Introduction
Pancreatic cancer, predominantly consisting of
pancreatic ductal adenocarcinoma (PDAC) cases, ranks among the most
lethal neoplasms with a 5-year survival rate of only 12% (1). Despite accounting for only 3% of all
tumors, PDAC is the fourth leading cause of cancer-related death
(2). PDAC exhibits extreme
resistance to traditional chemotherapy and radiotherapy.
Furthermore, while immunotherapy has achieved considerable
milestones in treating various tumors, standalone immunotherapy has
not exhibited an equal efficacy against pancreatic cancer (3). Therefore, exploring innovative targets
and mechanisms to enhance the therapeutic effects of chemotherapy
and immunotherapy is critical for improving the clinical prognosis
of patients with PDAC.
The inflammatory cytokine interleukin-17 (IL-17),
primarily released by T helper 17 (Th17) cells, is also produced by
other cell populations such as neutrophils, γδT cells,
CD8+ cytotoxic T cells and natural killer T cells (NKTs)
(4). IL-17 and its receptor,
IL-17R, play pivotal roles not only in allergic and autoimmune
diseases, inflammation (5) and in
response to COVID-19 (6), but also
in the microenvironment of various tumors, including PDAC (7–9). The
activation of IL-17/IL-17R and downstream signaling pathways,
including the nuclear factor κB (NF-κB) and mitogen-activated
protein kinase (MAPK) pathways, regulates tumor progression and
resistance to chemotherapy in several tumor types (10–12).
Due to the functions and properties of IL-17 in diverse
malignancies, comprehensive investigations into the role and
mechanisms of IL-17/IL-17R signaling in the pathogenesis of PDAC
could reveal a potentially beneficial strategy for improving
therapeutic outcomes.
This review presents a comprehensive exploration of
the biology of IL-17/IL-17R signaling and its associated pathogenic
mechanisms in pancreatic cancer. It provides an in-depth review of
the substantial effects of IL-17/IL-17R in PDAC from the
perspective of the tumor microenvironment (TME) and treatment
responsiveness at the cellular and molecular levels. The present
review aims to offer novel insight into the therapeutic management
of pancreatic cancer.
IL-17/IL-17R family: Structure, function and
signaling pathways
IL-17 family
The cytokine family IL-17 comprises six distinct
members: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (also known as
IL-25) and IL-17F. Among this family, IL-17A [or IL-17,
alternatively known as cytotoxic T-lymphocyte-associated protein
(CTLA)-8], is the prototypical and most extensively studied member
(5,13,14).
Besides Th17 cells, IL-17 is produced by other cell types including
Type 17 CD8+ cytotoxic T cells (Tc17), NKTs, innate
lymphoid cells and neutrophils (15,16).
Functioning as an immune effector cytokine, IL-17 is derived from
both adaptive and innate immune cells, facilitating an integral
nexus between adaptive and innate immunity.
Serving as the hallmark of Th17 cells, IL-17A plays
a pivotal role in the development of autoimmune diseases,
inflammation and tumorigenesis (5).
Increased levels of IL-17A have been implicated as a key driver in
a series of autoimmune diseases (17) including psoriasis (18), rheumatoid arthritis (19) and multiple sclerosis (20), which are primarily characterized by
chronic inflammation. In previous years, an increasing number of
studies have focused on the role of IL-17A in tumors. For instance,
in lung cancer cell lines, IL-17A stimulation triggers the
phosphorylation and activation of extracellular signal-regulated
kinase (ERK)1/2, which subsequently phosphorylates a myriad of
cytoplasmic proteins, thereby regulating various crucial cellular
processes such as proliferation, differentiation, invasion and
apoptosis (21). A recent study
indicated that the transcriptional activation of IL-17A by Oct4
regulates the p38 signaling pathway and encourages the polarization
of M2 macrophages, thereby promoting the metastasis of cervical
cancer (22). IL-17B is widely
expressed across diverse tissues, including the stomach, intestine
and pancreas, and is intimately correlated with tumorigenesis and
the progression of tumors, as observed in gastric and pancreatic
cancer (23). This underscores an
emerging field of research into the IL-17 family. Additionally,
IL-17B expression is positively correlated with an unfavorable
prognosis in breast (24), lung
(25) and pancreatic (26) cancer. IL-17C is predominantly
localized within the mucosal epithelium of the oral cavity, skin
and respiratory tract (27), and
responds to various cytokines and pathogenic stimuli at the mucosal
surfaces, thereby orchestrating inflammation, autoimmune diseases
and bacterial infections (28,29).
Furthermore, in lung cancer, IL-17C contributes to the growth and
proliferation of tumor cells by recruiting neutrophils into the TME
(30). Conversely, few studies have
investigated IL-17D; although its receptor remains obscure,
evidence suggests that it may play an immunoregulatory role in
tumors and infectious diseases (31). IL-17E, also known as IL-25, is
extensively distributed in a number of tissues (32), exerts potent proinflammatory
effects, and stimulates Th2 responses. Additionally, it induces
IL-4 and IL-13 production in various tissues, and fosters
eosinophil expansion by inducing IL-5 release (33). IL-17F, the last member of the IL-17
family, shares >50% amino acid sequence homology with IL-17A.
Typically produced by the same cells, IL-17F and IL-17A unite to
create homo or heterodimers and execute similar functions (5). IL-17F serves as a less potent inducer
of inflammation than IL-17A, displaying notably reduced signaling
intensity and downstream gene activation (34).
IL-17R
The cytokine, IL-17, interacts with IL-17R to
transmit upstream signals. The IL-17R family consists of five
members, ranging from IL-17RA to IL-17RE. The IL-17R transmembrane
protein comprises a fibronectin type III domain situated
extracellularly and a conserved Sef/IL-17R (SEFIR) domain at the
cytoplasmic terminus (5,34). During this process, the NF-κB
activator 1, Act1, is recruited downstream of the IL-17R complex
upon recognizing the SEFIR domain, thereby relaying IL-17-induced
inflammatory response signals (35,36).
Mechanistically, the interaction between IL-17 and
IL-17R is not straightforward; it involves the formation of
homodimers or heterodimers between IL-17Rs. For instance, IL-17RA
functions as a common receptor of the IL-17 family, forming
heterodimers with IL-17RC to transmit upstream IL-17A and IL-17F
signals in most cases, which is a characteristic feature of
IL-17/IL-17R signaling (34,35).
Similarly, IL-17RA can form heterodimers with IL-17RB and IL-17RE,
which recognize homodimeric IL-17B/IL-17E and IL-17C proteins,
respectively (37).
IL-17RB, often referred to as IL-25R, serves as a
receptor for IL-17B and IL-17E and exhibits widespread expression
across various organs and tissues, including the liver, kidney and
mucosal epithelium (25). IL-17B
signaling via IL-17RB can promote tumor growth, migration and
invasion. Furthermore, activating IL-17B/IL-17RB directly
stimulates the proliferation and migration of malignant cells and
induces resistance to conventional chemotherapy (38). The downstream signaling pathway of
IL-17RB is unclear. However, a recent study revealed the proximal
signal transduction pathway of IL-17RB when stimulated by IL-17B in
pancreatic cancer. This involves IL-17RB recruiting mixed-lineage
kinase 4 to phosphorylate the Y447 residue of IL-17RB.
Subsequently, phosphorylated IL-17RB recruits a ubiquitin ligase,
tripartite motif containing 56, which adds K63-linked ubiquitin
chains onto the K470 residue of IL-17RB. Mutation of either the
Y447 or K470 of IL-17RB subsequently propagates downstream
oncogenic signaling through assembling Act1 and other factors
(39). IL-17RC, also known as
IL-17RL, serves a crucial functional role in modulating
IL-17-mediated responses, particularly through interactions with
IL-17A and IL-17F (40). A recent
study suggested that IL-17RC could be a key factor in IL-17-induced
responses in tumor cells by regulating the activation of classical
IL-17A target genes and proteins such as C-X-C motif chemokine
ligand 1 (CXCL1), colony stimulating factor (CSF)1 and programmed
death-ligand 1 (PD-L1) at a molecular level. Thus, IL-17RC may
serve as a possible indicator of the impact of IL-17/IL-17R
signaling on tumor advancement (41).
New evidence shows that IL-17RD forms a heterodimer
with IL-17RA, exclusively binding to the IL-17A homodimer instead
of the IL-17A/F or IL-17F/F heterodimers (42). This updates the previous
understanding that IL-17RD functions as an orphan receptor. The
downregulation and loss of IL-17RD have been documented in a range
of malignancies, with mounting evidence indicating its involvement
in tumorigenesis (43).
Mechanistically, the expression of IL-17RD may regulate the
aggressiveness of tumor cells by modulating the biological
responses to EGF, FGF and Wnt signaling pathways (43). IL-17RE has been less investigated
compared with the other IL-17 receptors. However, previous studies
revealed interactions between IL-17RE and IL-17C in inflammatory
and immune responses (44,45). Additionally, an increased IL-17RE
expression level corresponds to a poor prognosis in patients with
hepatocellular carcinoma (46).
IL-17/IL-17R signaling
As a pivotal proinflammatory cytokine, the
physiological functions of IL-17 are predominantly mediated via the
initiation of downstream signaling cascades upon its interaction
with IL-17R. In the process of IL-17/IL-17R signal transduction,
the intracellular domain of IL-17R includes a conserved SEFIR
domain that orchestrates an interaction with the corresponding
SEFIR motif on Act1, thereby activating a wide range of downstream
signaling pathways (47). Act1
harbors a TNF receptor-associated factor (TRAF) binding site,
facilitating interaction with TRAF family proteins (35,36).
The binding of TRAF6 to Act1 effectively activates the downstream
NF-κB and MAPK pathways, thereby activating a cascade of
transcriptional regulatory alterations (48). Additionally, NF-κB participates in a
series of physiological and pathological processes, including
inflammation, oxidative stress, cell metabolism, proliferation and
apoptosis, and exerts pervasive regulatory effects (49–51).
Furthermore, the binding of TRAF2 and TRAF5 to Act1 enhances
posttranscriptional mRNA stability through the modulation of
diverse RNA-binding proteins, such as human antigen R (HuR) and
AT-rich interactive domain-containing protein 5a (Arid5a) (5).
