Introduction
Acute myeloid leukemia (AML), a prevalent form of
leukemia, poses significant treatment challenges and is frequently
associated with specific chromosomal abnormalities. Symptoms
predominantly include fever, bone pain and bleeding (1,2).
Despite advancements in personalized therapy (3) improving diagnosis and treatment
(4–6), central nervous system (CNS)
involvement remains a significant contributor to severe
complications and mortality in patients with AML, hindering
effective long-term disease management (7–9).
Although CNS infiltration and tumor formation are rare in adult
patients with AML (10,11), they occur more commonly in pediatric
patients (12). In adults, CNS
involvement incidence ranges from 0.6–3% (13), with a recurrence rate of ~2.9–4.1%
(10,13,14).
However, the rate of CNS infiltration by AML cells
in adults may be significantly underestimated due to detection
challenges (7,10). Previous studies indicated that up to
32% of cerebrospinal fluid (CSF) samples and 46% of autopsy
specimens from adult patients with AML reveal CNS involvement
(15,16). Paul and Short (17) reported a CNS involvement rate of
3.2%, with 52% of patients with AML who underwent CSF screening
testing positive for leukemia cells. In a recent study, flow
cytometry detected AML cells in the CSF of 645 patients with AML,
showing 41.7% positivity among those without neurological symptoms
(18). This substantial disparity
arises primarily because lumbar puncture (LP) assessments are
typically performed only on patients with neurological symptoms
(19). Although clinical symptoms
and neuroimaging can indicate CNS involvement, their sensitivity
and specificity vary, with LP remaining the diagnostic gold
standard (20).
In patients with AML, 58% exhibit clinical symptoms
such as headaches, vomiting, fatigue, cognitive changes, seizures
and various cranial nerve palsies, which are often subtle and
difficult to differentiate from drug side effects (21–23).
Although CNS radiological imaging techniques, including magnetic
resonance imaging and computer tomography, demonstrate high
sensitivity, their specificity remains limited (20,24).
Treatment for CNS infiltration involves systemic chemotherapy
capable of reaching the CNS, augmented by intrathecal chemotherapy
and radiotherapy (25–27).
Despite their potential efficacy, these approaches
are associated with relatively high recurrence rates and adverse
effects (28–30). Moreover, the unique CNS
microenvironment serves as a reservoir for AML cells, influencing
disease biology and chemotherapy resistance, which contributes to
BM relapse and reduced survival rates (31,32).
This emphasizes the necessity of deeper understanding of the
specific pathways and mechanisms underlying CNS involvement in
AML.
The infiltration of AML cells into the CNS involves
two primarily phases: Escaping the BM and migrating to the CNS
(1). AML cells must overcome two
barriers-the marrow-blood barrier (MBB) and the brain barrier to
reach the CNS. This process resembles leukocytes migrating from the
BM into the vasculature and subsequently into the tissues, relying
on interactions between migratory cells, vascular endothelial cells
and stromal cells. However, the mechanisms regulating the entry of
AML cells and leukocytes into the vasculature and CNS differ. In
normal hematopoiesis, myeloid progenitor cells, which have not yet
matured into myeloid cells, encounter obstacles crossing into the
CNS, an immune-privileged organ. By contrast, AML cells,
originating from a single myeloid progenitor cells with mutations,
acquire malignant traits that facilitate invasion and migration
(2). Additionally, changes in the
vascular anatomy within the tumor microenvironment during AML
development enable AML cells to escape from the BM (3,4).
The traversal of AML cells through the MBB and brain
barrier is driven by chemotactic factors, governed by cell adhesion
molecules (CAM), and facilitated by proteolytic enzymes (33,34).
Previous literature was analyzed to elucidate the pathways and
mechanisms by which AML cells evade the BM and migrate to the CNS.
Additionally, similarities and differences in escape mechanisms of
AML cells and leukocytes from the BM are discussed. The present
comprehensive overview aimed to assist clinicians and researchers
in developing novel diagnostic and therapeutic strategies. To
identify relevant studies, PubMed was searched for original
articles and reviews up to May 1, 2024, using the search terms
‘AML’, ‘CNS’, ‘peripheral blood’ and ‘therapeutic strategies’.
Studies were eligible if they met the following criteria: i)
Provided detailed descriptions of the pathways and molecular
mechanisms through which AML cells escape from the BM and
infiltration into the CNS; ii) therapeutic approaches targeting
molecules associated with AML cells' egress from BM and entry into
the CNS; iii) were written in English and published in
peer-reviewed journals. Studies were excluded if they solely
identified an association of a molecule with increased leukocyte
count in patients with AML or CNS infiltration without validation
in vivo or in vitro. Additionally, conference
articles lacking original research and studies for which full-text
access was unavailable were excluded.
The escape from the BM
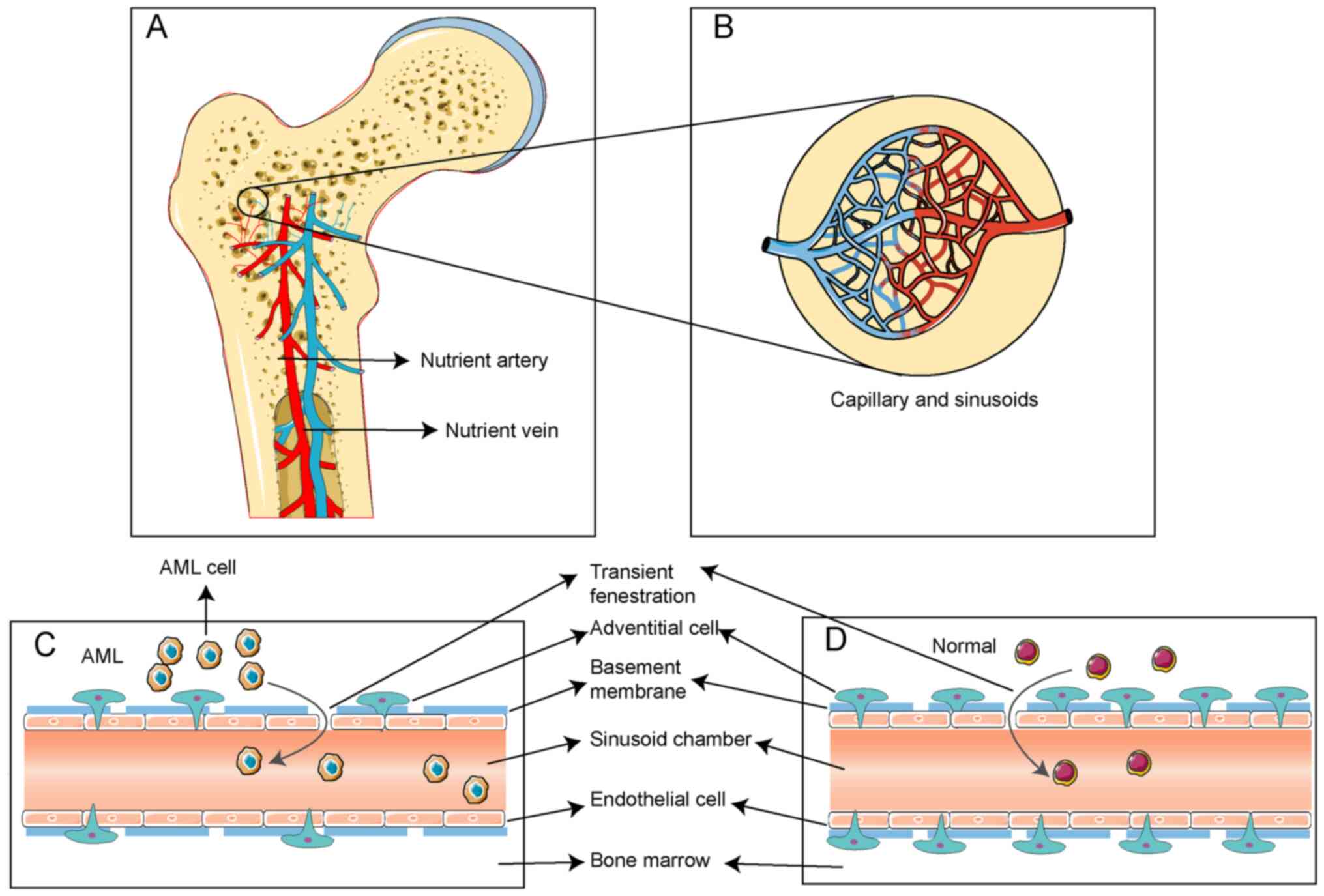
Anatomical structures involved in AML
cells' escape
AML cells originate within the extravascular niche,
necessitating their traversal through the MBB to access the
bloodstream. The hematopoietic blood supply in the BM primarily
derives from nutrient arteries, which return via nutrient veins
situated in the central trabecular bone of long bones (Fig. 1A) (35). Within the BM, nutrient arteries
bifurcate into arterioles, which further subdivide into capillaries
and sinusoidal capillaries, also referred to as blood sinuses
(Fig. 1B) (36). These blood sinuses constitute the
MBB, a barrier delineating the hematopoietic compartment from the
bloodstream (37). The MBB is
composed of endothelial cells, a basement membrane and adventitial
cells, sequentially arranged from the interior outward (Fig. 1C and D) (38).
