Introduction
GBM is the most common and lethal primary brain
tumor in adults, characterized by a complex and diverse
pathogenesis. In recent years, high-throughput sequencing
technologies and multi-omics analyses have revealed intricate
molecular features in GBM, including genetic mutations [such as
epidermal growth factor receptor (EGFR) amplification, TP53
mutations and isocitrate dehydrogenase (IDH)1/2 mutations],
epigenetic alterations (such as DNA methylation and histone
modifications), the immunosuppressive nature of the tumor
microenvironment and the regulatory roles of non-coding (nc)RNAs
[such as circular (circ)RNAs and micro (mi)RNAs], as well as
abnormal activation of multiple signaling pathways (such as the
PI3K/AKT/mTOR and MAPK pathways) (1). These molecular changes not only drive
tumor formation and progression but also influence the tumor's
response to treatment.
Traditional GBM treatment strategies are primarily
surgery-based. However, due to the highly invasive and diffuse
nature of GBM, complete resection is often unachievable.
Chemotherapeutic agents inhibit tumor growth by damaging tumor cell
DNA, but many patients develop drug resistance, reducing
therapeutic efficacy. In terms of targeted therapy, drugs targeting
key molecules such as EGFR and vascular endothelial growth factor
(VEGF) have been developed, but clinical outcomes remain
unsatisfactory (2). Recent research
has increasingly focused on immunotherapies, such as chimeric
antigen receptor T-cell (CAR-T) therapy and checkpoint inhibitors,
aiming to enhance the body's immune response to combat the tumor
(3). In addition, gene therapy and
physical therapies were shown to be potential applications. Despite
significant progress in both basic research and clinical treatment
for GBM, patient prognosis remains poor, highlighting the urgent
need for further research to uncover more molecular mechanisms and
develop more precise and effective treatment strategies. In the
future, multidisciplinary collaboration and personalized therapy
may be crucial directions for improving GBM treatment outcomes.
Therefore, a deeper understanding of GBM's molecular mechanisms and
the development of novel therapeutic strategies have become key
focuses of current research.
Literature selection
In the literature search for the present study
titled ‘Transitioning from molecular methods to therapeutic
methods: An in-depth analysis of glioblastoma’, to enhance
transparency, the selection of references adhered to strict
inclusion and exclusion criteria. The search was conducted in the
PubMed database (https://pubmed.ncbi.nlm.nih.gov/) and the search terms
were as follows: ‘Glioblastoma’, ‘molecular mechanisms’ and
‘treatment’.
The literature inclusion and exclusion criteria were
as follows: i) Selection of studies directly related to the
transition of GBM from molecular mechanisms to therapeutic
approaches to ensure the relevance of the research topic; ii)
Selection of papers published in authoritative peer-reviewed
academic journals to ensure the credibility and scientific rigor of
the studies; iii) Rigorous research methodology, reliable data,
clear study design, and reasonable data analysis; iv) Recent
publications to reflect the field's (of which 68% are published in
the last five years). (68% of which are references from the last
five years).
By specifying and strictly adhering to these
inclusion and exclusion criteria, the high quality, relevance, and
reliability of the selected references were ensured, thus providing
a solid foundation for the study and enhancing the scientific
validity of our manuscript.
Comprehensive overview of GBM
Essential clinical characteristics and
diagnostic directions for GBM
Globally, GBM stands as the leading invasive primary
malignant brain tumor, accounting for ~15% of intracranial and 45
to 50% of primary malignant brain tumors (4). The primary clinical signs of GBM are
primarily linked to the tumor's substantial impact on the brain's
nearby activities, including symptoms such as headaches, epilepsy,
changes in emotions or personality, speech modifications,
diminished sense of touch, hearing and smelling abilities, and
compromised physical coordination and balance (5).
Traditional methods for identifying GBM require
histopathological analysis, using the observation or
non-recognition of pathological features such as microvascular
expansion and necrosis as benchmarks. In 2021, The World Health
Organization (WHO) updated its Classification of Central Nervous
System Tumors, 5th Edition (CNS5) system, revising the diagnostic
criteria for GBM (6): The
diagnostic requirements for GBM are based on the absence of
isocitrate dehydrogenase and histone 3 mutations as cornerstones,
and the IDH mutation category has been eliminated.. While various
low-grade (WHO grade 2 or 3) diffuse astrocytomas do not have these
histological features, their clinical presentations also reflect
those of GBM. As a result, in 2021, the WHO's categorization
brought forth new molecular benchmarks: Simultaneous amplification
of the entire chromosome 7 and the eradication of the full
chromosome 10 (+ 5/10); mutations in the telomerase reverse
transcriptase (TERT) promoter; and the enhancement of the EGFR. The
aforementioned mutations in WHO grade 2 or 3 tumors could upgrade
them to the level of WHO grade 4 (molecular GBM) (4).
Details regarding epidemiology and
risk elements
Epidemiological data indicate that the global yearly
incidence of GBM is ~3–4 cases per 100,000 individuals. Each year,
the US sees an approximate GBM occurrence of 3.19 in every 100,000
individuals (7). As age progresses,
the incidence of GBM significantly increases, mainly among the 45
to 70 age bracket, with men having higher frequencies than women,
exhibiting a gender ratio of ~1.6:1 (8). The prognosis for GBM is dismal, with a
median survival duration of 12–15 months following diagnosis, and a
5-year survival rate close to 5% (9).
The development of GBM is linked to a range of
genetic and environmental factors. The presence of a familial
lineage, coupled with distinct genetic changes, significantly
increases the likelihood of developing the disease. A close link
exists between genetic conditions such as neurofibromatosis type 1
(NF1) and Li-Fraumeni syndrome and the development of GBM (10). In addition, changes in genes such as
TP53, phosphatase and tensin homolog (PTEN) and EGFR are more
commonly observed in patients with GBM (11). Considering diverse environmental
factors, elevated concentrations of ionizing radiation are
recognized as a critical risk element for GBM, while elements such
as mobile phone use and exposure to electromagnetic fields lack a
direct connection to GBM (12).
Investigations focusing on grasping the epidemiological
characteristics and risk facets of GBM are vital for understanding
its evolution and devising effective prevention and treatment
strategies. Future studies should explore more profoundly the exact
part that genetics and environmental factors play in the
development of GBM, offering new viewpoints for the prevention and
therapy of this malignant neoplasm (Fig. 1).
Molecular mechanisms
Process of molecular typing in
GBM
According to the molecular characteristics of GBM,
researchers have classified it into the following subtypes: The
classical type is represented by EGFR amplification, TP53 mutation
and PTEN deletion; the neural type has cellular characteristics
similar to normal nerve cells and shows high levels of
neurodevelopment at the molecular level; the mesenchymal type
possesses higher stem cell characteristics and proliferation
ability, often accompanied by NF1 mutation and upregulated
expression of immune-related genes; and the primary type is more
frequently seen in young patients, along with IDH1 mutation and
TP53 mutation (13).
Alterations in genetics and epigenetic
studies
Studies reveal that various vital genetic
alterations in GBM, including TP53, IDH1/2 and EGFR, have a
significant impact on the development, progression and treatment of
tumors (1).
Changes in TP53 are considered to be among the
prevalent mutations found in GBM. P53, the protein encoded by the
TP53 gene and known for tumor suppression, acts as a stimulant for
the cell cycle and apoptosis, with its reduced function leading to
rampant cell proliferation and tumor development (14). Approximately 80% of GBM cases show
mutations in TP53 (15).
IDH is the chief source of nicotinamide adenine
dinucleotide phosphate in the cytosol of brain cells, where its
variants, the cytoplasmic IDH1 and mitochondrial IDH2, reduce DNA
damage and lipid oxidation (16).
Despite the WHO CNS5 having discontinued IDH as a conclusive sign
of GBM, changes in the IDH1 and IDH2 genes are equally influential
in triggering GBM (17). Cancerous
growths carrying IDH mutations demonstrate a prolonged survival
period, unlike those in IDH-wild-type tumors that lack IDH
mutations (18). The proteins IDH1
and IDH2 function to convert isocitric acid into α-ketoglutarate
(AG). When altered, AG transforms into 2-hydroxyglutarate, an
essential metabolite of the gliomagenogenesis process (19), triggering changes in epigenetics and
the onset of tumors.
The EGFR is part of the receptor tyrosine kinase
(RTK) category, which includes four specific receptors [human EGFR
(HER)1-4/ErbB1-4]. This factor is crucial for maintaining cellular
existence, proliferation, migration and averting cell demise,
intricately connected to pathways such as PI3K/AKT, rat sarcoma
(RAS)/MAPK/ERK, phospholipase C (PLC)/protein kinase C (PKC) and
JAK/STAT (2). Changes and increased
expression of the EGFR gene are frequently found in GBM (20), accounting for ~40% of all primary
GBM cases (11).
These modifications result in an uneven rise in
receptor activity, promoting tumor cell growth and longevity,
aiding in tumor blood vessel formation and reducing the
effectiveness of chemotherapy and radiotherapy for GBM (2).
PTEN, located on chromosome 10q23.31, is a tumor
suppressor gene encoding a phosphatase protein. PTEN plays a vital
role in regulating cellular growth, multiplication and survival
processes, and is significantly involved in the molecular evolution
of gliomas (21). Xia et al
(22) found that eliminating or
modifying the PTEN gene activates the PI3K/AKT pathway
continuously, leading to the activation of mTOR and suppression of
glycogen synthase kinase (GSK)-3β, thereby increasing the movement
and infiltration of GBM cells (23). In addition, alterations or decreases
in PTEN expression levels are linked to increased severity of
disease, less positive results and lower overall survival rates
(21). Apart from genetic
modifications, key genetic alterations, including DNA methylation,
histone changes and other epigenetic shifts governed by ncRNA, play
a crucial role in shaping GBM (24).
DNA methylation is a frequent alteration found in
epigenetic sequences. Typically, methylation processes occur in CpG
Islands, which impact the gene's role in transcription. The state
of methylation in the O6-methylguanine-DNA methyltransferase (MGMT)
promoter of GBM is linked to the effectiveness of alkylation
chemotherapy in patients with GBM, acting as a vital measure for
both prognosis and the success of the treatment.
DNA methylation represents a prevalent epigenetic
modification, predominantly occurring at CpG islands within gene
promoter regions, thereby exerting regulatory control over gene
transcription. In GBM, the methylation status of the MGMT gene
promoter has been established as a critical biomarker,
demonstrating a significant association with the therapeutic
response to alkylating agents and serving as an independent
prognostic factor for patient outcomes (25). Modifying the methylation of the MGMT
promoter initiates gene suppression, leading to reduced DNA repair
capability and higher sensitivity of tumor cells to chemotherapy.
In addition, methylation at the promoter region of key
tumor-inhibiting genes such as retinoblastoma (Rb)1,
cyclin-dependent kinase inhibitor A and PTEN is observed in GBM,
highlighting their vital contribution to tumor growth (26).
Changes in histones, crucial to epigenetic
activities such as acetylation and phosphorylation, significantly
influence gene expression by influencing chromatin architecture
(27). Frequently, the erratic
functions of histone deacetylase (HDAC) and DNA methyltransferase
have been noted in instances of GBM (28). The ability of HDAC inhibitors to
stop GBM cell proliferation and induce cell death suggests their
potential application in the treatment of GBM (29).
Lately, ncRNAs have garnered substantial attention
as essential regulators. NcRNAs include a set of RNA entities
lacking proteins, notably miRNAs, long ncRNAs (lncRNAs) and
circRNAs (30). NcRNAs play a
pivotal role in regulating gene activity, fostering cellular
development, triggering apoptosis and forming the surrounding
microenvironment of tumors (31).
Furthermore, it is a key factor in the epigenetic regulation of GBM
(32).
MiRNAs are types of small RNA molecules of ~22
nucleotides in length, which serve to either inhibit translation or
assist in its degradation through attachment to specific gene
mRNAs. Within GBM, alterations in the expression levels of specific
miRNAs influence the growth and spread of tumors (33). For instance, miR-21, which is
upregulated in GBM, has the ability to suppress the expression of
genes such as PTEN and p53, thereby boosting cell proliferation,
survival, proliferation and infiltration (34). Furthermore, miRNAs such as miR-10b,
which is upregulated in GBM cells, contribute to the incursion and
proliferation of these cancerous cells (35). Tan et al (36) uncovered that modifications in miRNA
levels impact the biological functions of GBM cells, proposing new
therapeutic ideas.