The binding of IL-17R to Act1 via the SEFIR domain
is the initial step in IL-17/IL-17R signal transduction (5,52). By
serving as an E3 ubiquitin ligase, Act1 recruits and ubiquitinates
TRAF6, subsequently recruiting and activating transforming growth
factor β-activated kinase 1 and inhibitor of NF-κB kinase (IKK).
The activated IKK complex promotes the phosphorylation and
degradation of the IκB subunit on the NF-κB/IκB complex, resulting
in the exposure of the nuclear localization signal on NF-κB. This
process enables the rapid translocation of NF-κB to the nucleus and
the induction of inflammatory gene transcription (52). Furthermore, activated TRAF6 triggers
the phosphorylation and activation of the MAPK pathway (5,52).
Notably, CCAAT/enhancer-binding protein β (C/EBPβ) is another
significant transcription regulatory factor activated directly by
IL-17R through the C/EBPβ activation domain. C/EBPβ activation can
also occur as a secondary response to the activation of the
aforementioned MAPK pathway (5,52).
Compared with the transcriptional regulation
mediated by transcription factors, IL-17 triggers
posttranscriptional regulation by stabilizing specific mRNAs and
proteins, which can be more potent but less well-defined. The
complex formed by TRAF2 and TRAF5 binding with Act1 acts as an
RNA-binding protein, interacting with target mRNAs, such as CSF2
and CXCL2, and facilitating their fates (53). Consequently, the complex directly
influences mRNA metabolism, stability and translation.
Additionally, Act1 promotes the binding of HuR to mRNA, enabling
mRNA to translocate to polysomes for translation (52). Similarly, the expression of Arid5a,
an RNA-binding protein, is upregulated by IL-17, which competes
with Regnase-1 to stabilize IL-17-induced transcripts by binding to
TRAF2 (54). In addition, IL-17
promotes the interaction between Act1, IKK and TANK-binding kinase
1, resulting in the translocation of these proteins to the nucleus
and the phosphorylation of splicing factor 2 (SF2), which in turn
inhibits mRNA degradation mediated by SF2 (53). Notably, the IL-17-induced
posttranscriptional regulation of mRNA is a component of its
self-enhancement mechanisms, amplifying the activity of IL-17
(55).
Overall, the IL-17/IL-17R signaling cascade is
centered on Act1. On the one hand, it activates downstream
signaling primarily through the NF-κB and MAPK pathways, thereby
regulating the expression of corresponding target genes
transcriptionally. On the other hand, it exerts a robust
proinflammatory effect through posttranscriptional regulation,
principally by enhancing the stability of target mRNAs and protein
translation. The classification and structure of IL-17 cytokine
family members and the corresponding downstream IL-17R, as well as
the intracellular IL-17/IL-17R signal transduction pathways are
summarized in Fig. 1.
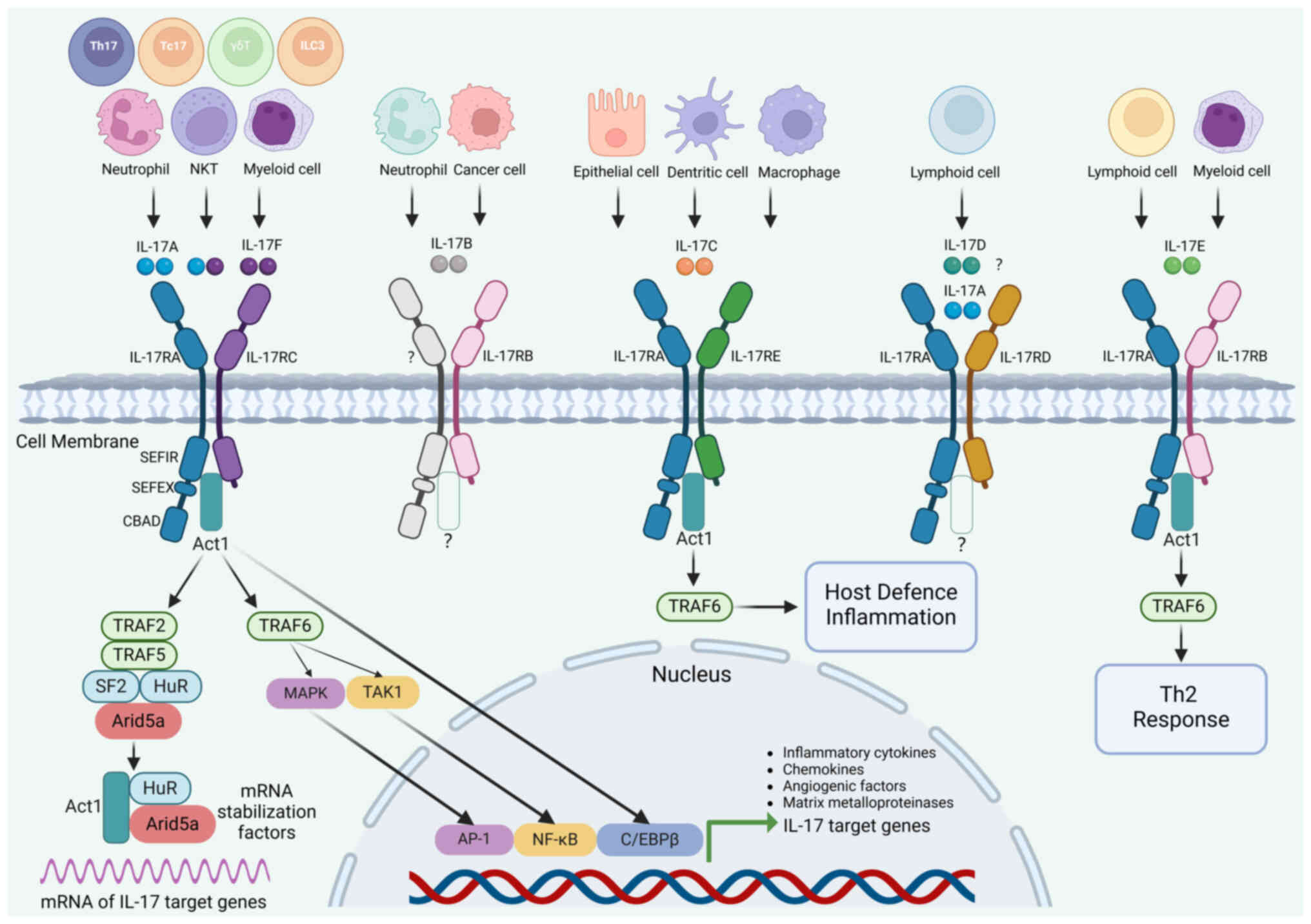 | Figure 1.IL-17 family members and
intracellular signal transduction pathways. The IL-17 family
comprises six cytokine members (IL-17A to IL-17F) and five receptor
members (IL-17RA to IL-17RE). In addition to Th17 cells,
IL-17-producing cells include NKT cells, Tc17 cells, ILCs and
neutrophils. A homodimer or heterodimer formed by IL-17A and IL-17F
binds to the IL-17RA/RC complex, functioning as a hallmark of
IL-17/IL-17R signaling. The downstream signaling pathways of
IL-17B/IL-17RB have not been thoroughly investigated. After
recognizing the IL-17RA/RE complex, the IL-17C homodimer triggers
downstream signaling pathways involved in host defense and
inflammation. Although the receptor for the IL-17D homodimer
remains unidentified, the IL-17RA/RD complex exclusively binds to
the IL-17A homodimer. The IL-17E homodimer binds to the IL-17RA/RB
complex, activating downstream Th2 responses. The intracellular
signaling pathways and critical signaling molecules downstream of
the activation of the IL-17RA/RC complex are summarized. Act1,
NF-κB activator 1; AP-1, activator protein-1; Arid5a, AT-rich
interactive domain-containing protein 5a; C/EBP, CCAAT
enhancer-binding protein; CBAD, C/EBPβ activation domain; HuR,
human antigen R; IL, interleukin; IL-R, IL-receptor; ILC, innate
lymphoid cell; MAPK, mitogen-activated protein kinase; NF-κB,
nuclear factor κB; NKT, natural killer T cell; SEFIR, SEF/IL-17
receptor; SEFEX, SEFIR-extension sequences; Tc17, Type 17
CD8+ cytotoxic cell; Th17, T helper-17 cell; TRAF,
TNF-receptor associated factor; TAK1, transforming growth
factor-β-activated kinase-1. |
IL-17/IL-17R signaling in tumors
IL-17/IL-17R signaling serves a crucial role in the
tumorigenesis and progression of diverse neoplasms by stimulating
distinct pathways and downstream effectors. For instance, IL-17
upregulates zinc finger E-box binding homeobox 1, a pivotal
regulator of epithelial-mesenchymal transition (EMT) through NF-κB,
thus fostering tumor cell invasion and metastasis by precipitating
EMT (11). Additionally, it has
been demonstrated that IL-17 expression is positively correlated
with the activation of STAT3 (56).
Facilitated by the intermediary IL-6, IL-17 can accelerate the
activation of the STAT3 pathway within tumor cells. This leads to
resistance to apoptosis and an increase in angiogenic factors,
consequently promoting tumor cell growth and angiogenesis, thereby
advancing tumor progression (57,58).
IL-17/IL-17R signaling not only has protumor effects
but can also achieve antitumor effects by regulating adaptive
immune responses. These include the recruitment of T lymphocytes,
the enhancement of NKT activity and the stimulation of cytotoxic T
lymphocyte (CTL) production (59–61).
For instance, mice lacking IL-17 displayed reduced levels of
IFN-γ+ T cells infiltrating colon cancer cells (62). This resulted in an increased tumor
volume and number of metastases. Similarly, there is an inverse
relationship between the expression of IL-17 and tumor invasion in
esophageal squamous cell carcinoma. By contrast, a positive
correlation was observed between IL-17 expression and the abundance
of CTLs, NKTs and dendritic cells (DCs) (59). The different roles and corresponding
mechanisms of IL-17/IL-17R signaling in various tumors are
summarized in Table I.
 | Table I.Functions and mechanisms of
IL-17/IL-17R signaling in tumors. |
Table I.
