability by ELAVL1. J Cell Biochem. 121:4043–4051. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lu X, Qiao L and Liu Y: Long noncoding RNA
LEF1-AS1 binds with HNRNPL to boost the proliferation, migration,
and invasion in osteosarcoma by enhancing the mRNA stability of
LEF1. J Cell Biochem. 121:4064–4073. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang M, Sun Y, Huang CP, Luo J, Zhang L,
Meng J, Liang C and Chang C: Targeting the Lnc-OPHN1-5/androgen
receptor/hnRNPA1 complex increases enzalutamide sensitivity to
better suppress prostate cancer progression. Cell Death Dis.
12:8552021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dos Santos AF, Fazeli G, Xavier da Silva
TN and Friedmann Angeli JP: Ferroptosis: Mechanisms and
implications for cancer development and therapy response. Trends
Cell Biol. 33:1062–1076. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Qi R, Bai Y, Li K, Liu N, Xu Y, Dal E,
Wang Y, Lin R, Wang H, Liu Z, et al: Cancer-associated fibroblasts
suppress ferroptosis and induce gemcitabine resistance in
pancreatic cancer cells by secreting exosome-derived
ACSL4-targeting miRNAs. Drug Resist Updat. 68:1009602023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang Q, Deng T, Zhang H, Zuo D, Zhu Q,
Bai M, Liu R, Ning T, Zhang L, Yu Z, et al: Adipocyte-derived
exosomal MTTP suppresses ferroptosis and promotes chemoresistance
in colorectal cancer. Adv Sci (Weinh). 9:e22033572022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang C, Liu X, Jin S, Chen Y and Guo R:
Ferroptosis in cancer therapy: A novel approach to reversing drug
resistance. Mol Cancer. 21:472022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang Y, Wu X, Ren Z, Li Y, Zou W, Chen J
and Wang H: Overcoming cancer chemotherapy resistance by the
induction of ferroptosis. Drug Resist Updat. 66:1009162023.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Luo J, Bai R, Liu Y, Bi H, Shi X and Qu C:
Long non-coding RNA ATXN8OS promotes ferroptosis and inhibits the
temozolomide-resistance of gliomas through the ADAR/GLS2 pathway.
Brain Res Bull. 186:27–37. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gowravaram M, Schwarz J, Khilji SK, Urlaub
H and Chakrabarti S: Insights into the assembly and architecture of
a Staufen-mediated mRNA decay (SMD)-competent mRNP. Nat Commun.
10:50542019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yadav DK, Zigáčková D, Zlobina M, Klumpler
T, Beaumont C, Kubíčková M, Vaňáčová Š and Lukavsky PJ: Staufen1
reads out structure and sequence features in ARF1 dsRNA for target
recognition. Nucleic Acids Res. 48:2091–2106. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lu D, Di S, Zhuo S, Zhou L, Bai R, Ma T,
Zou Z, Chen C, Sun M, Tang J and Zhang Z: The long noncoding RNA
TINCR promotes breast cancer cell proliferation and migration by
regulating OAS1. Cell Death Discov. 7:412021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao L, Jiang L, Zhang M, Zhang Q, Guan Q,
Li Y, He M, Zhang J and Wei M: NF-κB-activated SPRY4-IT1 promotes
cancer cell metastasis by downregulating TCEB1 mRNA via
Staufen1-mediated mRNA decay. Oncogene. 40:4919–4929. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Eken JA, Koning MT, Kupcova K, Sepúlveda
Yáñez JH, de Groen RAL, Quinten E, Janssen J, van Bergen CAM,
Vermaat JSP, Cleven A, et al: Antigen-independent, autonomous B
cell receptor signaling drives activated B cell DLBCL. J Exp Med.
221:e202309412024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schmitz R, Wright GW, Huang DW, Johnson
CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer
AL, et al: Genetics and pathogenesis of diffuse large B-cell
lymphoma. N Engl J Med. 378:1396–1407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu QH, Dai GR, Wu Y, Wang XN, Song MY, Li
XD, Wu Z and Xia RX: LncRNA FIRRE stimulates PTBP1-induced Smurf2
decay, stabilizes B-cell receptor, and promotes the development of
diffuse large B-cell lymphoma. Hematol Oncol. 40:554–566. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nag S, Goswami B, Das Mandal S and Ray PS:
Cooperation and competition by RNA-binding proteins in cancer.