LncRNAs, a category exceeding 200 nucleotides in
length, play a critical role in regulating gene expression and
cellular functions. In GBM, a notable expression pattern of lncRNAs
is intimately associated with the evolution and progression of
tumors (37). LncRNAs affect gene
expression through their interaction with transcription factors and
enzymes that modify chromatin structures. In GBM, LncRNAH19
demonstrates considerable levels of expression, contributing to the
growth of tumor cells by inhibiting the function of tumor
suppressor genes (38). LncRNAs
contribute to tumor avoidance in immune responses by altering
immune cells' functions in their immediate environments, and
lncRNAMALAT1 aids in tumor avoidance by changing T-cell functions
(39). In addition, lncRNAs such as
HOTAIR aid in modifying H3K27me3 by interacting with the polycomb
repressive complex 2, causing a reduction in chromatin and gene
suppression, which in turn affects the proliferation and locomotion
of GBM cells (40).
CircRNA is a special type of ncRNA that forms a
circular structure through head-to-tail ligation. This circular
structure endows circRNA with higher stability and specificity. It
has been demonstrated that the levels of oncogenic circRNAs are
elevated in GBM samples (41),
thereby promoting GBM proliferation, invasion, glycolysis and
epithelial-mesenchymal transition (EMT) (42). In addition, circRNAs can influence
the transcription process by interacting with transcription
factors, thereby regulating the biological behaviors of tumor cells
(43).
Apart from DNA methylation, histone modifications
and ncRNA alterations, other epigenetic phenomena such as chromatin
alteration and RNA shifts play a crucial role in the processes of
GBM. Changes and anomalies in chromatin remodeling structures, such
as the SWI/SNF complex, often arise in GBM, with these structures
governing gene expression by altering nucleosome locations and
chromatin structure (44).
Modifications in RNA, such as the N6-methyladenosine change, are
found in GBM, affecting tumor-specific gene expression via effects
on RNA balance, translation efficiency and splicing processes
(45).
The emergence and development of GBM originate from
a blend of numerous genetic mutations and epigenetic shifts.
Alterations in genetics and epigenetics provide insight into the
molecular dynamics of GBM and pave the way for creating alternative
diagnostic and treatment strategies. Further studies should
thoroughly investigate the exact mechanisms of these mutations and
alterations to strengthen the basis for tailored GBM therapies.
Paths of signal transmission
RTKs, a type of transmembrane receptor protein, form
part of an extracellular structure linking with specific ligands
such as EGF, platelet-derived growth factor (PDGF), VEGF,
fibroblast growth factor (FGF) and hepatocyte growth factor, while
their intracellular part shows activity in tyrosine kinase. When
ligands bind to RTKs, they initiate either dimerization or
multimerization, subsequently activating their tyrosine kinase
activities. Upon activation, RTKs begin the autophosphorylation
process, phosphorylating tyrosine residues and acting as docking
sites to draw in and activate different downstream signaling
proteins.
Pathway involving PI3K/AKT/mTOR
Phosphorylation of tyrosine components initiates
RTKs, which activate the PI3K/AKT/mTOR pathway, vital for the
proliferation, endurance, motility and metabolic processing of GBM
cells. An alarming 86% of individuals with GBM exhibit genetic
alterations in their RTK/PI3K pathway (46).
Upon activation of a cell surface receptor (e.g.,
growth factor receptor or insulin receptor), PI3K is recruited to
the membrane via its regulatory subunit, and its catalytic subunit
(p110) subsequently phosphorylates the substrate PIP2
(phosphatidylinositol-4,5-bisphosphate) to produce
phosphatidylinositol-3,4,5-trisphosphate (PIP3). PIP3 acts as a
second messenger and specifically binds to the PH domain of AKT to
recruit AKT to the cell membrane. PIP3 acts as a second messenger
and specifically binds to the PH domain of AKT to recruit AKT to
the cell membrane. Activated AKT regulates key biological processes
such as cell proliferation, survival, metabolism and apoptosis by
phosphorylating downstream effector molecules, mTOR and GSK3β
(47).
MDM2, subsequently targeted by AKT, advances to the
nucleus post-AKT phosphorylation, attaching to p53 with the
objective of dismantling tumor suppressor genes, resulting in
changes to MDM2, noted in 87% of GBM patients (14). The activation of AKT promotes the
cell cycle by phosphorylating and inhibiting the inhibitors p27 and
p21. This action leads to the stabilization and proliferation of
cyclin D1/D3 (48).
A vital molecule in the PI3K/AKT signaling pathway,
mTOR, is divided into two separate complexes: mTORC1 and mTORC2.
Such complexes boost cellular growth, fat generation and nucleotide
generation by phosphorylating and inhibiting lipin-1 in
nutrient-rich and growth factor-rich settings. Furthermore, mTORC1
acts to activate hypoxia-inducible factor 1α (HIF-1α). Furthermore,
mTORC1 enhances the mitochondrial tetrahydrofolate cycle's
metabolic rate, particularly in the purine synthesis pathway, by
boosting the amounts of active transcription factor 4 and
methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 2,
methenyltetrahydrofolate cyclohydrolase (49).
mTORC2′s phosphorylation and activation of AKT
trigger overlapping positive feedback loops in the PI3K route,
indicating a possible critical function in GBM's resistance against
PI3K/AKT/mTOR suppression. mTORC2′s evolving roles encompass
regulating glycolysis driven by AKT and MYC, managing lipid
processing and regulating glutamine metabolism within GBM. By
contrast, mTORC2 plays a crucial role in regulating the longevity
of cells and restructuring the cytoskeleton, thus enhancing the
migration and penetration of GBM cells (50).
Distinct from PI3K/AKT signaling, the
EGFRvIII-facilitated mTORC2 mechanism initiates phosphorylation and
inhibits the class IIa HDAC complex, leading to the deacetylation
of forkhead box (FOX)O transcription factors. Inhibiting FOXO
activity leads to heightened MYC levels, decreased gluconeogenesis
and improved glycolysis (51).
Therefore, the RTK/PI3K/AKT/mTOR mechanism impacts diverse
functions, including governing the cell cycle, metabolic
activities, cellular expansion and various epigenetic regulatory
roles.
Ras/Raf/MEK/ERK
The MAPK/ERK pathway begins at the cellular RTKs
stage (52). Once the receptor is
activated, RAS proteins detect signals via the growth factor
receptor-bound protein 2/SOS complex. RAS triggers RAF kinase
family proteins, such as BRAF, through their attachment to GTP.
After activation, RAF persistently phosphorylates and MAPK/ERK
kinase (MEK), which then triggers ERK phosphorylation, prompting
its migration to the nucleus and activation of multiple
transcription factors and their respective effector molecules
(53).
Aberrant activation of the MAPK/ERK signaling
pathway in GBM is closely associated with a variety of molecular
events (54), Dysregulation of this
pathway is usually driven by overactivation or mutation of upstream
receptor tyrosine kinases (RTKs, such as EGFR) (e.g., EGFRvIII),
oncogenic mutations in RAS or RAF genes, resulting in sustained
phosphorylation of ERK proteins. Upon translocation into the
nucleus, activated ERK promotes cell cycle progression (e.g.,
up-regulation of Cyclin D1, CDK4), cell growth and proliferation
through the regulation of key transcription factors (e.g., c-Myc,
CREB, AP-1, etc.), as well as inhibits the expression of
pro-apoptotic genes (e.g., BAX, PUMA) and proteins. In addition
MAPK/ERK signaling prolongs tumor cell survival and enhances drug
resistance by activating anti-apoptotic pathways (e.g. BCL-2 family
proteins) and telomerase activity, ultimately leading to malignant
progression of GBM (55). Within
the Raf protein family, BRAF stands out as the main factor
contributing to cancer development. In BRAF, the class I point
mutation known as BRAFV600E is recognized as the primary mutation.
The mutation in question, unique among BRAF mutations in GBM, is
found in a small number but appears predominantly in children,
young adults and epithelioid GBM. Changes in BRAF trigger its
inherent activation, resulting in extended stimulation of its
ensuing effectors MAPK, MEK1/2 and ERK1/2. Furthermore, ERK and
AKT/mTOR collaboratively focus on proteins like MYC and HIF1α,
collectively providing cancer cells with the essential proteins and
energy for their development and vigorous proliferation.
Furthermore, the MAPK/ERK pathway enhances the
movement and aggressive capability of GBM cells by altering the
extracellular matrix (ECM) and the cytoskeleton's structure
(56). Activating ERK augments the
generation of matrix metalloproteinases (MMPs), resulting in the
collapse of ECM components and the promotion of tumor cell spread.
Furthermore, the MAPK/ERK pathway is instrumental in angiogenesis,
regulating VEGF levels, helping establish new blood vessels in
tumors and providing GBM cells with sufficient nourishment and
oxygen (57).
PLC-γ is a member of the PLC family, encompassing 13
separate subtypes. Typically, this is activated by RTK or G
protein-coupled receptor, particularly post-RTK activation, through
phosphorylation at Y sites (tyrosine residues), which in turn
activates PLC-γ. The PLC-γ enzyme plays a role in decomposing
phosphatidylinositol 4,5-bisphosphate (PIP2), leading to the
formation of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol
(DAG). IP3 latches onto IP3 receptors on the endoplasmic reticulum,
producing calcium ions (Ca2+) and subsequently
increasing cellular calcium ion concentrations. DAG interacts with
PKC, consequently activating PKC (serine/threonine protein kinase).
Upon activation, PKC can phosphorylate various downstream proteins,
including transcription factors, kinases and structural proteins,
thereby controlling biological functions such as cellular growth,
survival, mobility and apoptosis (58).
In GBM, it is frequently observed that mutations or
the overproduction of RTKs such as EGFR are prevalent (59). The erratic activation of such RTKs
governs cytoskeletal remodeling via the phosphorylation of
associated proteins, such as actin-binding proteins, facilitating
the movement and penetration of GBM. PKCα aids in the expansion and
maintenance of glioma cells through the EGFR/mTORC pathway. Under
hypoxic conditions, PKCβ activation promotes tumor angiogenesis by
enhancing the migratory and proliferative capacity of brain
endothelial cells. Therefore, PKCβ may contribute to the uneven
vascular formation observed in GBM development, highlighting its
significance in therapy (60).
Although the PI3K and MAPK pathways are separate drivers of the
evolution and progression of GBM, PKC pathways cross these paths,
uncovering a complex web of signals within GBM cells.
Furthermore, PKC is capable of regulating MMP
activities and VEGF expression, allowing tumor cells to penetrate
the neighboring matrix, infiltrate adjacent tissues and enhance the
development of tumor blood vessels, thus providing essential
nutrients and oxygen for tumor growth (61).
Mechanism involving NF-κB
signalling
NF-κB, a type of transcription factor, is made up of
heterodimers formed by five elements within the family: p50, p52,
RelA, RelB and c-Rel. Activation usually takes place when surface
receptors such as TNF-α receptor 1 or IL-1 receptor are stimulated
(62). Triggering NF-κB promotes
tumor growth and proliferation, hinders programmed cell death and
increases treatment tolerance (63).
NF-κB's extensive cancer-causing effects include
regulating gene transcription to avert cell death, boosting cyclins
to fast-track the cell cycle and initiating the synthesis of
proteins associated with cellular invasion and angiogenesis, such
as MMP and VEGF. Changes in the NF-κB gene's genetics, dysfunctions
or disruptions in the mechanisms governing NF-κB dimer activation
can lead to various forms of cancer. Regarding GBM, there is a
regular occurrence of erratic activation of NF-κB, with several
processes associated with diminished NF-κB signaling in gliomas
(64). In the context of GBM, both
EGFR and PDGFR display inconsistent functions, and the pathways
triggering cancer through EGFR and PDGFR play a key role in the
growth and penetration of tumor cells. Furthermore, the reduction
of PTEN and NF1 is linked to atypical NF-κB activity in GBM,
leading to increased PI3K activity. The lack of Krueppel-like
factor 6, known for inhibiting NF-κB, triggers its activation in
GBM (65).