Functions and mechanisms of
IL-17/IL-17R signaling in tumors.
| Effect | Tumor | Mediator | Mechanism | (Refs.) |
|---|
| Pro-tumor | Lung cancer | IL-17 | Induces EMT via the
IL-17/NF-κB/ZEB1 pathway and promotes invasion and metastasis | (11) |
|
|
| IL-17A | Elicits the
phosphorylation activation of ERK1/2 and modulates cell
proliferation and invasion | (21) |
|
| Liver cancer | IL-17 | Facilitates
angiogenesis and tumor progression via the IL-6/STAT3 pathway | (57) |
|
|
| IL-17 | Induces the
transformation of LPCs into CSCs through the downregulation of
miR-122 activity | (102) |
|
| Pancreatic
cancer | IL-17A | Accelerates the
development of ADM and PanIN | (65,103) |
|
|
| IL-17B/IL-17RB | Facilitates the
recruitment of neutrophils, lymphocytes and endothelial cells | (26,89) |
|
|
| Tc17 | Mediates Tc17-iCAFs
interaction, alters the transcriptional profile of tumor cells and
promotes proliferation and metabolism | (58) |
|
| Colorectal
cancer | IL-17 | Potentiates tumor
progression and resistance to chemotherapy via the
IL-17/CXCL17/GPR35 axis | (104) |
|
|
| IL-17A | Induces
mitochondrial dysfunction and cell pyroptosis via the
ROS/NLRP3/capsase4/GSDMD pathway | (10) |
|
| Cervical
cancer | IL-17A | Encourages
polarization of M2 macrophages and promotes metastasis through the
OCT4/IL-17A/p38 pathway | (22) |
|
| Breast cancer | IL-17RB | Promotes
tumorigenesis through the inhibition of cell apoptosis mediated by
NF-κB | (24) |
|
| Gastric cancer | IL-17B/IL-17RB | Drives tumor cell
proliferation and migration via AKT-β-catenin activation | (105) |
|
| Prostate
cancer | IL-17 | Attracts M2
macrophages and promotes tumor growth | (106) |
|
| Oral squamous cell
carcinoma | IL-17A | Co-mediates the
protumor phenotype of neutrophils and EMT with TGF-β1 | (107) |
| Antitumor | Esophageal squamous
cell carcinoma | IL-17 | Enhances the
cytotoxic activity of NKTs and the migration of T cells and
DCs | (59) |
|
| Melanoma | Th17 | Stimulates
CD8+ cytotoxic T lymphocyte responses | (60) |
|
| Thymoma | Th17 | Facilitates diverse
inflammatory leukocyte recruitment (CD4+, CD8+ T cells
and DCs) | (61) |
IL-17/IL-17R signaling in the pathogenesis
of PDAC
PDAC, which is derived from pancreatic epithelial
cells, is the most common exocrine tumor of the pancreas and
accounts for >90% of all pancreatic cancer cases (63). In the presence of oncogenic Kras
mutations and acute or chronic inflammatory stimuli, mature
pancreatic acinar cells display marked plasticity and undergo
differentiation into duct-like cells with ductal features. This
transformation, known as acinar-to-ductal metaplasia (ADM),
subsequently progresses to pancreatic intraepithelial neoplasia
(PanIN) and ultimately gives rise to PDAC (63). A notable increase in the levels of
Th17 and IL-17 in the peripheral blood of patients with PDAC is
positively correlated with the tumor stage. Moreover, the abundance
of Th17 and IL-17A within the TME of patients with PDAC
significantly exceeds their level in peripheral blood and adjacent
normal tissue of the same patients (64). IL-17/IL17R signaling involves a
multitude of pathogenetic processes in PDAC, including ADM, PanIN
and advanced tumor progression (65–67).
During the precancerous stages of PDAC (ADM and
PanIN), IL-17A can accelerate the process of pancreatic ADM while
also assisting in the maintenance of stem cell features in tumor
cells and the recruitment of immune-suppressive granulocytes
(65). IL-17 released from Th17 and
γδT17 cells interacts with the IL-17RA located in PanIN epithelial
cells, resulting in the expansion of the tumor stroma and the
development of PanIN, which can be effectively suppressed
pharmacologically by inhibiting the signal transduction of IL-17
(65). In pancreatic epithelial
cells with the KrasG12D mutation, IL-17A can directly
stimulate the pancreatitis mediator, regenerating islet-derived
3-β, leading to the activation of STAT3, thereby furthering the
development of ADM and PanIN (68).
Moreover, IL-17/IL-17R signaling acts synergistically with the
Notch pathway and aids the differentiation of Th17 cells, to also
promote PanIN and PDAC development (68). Furthermore, IL-17 can modulate the
stem cell features of PanIN, leading to an enhanced embryonic stem
cell signature represented by doublecortin like kinase 1, POU class
2 homeobox 3 and aldehyde dehydrogenase 1 family member A1 and an
increased expression level of IL-17RC through the canonical NF-κB
and MAPK pathways. This ultimately promotes the initiation and
progression of PanIN (69).
Similarly, the impact of IL-17 on promoting the tumorigenic
potential of cancer stem-like cells has also been observed in
ovarian cancer (70).
As PDAC progresses, IL-17/IL-17R signaling also
plays a pivotal role. For instance, IL-17A collaborates with IL-4
to activate NF-κB within PDAC tumor cells, thereby inducing the
expression of dual oxidase 2 (DUOX2), triggering the accumulation
of extracellular hydrogen peroxide and reactive oxygen species
(ROS)-induced oxidative stress-related DNA damage and consequently
fostering tumor progression (71).
Additionally, activating IL-17B/RB on tumor cells can subsequently
phosphorylate and activate ERK1/2, enhancing the production of a
series of chemokines, such as C-C motif chemokine ligand (CCL)20,
CXCL1, IL-8 and trefoil factor 1, ultimately facilitating the
recruitment of neutrophils, lymphocytes and endothelial cells,
which assists the invasion and metastasis of PDAC (26). Moreover, activating the IL-17B/RB
pathway in activated pancreatic stellate cells can also enhance the
metabolism and proliferation of PDAC tumor cells (72). Furthermore, in a KPC-OG murine model
designed to study antigen-specific immune responses in the context
of PDAC, enhanced inflammation, fibrosis and neovascularization
were observed in KPC-OG lesions after IL-17 stimulation through the
phosphorylation and activation of ERK, STAT3, and EGFR (73).
In general, these findings underscore the
significant role of IL-17/IL-17R signaling and its associated
pathways in the tumorigenesis and progression of PDAC, suggesting
that IL-17/IL-17R is a promising target for therapeutic
interventions against PDAC. In particular, studies on the effect of
IL-17 on PDAC tumor cells have focused primarily on the initial
phases of the tumor, wherein IL-17 modulates the stem cell features
of tumor cells, and on the inflammation-cancer transformation of
epithelial cells. Therefore, it is imperative to delve further into
the involvement of IL-17/IL-17R signaling in the advanced stages of
PDAC, as this will provide more compelling evidence for the
targeting of IL-17 in PDAC. IL-17/IL-17R signaling in the
pathogenesis of PDAC is summarized in Fig. 2.
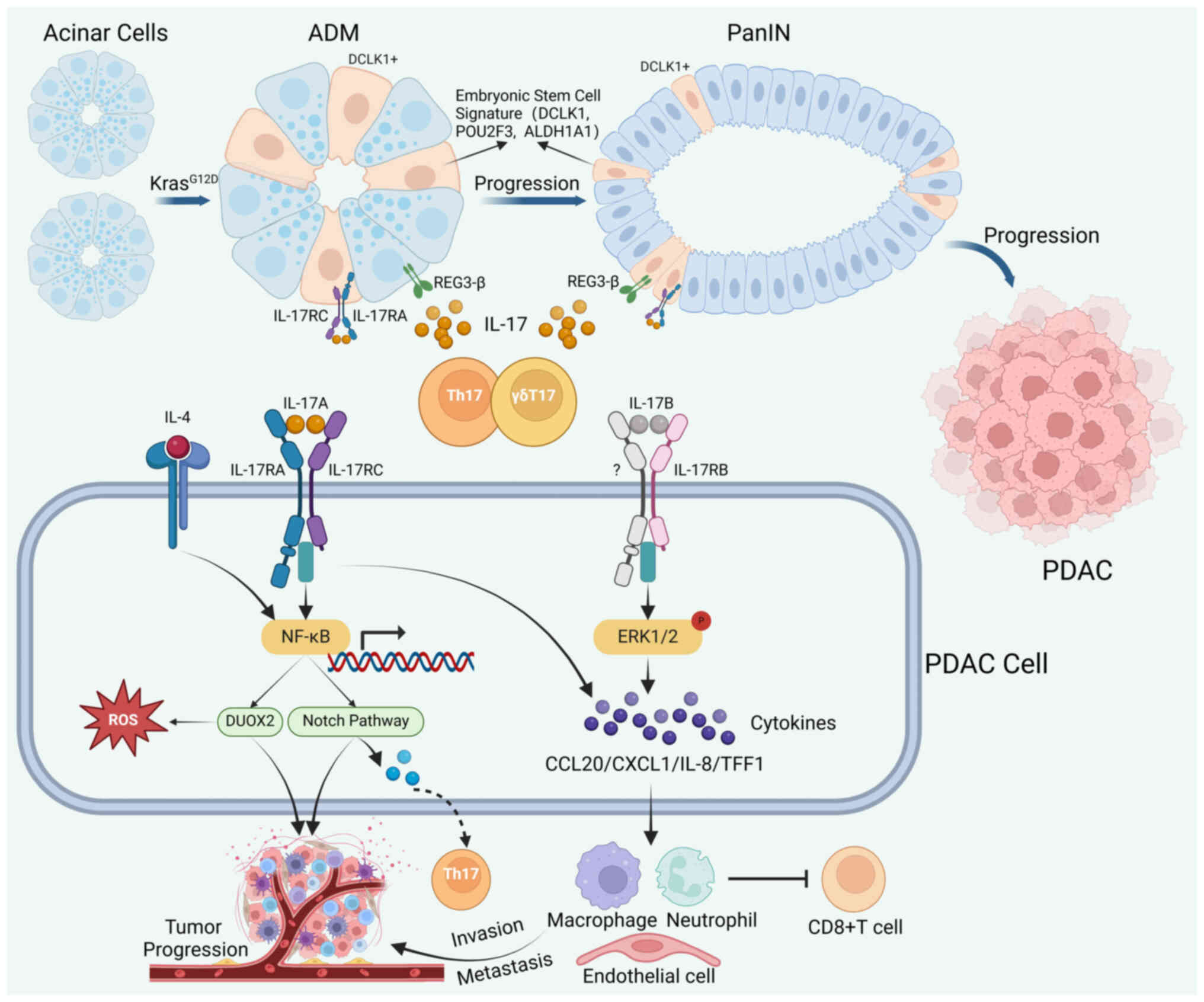 | Figure 2.IL-17/IL-17R signaling in the
pathogenesis of PDAC. In the presence of the oncogenic
KrasG12D mutation, mature pancreatic acinar cells can
differentiate into duct-like cells. These duct-like cells
subsequently progress through stages of PanIN leading to PDAC.