Endothelial cells form a monolayer lining the inner
wall of blood sinuses essential for cellular trafficking within the
MBB. They connect via tight junctions and exhibit transient
fenestrations that permit cell passage (38). The subendothelial basement membrane
comprises a network of laminin and type IV collagen interacting
with other extracellular matrix (ECM) molecules such as collagen
and fibronectin to facilitate vascular wall development (39,40).
Unlike typical basement membranes, the blood sinus basement
membrane is discontinuous and lacks a reticular structure.
Additionally, it contains an exceptionally high concentration of
sulfated glycosaminoglycans, facilitating communication and
adhesion between hematopoietic cells and vascular endothelial cells
(41). Adventitial cells form the
outermost layer of the blood sinus (42), partially encircling the outer wall
with an adventitial sheath covering ~2/3 of its surface.
The adventitial cell coverage ratio (ACCR)
represents the proportion of adventitial cells enveloping
endothelial cells. A reduced ACCR signifies an increased propensity
for AML cells to enter the bloodstream. Advanced morphometric
analysis demonstrated a substantial decline in ACCR, from 53 to
14%, in a transplantable monomyelocytic leukemia murine model, with
no alterations in the endothelial cell area or perimeter (43). Transmission electron microscopy
revealed that ACCR dropped to 40% among 24 untreated patients with
various forms of acute leukemia, compared with controls.
Furthermore, no abnormal translocation of leukemia cells at
intercellular junctions within blood sinuses was observed (44,45).
In the blood sinuses of patients with AML, leukemia
cells coexist with normal hematopoietic cells. The marrow stroma
also comprises stromal cells, soluble molecules and ECM components
(46). Specific ECM proteins of
leukemic cells can bind leukocyte-specific proteins, thereby
limiting leukocyte migration from the BM (38).
Mechanisms involved in AML cells'
escape
The traversal of the MBB by AML cells hinges on
alterations surface chemokine receptor expression and adhesion
molecules profiles. Endothelial cells critically mediate
interaction between AML cells and the barrier. This section delves
into intrinsic changes within AML cells and their interactions with
the tumor microenvironment, particularly examining how these
interactions facilitate BM escape.
Chemokines and their receptor, such as stromal
cell-derived factor 1 (SDF-1), also known as C-X-C motif chemokine
12 (CXCL12) and chemokine receptor 4 (CXCR-4) (47), are fundamental for the AML cells'
egress from the BM. A previous study by Möhle et al
(48) demonstrated that CXCR-4
expression modulates AML cells' traversal of the MBB through the
chemotactic response to SDF-1. Specifically, AML subtypes M4 and
M5, marked by monocytic differentiation, show heightened CXCR4
expression, enabling SDF-1 to induce rapid intracellular calcium
flux and significantly enhance extramedullary migration (49). Nonetheless, the mechanisms governing
SDF-1 upregulation and its cellular sources remain to be
elucidated.
Cluster of differentiation 31 (CD31), or platelet
endothelial cell adhesion molecule 1, is a member of the
immunoglobulin superfamily (50).
Present in both endothelial and AML cells, CD31 significantly
contributes to trans-endothelial migration via homophilic binding
(51). Its expression is markedly
elevated in the M4/M5 subgroups, which exhibit high metastatic
potential (50). Cluster of
differentiation 38 (CD38), a transmembrane glycoprotein, is also
expressed on AML cells (52,53).
The interactions of CD31 with endothelial cells and CD38 with
hyaluronic acid in the ECM influence the equilibrium between AML
cells release and retention. A CD31/CD38 ratio exceeding 1
facilitates homophilic interactions with the vasculature, enhancing
trans-endothelial migration. Conversely, a ratio below 1 promotes
retention of AML cells in the BM via interactions with hyaluronic
acid (54).
Elevated levels of lymphocyte function-associated
antigen-1 (LFA-1), an integrin superfamily member, can facilitate
the migration of normal immature progenitor cells (CD34+
precursor cells) into the bloodstream (55,56),
likely by enhancing their trans-endothelial migration (57,58).
LFA-1 acts as the primary receptor for intercellular CAM-1 (ICAM-1,
CD54). During leukocytosis, AML cells induce endothelial cells to
secrete ICAM-1, thereby enhancing adhesion via LFA-1 (59). Notably, LFA-1 expression is
significantly elevated in patients with AML with extramedullary
organ infiltration compared with those without, yet no substantial
difference in LFA-1 expression is evident between AML cells in the
BM microenvironment and those in the bloodstream. A previous study,
however, suggested that LFA-1 may not be crucial for the release of
AML cells into the bloodstream (60).
The heterodimeric integrin very late antigen 4,
consisting of CD29 subunits, is another integrin family member.
Vascular CAM-1 (VCAM-1), an immunoglobulin superfamily member, is
primarily a membrane-bound transmembrane type I sialic acid
glycoprotein with multiple Ig-like domains connected by disulfide
bonds (61). Soluble VCAM-1,
predominantly produced in response to pro-inflammatory cytokines in
the vascular endothelium, facilitates the recruitment, adhesion and
migration of monocytes, lymphocytes, eosinophils and basophils
(62,63). Although AML cells can adhere to
endothelial cells through the rate-limiting α-chain of the
CD49d/CD29 integrin heterodimer very late antigen-4 (CD49d)/VCAM-1
adhesion mechanism, CD49d expression is not essential for AML cell
release from the BM (60).
L-selectin (CD62L), a CAM in the selectin family, is
highly expressed on CD34+ precursor AML cells,
contrasting with its low expression on normal CD34+
cells. This expression contributes to aggregation of AML cells in
peripheral blood (55,64).
Matrix metalloproteinases (MMPs), zinc
(II)-dependent endopeptidases, are secreted by AML cells and the
tumor microenvironment to remodel the ECM (65,66).
By facilitating ECM degradation, MMPs enable the extensive release
of AML cells into the bloodstream, resulting in widespread
infiltration (67). The persistent
expression of tumor necrosis factor-α in AML cells elevates MMP-9
expression, which is crucial for their early release into
circulation (68). MMP-9 and/or
MMP-2 mRNA were expressed in all samples from patients with AML and
AML cell lines examined by Janowska-Wieczorek et al
(69) but not in normal
CD34+ precursor cells. Interestingly, mature monocytes
in the BM express and secrete MMP-9, suggesting its potential role
in monocyte migration into the bloodstream (69). Consequently, AML and white blood
cells may employ similar mechanisms for entering the
bloodstream.
Elastase, a protease degrading elastin in the ECM,
is excessively overexpressed on the cell surface of patients with
AML and correlates with AML cell counts in the circulation. SDF-1
can upregulate the expression of cell surface elastase in AML
cells. Inhibition of cell surface elastase diminishes migration and
adhesion of AML cell lines in vitro, while administration of
elastase inhibitors in AML mice significantly reduces circulating
AML cell levels (70).
Chemokine (SDF-1), CAMs (CD31/CD38 ratio, CD62L),
MMPs and elastase have been corroborated by in vitro and
in vivo experiments for their roles in AML cells' egress
from the bone marrow. However, the role of CAMs (LFA-1, CD49d)
remains contentious and warrants further investigation. The release
of AML cells into the bloodstream is influenced by their affinity
for two blood sinuses components: Endothelial cells and the ECM.
AML cells with higher endothelial affinity are more likely to enter
the bloodstream, while those with a higher ECM affinity remain
anchored within the blood sinus. Adhesion molecules (for example,
CD31) and chemokines (for example, CXCL12) facilitate AML cells
adhesion and migration, while elevated levels of proteases (such as
MPP family) can degrade the ECM to enable cell movement (Fig. 2).