Semin Cancer Biol. 86:286–297. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu Y, Xue M, Du S, Feng W, Zhang K, Zhang
L, Liu H, Jia G, Wu L, Hu X, et al: Competitive endogenous RNA is
an intrinsic component of EMT regulatory circuits and modulates
EMT. Nat Commun. 10:16372019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhou L, Jiang J, Huang Z, Jin P, Peng L,
Luo M, Zhang Z, Chen Y, Xie N, Gao W, et al: Hypoxia-induced lncRNA
STEAP3-AS1 activates Wnt/β-catenin signaling to promote colorectal
cancer progression by preventing m6A-mediated
degradation of STEAP3 mRNA. Mol Cancer. 21:1682022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tao W, Ma J, Zheng J, Liu X, Liu Y, Ruan
X, Shen S, Shao L, Chen J and Xue Y: Silencing SCAMP1-TV2 inhibited
the malignant biological behaviors of breast cancer cells by
interaction with PUM2 to facilitate INSM1 mRNA degradation. Front
Oncol. 10:6132020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang H, Ma P, Liu P, Guo D, Liu Z and
Zhang Z: lncRNA SNHG6 promotes hepatocellular carcinoma progression
by interacting with HNRNPL/PTBP1 to facilitate SETD7/LZTFL1 mRNA
destabilization. Cancer Lett. 520:121–131. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
He J, Zuo Q, Hu B, Jin H, Wang C, Cheng Z,
Deng X, Yang C, Ruan H, Yu C, et al: A novel, liver-specific long
noncoding RNA LINC01093 suppresses HCC progression by interaction
with IGF2BP1 to facilitate decay of GLI1 mRNA. Cancer Lett.
450:98–109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Puertollano R, Ferguson SM, Brugarolas J
and Ballabio A: The complex relationship between TFEB transcription
factor phosphorylation and subcellular localization. EMBO J.
37:e988042018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shen Y, Zou Y, Li J, Chen F, Li H and Cai
Y: CDK5RAP3, a novel nucleoplasmic shuttle, deeply regulates
hsf1-mediated heat stress response and protects mammary epithelial
cells from heat injury. Int J Mol Sci. 21:84002020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen J, Liu L, Wei G, Wu W, Luo H, Yuan J,
Luo J and Chen R: The long noncoding RNA ASNR regulates degradation
of Bcl-2 mRNA through its interaction with AUF1. Sci Rep.
6:321892016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang Y, Li Z, Xu S, Li W, Chen M, Jiang M
and Fan X: LncRNA FIRRE functions as a tumor promoter by
interaction with PTBP1 to stabilize BECN1 mRNA and facilitate
autophagy. Cell Death Dis. 13:982022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
He PC, Wei J, Dou X, Harada BT, Zhang Z,
Ge R, Liu C, Zhang LS, Yu X, Wang S, et al: Exon architecture
controls mRNA m6A suppression and gene expression.
Science. 379:677–682. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Deng X, Qing Y, Horne D, Huang H and Chen
J: The roles and implications of RNA m6A modification in
cancer. Nat Rev Clin Oncol. 20:507–526. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Uzonyi A, Dierks D, Nir R, Kwon OS, Toth
U, Barbosa I, Burel C, Brandis A, Rossmanith W, Le Hir H, et al:
Exclusion of m6A from splice-site proximal regions by the exon
junction complex dictates m6A topologies and mRNA stability. Mol
Cell. 83:237–251.e7. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen J, Zhang H, Xiu C, Gao C, Wu S, Bai
J, Shen Q and Yin T: METTL3 promotes pancreatic cancer
proliferation and stemness by increasing stability of ID2 mRNA in a
m6A-dependent manner. Cancer Lett. 565:2162222023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Fagre C and Gilbert W: Beyond reader
proteins: RNA binding proteins and RNA modifications in
conversation to regulate gene expression. Wiley Interdiscip Rev
RNA. 15:e18342024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Elcheva IA, Gowda CP, Bogush D,
Gornostaeva S, Fakhardo A, Sheth N, Kokolus KM, Sharma A, Dovat S,
Uzun Y, et al: IGF2BP family of RNA-binding proteins regulate
innate and adaptive immune responses in cancer cells and tumor
microenvironment. Front Immunol. 14:12245162023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Stowell JAW, Webster MW, Kögel A, Wolf J,
Shelley KL and Passmore LA: Reconstitution of targeted
deadenylation by the Ccr4-not complex and the YTH domain protein
Mmi1. Cell Rep. 17:1978–1989. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sikorski V, Selberg S, Lalowski M,
Karelson M and Kankuri E: The structure and function of YTHDF
epitranscriptomic m6A readers. Trends Pharmacol Sci.