During this process, IL-17A released from Th17 and γδT cells
stimulates REG3-β in pancreatic epithelial cells to promote the
progression of ADM and PanIN. IL-17A helps to maintain the stem
cell features of PanIN cells, leading to an enhanced embryonic stem
cell signature and expression of IL-17RC. IL-17A collaborates with
IL-4 to activate NF-κB in PDAC, thus inducing the expression of
DUOX2 and consequently promoting tumor progression by triggering
the ROS signature. The activation of NF-κB leads to overexpression
of the Notch pathway in PanIN and PDACs. This promotes the
progression of PanIN and PDAC and facilitates the differentiation
of Th17 cells. Activating IL-17B/RB in PDAC tumor cells can
subsequently phosphorylate and activate ERK1/2, enhancing the
production of a series of chemokines and facilitating the
recruitment of neutrophils, lymphocytes, and endothelial cells,
which ultimately contributes to the invasion and metastasis of
PDAC. ADM, acinar duct metaplasia; DUOX2, dual oxidase 2; ERK,
extracellular signal-regulated kinase; IL, interleukin; IL-R,
IL-receptor; NF-κB, nuclear factor κB; PanIN, pancreatic
intraepithelial neoplasia; PDAC, pancreatic ductal adenocarcinoma;
ROS, reactive oxygen species; Th17, T helper-17 cell; REG3-β,
regenerating islet-derived 3β; DCLK1, doublecortin like kinase 1;
POU2F3, POU class 2 homeobox 3; ALDH1A1, aldehyde dehydrogenase 1
family member A1; CCL20, C-C motif chemokine ligand 20; CXCL1,
C-X-C motif chemokine ligand 1; TFF1, trefoil factor 1. |
IL-17/IL-17R signaling in the PDAC TME
The TME of PDAC, characterized by markedly high
stromal density and spatial heterogeneity, encompasses a plethora
of cell components, such as fibroblasts, endothelial cells and
immune cells, that are surrounded by an extracellular matrix and
different types of signaling molecules (74). Compared with those of the normal
pancreas, the distinct milieu of the PDAC TME exhibits a series of
changes in immune cells and immunomodulatory molecules. A study has
shown a reduction in regulatory T (Treg) cells and increased IL-17A
expression levels in the TME of patients with PDAC in the stable or
remission phases. Conversely, patients with unresectable advanced
PDAC exhibit elevated Treg infiltration and reduced expression
levels of IL-17A in the TME (8).
Growing evidence indicates that tumor cells within the PDAC
microenvironment promote cell proliferation and immune escape by
engaging neighboring immune cells and the tumor stroma (74,75).
Activation of IL-17/IL-17R signaling and downstream pathways occurs
to varying degrees in different cells within the TME and
contributes to shaping an immunosuppressive microenvironment
together, thus promoting the progression of PDAC (9,76).
Th17 differentiation from naïve CD4+ T
cells can be induced by DCs located in the TME of PDAC. This is
achieved through the secretion of cytokines, including IL-23, IL-6
and TGF-β (76). Consequently, Th17
differentiation stimulates the synthesis of IL-17 and the
progression of PDAC. Notably, IL-17-stimulated tumor cells can
recruit neutrophils through the release of several cytokines and
chemokines (CCXL5, CXCL3, CSF3, CCL20 and CCXL1), triggering
neutrophil extracellular traps (NETs) and cytotoxic CD8+
T-cell exclusion and maintaining the immunosuppressive
microenvironment (12).
Nonetheless, research has indicated that neutrophils can suppress
IL-17+ γδT cells through ROS signaling, thereby exerting
antitumor effects (77). A recent
study indicated that eliminating IL-17RA in PDAC tumor epithelial
cells could lead to the enhanced infiltration of CD8+ T
cells into the TME. Mechanistically, IL-17/IL-17R signaling
regulates immune responses via the expression of the co-stimulatory
molecule, B7-H4, in tumor cells, hence promoting an
immunosuppressive microenvironment (78).
A prominent feature of the PDAC TME is its high
stromal density, where cancer-associated fibroblasts (CAFs)
contribute greatly (76). A
previous study has demonstrated that IL-17 can activate fibroblasts
through metabolic reprogramming and proliferation enhancement via
the induction of hypoxia inducible factor 1 subunit α (HIF1α)
expression (7). Accordingly,
depletion of IL-17A in the TME of PDAC can reshape the features of
CAFs and the tumor stroma, resulting in increased IL-17F levels in
the serum and elevated IL-17R expression levels in CAFs, ultimately
fostering an antitumor microenvironment. Specifically, the presence
of IL-17A leads to collagen accumulation and a progressive increase
in stiffness in the TME, thus hindering immune cell infiltration
and promoting EMT (9). Conversely,
IL-17A depletion in PDAC causes enhanced infiltration of α smooth
muscle actin (αSMA)+ fibroblasts, macrophages
(especially CD80+ macrophages), CD3+ T cells
and CD8+ T cells and downregulated infiltration of Treg
cells (9). The influence of
IL-17/IL-17R signaling on CAFs is also evident in the interaction
between Tc17 cells and inflammatory CAFs (iCAFs). IL-17A secreted
by Tc17 cells may synergistically promote the differentiation of
protumor iCAFs upon TNF stimulation, and activated iCAFs can in
turn promote the differentiation of Tc17 cells through an IL-6
positive feedback loop. iCAFs activated by Tc17 cells can transform
PDAC tumor cells toward a ‘basal-like’ transcriptional subtype
signature, with enhanced proliferation and metabolism (58).
Macrophages also play an essential role in
modulating the immunosuppressive microenvironment of PDAC.
Depletion of IL-17A in the TME can reshape macrophage
characteristics through metabolic reprogramming, with reduced
tumor-associated macrophage (TAM) and Treg infiltration and
increased CD8+ T-cell infiltration (79). Notably, receptor-interacting
serine/threonine-protein kinase 1 suppression in TAMs can trigger a
distinctive ‘mixed’ Th1/Th17 cell phenotype in PDAC, which is
associated with the superior efficacy of immunotherapy (80).
The influence of the gut microbiome on the TME has
been extensively documented (81).
Recent research has indicated that gut dysbiosis triggered by
enteric IL-17RA deficiency results in the expansion and
infiltration of Th17 and CD20+ B cells in PDAC.
Specifically, there exists a compensatory IL-17 loop between
extra-tumoral sites, such as the gut, and the PDAC TME. In this
context, the deficiency of IL-17RA in enteric epithelial cells
leads to gut microbial changes, which in turn drive systemic IL-17
production and promote the growth of pancreatic tumors through
IL-17RA signaling pathways within tumor cells. In tumor cells,
IL-17/IL-17RA signaling stimulates tumor growth through the
activation of the oxidative stress-related gene, DUOX2 (82). Another study demonstrated that the
inhibitory effect of gut microbiome depletion by antibiotics on
pancreatic tumor growth was mitigated in mice following treatment
with an IL-17 neutralizing antibody (83). This underscores the significant role
of IL-17 signaling in the interaction between the gut microbiome
and pancreatic cancer progression. A potential mechanism of IL-17
signaling in the progression of pancreatic cancer mediated by
alterations in gut microbiota involves the elevation of the
Bacteroides phylum, as observed in mice with enteric
epithelial IL-17RA deficiency [Il17ra(−/-) and Il17ra(fl/fl);
Villin-Cre mice] (82). Similarly,
it has been reported that a reduction in the levels of
Bacteroides phylum in the gut microbiome can impede Th17
cell differentiation and IL-17 production, resulting in the
suppression of pancreatic tumor growth (84).
These findings provide significant evidence for the
role of IL-17 in shaping the immunosuppressive environment of PDAC.
Although the specific underlying mechanisms remain intricate and
require further exploration, previous studies on neutrophils
(12,65) and CAFs (58) have established a strong basis for
understanding the potential immunosuppressive effects of
IL-17/IL-17R signaling in the TME. Future research should place
more emphasis on specific cell subtypes in the microenvironment to
uncover the precise mechanisms by which IL-17/IL-17R signaling
influences the modulation of the PDAC TME. This detailed
exploration will enhance the understanding of the PDAC TME and pave
the way for novel immunotherapeutic strategies for PDAC.
IL-17/IL-17R signaling involved in the PDAC TME is summarized in
Fig. 3.