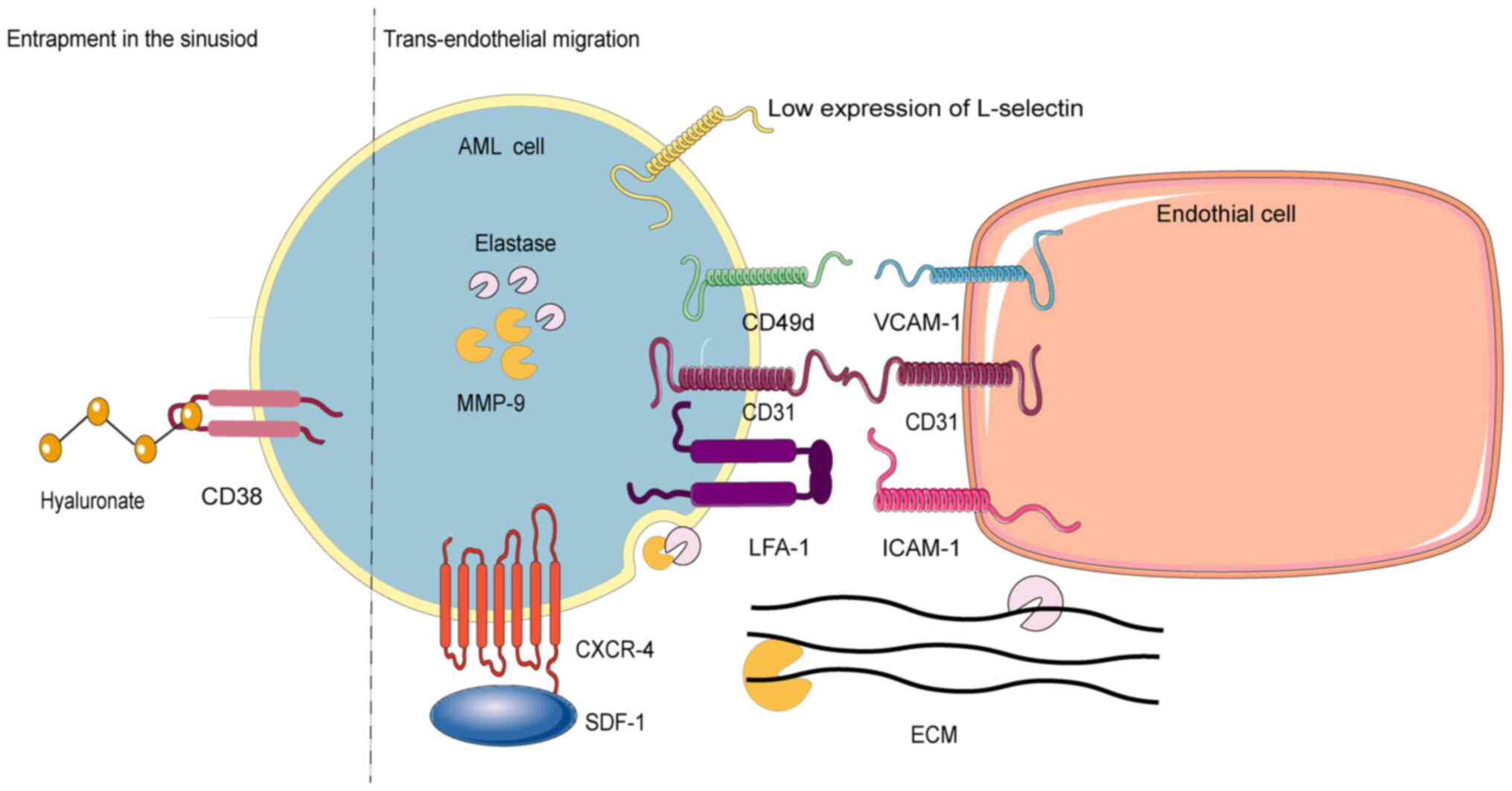 | Figure 2.Escape mechanisms of AML cells. This
figure illustrates two distinct processes: On the left side of the
dotted lines-a mechanism underlying the capture of the AML cells
within the sinusoid. On the right side of the dotted lines-a
mechanism facilitating the transportation of AML cells into
circulation. AML, acute myeloid leukemia; ECM, extracellular
matrix; CD38, cluster of differentiation 38; CD49d, the
rate-limiting α-chain of the CD49d/CD29 integrin heterodimer very
late antigen-4; VCAM-1, vascular cell-adhesion molecule 1; MMP-9,
matrix metalloproteinase-9; CD31, cluster of differentiation 31;
LFA-1, lymphocyte function associated antigen-1; ICAM-1,
intercellular cell adhesion molecule-1; SDF-1, stromal cell-derived
factor 1; CXCR-4, chemokine receptor 4. |
AML cell infiltration of the CNS
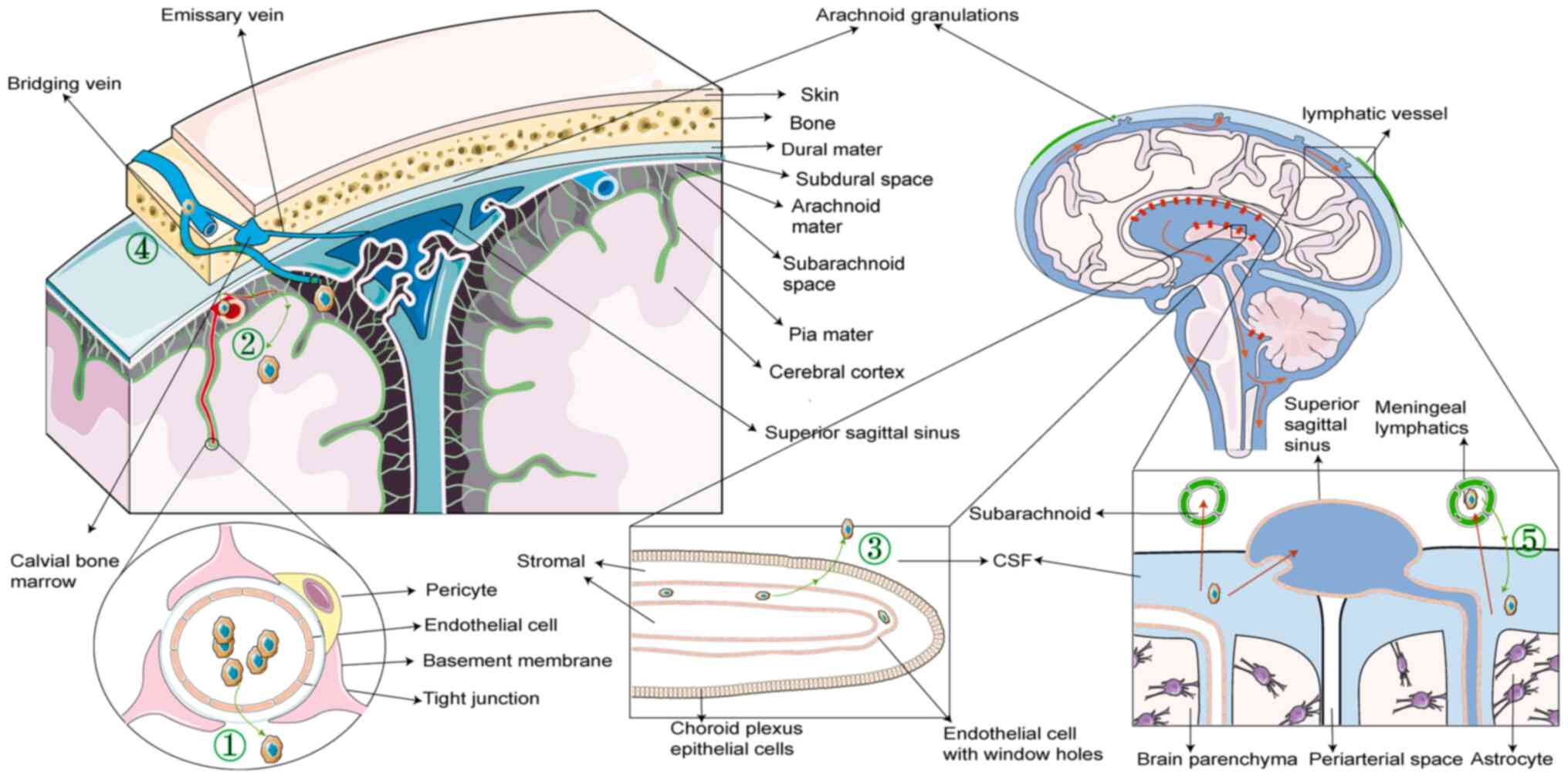
Potential routes and anatomical
structures involved
Tumors metastasize primarily through four routes:
Lymphatic metastasis, hematogenous metastasis, implantation
metastasis and direct extension. Conventionally, AML cells are
considered to enter the CNS predominantly through hematogenous
metastasis, necessitating traversal of the brain barrier. However,
a previous study suggested that AML cells may also infiltrate via
the bridging veins (1). Although
the CNS was once considered to possess immune privilege, recent
insights into its lymphatic system challenge this view, potentially
offering a more comprehensive understanding of how AML cells
infiltrate into the CNS.
Hematogenous metastasis
The CNS receives its blood supply from the vertebral
and internal carotid arteries. The interface between the
vasculature and the structural components of the CNS forms the
brain barrier, encompassing the blood-brain barrier (BBB), the
blood-leptomeningeal barrier (BLMB) and the blood-CSF barrier
(BCSFB) (71). This intricate
system regulates the physiological entry of molecules and cells
into the CNS, maintaining internal stability.
The BBB, located between the vasculature and the
CNS, consists of three primary layers arranged from inner to outer:
i) Capillary endothelial cells; ii) capillary basement membrane;
and iii) astrocytes. The endothelial cells are tightly joined and
lack fenestrations, limiting the passage of large molecules.
However, in specific CNS regions, such as the circumventricular
organs, pineal gland and neurohypophysis, the capillary endothelial
cells have fenestrations and are connected by gap junctions,
permitting the free movement of proteins and large molecules
(Fig. 3, route 1).
Histopathological evidence suggests that in advanced CNS
involvement, AML cells may proliferate along perivascular spaces
(or Virchow-Robin spaces) and infiltrate the brain parenchyma
(72).
The BLMB comprises a layer of leptomeningeal cells
encircling microvessels within the subarachnoid space (73). AML cells infiltrate the
leptomeninges through trans-endothelial migration or direct
vascular endothelium disruption (74). Once within, AML cells may infiltrate
brain parenchyma by invading the perivascular spaces (74) (Fig.
3, route 2).
The BCSFB, situated in the choroid plexus of the
brain ventricles, separates blood from the CSF. It is formed by
choroid plexus epithelial cells interconnected by tight junctions
and postcapillary venules of the meningeal microvasculature.