Wnt pathway
The influence of the Wnt signaling pathway is
crucial in shaping essential cell operations throughout the
development stages of the central nervous system. A strongly
established connection exists between the overactivation of Wnt
receptors and the promotion of harmful alterations, resulting in
the development of brain tumors (66).
GBM displays heightened activity in its Wnt pathway
(67). It is recognized that the
unconventional WNT5A molecule enhances neuronal cell
differentiation and plays a substantial role in cellular
multiplication. Knockdown of WNT5A in GBM cells using short hairpin
RNA (shRNA) significantly reduced their proliferation rate,
suggesting a pro-tumorigenic role for WNT5A. By contrast,
activation of the uncommon WNT signaling pathway is closely
connected to the aggressive characteristics of GBM cells. The
existence of atypical elements such as WNT5A and frizzled class
receptor 2 has a significant impact on cell penetration in GBM,
markedly affecting the outlook (68). In addition, a variety of minimally
present cell adhesion substances (such as cadherins and connectors)
hinder the adhesion of tumor cells, thereby affecting the
Wnt/β-catenin pathway, notably by enhancing the role of β-catenin,
thus bolstering the tumor's propensity to penetrate (69).
Minor surroundings of tumors
GBM represents the gravest onset of brain cancer,
exhibiting a significant variation among and within the tumors, a
limited lymphocyte count and a plethora of myeloid subgroups in
both malignant and non-malignant ventricular regions, resulting in
an environment primarily conducive to tumor growth and immune
suppression (70). In contrast to
conventional ECM, the GBM ECM is enriched with hyaluronic acid,
collagen, glycoprotein-1, neuroglycan (NG), chondroitin sulfate
proteoglycan 4 (CSPG4/NG2), versican, and tenascin-C, collectively
fostering tumor invasion and therapy resistance (71). In addition, numerous GBM cells
infiltrate adjacent brain layers, modifying the neural environment
to promote neuron electrochemical interactions and the metabolic
connection with benign astrocytes, thereby encouraging growth
(72).
Within the GBM ECM, tumor growth, including
microglia, neutrophils, dendritic cells, bone marrow and myeloid
suppressor cells, constitutes 50% of total tumor growths (73). Neutrophils, myeloid-derived
suppressor cells and bone marrow-derived macrophages in this
category are associated with negative outcomes, decreased survival
rates and a higher chance of recurrence in GBM (74). Tumor-associated macrophages (TAMs)
play a pivotal role in shaping the immune-suppressing environment
during GBM's pathological stages. TAMs are primarily categorized
into two types: Conventional macrophages (M1 type) and those
activated through different pathways (M2 type). In the GBM context,
TAMs often exhibit the M2 phenotype, which is recognized for
boosting tumor cell growth, invasion and the creation of new blood
vessels, while also inhibiting immune responses that combat
cancer.
TAMs regulate the immunosuppressive state of the
tumor microenvironment by discharging a range of cytokines and
chemokines, such as IL-10, TGF-β and VEGF (75). These components obstruct the
function of effector T cells and promote the accumulation of
regulatory T cells, thus exacerbating the immunosuppressive
environment. In addition, TAMs display a complex interaction with
GBM cells. Within GBM cells, increased concentrations of
colony-stimulating factor (CSF)-1 and C-C motif chemokine ligand
(CCL)2 boost TAM attraction and polarization, and the dissemination
through CSF-1R and CCR2 elevates the survival and efficacy of TAMs.
Cytokines improve the intrusion and mobility of tumor cells and
bolster their toughness, thus intensifying the progression of GBM
(76). Deterioration of the
blood-brain barrier (BBB), mainly due to inflammation and pressure
from tumors, and the formation of new blood vessels, largely
attributed to significant VEGF, leads to increased GBM blood flow.
The presence of hypoxia and macrophages plays a role in harming the
BBB by fostering immune suppression via the CCL4-CCR5 axis and the
invasion by GBM (77). Recently,
there has been a notable escalation in attention towards therapies
targeting TAMs within the immunosuppressive microenvironment
(78).
Comprehending angiogenesis via the
VEGF signaling process
The distinctive characteristic separating GBM is its
widespread vascularization (79).
Inside the vicinity of a tumor, cancer cells promote development,
mobility and the formation of new blood vessel networks by
releasing various pro-angiogenic substances that meet the demand
for oxygen and vitamins, providing pathways for the spread of
cancer cells. A considerable quantity of VEGF predominantly gets
activated by oxygen scarcity and a variety of cytokines in the area
encircling the tumor. In environments with insufficient oxygen, the
activation of VEGF gene transcription by HIF-1α results in a rise
in VEGF expression levels. Tumor cells, macrophages and nearby
cells in the microenvironment also release VEGF and other elements
that facilitate the process of angiogenesis. Research suggests that
GBM cells are high in VEGF production, triggering the following
PI3K/AKT and Ras/MAPK pathways when receptors attach (80). This process enhances endothelial
cell proliferation and migration, facilitates ECM remodeling, and
promotes cell-cell adhesion, ultimately driving the formation and
maturation of new blood vessels. VEGF, through its autocrine and
paracrine mechanisms, provides tumors with essential blood and
nutrients, enhancing the tumor cells' resilience and invasive
capacities to create a complex network that benefits the tumor.
Furthermore, VEGF increases the permeability of blood vessels,
leading to swelling adjacent to the tumor and greatly affecting its
growth and spread (Fig. 2).
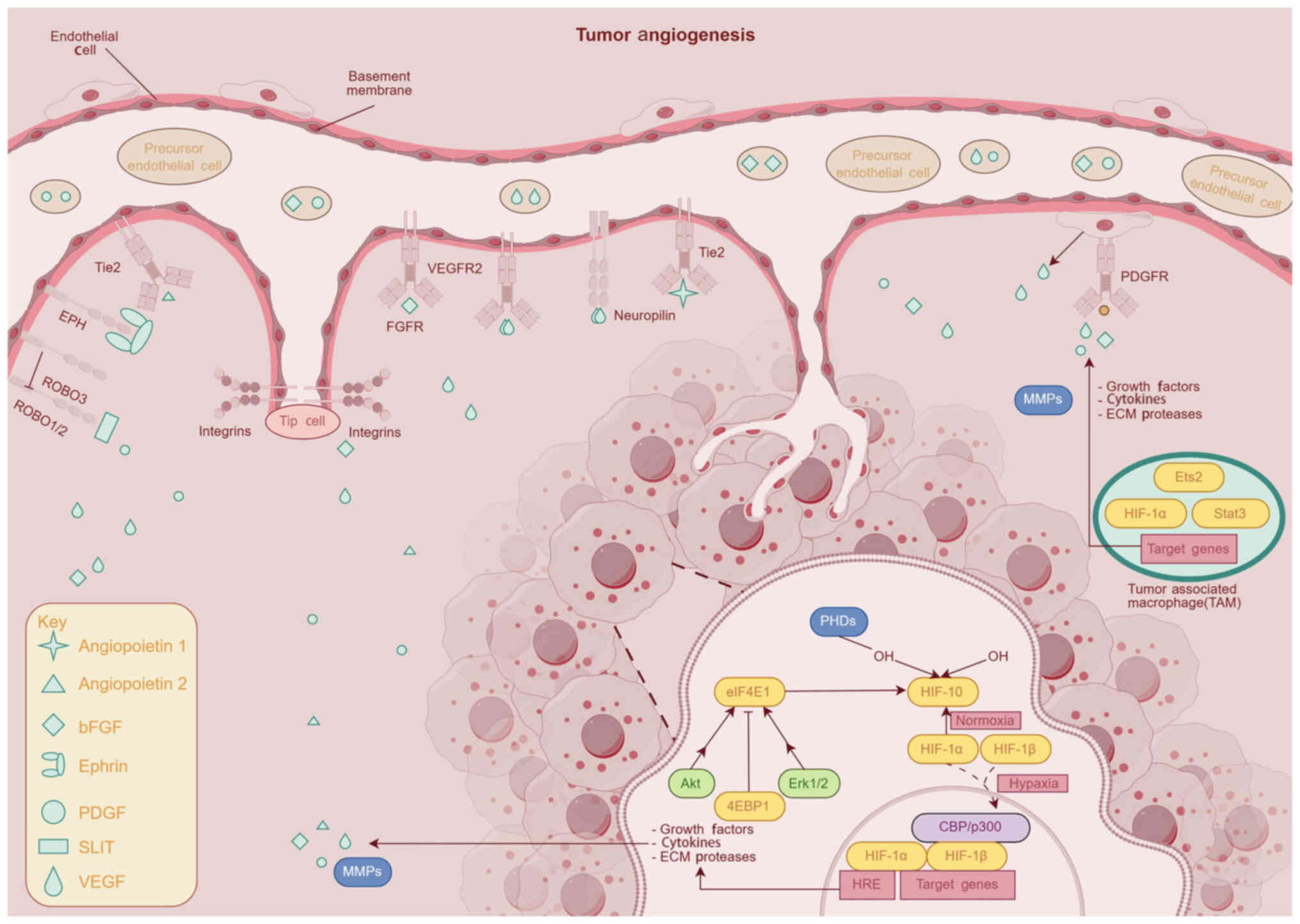 | Figure 2.Schematic depicting the angiogenesis
of tumors (generated with Figdraw) Tie2, Tie2 receptor tyrosine
kinase; EPH, erythropoietin-producing hepatocellular receptor;
ROBO, roundabout guidance receptor; PDGF, platelet-derived growth
factor; VEGF, vascular endothelial growth factor, PHDs, prolyl
hydroxylase domain proteins; eIF4E, eukaryotic initiation factor
4E, Akt, protein kinase B; Erk1/2, extracellular signal-regulated
kinase 1/2, 4EBP1, eukaryotic translation initiation factor
4e-binding protein 1; MMPs, matrix metalloproteinases; FGFR,
fibroblast growth factor receptor, VEGFR2, vascular endothelial
growth factor receptor 2; HIF, hypoxia-inducible factor; HRE,
hypoxia response element; PDGFR, platelet-derived growth factor
receptors; Ets2, endothelial transcription factor 2. |
Apart from its reliance on the creation of
endothelial cell blood vessels, GBM also demonstrates a behavior
known as vasculogenic mimicry (81). This relates to cancer cells creating
formations similar to blood vessels, separate from endothelial
cells. Cancer cells, by creating a microcirculation network, aid in
blood flow, particularly in cases of restricted angiogenesis,
thereby meeting the tumor's dietary needs. The progression of
tumors heavily relies on this procedure.
Bevacizumab, an antibody targeting VEGF, impedes the
process of angiogenesis by counteracting VEGF and hindering its
adherence to VEGFR (82). Studies
in medical environments suggest that bevacizumab may partly slow
down the development of GBM and improve patient survival. However,
the treatment targeting VEGF faces significant obstacles,
particularly regarding resistance to treatment and the adjustment
of the tumor. Tumor cells can combat anti-VEGF therapies by
boosting other angiogenic components (such as basic FGF) or
activating alternative pathways like Ang-2 signaling.
Dynamic interplay between stromal
cells and GBM
In GBM, the complex dynamics between stromal tissue
and tumor growth markedly affect how tumors evolve, develop and
resist treatments. Stromal cells include a range of cells such as
fibroblasts, endothelial and smooth muscle cells, and immune cells
like macrophages and lymphocytes, to name a few. These cells
interact with GBM cells through several techniques, encompassing
direct cellular communication, expulsion of signaling molecules and
reorganization of the ECM. Cancer-associated fibroblasts (CAFs)
amplify tumor cell growth, infiltration and transit by
disseminating different growth signals and cytokines, including
TGF-β, MMPs and FGFs (83).
Furthermore, CAFs encourage tumor propagation by altering the ECM
and rearranging the stroma structure. Endothelial cells facilitate
tumor growth and spread by discharging substances like nitric oxide
and prostaglandins and by regulating blood flow in the vicinity and
stimulating stromal cells. Apart from the earlier referenced cell
types, astrocytes augment the development and mobility of GBM cells
by secreting various neurotrophic components and cytokines, such as
glial cell-derived neurotrophic factor and CXCL12 (84). By contrast, oligodendrocytes
influence the growth and maturation stages of GBM cells through
direct engagement (85).