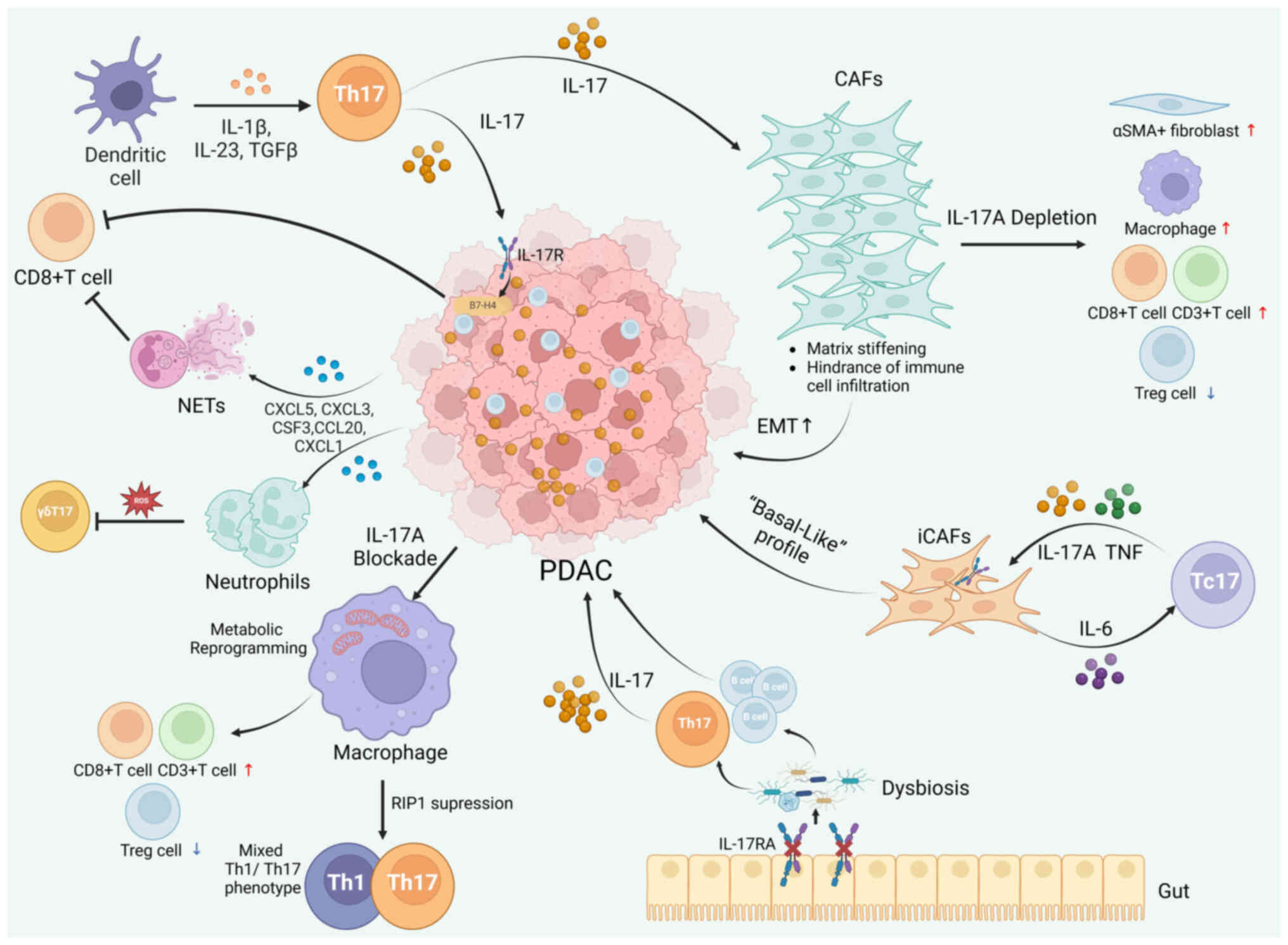 | Figure 3.IL-17/IL-17R signaling in the PDAC
tumor microenvironment. A schematic diagram depicting the role of
IL-17 in maintaining the immunosuppressive microenvironment of
PDAC. IL-23, IL-6 and TGF-β released by dendritic cells stimulate
the differentiation of Th17 cells. IL-17-stimulated tumor cells
recruit neutrophils through the release of several cytokines and
chemokines, triggering NETs and cytotoxic CD8+ T-cell
exclusion. Neutrophils in the TME can suppress IL-17+ γδ
T cells via ROS signaling. IL-17/IL-17R regulates the expression of
the co-stimulatory molecule B7-H4 in PDAC tumor epithelial cells,
thereby downregulating the infiltration of CD8+ T cells.
IL-17A in the TME can reshape the PDAC stromal microenvironment and
CAF characteristics, with a progressive increase in stiffness in
the presence of IL-17A. Conversely, IL-17A depletion causes the
enhanced infiltration of αSMA+ fibroblasts, macrophages,
CD3+ T cells and CD8+ T cells and
downregulated infiltration of Treg cells. Tc17 cells interact with
iCAFs through a positive feedback loop featuring IL-17A, TNF and
IL-6. Depletion of IL-17A in the TME can also reshape macrophage
characteristics through metabolic reprogramming and the recruitment
of effector T cells. RIP1 suppression in PDAC tumor-associated
macrophages can trigger a distinctive ‘mixed’ Th1/Th17 cell
phenotype. Additionally, gut dysbiosis triggered by enteric IL-17RA
deficiency induces tumor-promoting Th17 and B cells, with systemic
increases in IL-17 levels and the subsequent growth of PDAC. CAFs,
cancer-associated fibroblasts; iCAFs, inflammatory CAFs; IL,
interleukin; IL-R, IL-receptor; EMT, epithelial-mesenchymal
transition; NETs, neutrophil extracellular traps; TME, tumor
microenvironment; CCL20, C-C motif chemokine ligand 20; CXCL, C-X-C
motif chemokine ligand; PDAC, pancreatic ductal adenocarcinoma;
ROS, reactive oxygen species; Th17, T helper-17 cell; αSMA, α
smooth muscle actin; RIP1, receptor-interacting
serine/threonine-protein kinase 1; Treg, regulatory T cell. |
IL-17/IL-17R signaling in PDAC cancer
therapy
Chemotherapy
Chemotherapy remains the cornerstone of non-surgical
treatment for patients with PDAC, and gemcitabine is one of the
first-line chemotherapeutic agents (85). Nevertheless, the clinical efficacy
of gemcitabine is often limited by the rapid emergence of
resistance that arises within a brief timeframe for a number of
patients (86). Studies have
validated the contribution of cytokines in the TME, such as
interleukins and TNF, to the emergence of chemotherapy resistance
(87). Notably, IL-17 has been
identified as a promoter of resistance to chemotherapy across
various tumor types (88). Within
PDAC, elevated IL-17RB levels are closely associated with the
enhanced expression of mucin (MUC)1 and MUC4. The upregulated
production of these MUCs by IL-17RB contributes to the enhancement
of tumor stem cell-like features and resistance to gemcitabine
(38). Moreover, another study
revealed that IL-17RB may serve as a predictive marker for the
prognosis of patients with surgically resectable PDAC and the
efficacy of gemcitabine therapy (89). Therefore, targeting IL-17RB and
MUC1/4 may represent a viable strategy for overcoming resistance to
gemcitabine (38). Another
noteworthy discovery is that the combination of the IL-17A antibody
with gemcitabine can induce a distinctive ‘M1-like’ phenotype in
macrophages, with an increased phagocytosis rate and enhanced
antitumor response (79). Notably,
IL-17F may be linked to improved outcomes for patients with PDAC
treated with gemcitabine (90).
Consequently, improving the efficacy of chemotherapy in PDAC may
involve the administration of gemcitabine in conjunction with
IL-17A blockade therapies, including either neutralizing antibodies
or small molecule inhibitors.
Indeed, these discoveries are currently insufficient
to overcome the major challenge of overcoming chemotherapy
resistance in patients with PDAC. Further investigations are
required to explore the potential mechanisms and contributors to
IL-17-mediated chemoresistance and the development of novel PDAC
chemotherapeutic strategies.
Immune checkpoint inhibitors
(ICIs)
The combination of immunotherapy approaches, chiefly
based on ICIs, has yielded noteworthy therapeutic advantages for
patients with PDAC in recent years (91). According to recent research, members
of the IL-17 family are indicators of the potential therapeutic
effect of ICIs and are related to the infiltration of immune cells
in tumors (92). The impact of
IL-17 and Th17 cells on the therapeutic efficacy of ICIs has been
reported in different types of tumor (7,93,94).
For instance, in cutaneous squamous cell carcinoma, the
IL-17-Act1-HIFα pathway facilitates the proliferation and collagen
deposition of CAFs, promoting resistance to αPD-L1 (7). In colorectal cancer, a similar
increase in resistance to αPD-L1 is attributed to the activation of
the p65/nuclear respiratory factor 1/miRNA-15b-5p axis induced by
IL-17A (94). PD-L1/programmed
death-1 (PD-1) expression levels are reduced after IL-17 depletion,
which modulates the function and frequency of myeloid-derived
suppressor cells and enhances antitumor responses in breast cancer
(93). In essence, anti-IL-17
sensitization to ICI immunotherapy is effective across different
types of cancer.
In PDAC, the function of CD8+ T cells can
be inhibited by CD25+ Th17 cells through
CTLA-4-dependent mechanisms, thereby decreasing the concentration
of IL-17. This finding indicates the potential role of Th17 and
IL-17 in PDAC immunotherapy (95).
Coincidentally, the combined use of IL-17 blockade with αPD-1 or
αCTLA-4 effectively reduces pancreatic tumor volume by activating
CD8+ T cells and mitigating the resistance caused by the
single use of the aforementioned drugs (12). This observation underscores the
promising clinical application of such combination strategies in
PDAC. The relevance of Th17 cells to therapeutic efficacy was
demonstrated in a preclinical study that investigated combination
immunotherapy for PDAC. This study revealed that Th17 levels
increased in a pancreatic cancer mouse model following anti-PD-L1
immunotherapy. Notably, Th17 cells increased both at the onset of
PDAC and post-immunotherapy, indicating the context dependency of
this signaling pathway, as Th17 cells play distinct roles across
different stages of PDAC (96).
Mechanistically, research has indicated that although IL-17
regulates the expression of PD-L1 in myeloid cells, it is not
directly responsible for the expression of PD-L1 in tumor cells
(97). This discovery suggests that
IL-17 mediates the sensitivity of PDAC to anti-PD-L1 immunotherapy
through complex interactions with components of the TME. Further
comprehensive studies are required to elucidate these
interactions.
In a recent study, the application of virus-like
vesicles for the delivery of three immunomodulators (IL-12, PD-L1
short hairpin RNA and dnIL17-RA), in combination with IL-17
blockade and αPD-L1, decreased tumor growth and prolonged the
overall survival of mice (98). The
future implementation of innovative immunotherapies targeting IL-17
in combination with immune checkpoint molecules holds significant
potential and prospects for PDAC treatment.
Innovative immunotherapy
strategies
Tumor vaccines have represented a burgeoning field
in tumor immunotherapy in recent years. A previous study has
indicated that IL-17 may inhibit the therapeutic efficacy of tumor
vaccines by restricting the development of efficient antitumor
CD8+ T-cell responses (98). A previous study on the application
of the GM-CSF-modified tumor cell vaccine (GVAX) in patients with
PDAC revealed that following GVAX vaccine immunotherapy, Th17
signaling was enhanced, while Treg signaling was weakened in
tertiary lymphoid structures (TLSs), which was correlated with
prognosis (99). Moreover, a study
has confirmed that Th17 signaling can stimulate the production and
development of TLSs in PDAC (100). Thus, the IL-17/IL-17R
signaling-based Th17 signature could serve as a prognostic
indicator for patients with PDAC after GVAX vaccine
immunotherapy.