However, choroid plexus capillary endothelial cells have
fenestrations, providing some permeability. CSF is produced in the
choroid plexuses of each brain ventricle, flowing from the lateral
to the third ventricle, passes through the cerebral aqueduct of
Sylvius into the fourth ventricle, and merging with the CSF
generated by the third and fourth ventricles. It then enters the
subarachnoid space through the median aperture (Magendie's foramen)
of the fourth ventricle and is absorbed by the Pacchionian
granulations (arachnoid granulations) into the dural venous
sinuses, returning to the bloodstream. Leukemic cells can cross the
BCSFB to enter the CSF (Fig. 3,
Route 3).
Invasion through bridging veins,
analogous to direct extension
The CNS is protected by four anatomical layers: Pia
mater, arachnoid mater, dura mater and the cranial and vertebral
bones, arranged from innermost to outermost. The BM within cranial
and vertebral bones is hypothesized to serve as a crucial reservoir
of marrow cells supporting the CNS. These reservoirs directly
supply monocytes and neutrophils to the brain and spinal cord
through dura mater-BM connections formed by vascular bridging veins
(75), potentially serving as
routes for infiltration of AML cells into the CNS (Fig. 3, Route 4).
A study examining 31 cases of CNS involvement in
acute leukemia reported frequent dural infiltration, whereas
arachnoid infiltration without dural involvement was relatively
rare, occurring in only 9% of cases. Anatomical evidence robustly
supports the theory that AML cells infiltrate the CNS through veins
connecting the dura mater and BM (76).
Lymphatic metastasis
The CNS was considered an immune-privileged site;
however, previous murine studies have identified a lymphatic system
within the dura mater that drains CSF from deep brain parenchyma,
revealing new communication routes between the CNS and the
circulatory system (77,78). This unique dural lymphatic vascular
network extends along the dural sinuses, providing a unidirectional
absorption and transport system (79). Additionally, the CNS contains the
glymphatic system, which, unlike traditional lymphatic systems,
comprises perivascular spaces between capillaries and astrocytes.
CFS enters through perivascular spaces around arterial vessels,
exits around venous vessels, and subsequently joins the dural
lymphatic network near the dural sinuses (80).
Typically, CSF flow through the lymphatic system is
unidirectional; however, retrograde flow can occur if the distal
lymphatic system is obstructed, leading to the accumulation of
cells, such as AML cells, in the lymphatic system and their
subsequent entry into the CNS. This retrograde flow contributes to
the increased risk of CNS infiltration in patients with high white
blood cell counts, who are more susceptible to venous and lymphatic
stasis, a common complication in leukocytosis (Fig. 3, Route 5).
Other routes
AML cells infiltrate the CNS through multiple
routes. One involves traversing nerve roots through neural foramina
into the epidural space. Another route is the infiltration of AML
cells into the CNS during intracerebral hemorrhage. Additionally,
CNS infiltration can occur iatrogenically when AML cells are
introduced into the CFS during LP procedures (28).
Mechanisms of AML cells'
infiltration
Current research on AML cells entry into the CNS
focuses primarily on their traversal of the BBB. This discussion
emphasizes this aspect. Interactions between AML and endothelial
cells not only facilitate migration of AML cells but also induce
endothelial cells necroptosis, thereby promoting AML cells'
extravasation (81). Furthermore,
astrocytes, as critical component of the BBB, significantly
influence AML cell infiltration into the CNS.
Endothelial cells and astrocytes, components of the
BBB, express ICAM-1 and VCAM-1, respectively, facilitating AML cell
transmigration through interacting with LFA-1 and CD49d on AML
cells (82,83). Inhibiting the VCAM1-CD49d pathway
diminishes AML cells invasion into the brain and decelerates
disease progression. Furthermore, the loss of interferon regulatory
factor 7 promotes VCAM1-CD49d-mediated intracranial invasion by
downregulating TG-interacting factor 1 (84). The expression of cluster of
differentiation 56 on endothelial cells also plays a crucial role
in AML cell passage through the BBB (74) and is associated with CNS relapse
(85,86).
Immunoglobulin-like receptor B4 (LILRB4), part of
the leukocyte Ig-like receptor B subfamily, is crucial for CNS
infiltration by AML cells (87).
Antibody blockade of LILRB4 can reduce CNS infiltration. The APOE
protein (APOE-POPC) binds to LILRB4, suppressing immune responses
in patients with AML and facilitating dissemination of AML cells
(88). A study involving 56
patients with AML revealed that 91% of those with CNS infiltration
exhibited high LILRB4 expression, compared with 38% without CNS
involvement. Additionally, LILRB4 expression is elevated in M4 and
M5 AML subtypes, which are more susceptible to CNS infiltration
(89). The significant role of
LILRB4 in CNS infiltration highlights its potential as a target for
antibody-drug conjugates designed to eliminate AML cells and reduce
CNS involvement (87).
Macrophage-secreted MMP-9 disrupts the vascular
endothelial cells of the BBB, promoting secondary intracranial
infections and brain lesions (90).
Furthermore, MMP-mediated loss of endothelial integrity facilitates
AML cells' extravasation into tissues (91,92).
Given their ability to compromise the BBB and endothelial cells and
their high expression in AML, MMPs likely play a significant role
in CNS invasion.
Mutations in exon 18 of DNMT3A (D3Amut) enhance the
monocyte invasiveness and migration of AML cells, leading to
meningeal leukemia in mice (93).
Additionally, D3Amut induces demethylation and upregulation of the
TWIST1 gene in AML cells, facilitating CNS infiltration
(94). Overexpression of the human
TIMP-2 gene in mice results in increased tumor formation
across various organs and severe CNS infiltration (95). These mutations are prevalent in the
AML subtypes M4 or M5, which are more prone to CNS infiltration
(96).
In conclusion, enhanced expression of adhesion
molecules facilitates the interaction between AML cells and the BBB
components. Additionally, enzyme and gene mutations significantly
contribute to CNS infiltration in AML (Fig. 4).
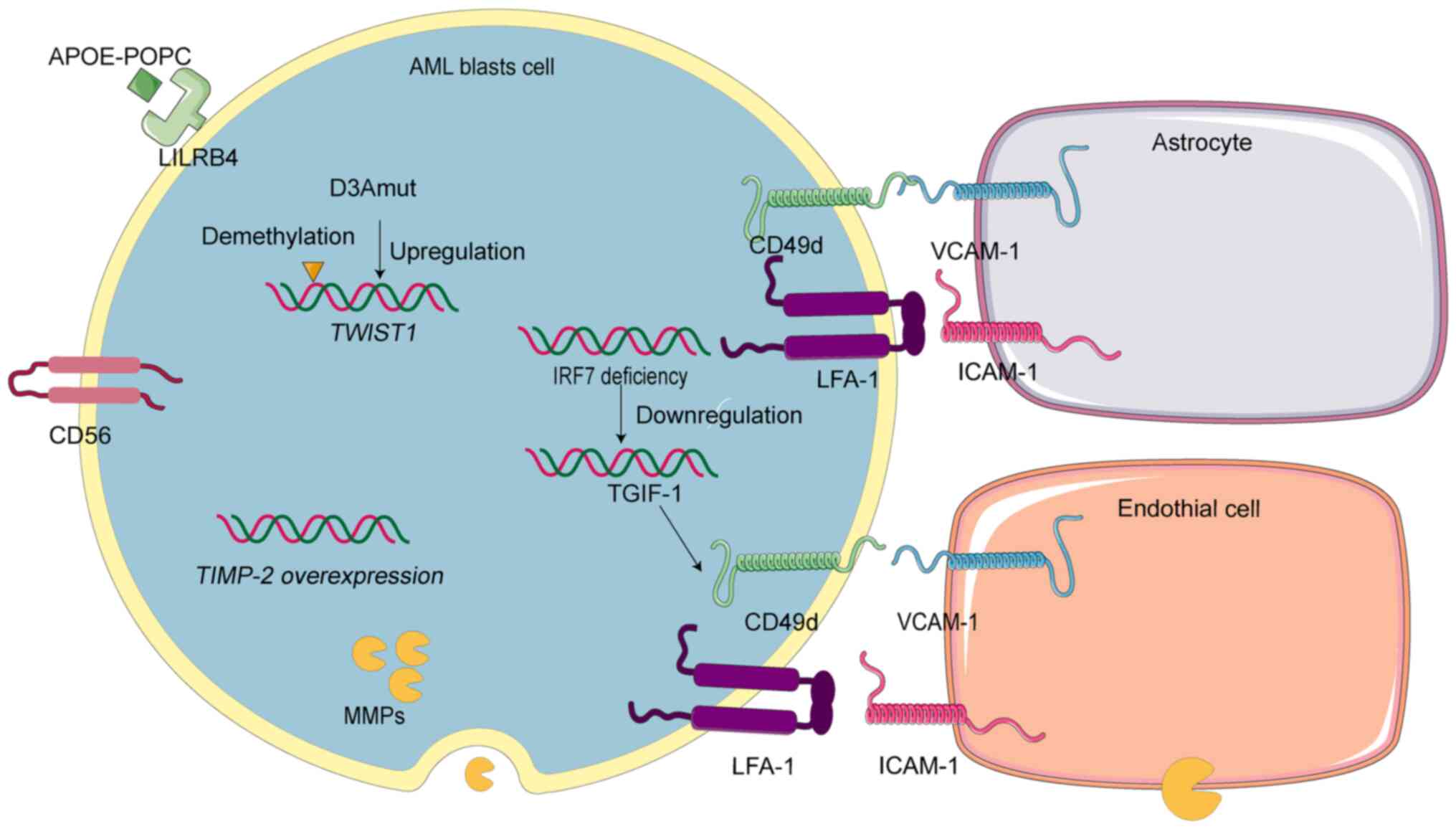 | Figure 4.Mechanisms of the central nervous
system exploited by AML cells. AML, acute myeloid leukemia;
APOE-POPC, liposome-reconstituted APOE protein; LILRB4,
immunoglobulin-like receptor B4; D3Amut, genetic mutation in exon
18 of DNMT3A; TWIST1, TWIST1 gene; TIMP-2, TIMP-2 gene; IRF7,
interferon regulatory factor 7; TGIF-1, TG-interacting factor 1;
CD56, cluster of differentiation 56. MMPs, matrix
metalloproteinases; CD49d, rate-limiting α-chain of the CD49d/CD29
integrin heterodimer very late antigen-4; VCAM-1, vascular
cell-adhesion molecule 1; LFA-1, lymphocyte function-associated
antigen-1; ICAM-1, intercellular cell adhesion molecule-1. |
Relevant molecules and genes for the
treatment in AML
The absence of specific surface antigens on AML
cells poses a significant challenge to developing targeted
therapies and cell-based treatments for AML. While the
aforementioned molecules and genes involved in AML cells' escape
and CNS infiltration hold potential as therapeutic targets, their
clinical application has been constrained by adverse effects.