Tumor stem cells
Markers within GBM stem cells (GSCs)
GSCs, a subpopulation of undifferentiated cells
within GBM, are characterized by their dual capacity for
self-renewal and multilineage differentiation into heterogeneous
tumor cells. GSCs drive tumor initiation, progression, recurrence,
and resistance to therapy by serving as a persistent reservoir of
therapy-resistant cells (86).
Morphologically, GSCs typically appear as diminutive, compact cell
masses, marked by sparse cytoplasm, widespread nuclei, evenly
dispersed nuclear chromatin and separate nucleoli. GSCs demonstrate
a notable capacity for enduring and adaptable differentiation
abilities, in addition to their strong aggressive nature and
resistance to medication. Understanding the role of GSCs in GBM is
vital for devising effective treatment strategies (Table I).
 | Table I.GSCs markers and their functions. |
Table I.
GSCs markers and their functions.
| Surface markers of
GSCs | Molecular type | Function | Detection
method | (Refs.) |
|---|
| CD133 | Membrane
protein | Stem cells maintain
and self-renew | Flow cytometry,
immunohistochemistry | (159) |
| Sox2 | Transcription
factor | Maintain
pluripotency and self-renewal | qPCR,
immunohistochemistry | (160) |
| OLIG2 | Transcription
factor | Promote the
differentiation of glial cells | Western blot,
immunohistochemistry | (161) |
| CD44 | Mucin
glycoprotein | Cell-cell and
cell-matrix interactions | Flow cytometry,
immunohistochemistry | (162) |
| A2B5 | Ganglioside | Glial precursor
markers | Flow cytometry,
immunohistochemistry | (163) |
| ALDH1A3 | Enzyme | Metabolic
regulation, self-renewal | Flow cytometry,
qPCR | (164) |
| L1CAM | Mucin
glycoprotein | Cell migration and
invasion | Flow cytometry,
immunohistochemistry | (165) |
| Nestin | Fibroin | Neural stem cell
markers, involved in cytoskeleton remodeling | Western blot,
immunohistochemistry | (166) |
Role of stem cells in tumor occurrence
and development
GSCs are acknowledged as the essential cells
responsible for the onset of GBM. These components maintain tumor
growth and variety through their self-regeneration and conversion
into various cell types. Ishii et al (87) effectively separated a group of GBM
cells with stem cell properties, proficient in forming neurospheres
in vitro and triggering highly invasive tumors in living
organisms. Further research has shown that GSCs can maintain their
stem cells and self-reproduction functions by triggering numerous
signaling pathways, such as Notch, Wnt and Sonic hedgehog. In these
pathways, the role of the Notch signaling pathway is pivotal for
the self-replacement and endurance of GSCs. Ryskalin et al
(88) found that obstructing the
Notch signaling pathway significantly reduces GSCs' capacity for
self-regeneration and potential to develop tumors.
GSCs have an increased capacity for mobility and
penetration, aiding their expansion in brain tissues and the
formation of new tumor sites. Research suggests that GSCs aid in
tumor angiogenesis and matrix reconstruction by discharging various
MMPs and VEGF, interacting with different cells in the tumor's
surrounding environment to accelerate tumor development (89).
Furthermore, GSCs display a pronounced ability to
form blood vessels. Releasing Notch1 signaling units via exosomes
improves the multiplication of nearby cells and blood vessel
generation, altering the development of tumors and the outcomes of
treatments (90). Although
therapies like surgery, radiation therapy and chemotherapy may
momentarily shrink tumors, GSCs demonstrate significant resistance
and extended viability. The cells demonstrate immunity to
apoptosis, a result of both radiotherapy and chemotherapy, by
enabling multiple anti-apoptotic pathways, including PI3K/Akt and
Bcl-2. Furthermore, they enhance drug resistance through the
production of varied drug outflow proteins such as ATP binding
cassette subfamily G member 2 and multidrug resistance 1, reducing
the accumulation of chemotherapy drugs in cells, which may lead to
tumor recurrence (91).
Pathological mechanisms
Characteristics of organizational
pathology
A hallmark of GBM is its cellular heterogeneity,
characterized by diverse morphologies, dysregulated signaling
pathways (92). Nuclei display
considerable pleomorphic traits, differing in dimensions and
shapes, and show an uneven distribution of chromatin, often leading
to hyperchromatic and multinucleated instances. Within GBM, mitotic
figures are common, indicating a significant rate of cell
proliferation. In addition, the occurrence of large and
multinucleated giant cells is evident, underscoring the vast
variety found within cancer cells (93).
GBM is primarily characterized by microvascular
development and areas of necrosis (94). Under the microscope, tumor tissues
often exhibit microvascular proliferation, marked by enlarged
capillary walls and clusters of endothelial cells, termed
‘glomeruloid bodies’. Tumor arteries, recently formed, provide a
plentiful blood source, aiding the rapid proliferation and spread
of tumor cells (95). Tumorous
areas of the tissue frequently exhibit necrotic features, typically
appearing as unevenly shaped zones encircled by cancer cells in a
way that mimics pseudopalisading, leading to the unique
‘pseudopalisading necrosis’ state. The formation of necrotic areas
is closely connected to localized oxygen deficiency and poor
nutrient supply, a consequence of the tumor's rapid expansion.
Glial fibrillary acidic protein (GFAP), a specific
marker protein indicative of astrocytes, is profusely present in
GBM (96). Immunohistochemical
analysis shows inconsistent amounts of GFAP in GBM cells, with
certain areas exhibiting GFAP-positive cancer cells while others
have fewer. The variety noted may be associated with different
stages of differentiation and the functional attributes of cancer
cells. However, tumor cells devoid of GFAP typically show increased
mitotic activity and invasive ability, suggesting a heightened
likelihood of being malignant.
Besides GFAP, other markers such as
microtubule-associated protein 2 (MAP2) and neuron-specific enolase
(NSE) in neurons and glia have also been observed in GBM cells
(97). The expression of these
indicators further reveals the multifaceted potential and variety
inherent in GBM neurons. Primarily existing in neuronal cells, MAP2
is also observed in certain GBM cells, suggesting a high
probability of these cells to display features akin to neuron
differentiation. NSE may serve as an additional neural marker, with
its expression in GBM representing the multifaceted nature and
variety of the cancerous cells.
Typically, GBM tumors exhibit significant swelling.
Swelling arises due to cancer cells releasing active vascular and
permeability-enhancing substances. Peritumoral edema exacerbates a
patient's neurological issues and additionally assists in the
spread and penetration of cancer cells. GBM cells possess the
capability to secrete various cytokines and enzymes, such as MMPs
and VEGF. These components disrupt the BBB, increase the
permeability of blood vessels and promote the emergence of a tumor
microenvironment (98).
GBM cell biological activity
GBM represents a gravely malignant tumor located
within the central nervous system. Rapid proliferation, a lack of
tolerance and the aggressive behavior of this organism play major
roles in the obstacles encountered in its therapeutic approach.
The amplification and alteration of the EGFR gene in
GBM often result in the growth of cancer cells by enhancing the
PI3K/AKT signaling pathway downstream, thus increasing the
dependence on growth signals. GBM cells exhibit a significant
ability to resist cellular demise, primarily attributed to the
modification and reduced function of the p53 gene. In GBM cells,
deactivating the function of p53 leads to the suppression of
apoptotic pathways, thus improving the survival rates of the tumor
cells. Furthermore, in the context of GBM, activating the NF-κB
signaling route is vital for combating apoptosis.
Triggering the PI3K/AKT pathway promotes the
creation of GBM by reducing cell death, accelerating the cell
cycle, enhancing tumor cell multiplication and facilitating
metastasis (99). In addition, the
athanogene 3 (BAG3), associated with Bcl2, belongs to the BAG
family. Under low-oxygen circumstances, there is an observed
increase in tumor cells' production of the BAG3 protein, similar to
the reaction to HIF-1α. A study showed that lower BAG3 expression
leads to decreased HIF-1α in both normoxic and hypoxic states,
causing a pause in GBM growth and a rise in apoptosis (100). The CD2-associated protein (CD2AP)
acts as an adaptable protein structure, overseeing cell adhesion
and diverse modes of communication. Zhang et al (101) found that CD2AP improves the onset
of GBM by activating tripartite motif containing 5-driven NF-κB
signals.
MMPs, a group of zinc-dependent endopeptidases, play
a crucial role in the progression of GBM (102). Commonly, GBM displays an increased
presence of subtypes like MMP-2, MMP-9 and MMP-14 (103). MMPs possess the ability to
decompose various components of the ECM, including collagen,
laminin and fibronectin, among others. The impairment of the ECM
interferes with the robust structure of normal tissue, permitting
the movement and infiltration of cancerous cells. For instance,
MMP-9 can break down type IV collagen, essential for the basement
membrane, allowing tumor cells to penetrate the vessel walls and
enter the bloodstream, consequently increasing the likelihood of
metastasis. The expression and activity of MMPs are governed by
various signals that exist inside and outside the cell. Factors
like hypoxia, inflammatory cytokines (TNF-α, IL-1β) and growth
components (including EGF, PDGF) can trigger GBM cells to release a
higher quantity of MMPs.
EMT refers to a biological process in which
epithelial cells transform deliberately to become cells that
display mesenchymal traits. During this stage, cancer cells lose
their adhesion ability, their epithelial polarity vanishes, and
their invasion and migration potential is eventually enhanced.
Principal characteristics include reduced synthesis of cell
adhesion agents (such as E-cadherin), the transformation of the
cytoskeleton from a cytokeratin-focused to a vimentin-focused form,
and the structural qualities of mesenchymal cells. The activation
of EMT is driven by starting elements, a range of transcription
factor families and several signals transmitted via channels such
as TGF-β/Smad and Wnt/β-catenin, along with related genes, all
contributing to the incursion and progression of glioma (104).
Concentrating on the invasive processes of GBM
requires altering the cytoskeleton, relocating cells and
dismantling the extracellular matrix (105). Elements within the Rho GTPase
family, namely ras homolog family member A (RhoA), Rac family small
GTPase 1 and cell division cycle (CDC)42, are crucial for managing
cytoskeletal restructuring processes (106). The serine/threonine kinases
Rho-associated coiled-coil containing protein kinase 1 (ROCK1) and
ROCK2 are essential downstream components of RhoA, influencing
mechanisms such as cell invasion, proliferation and muscle
contraction (107). Upon
activation of RhoA, its interaction with downstream effector
proteins (e.g., ROCK) enhances contraction of the intracellular
actin-myosin cytoskeleton. This contraction remodels cellular
morphology and generates mechanical forces that promote tumor cell
motility (e.g., migration and invasion).. Studies reveal that the
teneurin transmembrane protein 1 (TENM1) aids in altering the
cytoskeleton and parenchymal invasion in GBM cells by triggering
the RhoA-ROCK pathway (108).
CDC42, part of the Rho GTPase group, functions as a
trigger within cells. The element under study shows elevated levels
in various human cancerous formations and plays a crucial role in
the advancement of tumor growth (109). Upon activation, CDC42 initiates
actin polymerization, resulting in filopodia that help cells locate
their external surroundings and choose migration points. Elevated
CDC42 levels are associated with an unfavorable prognosis for
patients with glioma and boost CDC42-induced cell death in tumors
by blocking the p21 (RAC1) activated kinase/AKT/MDM2/p53 pathway in
these cells (110).
The PI3K/AKT pathway is vital for cellular
infiltration and mobility in GBM (111). Activation of PI3K leads to the
production of PIP3, which then activates AKT. The mechanism
inhibits GSK-3β, prompting β-catenin accumulation and nuclear
entry, subsequently activating genes associated with cellular
proliferation and movement.
The function of focal adhesion kinase (FAK) is
essential for signal transmission in the interaction between cells
and the ECM (97). The attachment
of the ECM to integrins activates FAK, sparking a series of signal
transmissions through phosphorylation and interactions with other
molecules such as Src family kinases and PI3K, thereby regulating
cellular adherence, locomotion and penetration.