44:335–353. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yang J, Qian X, Qiu Q, Xu L, Pan M, Li J,
Ren J, Lu B, Qiu T, Chen E, et al: LCAT1 is an oncogenic LncRNA by
stabilizing the IGF2BP2-CDC6 axis. Cell Death Dis. 13:8772022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhu P, He F, Hou Y, Tu G, Li Q, Jin T,
Zeng H, Qin Y, Wan X, Qiao Y, et al: A novel hypoxic long noncoding
RNA KB-1980E6.3 maintains breast cancer stem cell stemness via
interacting with IGF2BP1 to facilitate c-Myc mRNA stability.
Oncogene. 40:1609–1627. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang Y and Chen Z: Long noncoding RNA
UBA6-AS1 inhibits the malignancy of ovarian cancer cells via
suppressing the decay of UBA6 mRNA. Bioengineered. 13:178–189.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yang H, Hu Y, Weng M, Liu X, Wan P, Hu Y,
Ma M, Zhang Y, Xia H and Lv K: Hypoxia inducible lncRNA-CBSLR
modulates ferroptosis through m6A-YTHDF2-dependent modulation of
CBS in gastric cancer. J Adv Res. 37:91–106. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Afrin H, Geetha Bai R, Kumar R, Ahmad SS,
Agarwal SK and Nurunnabi M: Oral delivery of RNAi for cancer
therapy. Cancer Metastasis Rev. 42:699–724. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chan YT, Lu Y, Wu J, Zhang C, Tan HY, Bian
ZX, Wang N and Feng Y: CRISPR-Cas9 library screening approach for
anti-cancer drug discovery: Overview and perspectives.
Theranostics. 12:3329–3344. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fan J, Xu Y, Wen X, Ge S, Jia R, Zhang H
and Fan X: A cohesin-mediated intrachromosomal loop drives
oncogenic ROR lncRNA to accelerate tumorigenesis. Mol Ther.
27:2182–2194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang R, Sun Y, Li L, Niu Y, Lin W, Lin C,
Antonarakis ES, Luo J, Yeh S and Chang C: Preclinical study using
Malat1 small interfering RNA or androgen receptor splicing variant
7 degradation enhancer ASC-J9® to suppress
enzalutamide-resistant prostate cancer progression. Eur Urol.
72:835–844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu X, Ramesh R, Wang J, Zheng Y, Armaly
AM, Wei L, Xing M, Roy S, Lan L, Gao FP, et al: Small molecules
targeting the RNA-binding protein HuR inhibit tumor growth in
xenografts. J Med Chem. 66:2032–2053. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Weng H, Huang F, Yu Z, Chen Z, Prince E,
Kang Y, Zhou K, Li W, Hu J, Fu C, et al: The m6A reader
IGF2BP2 regulates glutamine metabolism and represents a therapeutic
target in acute myeloid leukemia. Cancer Cell. 40:1566–1582.e10.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xu F, Li J, Ni M, Cheng J, Zhao H, Wang S,
Zhou X and Wu X: FBW7 suppresses ovarian cancer development by
targeting the N6-methyladenosine binding protein YTHDF2.
Mol Cancer. 20:452021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Katsushima K, Lee B, Kunhiraman H, Zhong
C, Murad R, Yin J, Liu B, Garancher A, Gonzalez-Gomez I, Monforte
HL, et al: The long noncoding RNA lnc-HLX-2-7 is oncogenic in group
3 medulloblastomas. Neuro Oncol. 23:572–585. 2021. View Article : Google Scholar : PubMed/NCBI
|