Recent advancements in drug synthesis and novel delivery systems
have enabled more precise targeting of these drugs, mitigating side
effects. Subsequently, the present discussion delved into the
practical implications and current limitations of these therapeutic
strategies.
The chemokine axis CXCR4/SDF-1 serves as a potential
therapeutic target in AML, with the efficacy of related inhibitors
validated in both in vitro and in vivo experiments.
Some inhibitors have advanced to clinical trials. In vitro
studies have shown promising results in AML treatment through
reducing CXCR4 expression using lipid polymer/siRNA complexes
(97). Currently, CXCR4 inhibitors
such as AMD3100 are under investigation in Phase I/II clinical
trials combined with chemotherapy for refractory/relapsed and newly
diagnosed patients with AML (98,99).
Although direct evidence is lacking, targeted CXCR4 therapy may
potentially reduce CNS involvement in AML. In vivo
experiments have shown that the chemically synthesized CXCR4
antagonistic peptide E5, formulated as micelles (M-E5),
significantly inhibits AML cells engraftment in the spleen, thereby
reducing organ burden (100). Due
to their widespread presence across various cells types, CAMs have
been limited as targets for AML therapy. However, recent studies
have opened new avenues for preclinical investigations. For
instance, combining all-trans retinoic acid with daratumumab, an
anti-CD38 antibody-conjugated polymer sulfate deoxycholic acid,
effectively eliminated circulating leukemia cells and reduced organ
invasion in AML models with low CD38 expression (101,102). Additionally, low and non-toxic
doses of the microtubule destabilizer combretastatin-A4-phosphate
(CA4P) downregulated CAM (VCAM-1), targeting circulating leukemia
cells without inducing hematologic toxicity, providing an effective
approach for treating refractory organ-infiltrative leukemia
(103).
LILRB4, extensively expressed on monocyte AML cells,
represents a promising tumor-associated antigen for
immunotherapeutic targeting. A novel anti-LILRB4 CAR-T cell,
exhibiting high affinity and specificity, has shown potent
cytotoxicity against LILRB4-positive AML cells in both in
vitro and in vivo models (104).
MMPs, as potential therapeutic targets for various
diseases, have attracted substantial research interest. These
enzymes are pivotal in extracellular matrix degradation and are
regarded as primary drivers of cancer invasion and metastasis.
Consequently, MMPs have become significant targets in anticancer
drug development (105–108). Studies have explored the in
vitro anti-AML activity of METVAN
[bis(4,7-dimethyl-1,10-phenanthroline) sulfatooxovanadium(IV);
VO(SO(4))(Me(2)-Phen)(2)], which inhibits the expression and
gelatinolytic activity of MMP-2 and MMP-9 proteins, thereby
inducing apoptosis in AML cells (109).
DNMT3A, frequently mutated in AML, functions as a
DNA cytosine methyltransferase and a key epigenetic driver of
transcriptional silencing, commonly dysregulated in cancer.
Hypomethylating agents, such as the cytidine analogs decitabine and
azacitidine, have demonstrated clinical benefits in hematologic
malignancies. However, their significant toxicity to normal blood
cells restricts clinical dosing (110). A targeted delivery system using
endogenous anti-CD33 antibody-fused protein conjugates to deliver
DNMT3A-targeting siRNA into AML cells has been recently
investigated, showing therapeutic efficacy both in vitro and
in vivo (111).
Advancements in drug synthesis and delivery
technologies have validated the therapeutic roles of relevant
molecules and genes in AML through in vitro and in
vivo experiments, and preclinical studies. While existing
research primarily addresses extramedullary infiltration, specific
data on inhibiting CNS involvement in AML remains scarce.
Conclusion and future considerations
The objective of the present review was to enhance
the understanding of AML cells' invasion into the CNS, a
significant concern due to its association with relapse and
mortality in patients with AML. Preventing CNS relapse is a key
component of AML treatment, yet it remains a formidable issue.
Elucidating the molecular mechanisms of CNS infiltration in AML can
pave the way for innovative diagnostic and therapeutic strategies.
AML cells migrate to the bloodstream through sinusoids under the
influence of chemotactic factors, CAMs, MMPs and elastase. They
subsequently cross the BBB into the CNS due to gene mutations,
CAMs, MMPs and LILRB, or infiltrate the CNS via bridging veins and
lymphatics. The present review highlights the role of CAMs and gene
mutations in CNS infiltration, suggesting that immunotherapy and
targeted therapy could offer effective therapeutic options.
However, the precise pathways and mechanisms underlying AML cells'
escape from the BM and CNS invasion remain partially understood.
Current therapeutic approaches for CNS involvement in AML primarily
rely on systemic chemotherapy, intrathecal chemotherapy and
radiotherapy, which continue to exhibit high recurrence rates and
side effects due to the lack of molecularly targeted agents and
advanced cellular therapies. Further research is imperative to
clarify the routes and regulatory mechanisms of AML cells' egress
from the BM and CNS invasion. Additionally, detecting gene
mutations in AML cells within the CSF could provide a novel and
more effective diagnostic method. Future advances in drug synthesis
and delivery systems hold promise for developing therapies
targeting CAMs, MMPs, elastase and gene mutations, thereby offering
new treatment options for patients with AML with CNS
involvement.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Innovation Platform and
Talent Program (grant no. 2023TP1047), the Natural Science
Foundation of Hunan (grant no. 2023JJ30547), the Major Project of
Hunan Provincial Health Commission (grant no. W20241008) and the
Clinical Research 4310 Program of the First Affiliated Hospital of
the University of South China (grant no. 20214310NHYCG03).
Availability of data and materials
Not applicable.
Authors' contributions
LC wrote the original draft. PZ wrote and reviewed
the manuscript. HT performed investigation. GC conducted
validation. JX performed visualization. XY supervised the study. XL
conceptualized the study. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AML
|
acute myeloid leukemia
|
|
CNS
|
central nervous system
|
|
BM
|
bone marrow
|
|
CSF
|
cerebrospinal fluid
|
|
LP
|
lumbar puncture
|
|
MBB
|
marrow-blood barrier
|
|
CAM
|
cell adhesion molecules
|
|
ECM
|
extracellular matrix
|
|
ACCR
|
adventitial cell coverage ratio
|
|
SDF-1
|
stromal cell-derived factor 1
|
|
CXCL12
|
C-X-C motif chemokine 12
|
|
CXCR-4
|
chemokine receptor 4
|
|
CD31
|
cluster of differentiation 31
|
|
CD38
|
cluster of differentiation 38
|
|
LFA-1
|
lymphocyte function associated
antigen-1
|
|
ICAM-1
|
intercellular CAM-1
|
|
VCAM-1
|
vascular CAM-1
|
|
CD49d
|
the rate-limiting α-chain of the
CD49d/CD29 integrin heterodimer very late antigen-4
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
BBB
|
blood-brain barrier
|
|
BLMB
|
blood-leptomeningeal barrier
|
|
BCSFB
|
blood-CSF barrier
|
|
LILRB4
|
immunoglobulin-like receptor B4
|
References
|
1
|
Korn C and Méndez-Ferrer S: Myeloid
malignancies and the microenvironment. Blood. 129:811–822. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Munker R, Labopin M, Esteve J, Schmid C,
Mohty M and Nagler A: Mixed phenotype acute leukemia: Outcomes with
allogeneic stem cell transplantation. A retrospective study from
the Acute Leukemia Working Party of the EBMT. Haematologica.