Existing treatment methods
The surgical removal of an exceptionally cancerous
first-generation brain tumor can significantly reduce the tumor's
severity, decrease its symptoms and prolong life expectancy
(112). However, the unclear
boundaries of a tumor may result in residual tumor cells after
surgery, prompting their reemergence and leading to adverse
outcomes such as infections, bleeding and nerve damage. The
importance of these risks escalates if the tumor is located in a
brain area that is responsive to activity (113).
The considerable advantages of radiation therapy
originate from its unobtrusive character. The technique efficiently
eliminates minor lesions left after tumor removal, and with
diagnostic imaging like MRI, radiation is limited to a certain
location (114). The most
effective approach for individuals under 70 years of age or those
who are generally healthy is starting radiation treatment between 4
to 6 weeks post-surgery or before, in combination with
chemotherapy. Radiotherapy has the potential to change the tumor's
surrounding environment, alter the functioning of immune cells and
enhance the immune system's capacity to detect and direct tumors.
However, owing to either inherent or tumor environment resistance
to radiation, tumor recurrence remains inevitable (115).
The efficacy of chemotherapy lies in the immediate
damage to tumor cell DNA, leading to either their death or the
cessation of cellular proliferation. Currently, just three
chemotherapy drugs have been granted authorization by the US Food
and Drug Administration (FDA). The initial categorization includes
nitrosourea medications such as carmustine and lomustine; however,
their usage is typically halted in treatments due to liver and
kidney toxicity (116).
Temozolomide (TMZ) is second approved, standing as the only
chemotherapy drug sanctioned by the FDA for primary GBM treatment.
TMZ swiftly penetrates the BBB and modifies tumor cell DNA through
methylation, inflicting damage on DNA, hindering mismatch repair,
obstructing DNA replication and inducing apoptosis in rapidly
dividing cells (117).
A substantial blockage of the BBB hinders the
penetration of various medications into the nervous system. This
characteristic obstructs the distribution of chemotherapy drugs to
cancer cells, leading to a decrease in the drug's quantity and a
decline in treatment efficacy (118). Given the significant variation
both within and across tumors, a variety of tumor cell clusters
respond variedly to identical drugs, thus bypassing the effects of
chemotherapy (119). GSCs mainly
neutralize drug damage through the amplification of anti-apoptotic
proteins, initiating drug expulsion processes (such as
P-glycoprotein), along with other tactics (120). Within GBM tumors, the surrounding
environment markedly dampens the immune defense, as cancer cells
defy typical chemotherapy using the expulsion of immunosuppressive
components and manifestation of immune checkpoints such as
programmed cell death ligand 1 (PD-L1), among other ways (121). In the end, the Warburg effect
assists cancer cells in enduring conditions of limited oxygen and
nutrients, and metabolic increase can bolster drug resistance by
regulating cell signaling routes (119).
Emerging treatment strategies
In recent years, treatments such as surgery,
radiotherapy and chemotherapy have provided certain benefits, but
they have not significantly increased the overall survival duration
(9). The rise of molecular biology,
innovative therapeutic techniques and cutting-edge platforms has
catalyzed significant shifts in the methods for treating GBM.
Advancements in fields such as immunological checkpoint inhibitors,
cancer virus therapies, adoptive cell healing, nanoparticles,
convection-enhanced delivery, and boron neutron capture therapy
have fueled optimism for tackling GBM (122).
Targeted therapy
Creating targeted therapies for GBM has gained
research interest due to its unique characteristics and negligible
adverse effects. EGFR and VEGF are key players in various
malignancies, GBM included, and are central to therapeutic efforts
(2). EGFR inhibitors uniquely bind
with EGFR, thereby halting its ensuing signaling routes, which
consequently curtail the expansion and multiplication of cancer
cells. Compounds blocking EGFR, such as gefitinib and erlotinib,
have shown varying effectiveness, notably in patients with
EGFRvIII-mutant GBM. However, the diverse nature of tumors and
complex EGFR signaling mechanisms limit the efficacy of EGFR
inhibitors, which may result in resistance (2).
VEGF plays a crucial role in creating blood vessels
and shaping the tumor-surrounding environment in GBM (123). By using anti-VEGF agents to block
the binding of VEGF to its receptors, the growth and function of
tumor vasculature can be inhibited, thereby restricting the blood
supply to the tumor. The human-originated anti-VEGF monoclonal
antibody Bevacizumab has shown success in recurrent GBM management
in various clinical trials, thus extending the duration of patient
survival. However, the persistent efficiency and safety of VEGF
inhibitors demand further research and exploration.
Gene therapy and gene editing
technologies
Clustered regularly interspaced short palindromic
repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) technology,
famed for its significant gene editing power, has drastically
altered the disciplines of genetics and molecular biology (124). This technique precisely modifies
specific genes in cancer cells, providing novel possibilities for
GBM treatment (125).
CRISPR screening has been used in multiple steps of
studying GBM progression, including tumor initiation, tumor growth
and tumor invasion. To uncover the genetic factors regulating GBM
tumor initiation, Chow et al (126) developed an adeno-associated virus
(AAV)-mediated in vivo CRISPR screening approach.
They injected an AAV library targeting common
mutations in human cancers' tumor suppressor genes into the brains
of mice conditionally expressing Cas9 in astrocytes. Using this
method, they identified different mutation spectra in tumors and
driver combinations concurrently occurring in GBM, such as
beta-2-microglobulin, neurofibromin 1 and zinc finger CCCH-type
containing 13-retinoblastoma 1. Targeting key oncogenes or tumor
suppressor genes, such as TP53 and EGFR, inhibits tumor cell
growth, proliferation and invasion.
CRISPR screening has also been used to study other
aspects of GBM progression. Tu et al (127) used CRISPR screening to identify
the genetic vulnerability of TERT promoter mutations (TPMs) in GBM,
a genomic alteration present in >80% of GBM cases. While TPM
status is associated with differential gene expression and
dependence on ETS transcription factors such as E74-like ETS
transcription factor 1, ETS variant transcription factor 4 and GA
binding protein transcription factor, it is not particularly
related to TERT dependency. Lin et al (128) employed an in vivo and in
vitro CRISPR-Cas9 screening strategy to discover that ubiquitin
ligase RB binding protein 6, ubiquitin ligase promotes GSC
proliferation, self-renewal and tumor growth by regulating variable
polyadenylation through ubiquitination.
Immunotherapy
Immunotherapy aims to activate or fortify a
patient's immune defenses to identify and eliminate tumor cells.
Currently, immunotherapy leads the forefront in cancer treatments,
attracting attention through numerous methods including immune
checkpoint inhibitors, antibody-based therapies, cellular
therapies, cytokines, cancer vaccines, oncolytic viruses, and more,
with a special emphasis on therapies like mesenchymal stem cells
(MSCs) and adoptive cell transfer (ACT) (129).
MSCs are a type of adult stem cells designed for
self-renewal and varying lineage diversification, typically found
in regions such as the bone marrow, fat tissue and the umbilical
cord. Primarily, MSCs are intended for GBM treatment due to their
unique attraction to tumors and their capacity to alter immune
reactions. A study revealed that MSCs protect from rapid
degradation of medicinal substances through their response to
chemical components present in the tumor milieu, reducing
widespread side effects and enhancing treatment success by
precisely aiming at cancerous tissues for drug administration
(130).
By genetically altering MSCs, the distribution of
anti-cancer medications or genes to GBM lesions can enhance
treatment success and reduce extensive toxic responses.
Additionally, MSCs are crucial in regulating immunity, holding the
ability to curb inflammation and manage immune cells through the
release of various cytokines and growth factors. This type of
immunological adjustment intensifies the tumor's microenvironment,
strengthens immune responses to tumors and suppresses the spread of
tumors (131).
ACT is as a laboratory-based treatment approach,
boosting the immune cells in patients or donors and then
reintroducing them into the patient for an improved immune response
against cancer. During the treatment of GBM, research primarily
focuses on CAR-T, tumor-infiltrating lymphocytes (TILs) and
cytokine-triggered killer cells (CIKs).
CAR-T cell therapy involves genetically modifying T
cells in a patient through gene engineering, leading to the
production of CARs that detect tumor antigens, thereby enhancing
the T cells' ability to kill cancer. CAR-T is notably effective in
preventing blood cancers (3). For
the treatment of solid tumors, CAR-T cells targeting certain
antigens like EGFRvIII and IL13Rα2 have been developed to fight
GBM, proving successful through early and advanced clinical trials.
However, the vast variety in GBM and the tumor's internal
environment, which hinder immune reactions, remain the key barriers
in CAR-T cell therapy (132).
Utilizing TILs for treatment involves isolating
infiltrating lymphocytes from the patient's tumor, amplifying and
activating them externally to the body, and then reinserting them
into the patient to enhance the immune response. Using TILs as a
treatment has been shown to be effective in addressing solid
cancers, such as melanoma. Immune CIK cells, known for their
wide-ranging tumor-destroying capabilities, attain anti-cancer
effects through the release of various cytokines. Although
immunotherapy was proven to be effective in GBM treatment, its
lasting effects and safety remain unconfirmed by comprehensive
clinical studies (133).
The role of programmed cell death protein 1 (PD-1)
in tandem with PD-L1, a binding agent, plays a crucial role in
evading detection by the tumor's immune defense (134). Blocking PD-1/PD-L1 revitalizes
tumor T-cell activities by hindering their interaction,
demonstrating efficacy in treating a range of cancers such as
melanoma, non-small cell lung cancer and bladder cancer (135).
In the case of GBM, the extent of PD-1/PD-L1
expression is closely connected to the immune system of the tumor.
Preliminary clinical tests have shown promising results for PD-1
inhibitors such as pembrolizumab and nivolumab in managing GBM,
demonstrating prolonged impacts in several instances (136). However, the success of PD-1/PD-L1
inhibitors in managing GBM depends on various factors, such as the
tumor's environment and patient genetics, highlighting the need for
further biomarker studies to adjust patient decisions (137).
Nanometer drugs and drug delivery
systems
Nanoparticles show great potential in the field of
drug delivery due to their unique physicochemical properties
(138). By loading cytokines into
nanoparticles, the pharmacokinetics (e.g., extended half-life),
pharmacodynamics (e.g., targeted delivery), and overall therapeutic
efficacy of cytokines can be significantly improved. Specifically,
nanoparticles control cytokine release by anchoring cytokines or
utilizing mRNA-encoded cytokines and effectively activate immune
cells to enhance local immune responses (139). These innovations are expected to
overcome the blood-brain barrier for efficient drug delivery, break
the tumor immunosuppressive microenvironment, and enhance
anti-tumor immune responses.
Engineered nanomaterials are effective due to the
BBB's high porosity and restricted lymph flow within the GBM,
facilitating a buildup of medication in the brain. In addition, the
use of nanomaterials facilitates the extended and controlled
release of antigens or adjuvants, accurate targeting of BBB
endothelial cells and the cellular introduction of RNA-centric
vaccines, offering potential solutions to the challenges these
vaccines face (140).
Overcoming the dual barriers of the BBB and
blood-tumor barrier (BTB) in GBM remains a major therapeutic
challenge, due to their structural complexity and adaptive
resistance mechanisms. Nonetheless, pharmaceutical distributing
methods that employ nanoparticles for cell transport and virus
delivery, as well as accurate ultrasound, magnetic fields and nasal
medication administration, enhance the permeability of the BBB and
BTB, thus providing unique advantages for treating GBM (141).
Song et al (142) constructed a biomimetic nano-drug
delivery system targeting GBM [red blood cell
membrane-functionalized albumin nanoparticles (RFANPs)] to deliver
Lomitorpeptide (LMP), demonstrating that LMP RFANPs exhibited
excellent anti-GBM activity in tumor-bearing mice, significantly
improving targeted drug delivery efficiency. In a subsequent study
(143), the researchers engineered
a biomimetic nanotherapeutic system coated with GBM cell membranes.