The gut microbiome has garnered increasing attention
in the field of immunotherapy in recent years. Researchers have
shown that the gut microbiome and its products can promote the
progression of gastrointestinal tumors through interactions with
the host immune system (101). A
study has shown that the oral administration of broad-spectrum
antibiotics can reduce the number of IL-17-producing cells and
increase the number of IFN-γ+ T cells, thereby
inhibiting the growth of PDAC (83). Hence, regulating the gut microbiome
could become a new strategy for PDAC immunotherapy. The
IL-17/IL-17R signaling involved in different PDAC treatments is
summarized in Table II.
 | Table II.IL-17/IL-17R signaling in PDAC
treatment response. |
Table II.
IL-17/IL-17R signaling in PDAC
treatment response.
| Therapeutic
strategy | Mediator | Biological effect
and mechanism | (Refs.) |
|---|
| Gemcitabine | IL-17RB | Benefits
gemcitabine resistance by upregulating MUC1 and MUC4
expression | (38) |
| Gemcitabine | IL-17A | Remodels the
phenotype of macrophages, improving gemcitabine resistance with the
IL-17A antibody | (79) |
| Gemcitabine | IL-17F | Associated with
favorable outcomes in patients with PDAC undergoing gemcitabine
treatment | (90) |
|
Anti-PD-1/CTLA-4 | IL-17A/IL-17RA | Enhances the
responsiveness of ICIs via CD8+ T cell activation after
IL-17 blocking | (12) |
|
Anti-PD-1/CTLA-4 | Th17 | CD25+
Th17 impedes the function of Tc cells by overexpressing CTLA-4;
increases Th17 levels in mice following anti-PD-L1
immunotherapy | (95,96) |
| GVAX | Th17 | Stimulates the
production and development of tertiary lymph nodes in PDAC and
serves as a prognostic indicator for patients with PDAC post-GVAX
immunotherapy | (99) |
| Anti-intestinal
microbiome | IL-17 | Reduces the level
of IL-17-producing cells with oral broad-spectrum antibiotics, thus
inhibiting PDAC growth | (83) |
Conclusion and perspectives
In summary, the present review provides a detailed
discussion on the functional mechanisms involving the IL-17 family
and its receptors in the tumorigenesis and progression of
pancreatic cancer. These findings highlight the crucial roles
played by IL-17/IL-17R signaling and the associated pathways in
this exceptionally malignant tumor, when considering both the TME
and treatment responsiveness. Treating pancreatic cancer presents a
considerable challenge when focusing on a single target, such as
ICIs or IL-17, owing to the unique characteristics of the TME. An
enhanced understanding of the interplay among immune cells, tumor
cells and diverse regulatory factors of the immune system is
imperative for developing more efficacious therapeutic approaches.
At present, monoclonal antibodies targeting IL-17, such as
brodalumab and secukinumab, have received approval for treating
autoimmune diseases (5). We
consider that IL-17 blockade could serve as a propellant to
overcome chemotherapy resistance in various malignancies, including
PDAC, and will serve as a crucial component of future
immunotherapeutic innovations.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 82102940).
Availability of data and materials
Not applicable.
Authors' contributions
WL and XW contributed equally to this manuscript. WL
was responsible for the initial conceptualization of the review,
developed the research questions and coordinated the overall
writing process. XW also conducted the literature search, selected
relevant studies and drafted the initial section on ‘IL-17/IL-17R
signaling in the pathogenesis of PDAC’. XW assisted with the
writing of the background section and the implications of the
findings in the context of ‘IL-17/IL-17R signaling in PDAC cancer
therapy’. Additionally, XW assisted in the revision of the
manuscript and responded to reviewer comments. WW was responsible
for the overall coordination and supervision of the project,
ensured the integrity and coherence of the manuscript, facilitated
communication with the co-authors and managed the submission
process. WW also contributed to the writing of the introduction and
conclusion sections, providing a comprehensive overview of the
objectives and implications of the review. All authors have read
and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Act1
|
NF-κB activator 1
|
|
ADM
|
acinar-to-ductal metaplasia
|
|
CAF
|
cancer-associated fibroblast
|
|
iCAF
|
inflammatory CAF
|
|
CTLA-4/8
|
cytotoxic T-lymphocyte-associated
protein 4/8
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
GVAX
|
GM-CSF-modified tumor cell vaccine
|
|
ICI
|
immune checkpoint inhibitor
|
|
IL-17
|
interleukin-17
|
|
IL-17R
|
interleukin-17 receptor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor κB
|
|
NKT
|
natural killer T cell
|
|
PanIN
|
pancreatic intraepithelial
neoplasia
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
PD-1
|
programmed death-1
|
|
PD-L1
|
programmed death-ligand 1
|
|
ROS
|
reactive oxygen species
|
|
SEFIR
|
SEF/IL-17 receptor
|
|
Tc17
|
Type 17 CD8+ cytotoxic T
cell
|
|
Th17
|
T helper 17 cell
|
|
TLS
|
tertiary lymphoid structure
|
|
TME
|
tumor microenvironment
|
|
TRAF
|
TNF-receptor associated factor
|
|
Treg
|
regulatory T cell
|
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeLeo AB and Appella E: The p53 saga:
Early steps in the development of tumor immunotherapy. J Immunol.
204:2321–2328. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwanaga N and Kolls JK: Updates on T
helper type 17 immunity in respiratory disease. Immunology.
156:3–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McGeachy MJ, Cua DJ and Gaffen SL: The
IL-17 family of cytokines in health and disease. Immunity.
50:892–906. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pacha O, Sallman MA and Evans SE:
COVID-19: A case for inhibiting IL-17? Nat Rev Immunol. 20:345–346.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen X, Zhao J, Herjan T, Hong L, Liao Y,
Liu C, Vasu K, Wang H, Thompson A, Fox PL, et al: IL-17-induced
HIF1α drives resistance to anti-PD-L1 via fibroblast-mediated
immune exclusion. J Exp Med. 219:e202106932022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Cheng H, Luo G, Lu Y, Jin K, Guo M,
Ni Q and Yu X: Circulating regulatory T cell subsets predict
overall survival of patients with unresectable pancreatic cancer.
Int J Oncol. 51:686–694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mucciolo G, Curcio C, Roux C, Li WY,
Capello M, Curto R, Chiarle R, Giordano D, Satolli MA, Lawlor R, et
al: IL17A critically shapes the transcriptional program of
fibroblasts in pancreatic cancer and switches on their
protumorigenic functions. Proc Natl Acad Sci USA.
118:e20203951182021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng WQ, Zhang YC, Xu ZQ, Yu SY, Huo JT,
Tuersun A, Zheng MH, Zhao JK, Zong YP and Lu AG: IL-17A-mediated
mitochondrial dysfunction induces pyroptosis in colorectal cancer
cells and promotes CD8 + T-cell tumour infiltration. J Transl Med.
21:3352023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu K, Li MM, Shen J, Liu F, Cao JY, Jin S
and Yu Y: Interleukin-17-induced EMT promotes lung cancer cell
migration and invasion via NF-κB/ZEB1 signal pathway. Am J Cancer
Res. 5:1169–1179. 2015.PubMed/NCBI
|
|
12
|
Zhang Y, Chandra V, Riquelme Sanchez E,
Dutta P, Quesada PR, Rakoski A, Zoltan M, Arora N, Baydogan S,
Horne W, et al: Interleukin-17-induced neutrophil extracellular
traps mediate resistance to checkpoint blockade in pancreatic
cancer. J Exp Med. 217:e201903542020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu S, Song X, Chrunyk BA, Shanker S, Hoth
LR, Marr ES and Griffor MC: Crystal structures of interleukin 17A
and its complex with IL-17 receptor A. Nat Commun. 4:18882013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rouvier E, Luciani MF, Mattéi MG, Denizot
F and Golstein P: CTLA-8, cloned from an activated T cell, bearing
AU-rich messenger RNA instability sequences, and homologous to a
herpesvirus saimiri gene. J Immunol. 150:5445–5456. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marques HS, de Brito BB, da Silva FAF,
Santos MLC, de Souza JCB, Correia TML, Lopes LW, Neres NSM, Dórea
RSDM, Dantas ACS, et al: Relationship between Th17 immune response
and cancer. World J Clin Oncol. 12:845–867. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lückel C, Picard FSR and Huber M: Tc17
biology and function: Novel concepts. Eur J Immunol. 50:1257–1267.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuwabara T, Ishikawa F, Kondo M and
Kakiuchi T: The role of IL-17 and related cytokines in inflammatory
autoimmune diseases. Mediators Inflamm. 2017:39080612017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Z, Yu Q, Xu H, Dai X, Yu Y, Cui L, Chen
Y, Gu J, Zhang X, Guo C and Shi Y: IL-17A promotes
psoriasis-associated keratinocyte proliferation through
ACT1-dependent activation of YAP-AREG axis. J Invest Dermatol.
142:2343–2352. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hot A and Miossec P: Effects of
interleukin (IL)-17A and IL-17F in human rheumatoid arthritis
synoviocytes. Ann Rheum Dis. 70:727–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kolbinger F, Huppertz C, Mir A and Padova
FD: IL-17A and multiple sclerosis: Signaling pathways, producing
cells and target cells in the central nervous system. Curr Drug
Targets. 17:1882–1893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Wan J, Liu J, Xie W, Diao X, Xu J,
Zhu B and Chen Z: Increased IL-17-producing cells correlate with
poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer.
69:348–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bian Z, Wu X, Chen Q, Gao Q, Xue X and
Wang Y: Oct4 activates IL-17A to orchestrate M2 macrophage
polarization and cervical cancer metastasis. Cancer Immunol
Immunother. 73:732024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bastid J, Dejou C, Docquier A and Bonnefoy
N: The emerging role of the IL-17B/IL-17RB pathway in cancer. Front
Immunol. 11:7182020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang CK, Yang CY, Jeng YM, Chen CL, Wu
HH, Chang YC, Ma C, Kuo WH, Chang KJ, Shew JY and Lee WH:
Autocrine/paracrine mechanism of interleukin-17B receptor promotes
breast tumorigenesis through NF-κB-mediated antiapoptotic pathway.