102:2134–2140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sas V, Blag C, Zaharie G, Puscas E,
Lisencu C, Andronic-Gorcea N, Pasca S, Petrushev B, Chis I, Marian
M, et al: Transient leukemia of Down syndrome. Crit Rev Clin Lab
Sci. 56:247–259. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dima D, Oprita L, Rosu AM, Trifa A,
Selicean C, Moisoiu V, Frinc I, Zdrenghea M and Tomuleasa C: Adult
acute megakaryoblastic leukemia: Rare association with cytopenias
of undetermined significance and p210 and p190 BCR-ABL transcripts.
Onco Targets Ther. 10:5047–5051. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gafencu GA, Tomuleasa CI and Ghiaur G:
PARP inhibitors in acute myeloid leukaemia therapy: How a synthetic
lethality approach can be a valid therapeutic alternative. Med
Hypotheses. 104:30–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnston DL, Alonzo TA, Gerbing RB, Aplenc
R, Woods WG, Meshinchi S and Gamis AS: Central nervous system
disease in pediatric acute myeloid leukemia: A report from the
Children's Oncology Group. Pediatr Blood Cancer. 64:102017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Constantinescu C, Bodolea C, Pasca S,
Teodorescu P, Dima D, Rus I, Tat T, Achimas-Cadariu P, Tanase A,
Tomuleasa C and Einsele H: Clinical Approach to the Patient in
Critical State Following Immunotherapy and/or Stem Cell
Transplantation: Guideline for the On-Call Physician. J Clin Med.
8:8842019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goulart H, Sastow D, Moshier E, Martin L,
Mascarenhas J and Tremblay D: Systematic review and meta-analysis
evaluating clinical outcomes in adult acute myeloid leukemia
patients with central nervous system involvement. Leuk Res.
137:1074522024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alakel N, Stölzel F, Mohr B, Kramer M,
Oelschlägel U, Röllig C, Bornhäuser M, Ehninger G and Schaich M:
Symptomatic central nervous system involvement in adult patients
with acute myeloid leukemia. Cancer Manag Res. 9:97–102. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng CL, Li CC, Hou HA, Fang WQ, Chang
CH, Lin CT, Tang JL, Chou WC, Chen CY, Yao M, et al: Risk factors
and clinical outcomes of acute myeloid leukaemia with central
nervous system involvement in adults. BMC Cancer. 15:3442015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Felix A, Leblanc T, Petit A, Nelkem B,
Bertrand Y, Gandemer V, Sirvent A, Paillard C, Schmitt C, Rohrlich
PS, et al: Acute Myeloid Leukemia With Central Nervous System
Involvement in Children: Experience From the French Protocol
Analysis ELAM02. J Pediatr Hematol Oncol. 40:43–47. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ganzel C, Lee JW, Fernandez HF, Paietta
EM, Luger SM, Lazarus HM, Cripe LD, Douer D, Wiernik PH, Rowe JM,
et al: CNS involvement in AML at diagnosis is rare and does not
affect response or survival: Data from 11 ECOG-ACRIN trials. Blood
Adv. 5:4560–4568. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jabbour E, Guastad Daver N, Short NJ,
Huang X, Chen HC, Maiti A, Ravandi F, Cortes J, Abi Aad S,
Garcia-Manero G, et al: Factors associated with risk of central
nervous system relapse in patients with non-core binding factor
acute myeloid leukemia. Am J Hematol. 92:924–928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Del Principe MI, Buccisano F, Soddu S,
Maurillo L, Cefalo M, Piciocchi A, Consalvo MI, Paterno G, Sarlo C,
De Bellis E, et al: Involvement of central nervous system in adult
patients with acute myeloid leukemia: Incidence and impact on
outcome. Semin Hematol. 55:209–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bojsen-Møller M and Nielsen JL: CNS
involvement in leukaemia. An autopsy study of 100 consecutive
patients. Acta Pathol Microbiol Immunol Scand A. 91:209–216.
1983.PubMed/NCBI
|
|
17
|
Paul S and Short NJ: Central Nervous
System Involvement in Adults with Acute Leukemia: Diagnosis,
Prevention, and Management. Curr Oncol Rep. 24:427–436. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Virijevic M, Kraguljac-Kurtovic N,
Mitrovic M, Jakovic L, Bukumuric Z, Pantic N, Sabljic N, Pravdic Z,
Cvetkovic M, Knezevic V, et al: Incidence, risk factors, and
outcome of asymptomatic central nervous system involvement in adult
patients with acute myeloid leukemia. Hematol Oncol. 42:e32532024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bar M, Tong W, Othus M, Loeb KR and Estey
EH: Central nervous system involvement in acute myeloid leukemia
patients undergoing hematopoietic cell transplantation. Biol Blood
Marrow Transplant. 21:546–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bento LC, Correia RP, Alexandre AM, Nosawa
ST, Pedro EC, Vaz ADC, Schimidell D, Fernandes GBP, Duarte CAS,
Barroso RS and Bacal NS: Detection of Central Nervous System
Infiltration by Myeloid and Lymphoid Hematologic Neoplasms Using
Flow Cytometry Analysis: Diagnostic Accuracy Study. Front Med
(Lausanne). 5:702018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ranta S, Palomäki M, Levinsen M, Taskinen
M, Abrahamsson J, Hasle H, Jahnukainen K, Heyman M and Harila-Saari
A; Nordic Society of Pediatric Haematology Oncology (NOPHO), :
Presenting features and imaging in childhood acute myeloid leukemia
with central nervous system involvement. Pediatr Blood Cancer.
64:2017. View Article : Google Scholar
|
|
22
|
Reid JH, Perissinotti AJ, Benitez L, Bixby
DL, Burke P, Pettit K and Marini BL: Impact of prophylactic
intrathecal chemotherapy on CNS relapse rates in AML patients
presenting with hyperleukocytosis. Leuk Lymphoma. 61:862–868. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berg S and Nand S: Neurological
Complications of the Leukemias Across the Ages. Curr Neurol
Neurosci Rep. 17:132017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chamberlain MC, Glantz M, Groves MD and
Wilson WH: Diagnostic tools for neoplastic meningitis: Detecting
disease, identifying patient risk, and determining benefit of
treatment. Semin Oncol. 36 (4 Suppl 2):S35–S45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thakkar JP, Kumthekar P, Dixit KS, Stupp R
and Lukas RV: Leptomeningeal metastasis from solid tumors. J Neurol
Sci. 411:1167062020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duzova A and Bakkaloglu A: Central nervous
system involvement in pediatric rheumatic diseases: Current
concepts in treatment. Curr Pharm Des. 14:1295–1301. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ebadi M, Morse M, Gooley T, Ermoian R,
Halasz LM, Lo SS, Yang JT, Blau MH, Percival ME, Cassaday RD, et
al: Craniospinal irradiation for CNS leukemia: Rates of response
and durability of CNS control. J Neurooncol. 166:351–357. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siegal T, Benouaich-Amiel A and Bairey O:
Neurologic complications of acute myeloid leukemia. Diagnostic
approach and therapeutic modalities. Blood Rev. 53:1009102022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Q, Zhu XL, Zhao X, Liu X, Fu HX,
Zhang YY, Chen YH, Mo XD, Han W, Chen H, et al: Prognosis and risk
factors for central nervous system relapse after allogeneic
hematopoietic stem cell transplantation in acute myeloid leukemia.
Ann Hematol. 100:505–516. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu J, Su A, Liu X, Tong Z, Jiang Q and Yu
J: Effects of D-CAG chemotherapy regimen on cognitive function in
patients with acute myeloid leukaemia: A resting-state functional
magnetic resonance imaging study. Eur J Neurosci. 59:119–131. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang C, Zhong JF and Zhang X: Revealing
the molecular mechanism of central nervous system leukemia with
single-cell technology. Crit Rev Oncol Hematol. 153:1030462020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Si M, Jiao X, Li Y, Chen H, He P and Jiang
F: The role of cytokines and chemokines in the microenvironment of
the blood-brain barrier in leukemia central nervous system
metastasis. Cancer Manag Res. 10:305–313. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nourshargh S and Alon R: Leukocyte
migration into inflamed tissues. Immunity. 41:694–707. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ley K, Laudanna C, Cybulsky MI and
Nourshargh S: Getting to the site of inflammation: The leukocyte
adhesion cascade updated. Nat Rev Immunol. 7:678–689. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maloney MA, Forsyth RP and Patt HM: Bone
marrow blood flow after marrow removal or nutrient vessel ligation.
Proc Soc Exp Biol Med. 135:871–873. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kusumbe AP, Ramasamy SK and Adams RH:
Coupling of angiogenesis and osteogenesis by a specific vessel
subtype in bone. Nature. 507:323–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tavassoli M: The marrow-blood barrier. Br
J Haematol. 41:297–302. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petrides PE and Dittmann KH: How do normal
and leukemic white blood cells egress from the bone marrow?
Morphological facts and biochemical riddles. Blut. 61:3–13. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wight TN: Cell biology of arterial
proteoglycans. Arteriosclerosis. 9:1–20. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Owen M: Marrow stromal stem cells. J Cell
Sci Suppl. 10:63–76. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Inoue S and Osmond DG: Basement membrane
of mouse bone marrow sinusoids shows distinctive structure and
proteoglycan composition: A high resolution ultrastructural study.
Anat Rec. 264:294–304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bentley SA and Foidart JM: Some properties
of marrow derived adherent cells in tissue culture. Blood.