This platform co-delivered HuaChanSu and photoresponsive Cu2-xSe
nanoparticles across the blood-brain barrier. Upon near-infrared
irradiation, Cu2-xSe generated localized hyperthermia for
photothermal ablation, while HuaChanSu exerted cytotoxic effects by
arresting the cell cycle at G2/M phase and triggering mitochondrial
apoptosis. The GBM membrane coating not only enhanced
tumor-specific accumulation through homotypic binding but also
mitigated immune clearance, thereby amplifying the synergy between
chemotherapy and photothermal therapy. Ruan et al (144) utilized the Cas12a gene editing
function to create an effective CRISPR/Cas12a nanodrug-targeting
GBM therapy based on nanocapsules; The CRISPR/Cas12a system is able
to extend blood half-life, effectively cross the BBB, active tumor
targeting and selective release.
Physical therapy
The tumor treating (TT)Fields signify a
groundbreaking technique in the field of physical therapy.
Unveiling the results of the EF-14 clinical trials for electric
field therapy in 2017 ignited widespread enthusiasm and renewed
hope in the treatment of GBM (145).
TTFields employs a mild treatment strategy to avert
the division and multiplication of tumor cells, utilizing the soft,
varying electric fields present at the tumor site. The fundamental
idea involves applying electric fields to modulate the motion of
charged molecules, thereby obstructing the creation and
disintegration of microtubules during cell division, which in turn
leads to cell death by apoptosis. TTFields principally hinders the
gathering of microtubule proteins during cell division, thereby
preventing the formation of mitotic spindles and changes in cell
membrane potential caused by electric fields, which in turn
initiates the apoptosis signaling route (146).
Studies suggest that electrical fields selectively
induce cell cycle arrest and apoptosis in dividing cells, while
sparing non-dividing cells (147).
During metaphase, microtubules undergo changes in oscillation,
rotation and structure due to differing electric field pressures
from TTFields, affecting spindle formation, leading to mitotic
stops, delayed responses and irregular chromosome splitting. As a
result, tumor cells stray from their mitotic path, resulting in
reduced growth rates or the formation of non-diploid progeny cells
(148).
Focused ultrasound technology
(FUS)
FUS utilizes the placement of microbubbles and the
amplification of acoustic waves to create mechanical effects on
blood vessels, leading to a brief breach of the BBB and subsequent
biological repercussions. This method generates thermal and
mechanical effects in specified zones through intense concentrated
ultrasound, causing protein disintegration and cellular demise,
with the goal of eliminating tumor tissue.
Initial results suggested that specific ultrasound
may trigger an immune response. A study on mice with GBM models
demonstrated that under specific ultrasound, tumor antigens are
released, prompting immune-cell activation and shifting the tumor's
surrounding environment from frigid to warm states (149). Strong ultrasound may induce
radiosensitization due to tumor resistance in hypoxia; hence,
increased blood flow and oxygen concentration can heighten the
responsiveness to radiotherapy (150). In addition, localized
ultrasound-detecting microbubbles can obstruct tumor blood vessels,
leading to low oxygen levels and the demise of cancer cells
(151). By precisely excising
tumors using ultrasound, reducing their size, momentarily opening
the BBB and enhancing the effectiveness of chemotherapy, targeted
therapy and immunotherapy drugs, superior outcomes are achieved
(152).
Magnetic hyperthermia-mediated cancer
therapy (MHCT)
Magnetic hyperthermia is a term that describes the
generation of heat via the twisting of particles, reduction in Eddy
currents, hysteresis and the turning of magnetic particles within a
volatile magnetic field. MHCT utilizes magnetic nanoparticles in
cancer tissues exposed to a varying magnetic field, thereby heating
the tumor to achieve therapeutic goals (153). Studies showed that MHCT is an
effective treatment that reduces tumor cell growth and elevates
survival rates in initial tumor models. MHCT can be employed
independently as a therapy for GBM or in conjunction with
additional methods (154).
Currently, MHCT is regarded as a promising and non-intrusive
treatment method, adept at administering thermotherapy to tumors
requiring surgical intervention.
The thermal attributes of drug-treated nanocarriers
can temporarily breach the BBB, thereby increasing the amount of
medication injected at the targeted tumor site and enhancing the
therapeutic advantages of hyperthermia coupled with chemotherapy
(155). Research indicates that
MHCT increases the drug resistance of GBM to temozolomide, thereby
enhancing the absorption of the drug by cancerous cells with
decreased MGMT expression (156).
Thus, the utilization of hyperthermia may act as a tactic to
mitigate chemotherapy resistance in GBM, thereby improving the
effectiveness of chemotherapy.
Summary and outlook
GBM, as a highly malignant brain tumor, is
characterized by highly complex molecular profiles, IDH mutations,
MGMT promoter methylation status and other genetic alterations
(157). No major breakthroughs in
therapeutic outcomes have been achieved since the 2000s (158). The current state of the field is
characterised by the following: On the diagnostic side, imaging
techniques and molecular testing tools continue to advance, but
early and accurate diagnosis remains a challenge. Therapeutically,
combination therapy of surgical resection, radiotherapy and
chemotherapy is still the mainstay, but the efficacy is limited and
the median survival of patients remains short. Although research on
the molecular mechanisms of GBM has made some progress and
identified key gene mutations and signalling pathway abnormalities,
they have not yet been translated into effective clinical treatment
strategies.
In order to further break through the GBM treatment
dilemma, future research needs to focus on the following
directions: Developing sensitive and specific biomarkers based on
the molecular features of GBM; exploring the tumour
microenvironment of GBM in depth to develop more targeted
immunotherapy strategies; and integrating genomic, epigenomic,
proteomic and spatial transcriptomic data to construct dynamic
molecular typing frameworks to guide individualized treatment.
Immunometabolic regulation, engineered cell therapy, microbiome
intervention to explore the impact of gut-brain axis regulation on
the immune response of GBM, penetration of the BBB, development of
a real-time monitoring platform based on liquid biopsy (e.g.,
ctDNA, exosomes) and tracking the clonal evolution and drug
resistance mechanisms during the treatment process are also future
tasks. In addition, an international collaborative GBM multi-omics
database will be established and clinical images, pathological
sections and organoid drug sensitivity data will be integrated to
predict therapeutic responses using deep learning. The
cross-application of physics and synthetic biology will also be
explored. Advanced experimental techniques and models will be
further developed to more realistically simulate tumour properties.
This may bring substantial survival hope to patients with GBM.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Natural Science
Foundation of Gansu Province (grant no. 22JR5RA959/24JRRA928).
Availability of data and materials
Not applicable.
Authors' contributions
HXH and AD were responsible for the collection and
organization of literature. JL and HYH were accountable for the
analysis and assessment of data. PF was in charge of organizing and
writing the article. GT was tasked with drawing illustrations. YZ
and XL helped GT with the image conception and color mixing
software operation. HY and BZ were responsible for writing the
first draft of the manuscript. WL contributed by generating
Table I. GY was responsible for the
final revision and proofreading. All authors actively contributed
to different aspects of this study and jointly reviewed and
approved the content of the final manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
In the preparation of this work, chatgtp3.5 as well
as ERNIE Bot 4.0 were used to linguistically edit the already
written manuscript in order to make the manuscript more rigorous
and standardised in this one aspect of language. After using this
tool, the content was reviewed and edited as needed and the authors
take full responsibility for the content of the publication.
References
|
1
|
Richardson TE, Walker JM, Hambardzumyan D,
Brem S, Hatanpaa KJ, Viapiano MS, Pai B, Umphlett M, Becher OJ,
Snuderl M, et al: Genetic and epigenetic instability as an
underlying driver of progression and aggressive behavior in
IDH-mutant astrocytoma. Acta Neuropathol. 148:52024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ezzati S, Salib S, Balasubramaniam M and
Aboud O: Epidermal growth factor receptor inhibitors in
glioblastoma: Current status and future possibilities. Int J Mol
Sci. 25:23162024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Testa U, Castelli G and Pelosi E: CAR-T
Cells in the treatment of nervous system tumors. Cancers (Basel).
16:29132024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo X, Gu L, Li Y, Zheng Z, Chen W, Wang
Y, Wang Y, Xing H, Shi Y, Liu D, et al: Histological and molecular
glioblastoma, IDH-wildtype: A real-world landscape using the 2021
WHO classification of central nervous system tumors. Front Oncol.
13:12008152023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan AC, Ashley DM, López GY, Malinzak M,
Friedman HS and Khasraw M: Management of glioblastoma: State of the
art and future directions. CA Cancer J Clin. 70:299–312. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agarwal A, Edgar MA, Desai A, Gupta V,
Soni N and Bathla G: Molecular GBM versus Histopathological GBM:
Radiology-pathology-genetic correlation and the new WHO 2021
definition of glioblastoma. AJNR Am J Neuroradiol. 45:1006–1012.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ostrom QT, Cioffi G, Waite K, Kruchko C
and Barnholtz-Sloan JS: CBTRUS Statistical Report: Primary brain
and other central nervous system tumors diagnosed in the United
States in 2014–2018. Neuro Oncol. 23:iii1–iii105. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yi GZ, Zhang HY, Que TS, Qu SQ, Li ZY, Qi
ST, Feng WY and Huang GL: Identification of the clinical and
genetic characteristics of gliomas with gene fusions by integrated
genomic and transcriptomic analysis. Eur J Med Res. 30:492025.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ungan G, Pons-Escoda A, Ulinic D, Arús C,
Ortega-Martorell S, Olier I, Vellido A, Majós C and Julià-Sapé M:
Early pseudoprogression and progression lesions in glioblastoma
patients are both metabolically heterogeneous. NMR Biomed.
37:e50952024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thornton ZA, Andrews LJ, Zhao H, Zheng J,
Paternoster L, Robinson JW and Kurian KM: Brain multi-omic
Mendelian randomisation to identify novel drug targets for
gliomagenesis. Hum Mol Genet. 34:178–192. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karipidis K, Baaken D, Loney T, Blettner
M, Mate R, Brzozek C, Elwood M, Narh C, Orsini N, Röösli M, et al:
The effect of exposure to radiofrequency fields on cancer risk in
the general and working population: A systematic review of human
observational studies-Part I: Most researched outcomes. Environ
Int. 191:1089832024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Q, Hu B, Hu X, Kim H, Squatrito M,
Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, et al: Tumor
evolution of Glioma-intrinsic gene expression subtypes associates
with immunological changes in the microenvironment. Cancer Cell.
33:1522018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cancer Genome Atlas Research Network, .
Comprehensive genomic characterization defines human glioblastoma
genes and core pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo J, Junaid M, Hamid N, Duan JJ, Yang X
and Pei DS: Current understanding of gliomagenesis: From model to
mechanism. Int J Med Sci. 19:2071–2079. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Senhaji N, Squalli Houssaini A, Lamrabet
S, Louati S and Bennis S: Molecular and Circulating Biomarkers in
Patients with Glioblastoma. Int J Mol Sci. 23:74742022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grewal EP, Richardson LGK, Sun J,
Ramapriyan R, Martinez-Lage M, Miller JJ, Carter BS, Cahill DP,
Curry WT and Choi BD: Mutant IDH modulates suppressive myeloid
populations in malignant glioma. Clin Cancer Res. 30:4068–4076.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lucas CG, Al-Adli NN, Young JS, Gupta R,
Morshed RA, Wu J, Ravindranathan A, Shai A, Oberheim Bush NA,
Taylor JW, et al: Longitudinal multimodal profiling of IDH-wildtype
glioblastoma reveals the molecular evolution and cellular
phenotypes underlying prognostically different treatment responses.
Neuro Oncol. 27:89–105. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du X and Hu H: The Roles of
2-hydroxyglutarate. Front Cell Dev Biol. 9:6513172021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou Z, Wu H, Luo N, Li S, Zhang X, Dong S,
Zhu D, Zhang H and Tao R: Almonertinib combined with anlotinib and
temozolomide in a patient with recurrent glioblastoma with EGFR
L858R mutation. Oncologist. 28:449–452. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sai Krishna AVS, Sinha S, Satyanarayana
Rao MR and Donakonda S: The impact of PTEN status on glioblastoma
multiforme: A glial cell type-specific study identifies unique
prognostic markers. Comput Biol Med. 184:1093952025. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia Q, Zhang H, Zhang P, Li Y, Xu M, Li X,
Li X and Dong L: Oncogenic Smurf1 promotes PTEN wild-type
glioblastoma growth by mediating PTEN ubiquitylation. Oncogene.