Oncogene. 33:2968–2977. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang YF, Lee YC, Lo S, Chung YN, Hsieh YC,
Chiu WC and Yuan SF: A positive feedback loop of IL-17B-IL-17RB
activates ERK/β-catenin to promote lung cancer metastasis. Cancer
Lett. 422:44–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu HH, Hwang-Verslues WW, Lee WH, Huang
CK, Wei PC, Chen CL, Shew JY, Lee EY, Jeng YM, Tien YW, et al:
Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody
blocks pancreatic cancer metastasis by silencing multiple
chemokines. J Exp Med. 212:333–349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Samadi A, Kouri VP, Salem A, Ainola M,
Kaivosoja E, Barreto G, Konttinen YT, Hietanen J and
Häyrinen-Immonen R: IL-17C and its receptor IL-17RA/IL-17RE
identify human oral epithelial cell as an inflammatory cell in
recurrent aphthous ulcer. J Oral Pathol Med. 43:117–124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nies JF and Panzer U: IL-17C/IL-17RE:
Emergence of a unique axis in TH17 biology. Front Immunol.
11:3412020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramirez-Carrozzi V, Sambandam A, Luis E,
Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, et al:
IL-17C regulates the innate immune function of epithelial cells in
an autocrine manner. Nat Immunol. 12:1159–1166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jungnickel C, Schmidt LH, Bittigkoffer L,
Wolf L, Wolf A, Ritzmann F, Kamyschnikow A, Herr C, Menger MD,
Spieker T, et al: IL-17C mediates the recruitment of
tumor-associated neutrophils and lung tumor growth. Oncogene.
36:4182–4190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Sun S and Liu D: IL-17D: A less
studied cytokine of IL-17 family. Int Arch Allergy Immunol.
181:618–623. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borowczyk J, Shutova M, Brembilla NC and
Boehncke WH: IL-25 (IL-17E) in epithelial immunology and
pathophysiology. J Allergy Clin Immunol. 148:40–52. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu M and Dong C: IL-25 in allergic
inflammation. Immunol Rev. 278:185–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gaffen SL: Structure and signalling in the
IL-17 receptor family. Nat Rev Immunol. 9:556–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qian Y, Liu C, Hartupee J, Altuntas CZ,
Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al: The
adaptor Act1 is required for interleukin 17-dependent signaling
associated with autoimmune and inflammatory disease. Nat Immunol.
8:247–256. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herjan T, Hong L, Bubenik J, Bulek K, Qian
W, Liu C, Li X, Chen X, Yang H, Ouyang S, et al:
IL-17-receptor-associated adaptor Act1 directly stabilizes mRNAs to
mediate IL-17 inflammatory signaling. Nat Immunol. 19:354–365.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin
SD and Qian Y: IL-17RE is the functional receptor for IL-17C and
mediates mucosal immunity to infection with intestinal pathogens.
Nat Immunol. 12:1151–1158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsai LH, Hsu KW, Chiang CM, Yang HJ, Liu
YH, Yang SF, Peng PH, Cheng WC and Wu HH: Targeting interleukin-17
receptor B enhances gemcitabine sensitivity through downregulation
of mucins in pancreatic cancer. Sci Rep. 10:178172020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu HH, Tsai LH, Huang CK, Hsu PH, Chen MY,
Chen YI, Hu CM, Shen CN, Lee CC, Chang MC, et al: Characterization
of initial key steps of IL-17 receptor B oncogenic signaling for
targeted therapy of pancreatic cancer. Sci Transl Med.
13:eabc28232021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ho AW and Gaffen SL: IL-17RC: A partner in
IL-17 signaling and beyond. Semin Immunopathol. 32:33–42. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rodriguez C, Araujo Furlan CL, Tosello
Boari J, Bossio SN, Boccardo S, Fozzatti L, Canale FP, Beccaria CG,
Nuñez NG, Ceschin DG, et al: Interleukin-17 signaling influences
CD8+ T cell immunity and tumor progression according to the IL-17
receptor subunit expression pattern in cancer cells.
Oncoimmunology. 12:22613262023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Su Y, Huang J, Zhao X, Lu H, Wang W, Yang
XO, Shi Y, Wang X, Lai Y and Dong C: Interleukin-17 receptor D
constitutes an alternative receptor for interleukin-17A important
in psoriasis-like skin inflammation. Sci Immunol. 4:eaau96572019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pande S, Yang X and Friesel R:
Interleukin-17 receptor D (Sef) is a multi-functional regulator of
cell signaling. Cell Commun Signal. 19:62021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vella G, Lunding L, Ritzmann F, Honecker
A, Herr C, Wegmann M, Bals R and Beisswenger C: The IL-17 receptor
IL-17RE mediates polyIC-induced exacerbation of experimental
allergic asthma. Respir Res. 21:1762020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pätzold L, Stark A, Ritzmann F, Meier C,
Tschernig T, Reichrath J, Bals R, Bischoff M and Beisswenger C:
IL-17C and IL-17RE promote wound closure in a Staphylococcus
aureus-Based murine wound infection model. Microorganisms.
9:18212021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW,
Cai XY, Zhou J, Cheng YF, Fan J and Qiu SJ: High expression of
IL-17 and IL-17RE associate with poor prognosis of hepatocellular
carcinoma. J Exp Clin Cancer Res. 32:32013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gu C, Wu L and Li X: IL-17 family:
Cytokines, receptors and signaling. Cytokine. 64:477–485. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schwandner R, Yamaguchi K and Cao Z:
Requirement of tumor necrosis factor receptor-associated factor
(TRAF)6 in interleukin 17 signal transduction. J Exp Med.
191:1233–1240. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Napetschnig J and Wu H: Molecular basis of
NF-κB signaling. Annu Rev Biophys. 42:443–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mitchell S, Vargas J and Hoffmann A:
Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med.
8:227–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Oeckinghaus A, Hayden MS and Ghosh S:
Crosstalk in NF-κB signaling pathways. Nat Immunol. 12:695–708.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu NL, Huang DY, Tsou HN, Lin YC and Lin
WW: Syk mediates IL-17-induced CCL20 expression by targeting
Act1-dependent K63-linked ubiquitination of TRAF6. J Invest
Dermatol. 135:490–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tanaka H, Arima Y, Kamimura D, Tanaka Y,
Takahashi N, Uehata T, Maeda K, Satoh T, Murakami M and Akira S:
Phosphorylation-dependent Regnase-1 release from endoplasmic
reticulum is critical in IL-17 response. J Exp Med. 216:1431–1449.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Amatya N, Childs EE, Cruz JA, Aggor FEY,
Garg AV, Berman AJ, Gudjonsson JE, Atasoy U and Gaffen SL: IL-17
integrates multiple self-reinforcing, feed-forward mechanisms
through the RNA binding protein Arid5a. Sci Signal.
11:eaat46172018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gaffen SL, Jain R, Garg AV and Cua DJ: The
IL-23-IL-17 immune axis: From mechanisms to therapeutic testing.
Nat Rev Immunol. 14:585–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang L, Yi T, Kortylewski M, Pardoll DM,
Zeng D and Yu H: IL-17 can promote tumor growth through an
IL-6-Stat3 signaling pathway. J Exp Med. 206:1457–1464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gu FM, Li QL, Gao Q, Jiang JH, Zhu K,
Huang XY, Pan JF, Yan J, Hu JH, Wang Z, et al: IL-17 induces
AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in
hepatocellular carcinoma. Mol Cancer. 10:1502011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Picard FSR, Lutz V, Brichkina A, Neuhaus
F, Ruckenbrod T, Hupfer A, Raifer H, Klein M, Bopp T, Pfefferle PI,
et al: IL-17A-producing CD8+ T cells promote PDAC via induction of
inflammatory cancer-associated fibroblasts. Gut. 72:1510–1522.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lu L, Pan K, Zheng HX, Li JJ, Qiu HJ, Zhao
JJ, Weng DS, Pan QZ, Wang DD, Jiang SS, et al: IL-17A promotes
immune cell recruitment in human esophageal cancers and the
infiltrating dendritic cells represent a positive prognostic marker
for patient survival. J Immunother. 36:451–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ankathatti Munegowda M, Deng Y, Mulligan
SJ and Xiang J: Th17 and Th17-stimulated CD8+ T cells
play a distinct role in Th17-induced preventive and therapeutic
antitumor immunity. Cancer Immunol Immunother. 60:1473–1484. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Guéry L, Dubrot J, Lippens C, Brighouse D,
Malinge P, Irla M, Pot C, Reith W, Waldburger JM and Hugues S:
Ag-presenting CpG-activated pDCs prime Th17 cells that induce tumor
regression. Cancer Res. 74:6430–6440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kryczek I, Wei S, Szeliga W, Vatan L and
Zou W: Endogenous IL-17 contributes to reduced tumor growth and
metastasis. Blood. 114:357–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Storz P: Acinar cell plasticity and
development of pancreatic ductal adenocarcinoma. Nat Rev
Gastroenterol Hepatol. 14:296–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
He S, Fei M, Wu Y, Zheng D, Wan D, Wang L
and Li D: Distribution and clinical significance of Th17 cells in
the tumor microenvironment and peripheral blood of pancreatic
cancer patients. Int J Mol Sci. 12:7424–7437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
McAllister F, Bailey JM, Alsina J, Nirschl
CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H,
et al: Oncogenic Kras activates a hematopoietic-to-epithelial IL-17
signaling axis in preinvasive pancreatic neoplasia. Cancer Cell.
25:621–637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
McAllister F and Leach SD: Targeting IL-17
for pancreatic cancer prevention. Oncotarget. 5:9530–9531. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang X, Wang L, Mo Q, Dong Y, Wang G and
Ji A: Changes of Th17/Treg cell and related cytokines in pancreatic
cancer patients. Int J Clin Exp Pathol. 8:5702–5708.
2015.PubMed/NCBI
|
|
68
|
Loncle C, Bonjoch L, Folch-Puy E,
Lopez-Millan MB, Lac S, Molejon MI, Chuluyan E, Cordelier P, Dubus
P, Lomberk G, et al: IL17 Functions through the Novel
REG3β-JAK2-STAT3 inflammatory pathway to promote the transition
from chronic pancreatitis to pancreatic cancer. Cancer Res.
75:4852–4862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang Y, Zoltan M, Riquelme E, Xu H, Sahin
I, Castro-Pando S, Montiel MF, Chang K, Jiang Z, Ling J, et al:
Immune cell production of interleukin 17 induces stem cell features
of pancreatic intraepithelial neoplasia cells. Gastroenterology.
155:210–223.e3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xiang T, Long H, He L, Han X, Lin K, Liang
Z, Zhuo W, Xie R and Zhu B: Interleukin-17 produced by tumor
microenvironment promotes self-renewal of CD133+ cancer stem-like
cells in ovarian cancer. Oncogene. 34:165–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wu Y, Konaté MM, Lu J, Makhlouf H, Chuaqui
R, Antony S, Meitzler JL, Difilippantonio MJ, Liu H, Juhasz A, et
al: IL-4 and IL-17A cooperatively promote hydrogen peroxide
production, oxidative DNA damage, and upregulation of dual oxidase
2 in human colon and pancreatic cancer cells. J Immunol.