56:1006–1012. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leonardi GP, Manthos M, Orlic D and Lobue
J: Morphometric analysis of bone marrow sinus cell elements after
induction of monomyelocytic leukemia in BALB/c mice. Anat Rec.
224:331–335. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kuto F, Nagaoka T, Watanabe Y, Hayashi M,
Horasawa Y, Hirasawa Y and Tokuhiro H: Chronic myelocytic leukemia:
Ultrastructural histopathology of bone marrow from patients in the
chronic phase. Ultrastruct Pathol. 6:307–317. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nagaoka T, Kuto F, Watanabe Y, Fujino Y,
Hirasawa Y and Tokuhiro H: Bone marrow sinus and cell egress in
human leukaemia: a morphometric study of core biopsies using
wide-field electron microscopy. Br J Haematol. 63:737–747. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gordon MY: Extracellular matrix of the
marrow microenvironment. Br J Haematol. 70:1–4. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Oberlin E, Amara A, Bachelerie F, Bessia
C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM,
Clark-Lewis I, Legler DF, et al: The CXC chemokine SDF-1 is the
ligand for LESTR/fusin and prevents infection by
T-cell-line-adapted HIV-1. Nature. 382:833–835. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Möhle R, Bautz F, Rafii S, Moore MA,
Brugger W and Kanz L: The chemokine receptor CXCR-4 is expressed on
CD34+ hematopoietic progenitors and leukemic cells and mediates
transendothelial migration induced by stromal cell-derived
factor-1. Blood. 91:4523–4530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Möhle R, Schittenhelm M, Failenschmid C,
Bautz F, Kratz-Albers K, Serve H, Brugger W and Kanz L: Functional
response of leukaemic blasts to stromal cell-derived factor-1
correlates with preferential expression of the chemokine receptor
CXCR4 in acute myelomonocytic and lymphoblastic leukaemia. Br J
Haematol. 110:563–572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Newman PJ, Berndt MC, Gorski J, White GC
II, Lyman S, Paddock C and Muller WA: PECAM-1 (CD31) cloning and
relation to adhesion molecules of the immunoglobulin gene
superfamily. Science. 247:1219–1222. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Muller WA, Weigl SA, Deng X and Phillips
DM: PECAM-1 is required for transendothelial migration of
leukocytes. J Exp Med. 178:449–460. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Howard M, Grimaldi JC, Bazan JF, Lund FE,
Santos-Argumedo L, Parkhouse RM, Walseth TF and Lee HC: Formation
and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen
CD38. Science. 262:1056–1059. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nishina H, Inageda K, Takahashi K, Hoshino
S, Ikeda K and Katada T: Cell surface antigen CD38 identified as
ecto-enzyme of NAD glycohydrolase has hyaluronate-binding activity.
Biochem Biophys Res Commun. 203:1318–1323. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gallay N, Anani L, Lopez A, Colombat P,
Binet C, Domenech J, Weksler BB, Malavasi F and Herault O: The role
of platelet/endothelial cell adhesion molecule 1 (CD31) and CD38
antigens in marrow microenvironmental retention of acute
myelogenous leukemia cells. Cancer Res. 67:8624–8632. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dercksen MW, Gerritsen WR, Rodenhuis S,
Dirkson MK, Slaper-Cortenbach IC, Schaasberg WP, Pinedo HM, von dem
Borne AE and van der Schoot CE: Expression of adhesion molecules on
CD34+ cells: CD34+ L-selectin+ cells predict a rapid platelet
recovery after peripheral blood stem cell transplantation. Blood.
85:3313–3319. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Torensma R, Raymakers RA, van Kooyk Y and
Figdor CG: Induction of LFA-1 on pluripotent CD34+ bone marrow
cells does not affect lineage commitment. Blood. 87:4120–4128.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Möhle R, Moore MA, Nachman RL and Rafii S:
Transendothelial migration of CD34+ and mature hematopoietic cells:
An in vitro study using a human bone marrow endothelial cell line.
Blood. 89:72–80. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yong KL, Watts M, Shaun Thomas N, Sullivan
A, Ings S and Linch DC: Transmigration of CD34+ cells across
specialized and nonspecialized endothelium requires prior
activation by growth factors and is mediated by PECAM-1 (CD31).
Blood. 91:1196–1205. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang W, Zhang X, Fan X, Li D and Qiao Z:
Effect of ICAM-1 and LFA-1 in hyperleukocytic acute myeloid
leukaemia. Clin Lab Haematol. 28:177–182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu T, Liu X, Xiang J, Zou P, Zhou J, Chen
Y, Yu D and Li C: Study on the relationship between the expression
of adhesion molecules and the invasiveness of acute myeloid
leukemia cells. Zhonghua Xue Ye Xue Za Zhi. 18:29–31. 1997.(In
Chinese). PubMed/NCBI
|
|
61
|
Cook-Mills JM, Marchese ME and
Abdala-Valencia H: Vascular cell adhesion molecule-1 expression and
signaling during disease: Regulation by reactive oxygen species and
antioxidants. Antioxid Redox Signal. 15:1607–1638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Meigs JB, Hu FB, Rifai N and Manson JE:
Biomarkers of endothelial dysfunction and risk of type 2 diabetes
mellitus. JAMA. 291:1978–1986. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dessein PH, Joffe BI and Singh S:
Biomarkers of endothelial dysfunction, cardiovascular risk factors
and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther.
7:R634–R643. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
64
|
Watanabe T, Dave B, Heimann DG, Jackson
JD, Kessinger A and Talmadge JE: Cell adhesion molecule expression
on CD34+ cells in grafts and time to myeloid and platelet recovery
after autologous stem cell transplantation. Exp Hematol. 26:10–18.
1998.PubMed/NCBI
|
|
65
|
Jing M, Chen X, Qiu H, He W, Zhou Y, Li D,
Wang D, Jiao Y and Liu A: Insights into the immunomodulatory
regulation of matrix metalloproteinase at the maternal-fetal
interface during early pregnancy and pregnancy-related diseases.
Front Immunol. 13:10676612023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pirillo C, Birch F, Tissot FS, Anton SG,
Haltalli M, Tini V, Kong I, Piot C, Partridge B, Pospori C, et al:
Metalloproteinase inhibition reduces AML growth, prevents stem cell
loss, and improves chemotherapy effectiveness. Blood Adv.
6:3126–3141. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Song JH, Kim SH, Cho D, Lee IK, Kim HJ and
Kim TS: Enhanced invasiveness of drug-resistant acute myeloid
leukemia cells through increased expression of matrix
metalloproteinase-2. Int J Cancer. 125:1074–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ismair MG, Ries C, Lottspeich F, Zang C,
Kolb HJ and Petrides PE: Autocrine regulation of matrix
metalloproteinase-9 gene expression and secretion by tumor necrosis
factor-alpha (TNF-alpha) in NB4 leukemic cells: Specific
involvement of TNF receptor type 1. Leukemia. 12:1136–1143. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Janowska-Wieczorek A, Marquez LA,
Matsuzaki A, Hashmi HR, Larratt LM, Boshkov LM, Turner AR, Zhang
MC, Edwards DR and Kossakowska AE: Expression of matrix
metalloproteinases (MMP-2 and −9) and tissue inhibitors of
metalloproteinases (TIMP-1 and −2) in acute myelogenous leukaemia
blasts: comparison with normal bone marrow cells. Br J Haematol.
105:402–411. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tavor S, Petit I, Porozov S, Goichberg P,
Avigdor A, Sagiv S, Nagler A, Naparstek E and Lapidot T: Motility,
proliferation, and egress to the circulation of human AML cells are
elastase dependent in NOD/SCID chimeric mice. Blood. 106:2120–2127.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shechter R, London A and Schwartz M:
Orchestrated leukocyte recruitment to immune-privileged sites:
Absolute barriers versus educational gates. Nat Rev Immunol.
13:206–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Price RA: Histopathology of CNS leukemia
and complications of therapy. Am J Pediatr Hematol Oncol. 1:21–30.
1979.PubMed/NCBI
|
|
73
|
Spadoni I, Fornasa G and Rescigno M:
Organ-specific protection mediated by cooperation between vascular
and epithelial barriers. Nat Rev Immunol. 17:761–773. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Deak D, Gorcea-Andronic N, Sas V,
Teodorescu P, Constantinescu C, Iluta S, Pasca S, Hotea I, Turcas
C, Moisoiu V, et al: A narrative review of central nervous system
involvement in acute leukemias. Ann Transl Med. 9:682021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cugurra A, Mamuladze T, Rustenhoven J,
Dykstra T, Beroshvili G, Greenberg ZJ, Baker W, Papadopoulos Z,
Drieu A, Blackburn S, et al: Skull and vertebral bone marrow are
myeloid cell reservoirs for the meninges and CNS parenchyma.