39:5902–5915. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mazzoleni A, Awuah WA, Sanker V, Bharadwaj
HR, Aderinto N, Tan JK, Huang HYR, Poornaselvan J, Shah MH, Atallah
O, et al: Chromosomal instability: A key driver in glioma
pathogenesis and progression. Eur J Med Res. 29:4512024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghosh D, Pryor B and Jiang N: Cellular
signaling in glioblastoma: A molecular and clinical perspective.
Int Rev Cell Mol Biol. 386:1–47. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pham J, Cote DJ, Kang K, Briggs RG, Gomez
D, Prasad A, Daggupati S, Sisti J, Chow F, Attenello F, et al:
Treatment practices and survival outcomes for IDH-wildtype
glioblastoma patients according to MGMT promoter methylation
status: Insights from the U.S. National Cancer Database. J
Neurooncol. Feb 5–2025.doi: 10.1007/s11060-025-04952-y (Epub ahead
of print). View Article : Google Scholar
|
|
26
|
Fathi Kazerooni A, Bakas S, Saligheh Rad H
and Davatzikos C: Imaging signatures of glioblastoma molecular
characteristics: A radiogenomics review. J Magn Reson Imaging.
52:54–69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stazi G, Taglieri L, Nicolai A, Romanelli
A, Fioravanti R, Morrone S, Sabatino M, Ragno R, Taurone S,
Nebbioso M, et al: Dissecting the role of novel EZH2 inhibitors in
primary glioblastoma cell cultures: Effects on proliferation,
epithelial-mesenchymal transition, migration, and on the
pro-inflammatory phenotype. Clin Epigenetics. 11:1732019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bates SE: Epigenetic therapies for cancer.
N Engl J Med. 383:650–663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meng W, Wang B, Mao W, Wang J, Zhao Y, Li
Q, Zhang C, Tang Y and Ma J: Enhanced efficacy of histone
deacetylase inhibitor combined with bromodomain inhibitor in
glioblastoma. J Exp Clin Cancer Res. 37:2412018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan Z, Zhou Y, Gao S, Cheng Y and Li Z:
Homogeneous and sensitive detection of microRNA with ligase chain
reaction and lambda exonuclease-assisted cationic conjugated
polymer biosensing. ACS Appl Mater Interfaces. 6:6181–6185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai SM, Li FJ, Long HZ, Zhou ZW, Luo HY,
Xu SG and Gao LC: Relationship between miRNA and ferroptosis in
tumors. Front Pharmacol. 13:9770622022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Charbit H and Lavon I: Investigating
expression dynamics of miR-21 and miR-10b in glioblastoma cells in
vitro: Insights into responses to hypoxia and secretion mechanisms.
Int J Mol Sci. 25:79842024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang RJ, Li JW, Bao BH, Wu HC, Du ZH, Su
JL, Zhang MH and Liang HQ: MicroRNA-873 (miRNA-873) inhibits
glioblastoma tumorigenesis and metastasis by suppressing the
expression of IGF2BP1. J Biol Chem. 290:8938–8948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rhim J, Baek W, Seo Y and Kim JH: From
molecular mechanisms to therapeutics: Understanding MicroRNA-21 in
cancer. Cells. 11:27912022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Rabinovsky R, Wei Z, El Fatimy R,
Deforzh E, Luan B, Peshkin L, Uhlmann EJ and Krichevsky AM:
Secreted PGK1 and IGFBP2 contribute to the bystander effect of
miR-10b gene editing in glioma. Mol Ther Nucleic Acids. 31:265–275.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tan C, Wei J, Li Z, Tian N, Wang Z, Wang
G, Han L and Tian Y: Circ_0021350 plays an oncogene role by
regulating miR-1207-3p/PIK3R3 in glioblastoma. BMC Cancer.
23:8082023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu H, Li C, Yang J, Sun Y, Zhang S, Yang
J, Yang L, Wang Y and Jiao B: Long noncoding RNA
CASC9/miR-519d/STAT3 positive feedback loop facilitate the glioma
tumourigenesis. J Cell Mol Med. 22:6338–6344. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang G, Lin X, Han H, Zhang H, Li X, Feng
M and Jiang C: lncRNA H19 promotes glioblastoma multiforme
development by activating autophagy by sponging miR-491-5p.
Bioengineered. 13:11440–11455. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yao H, Huang C, Zou J, Liang W, Zhao Y,
Yang K, Zhong Z, Zhou S, Li J, Li Y, et al: Extracellular
vesicle-packaged lncRNA from cancer-associated fibroblasts promotes
immune evasion by downregulating HLA-A in pancreatic cancer. J
Extracell Vesicles. 13:e124842024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao J, Jin W, Yi K, Wang Q, Zhou J, Tan
Y, Xu C, Xiao M, Hong B, Xu F, et al: Combination LSD1 and
HOTAIR-EZH2 inhibition disrupts cell cycle processes and induces
apoptosis in glioblastoma cells. Pharmacol Res. 171:1057642021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Yu Y, Yu J, Wang C, Wang Y, Fu R
and Zhang C: The role of the dysregulation of circRNAs expression
in glioblastoma multiforme. J Mol Neurosci. 75:92025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alom MW, Jibon MDK, Faruqe MO, Rahman MS,
Akter F, Ali A and Rahman MM: Integrated gene expression
Data-driven identification of molecular signatures, prognostic
biomarkers, and drug targets for glioblastoma. Biomed Res Int.
2024:68102002024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang S, Gao S and Dong Z: CircVCAN
promotes glioma progression through the miR-488-3p/MEF2C-JAGGED1
axis. Environ Toxicol. 39:4417–4430. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kadoch C and Crabtree GR: Mammalian
SWI/SNF chromatin remodeling complexes and cancer: Mechanistic
insights gained from human genomics. Sci Adv. 1:e15004472015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun
G, Lu Z, Huang Y, Yang CG, et al: m6A RNA methylation
regulates the Self-renewal and tumorigenesis of glioblastoma stem
cells. Cell Rep. 18:2622–2634. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hashemi M, Etemad S, Rezaei S, Ziaolhagh
S, Rajabi R, Rahmanian P, Abdi S, Koohpar ZK, Rafiei R, Raei B, et
al: Progress in targeting PTEN/PI3K/Akt axis in glioblastoma
therapy: Revisiting molecular interactions. Biomed Pharmacother.
158:1142042023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen CH, Shaikenov T, Peterson TR,
Aimbetov R, Bissenbaev AK, Lee SW, Wu J, Lin HK and Sarbassov dos
D: ER stress inhibits mTORC2 and Akt signaling through
GSK-3β-mediated phosphorylation of rictor. Sci Signal. 4:ra102011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen W, Liu F, Lin X, Li L, Chen W, Zhang
T, Liu Y, Niu L, Zhang Y and Hu P: Cucurbitacin E inhibits the
proliferation of glioblastoma cells via FAK/AKT/GSK3β pathway.
Oncol Rep. 50:2212023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Şengelen A and Önay-Uçar E: Rosmarinic
acid attenuates glioblastoma cells and spheroids' growth and
EMT/stem-like state by PTEN/PI3K/AKT downregulation and ERK-induced
apoptosis. Phytomedicine. 135:1560602024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mekala JR, Kurappalli RK, Ramalingam P and
Moparthi NR: N-acetyl l-aspartate and Triacetin modulate tumor
suppressor MicroRNA and class I and II HDAC gene expression induce
apoptosis in Glioblastoma cancer cells in vitro. Life Sci.
286:1200242021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Harachi M, Masui K, Honda H, Muragaki Y,
Kawamata T, Cavenee WK, Mischel PS and Shibata N: Dual regulation
of histone methylation by mTOR complexes controls glioblastoma
tumor cell growth via EZH2 and SAM. Mol Cancer Res. 18:1142–1152.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Barrie U, Floyd K, Datta A and Wetzel DM:
MAPK/ERK activation in macrophages promotes Leishmania
internalization and pathogenesis. Microbes Infect. 26:1053532024.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ullah R, Yin Q, Snell AH and Wan L:
RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer
Biol. 85:123–154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Park AK, Kim P, Ballester LY, Esquenazi Y
and Zhao Z: Subtype-specific signaling pathways and genomic
aberrations associated with prognosis of glioblastoma. Neuro Oncol.
21:59–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Angom RS, Mondal SK, Wang F, Madamsetty
VS, Wang E, Dutta SK, Gulani Y, Sarabia-Estrada R, Sarkaria JN,
Quiñones-Hinojosa A and Mukhopadhyay D: Ablation of neuropilin-1
improves the therapeutic response in conventional drug-resistant
glioblastoma multiforme. Oncogene. 39:7114–7126. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li H, Niu X and Cheng R: Prevalence,
prognostic and clinicopathological value of HIF-1α in glioblastoma
patients: A systematic review and meta-analysis. Neurosurg Rev.
47:8602024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Karkon-Shayan S, Aliashrafzadeh H,
Dianat-Moghadam H, Rastegar-Pouyani N, Majidi M, Zarei M,
Moradi-Vastegani S, Bahramvand Y, Babaniamansour S and Jafarzadeh
E: Resveratrol as an antitumor agent for glioblastoma multiforme:
Targeting resistance and promoting apoptotic cell deaths. Acta
Histochem. 125:1520582023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun J, Wu S, Zhao W, Xue S, Zhang L and
Ren J: MAPK-activated protein kinase 2 is associated with poor
prognosis of glioma patients and immune inhibition in glioma. Front
Oncol. 14:13079922024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Golovin A, Dzarieva F, Rubetskaya K,
Shamadykova D, Usachev D, Pavlova G and Kopylov A: In silico born
designed Anti-EGFR aptamer gol1 has Anti-proliferative potential
for patient glioblastoma Cells. Int J Mol Sci. 26:10722025.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Geribaldi-Doldán N, Hervás-Corpión I,
Gómez-Oliva R, Domínguez-García S, Ruiz FA, Iglesias-Lozano I,
Carrascal L, Pardillo-Díaz R, Gil-Salú JL, Nunez-Abades P, et al:
Targeting protein Kinase C in glioblastoma treatment. Biomedicines.
9:3812021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hatipoglu OF, Nishinaka T, Nishibori M,
Watanabe M, Toyomura T, Mori S, Yaykasli KO, Wake H and Takahashi
H: Histamine promotes angiogenesis through a histamine H1
receptor-PKC-VEGF-mediated pathway in human endothelial cells. J
Pharmacol Sci. 151:177–186. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang LH, Wu CF, Rajasekaran N and Shin YK:
Loss of tumor suppressor gene function in human cancer: An
overview. Cell Physiol Biochem. 51:2647–2693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Solt LA and May MJ: The IkappaB kinase
complex: Master regulator of NF-kappaB signaling. Immunol Res.
42:3–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Latour M, Her NG, Kesari S and Nurmemmedov
E: WNT signaling as a therapeutic target for glioblastoma. Int J
Mol Sci. 22:84282021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sedgwick AE and D'Souza-Schorey C: Wnt
signaling in cell motility and invasion: Drawing parallels between
development and cancer. Cancers (Basel). 8:802016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cadigan KM and Waterman ML: TCF/LEFs and
Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol.
4:a0079062012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Desterke C, Fu Y, Bonifacio-Mundaca J,
Monge C, Pineau P, Mata-Garrido J and Francés R: Single-cell RNA
sequencing reveals LEF1-driven wnt pathway activation as a shared
oncogenic program in hepatoblastoma and medulloblastoma. Curr
Oncol. 32:352025. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guan R, Zhang X and Guo M: Glioblastoma
stem cells and Wnt signaling pathway: Molecular mechanisms and
therapeutic targets. Chin Neurosurg J. 6:252020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhou C, Zhao H, Wang S, Dong C, Yang F and
Zhang J: LncRNA ADAMTS9-AS1 knockdown suppresses cell proliferation
and migration in glioma through downregulating Wnt/β-catenin
signaling pathway. Bosn J Basic Med Sci. 22:395–402.