203:2532–2544. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li J, Wu X, Schiffmann L, MacVicar T, Zhou
C, Wang Z, Li D, Camacho OV, Heuchel R, Odenthal M, et al:
IL-17B/RB activation in pancreatic stellate cells promotes
pancreatic cancer metabolism and growth. Cancers (Basel).
13:53382021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hegde S, Krisnawan VE, Herzog BH, Zuo C,
Breden MA, Knolhoff BL, Hogg GD, Tang JP, Baer JM, Mpoy C, et al:
Dendritic cell paucity leads to dysfunctional immune surveillance
in pancreatic cancer. Cancer Cell. 37:289–307.e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sherman MH and Beatty GL: Tumor
microenvironment in pancreatic cancer pathogenesis and therapeutic
resistance. Annu Rev Pathol. 18:123–148. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hosein AN, Brekken RA and Maitra A:
Pancreatic cancer stroma: An update on therapeutic targeting
strategies. Nat Rev Gastroenterol Hepatol. 17:487–505. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Barilla RM, Diskin B, Caso RC, Lee KB,
Mohan N, Buttar C, Adam S, Sekendiz Z, Wang J, Salas RD, et al:
Specialized dendritic cells induce tumor-promoting IL-10+IL-17+
FoxP3neg regulatory CD4+ T cells in pancreatic carcinoma. Nat
Commun. 10:14242019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mensurado S, Rei M, Lança T, Ioannou M,
Gonçalves-Sousa N, Kubo H, Malissen M, Papayannopoulos V, Serre K
and Silva-Santos B: Tumor-associated neutrophils suppress
pro-tumoral IL-17+γδ T cells through induction of oxidative stress.
PLoS Biol. 16:e20049902018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Castro-Pando S, Howell RM, Li L, Mascaro
M, Faraoni EY, Le Roux O, Romanin D, Tahan V, Riquelme E, Zhang Y,
Kolls JK, Allison JP, Lozano G, Moghaddam SJ and McAllister F:
Pancreatic epithelial IL-17/IL-17RA signaling drives B7-H4
expression to promote tumorigenesis. Cancer Immunol Res. doi:
10.1158/2326-6066.CIR-23-0527, Published online June 6. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Roux C, Mucciolo G, Kopecka J, Novelli F,
Riganti C and Cappello P: IL17A depletion affects the metabolism of
macrophages treated with gemcitabine. Antioxidants (Basel).
10:4222021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang W, Marinis JM, Beal AM, Savadkar S,
Wu Y, Khan M, Taunk PS, Wu N, Su W, Wu J, et al: RIP1 kinase drives
macrophage-mediated adaptive immune tolerance in pancreatic cancer.
Cancer Cell. 34:757–774.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wong-Rolle A, Wei HK, Zhao C and Jin C:
Unexpected guests in the tumor microenvironment: Microbiome in
cancer. Protein Cell. 12:426–435. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chandra V, Li L, Le Roux O, Zhang Y,
Howell RM, Rupani DN, Baydogan S, Miller HD, Riquelme E, Petrosino
J, et al: Gut epithelial Interleukin-17 receptor a signaling can
modulate distant tumors growth through microbial regulation. Cancer
Cell. 42:85–100.e6. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sethi V, Kurtom S, Tarique M, Lavania S,
Malchiodi Z, Hellmund L, Zhang L, Sharma U, Giri B, Garg B, et al:
Gut Microbiota promotes tumor growth in mice by modulating immune
response. Gastroenterology. 155:33–37.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ivanov II, Frutos R de L, Manel N,
Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB and Littman DR:
Specific microbiota direct the differentiation of IL-17-producing
T-helper cells in the mucosa of the small intestine. Cell Host
Microbe. 4:337–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Grossberg AJ, Chu LC, Deig CR, Fishman EK,
Hwang WL, Maitra A, Marks DL, Mehta A, Nabavizadeh N, Simeone DM,
et al: Multidisciplinary standards of care and recent progress in
pancreatic ductal adenocarcinoma. CA Cancer J Clin. 70:375–403.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Amrutkar M and Gladhaug IP: Pancreatic
cancer chemoresistance to gemcitabine. Cancers (Basel). 9:1572017.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Senthebane DA, Rowe A, Thomford NE,
Shipanga H, Munro D, Mazeedi MAMA, Almazyadi HAM, Kallmeyer K,
Dandara C, Pepper MS, et al: The role of tumor microenvironment in
chemoresistance: To survive, keep your enemies closer. Int J Mol
Sci. 18:15862017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cochaud S, Giustiniani J, Thomas C,
Laprevotte E, Garbar C, Savoye AM, Curé H, Mascaux C, Alberici G,
Bonnefoy N, et al: IL-17A is produced by breast cancer TILs and
promotes chemoresistance and proliferation through ERK1/2. Sci Rep.
3:34562013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Song Y, Ji B, Jiang CX, Chen ZM, Yao NH,
Mukaida N and Huang H: IL17RB expression might predict prognosis
and benefit from gemcitabine in patients with resectable pancreatic
cancer. Pathol Res Pract. 215:1526502019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Innocenti F, Owzar K, Cox NL, Evans P,
Kubo M, Zembutsu H, Jiang C, Hollis D, Mushiroda T, Li L, et al: A
genome-wide association study of overall survival in pancreatic
cancer patients treated with gemcitabine in CALGB 80303. Clin
Cancer Res. 18:577–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Morrison AH, Byrne KT and Vonderheide RH:
Immunotherapy and prevention of pancreatic cancer. Trends Cancer.
4:418–428. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Han X, Ye J, Huang R, Li Y, Liu J, Meng T
and Song D: Pan-cancer analysis reveals interleukin-17 family
members as biomarkers in the prediction for immune checkpoint
inhibitor curative effect. Front Immunol. 13:9002732022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Moaaz M, Lotfy H, Motawea MA and Fadali G:
The interplay of interleukin-17A and breast cancer tumor
microenvironment as a novel immunotherapeutic approach to increase
tumor immunogenicity. Immunobiology. 226:1520682021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu C, Liu R, Wang B, Lian J, Yao Y, Sun
H, Zhang C, Fang L, Guan X, Shi J, et al: Blocking IL-17A enhances
tumor response to anti-PD-1 immunotherapy in microsatellite stable
colorectal cancer. J Immunother Cancer. 9:e0018952021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lang C, Wang J and Chen L: CD25-expressing
Th17 cells mediate CD8+ T cell suppression in CTLA-4 dependent
mechanisms in pancreatic ductal adenocarcinoma. Exp Cell Res.
360:384–389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li Y, Hong YK, Wang X, Pandit H, Zheng Q,
Yu Y, Shi X, Chen Y, Tan M, Pulliam Z, et al: Epigenetic modulation
enhances immunotherapy for pancreatic ductal adenocarcinoma. Clin
Transl Immunol. 11:e14302022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Du W, Menjivar RE, Donahue KL, Kadiyala P,
Velez-Delgado A, Brown KL, Watkoske HR, He X, Carpenter ES, Angeles
CV, et al: WNT signaling in the tumor microenvironment promotes
immunosuppression in murine pancreatic cancer. J Exp Med.
220:e202205032023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chen J, Madina BR, Ahmadi E, Yarovinsky
TO, Krady MM, Meehan EV, Wang IC, Ye X, Pitmon E, Ma XY, et al:
Cancer immunotherapy with enveloped self-amplifying mRNA CARG-2020
that modulates IL-12, IL-17 and PD-L1 pathways to prevent tumor
recurrence. Acta Pharm Sin B. 14:335–349. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G,
Soares K, Solt S, Dorman A, Wamwea A, Yager A, et al: Immunotherapy
converts nonimmunogenic pancreatic tumors into immunogenic foci of
immune regulation. Cancer Immunol Res. 2:616–631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Grogan JL and Ouyang W: A role for Th17
cells in the regulation of tertiary lymphoid follicles. Eur J
Immunol. 42:2255–2262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Shalapour S and Karin M: Cruel to be kind:
Epithelial, microbial, and immune cell interactions in
gastrointestinal cancers. Annu Rev Immunol. 38:649–671. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gasmi I, Machou C, Rodrigues A, Brouillet
A, Nguyen TC, Rousseau B, Guillot A, Rodriguez C, Demontant V,
Ait-Ahmed Y, et al: Interleukin-17 programs liver progenitor cell
transformation into cancer stem cells through miR-122
downregulation with increased risk of primary liver cancer
initiation. Int J Biol Sci. 18:1944–1960. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang X, Chen H, Jiang R, Hong X, Peng J,
Chen W, Jiang J, Li J, Huang D, Dai H, et al: Interleukin-17
activates and synergizes with the notch signaling pathway in the
progression of pancreatic ductal adenocarcinoma. Cancer Lett.
508:1–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bu J, Yan W, Huang Y and Lin K: Activation
of the IL-17 signalling pathway by the CXCL17-GPR35 axis affects
drug resistance and colorectal cancer tumorigenesis. Am J Cancer
Res. 13:2172–2187. 2023.PubMed/NCBI
|
|
105
|
Bie Q, Sun C, Gong A, Li C, Su Z, Zheng D,
Ji X, Wu Y, Guo Q, Wang S and Xu H: Non-tumor tissue derived
interleukin-17B activates IL-17RB/AKT/β-catenin pathway to enhance
the stemness of gastric cancer. Sci Rep. 6:254472016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Duan Z, Miller HD, Fu X, Ge D, Jin B,
Moustafa AA, Lan R, Zhang K, Chen Z and You Z: Th17 cells promote
tumor growth in an immunocompetent orthotopic mouse model of
prostate cancer. Am J Clin Exp Urol. 7:249–261. 2019.PubMed/NCBI
|
|
107
|
Yu T, Tang Q, Chen X, Fan W, Zhou Z, Huang
W and Liang F: TGF-β1 and IL-17A comediate the protumor phenotype
of neutrophils to regulate the epithelial-mesenchymal transition in
oral squamous cell carcinoma. J Oral Pathol Med. 50:353–361. 2021.
View Article : Google Scholar : PubMed/NCBI
|

