Science. 373:eabf78442021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Azzarelli V and Roessmann U: Pathogenesis
of central nervous system infiltration in acute leukemia. Arch
Pathol Lab Med. 101:203–205. 1977.PubMed/NCBI
|
|
77
|
Louveau A, Smirnov I, Keyes TJ, Eccles JD,
Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et
al: Structural and functional features of central nervous system
lymphatic vessels. Nature. 523:337–341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Aspelund A, Antila S, Proulx ST, Karlsen
TV, Karaman S, Detmar M, Wiig H and Alitalo K: A dural lymphatic
vascular system that drains brain interstitial fluid and
macromolecules. J Exp Med. 212:991–999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Louveau A, Plog BA, Antila S, Alitalo K,
Nedergaard M and Kipnis J: Understanding the functions and
relationships of the glymphatic system and meningeal lymphatics. J
Clin Invest. 127:3210–3219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng
W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et
al: A paravascular pathway facilitates CSF flow through the brain
parenchyma and the clearance of interstitial solutes, including
amyloid β. Sci Transl Med. 4:147ra1112012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Guerrini MM, Okamoto K, Komatsu N, Sawa S,
Danks L, Penninger JM, Nakashima T and Takayanagi H: Inhibition of
the TNF Family Cytokine RANKL prevents autoimmune inflammation in
the central nervous system. Immunity. 43:1174–1185. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Maeda A, Kobayashi Y, Saito T, Togitani K,
Kawahigashi N, Tanosaki R, Takaue Y, Takenaka T, Iwata N and
Tobinai K: Central nervous system relapse with multiple brain
masses in an acute promyelocytic leukemia patient treated with
all-trans retinoic acid. Rinsho Ketsueki. 40:1081–1086. 1999.(In
Japanese). PubMed/NCBI
|
|
83
|
Raanani P, Shpilberg O and Ben-Bassat I:
Extramedullary disease and targeted therapies for hematological
malignancies-is the association real? Ann Oncol. 18:7–12. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang H, Zhang D, Cui X, Dai Y, Wang C,
Feng W, Lv X, Li Y, Wang L, Ru Y, et al: Loss of IRF7 accelerates
acute myeloid leukemia progression and induces VCAM1-VLA-4 mediated
intracerebral invasion. Oncogene. 41:2303–2314. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chang H, Brandwein J, Yi QL, Chun K,
Patterson B and Brien B: Extramedullary infiltrates of AML are
associated with CD56 expression, 11q23 abnormalities and inferior
clinical outcome. Leuk Res. 28:1007–1011. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yang SW, Ma RJ, Yuan XL, Jiang L, Li YL,
Dong XY, Wang Z, Zhang L, Shang BJ, Lei PC and Zhu ZM: Correlation
analysis of central nervous system relapse and cell biological
characteristics in acute promyelocytic leukemia. Zhonghua Xue Ye
Xue Za Zhi. 42:517–520. 2021.(In Chinese). PubMed/NCBI
|
|
87
|
Bergstrom CP, Dahiya S, Chen W, Zhang CC,
Zhu H, Yan J, Madanat Y, Patel P, Vusirkala M, Ramakrishnan P, et
al: The association of leukocyte immunoglobulin-like receptor
subfamily B-4 expression in acute myeloid leukemia and central
nervous system involvement. Leuk Res. 100:1064802021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Deng M, Gui X, Kim J, Xie L, Chen W, Li Z,
He L, Chen Y, Chen H, Luo W, et al: LILRB4 signalling in leukaemia
cells mediates T cell suppression and tumour infiltration. Nature.
562:605–609. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li Z, Deng M, Huang F, Jin C, Sun S, Chen
H, Liu X, He L, Sadek AH and Zhang CC: LILRB4 ITIMs mediate the T
cell suppression and infiltration of acute myeloid leukemia cells.
Cell Mol Immunol. 17:272–282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bonoiu A, Mahajan SD, Ye L, Kumar R, Ding
H, Yong KT, Roy I, Aalinkeel R, Nair B, Reynolds JL, et al: MMP-9
gene silencing by a quantum dot-siRNA nanoplex delivery to maintain
the integrity of the blood brain barrier. Brain Res. 1282:142–55.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Stefanidakis M, Karjalainen K, Jaalouk DE,
Gahmberg CG, O'Brien S, Pasqualini R, Arap W and Koivunen E: Role
of leukemia cell invadosome in extramedullary infiltration. Blood.
114:3008–3017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Röllig C and Ehninger G: How I treat
hyperleukocytosis in acute myeloid leukemia. Blood. 125:3246–3252.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xu J, Wang YY, Dai YJ, Zhang W, Zhang WN,
Xiong SM, Gu ZH, Wang KK, Zeng R, Chen Z and Chen SJ: DNMT3A Arg882
mutation drives chronic myelomonocytic leukemia through disturbing
gene expression/DNA methylation in hematopoietic cells. Proc Natl
Acad Sci USA. 111:2620–2625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xu J, Zhang W, Yan XJ, Lin XQ, Li W, Mi
JQ, Li JM, Zhu J, Chen Z and Chen SJ: DNMT3A mutation leads to
leukemic extramedullary infiltration mediated by TWIST1. J Hematol
Oncol. 9:1062016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li ZJ, Chen ZX, Cen JN and He J:
Overexpression of tissue inhibitor of metalloprotease-2 promotes
proliferation and infiltration of human monocytic leukemia cells.
Zhonghua Yi Xue Za Zhi. 86:2409–2412. 2006.(In Chinese). PubMed/NCBI
|
|
96
|
Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y,
Shi JY, Zhu YM, Tang L, Zhang XW, et al: Exome sequencing
identifies somatic mutations of DNA methyltransferase gene DNMT3A
in acute monocytic leukemia. Nat Genet. 43:309–315. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Landry B, Gül-Uludağ H, Plianwong S,
Kucharski C, Zak Z, Parmar MB, Kutsch O, Jiang H, Brandwein J and
Uludağ H: Targeting CXCR4/SDF-1 axis by lipopolymer complexes of
siRNA in acute myeloid leukemia. J Control Release. 224:8–21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Uy GL, Rettig MP, Motabi IH, McFarland K,
Trinkaus KM, Hladnik LM, Kulkarni S, Abboud CN, Cashen AF,
Stockerl-Goldstein KE, et al: A phase 1/2 study of
chemosensitization with the CXCR4 antagonist plerixafor in relapsed
or refractory acute myeloid leukemia. Blood. 119:3917–3924. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Peled A and Tavor S: Role of CXCR4 in the
pathogenesis of acute myeloid leukemia. Theranostics. 3:34–39.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Meng J, Ge Y, Xing H, Wei H, Xu S, Liu J,
Yan D, Wen T, Wang M, Fang X, et al: Synthetic CXCR4 antagonistic
peptide assembling with nanoscaled micelles combat acute myeloid
leukemia. Small. 16:e20018902020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yue S, An J, Zhang Y, Li J, Zhao C, Liu J,
Liang L, Sun H, Xu Y and Zhong Z: Exogenous antigen upregulation
empowers antibody targeted nanochemotherapy of leukemia. Adv Mater.
35:e22099842023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sayitoglu EC, Luca BA, Boss AP, Thomas BC,
Freeborn RA, Uyeda MJ, Chen PP, Nakauchi Y, Waichler C, Lacayo N,
et al: AML/T cell interactomics uncover correlates of patient
outcomes and the key role of ICAM1 in T cell killing of AML.
Leukemia. 38:1246–1255. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Petit I, Karajannis MA, Vincent L, Young
L, Butler J, Hooper AT, Shido K, Steller H, Chaplin DJ, Feldman E
and Rafii S: The microtubule-targeting agent CA4P regresses
leukemic xenografts by disrupting interaction with vascular cells
and mitochondrial-dependent cell death. Blood. 111:1951–1961. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
John S, Chen H, Deng M, Gui X, Wu G, Chen
W, Li Z, Zhang N, An Z and Zhang CC: A Novel Anti-LILRB4 CAR-T Cell
for the Treatment of Monocytic AML. Mol Ther. 26:2487–2495. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Jiang H and Li H: Prognostic values of
tumoral MMP2 and MMP9 overexpression in breast cancer: A systematic
review and meta-analysis. BMC Cancer. 21:1492021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bharadwaj S, Sahoo AK and Yadava U:
Editorial: Advances in the therapeutic targeting of human matrix
metalloproteinases in health and disease. Front Mol Biosci.
10:11504742023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Levin M, Udi Y, Solomonov I and Sagi I:
Next generation matrix metalloproteinase inhibitors-Novel
strategies bring new prospects. Biochim Biophys Acta Mol Cell Res.
1864((11 Pt A)): 1927–1939. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Narla RK, Dong Y, Klis D and Uckun FM:
Bis(4,7-dimethyl-1,10-phenanthroline) sulfatooxovanadium(I.V.) as a
novel antileukemic agent with matrix metalloproteinase inhibitory
activity. Clin Cancer Res. 7:1094–1101. 2001.PubMed/NCBI
|
|
110
|
Pappalardi MB, Keenan K, Cockerill M,
Kellner WA, Stowell A, Sherk C, Wong K, Pathuri S, Briand J,
Steidel M, et al: Discovery of a first-in-class reversible
DNMT1-selective inhibitor with improved tolerability and efficacy
in acute myeloid leukemia. Nat Cancer. 2:1002–1017. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Bäumer N, Scheller A, Wittmann L, Faust A,
Apel M, Nimmagadda SC, Geyer C, Grunert K, Kellmann N, Peipp M, et
al: Electrostatic anti-CD33-antibody-protamine nanocarriers as
platform for a targeted treatment of acute myeloid leukemia. J
Hematol Oncol. 15:1712022. View Article : Google Scholar : PubMed/NCBI
|