2022.PubMed/NCBI
|
|
70
|
Butler M, Prasad S and Srivastava SK:
Targeting glioblastoma tumor microenvironment. Adv Exp Med Biol.
1296:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
DePalma TJ, Sivakumar H and Skardal A:
Strategies for developing complex multi-component in vitro tumor
models: Highlights in glioblastoma. Adv Drug Deliv Rev.
180:1140672022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Read RD, Tapp ZM, Rajappa P and
Hambardzumyan D: Glioblastoma microenvironment-from biology to
therapy. Genes Dev. 38:360–379. 2024.PubMed/NCBI
|
|
73
|
De Leo A, Ugolini A and Veglia F: Myeloid
cells in glioblastoma microenvironment. Cells. 10:182020.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lakshmanachetty S, Cruz-Cruz J, Hoffmeyer
E, Cole AP and Mitra SS: New insights into the multifaceted role of
Myeloid-derived suppressor cells (MDSCs) in High-grade gliomas:
From metabolic reprograming, immunosuppression, and therapeutic
resistance to current strategies for targeting MDSCs. Cells.
10:8932021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu Y, Chen X, Xu Y, Yang T, Wang H, Wang
Z, Hu Z, Chen L, Zhang Z and Wu Y: CTHRC1 promotes colorectal
cancer progression by recruiting tumor-associated macrophages via
up-regulation of CCL15. J Mol Med (Berl). 102:81–94. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ren J, Xu B, Ren J, Liu Z, Cai L, Zhang X,
Wang W, Li S, Jin L and Ding L: The Importance of M1-and
M2-Polarized macrophages in glioma and as potential treatment
targets. Brain Sci. 13:12692023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Guo M, Zhang J, Han J, Hu Y, Ni H, Yuan J,
Sun Y, Liu M, Gao L, Liao W, et al: VEGFR2 blockade inhibits
glioblastoma cell proliferation by enhancing mitochondrial
biogenesis. J Transl Med. 22:4192024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jia S, Bode AM, Chen X and Luo X:
Unlocking the potential: Targeting metabolic pathways in the tumor
microenvironment for cancer therapy. Biochim Biophys Acta Rev
Cancer. 1879:1891662024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Agnihotri TG, Salave S, Shinde T, Srikanth
I, Gyanani V, Haley JC and Jain A: Understanding the role of
endothelial cells in brain tumor formation and metastasis: A
proposition to be explored for better therapy. J Natl Cancer Cent.
3:222–235. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shimizu M, Shirakami Y and Moriwaki H:
Targeting receptor tyrosine kinases for chemoprevention by green
tea catechin, EGCG. Int J Mol Sci. 9:1034–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang C, Liu Y, Zuo Z, Cui D, Xu Y, Li L
and Jiang Y: Dual role of exosomal circCMTM3 derived from GSCs in
impeding degradation and promoting phosphorylation of STAT5A to
facilitate vasculogenic mimicry formation in glioblastoma.
Theranostics. 14:5698–5724. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang F, Liao W, Li C and Zhu L: Silencing
BMAL1 promotes M1/M2 polarization through the LDHA/lactate axis to
promote GBM sensitivity to bevacizumab. Int Immunopharmacol.
134:1121872024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Schor AM, Woolston AM, Kankova K, Harada
K, Aljorani LE, Perrier S, Felts PA, Keatch RP and Schor SL:
Migration stimulating factor (MSF): Its role in the tumour
microenvironment. Adv Exp Med Biol. 1329:351–397. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang B, Han X, Gao Q, Liu J, Li S, Zha W,
Wang X, Guo X and Gao D: Enhancer II-targeted dsRNA decreases GDNF
expression via histone H3K9 trimethylation to inhibit glioblastoma
progression. Brain Res Bull. 167:22–32. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Meier C and Bischoff A: Oligodendroglial
cell development in jimpy mice and controls. An
electron-microscopic study in the optic nerve. J Neurol Sci.
26:517–528. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Slepak TI, Guyot M, Walters W, Eichberg DG
and Ivan ME: Dual role of the adhesion G-protein coupled receptor
ADRGE5/CD97 in glioblastoma invasion and proliferation. J Biol
Chem. 299:1051052023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ishii H, Mimura Y, Zahra MH, Katayama S,
Hassan G, Afify SM and Seno M: Isolation and characterization of
cancer stem cells derived from human glioblastoma. Am J Cancer Res.
11:441–457. 2021.PubMed/NCBI
|
|
88
|
Ryskalin L, Gaglione A, Limanaqi F,
Biagioni F, Familiari P, Frati A, Esposito V and Fornai F: The
autophagy status of cancer stem cells in gliobastoma multiforme:
From cancer promotion to therapeutic strategies. Int J Mol Sci.
20:38242019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lathia JD, Mack SC, Mulkearns-Hubert EE,
Valentim CL and Rich JN: Cancer stem cells in glioblastoma. Genes
Dev. 29:1203–1217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Singh DK, Shivalingappa PKM, Sharma A,
Mondal A, Muzumdar D, Shiras A and Bapat SA: NSG-70, a new
glioblastoma cell line with mixed proneural-mesenchymal features,
associates NOTCH1-WNT5A signaling with stem cell maintenance and
angiogenesis. J Neurooncol. 157:575–591. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Comba A, Faisal SM, Varela ML, Hollon T,
Al-Holou WN, Umemura Y, Nunez FJ, Motsch S, Castro MG and
Lowenstein PR: Uncovering spatiotemporal heterogeneity of
High-grade gliomas: From disease biology to therapeutic
implications. Front Oncol. 11:7037642021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Griessmair M, Delbridge C, Ziegenfeuter J,
Jung K, Mueller T, Schramm S, Bernhardt D, Schmidt-Graf F, Kertels
O, Thomas M, et al: Exploring molecular glioblastoma: Insights from
advanced imaging for a nuanced understanding of the molecularly
defined malignant biology. Neurooncol Adv. 6:vdae1062024.PubMed/NCBI
|
|
94
|
Liu M, Ji Z, Jain V, Smith VL, Hocke E,
Patel AP, McLendon RE, Ashley DM, Gregory SG and López GY: Spatial
transcriptomics reveals segregation of tumor cell states in
glioblastoma and marked immunosuppression within the perinecrotic
niche. Acta Neuropathol Commun. 12:642024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rojiani AM and Dorovini-Zis K: Glomeruloid
vascular structures in glioblastoma multiforme: An
immunohistochemical and ultrastructural study. J Neurosurg.
85:1078–1084. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lista S, Imbimbo BP, Grasso M, Fidilio A,
Emanuele E, Minoretti P, López-Ortiz S, Martín-Hernández J, Gabelle
A, Caruso G, et al: Tracking neuroinflammatory biomarkers in
Alzheimer's disease: A strategy for individualized therapeutic
approaches? J Neuroinflammation. 21:1872024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang Y, Wang X, Hawkins CA, Chen K,
Vaynberg J, Mao X, Tu Y, Zuo X, Wang J, Wang YX, et al: Structural
basis of focal adhesion localization of LIM-only adaptor PINCH by
integrin-linked kinase. J Biol Chem. 284:5836–5844. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ainslie K: Modifying Post-surgical
immunity: Controlled release of TLR7/8 agonist for immune mediated
clearance of glioblastoma. Res Sq: rs.3.rs-5024510. 2024.doi:
10.21203/rs.3.rs-5024510/v1.
|
|
99
|
Zhang L, Liu Z, Dong Y and Kong L: E2F2
drives glioma progression via PI3K/AKT in a PFKFB4-dependent
manner. Life Sci. 276:1194122021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li J, Chen Q, Xu S, Wu J, Huang Q, Song P
and Duan F: Down-regulation of BAG3 inhibits proliferation and
promotes apoptosis of glioblastoma multiforme through
BAG3/HSP70/HIF-1α signaling pathway. Int J Clin Exp Pathol.
11:4305–4318. 2018.PubMed/NCBI
|
|
101
|
Zhang L, He J, Zhao W, Zhou Y, Li J, Li S,
Zhao W, Zhang L, Tang Z, Tan G, et al: CD2AP promotes the
progression of glioblastoma multiforme via TRIM5-mediated NF-kB
signaling. Cell Death Dis. 15:7222024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Taheri E and Raeeszadeh-Sarmazdeh M:
Effect of TIMPs and their minimally engineered variants in blocking
invasion and migration of brain cancer cells. bioRxiv.
2024.06.05.597644. 2024.
|
|
103
|
Ghaffari SH, Yousefi M, Dizaji MZ, Momeny
M, Bashash D, Zekri A, Alimoghaddam K and Ghavamzadeh A: Arsenic
trioxide induces apoptosis and incapacitates proliferation and
invasive properties of U87MG glioblastoma cells through a possible
NF-κB-Mediated mechanism. Asian Pac J Cancer Prev. 17:1553–1564.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Pham LC, Weller L, Gann CN, Schumacher KM,
Vlassak S, Swanson T, Highsmith K, O'Brien BJ, Nash S, Aaroe A, et
al: Prolonged complete response to adjuvant tepotinib in a patient
with newly diagnosed disseminated glioblastoma harboring
mesenchymal-epithelial transition fusion. Oncologist.
30:oyae1002025. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Huang Z, Wang M, Chen Y, Tang H, Tang K,
Zhao M, Yang W, Zhou Z, Tian J, Xiang W, et al:
Glioblastoma-derived migrasomes promote migration and invasion by
releasing PAK4 and LAMA4. Commun Biol. 8:912025. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Magalhaes YT and Forti FL: ROCK inhibition
reduces the sensitivity of mutant p53 glioblastoma to genotoxic
stress through a Rac1-driven ROS production. Int J Biochem Cell
Biol. 164:1064742023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mulherkar S and Tolias KF: RhoA-ROCK
signaling as a therapeutic target in traumatic brain injury. Cells.
9:2452020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Velasquez C, Gutierrez O, Carcelen M and
Fernandez-Luna JL: The invasion factor ODZ1 is upregulated through
an epidermal growth factor Receptor-induced pathway in primary
glioblastoma cells. Cells. 13:662024. View Article : Google Scholar
|
|
109
|
Xue Y, Bi F, Zhang X, Zhang S, Pan Y, Liu
N, Shi Y, Yao X, Zheng Y and Fan D: Role of Rac1 and Cdc42 in
hypoxia induced p53 and von Hippel-Lindau suppression and HIF1alpha
activation. Int J Cancer. 118:2965–2972. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Shi C, Luo W, Sun C, Yu L, Zhou X, Hua D,
Jiang Z, Wang Q and Yu S: The miR-29 family members induce
glioblastoma cell apoptosis by targeting cell division cycle 42 in
a p53-dependent manner. Eur J Clin Invest. 53:e139642023.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yin X, Xia K, Peng S, Tan B, Huang Y, Wang
M and He M: ABCF1/CXCL12/CXCR4 enhances glioblastoma cell
proliferation, migration, and invasion by activating the PI3K/AKT
signal pathway. Dev Neurosci. 46:210–220. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Horowitz MA, Ghadiyaram A, Mehkri Y,
Chakravarti S, Liu J, Fox K, Gendreau J and Mukherjee D: Surgical
resection of glioblastoma in the very elderly: An analysis of
survival outcomes using the surveillance, epidemiology, and end
results database. Clin Neurol Neurosurg. 245:1084692024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Montemurro N, Fanelli GN, Scatena C,
Ortenzi V, Pasqualetti F, Mazzanti CM, Morganti R, Paiar F,
Naccarato AG and Perrini P: Surgical outcome and molecular pattern
characterization of recurrent glioblastoma multiforme: A
single-center retrospective series. Clin Neurol Neurosurg.
207:1067352021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Vanhove C and Goethals I: Magnetic
resonance imaging-guided radiation therapy using animal models of
glioblastoma. Br J Radiol. 92:201807132019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Li R, Wang H, Liang Q, Chen L and Ren J:
Radiotherapy for glioblastoma: Clinical issues and nanotechnology
strategies. Biomater Sci. 10:892–908. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ashby LS, Smith KA and Stea B: Gliadel
wafer implantation combined with standard radiotherapy and
concurrent followed by adjuvant temozolomide for treatment of newly
diagnosed high-grade glioma: A systematic literature review. World
J Surg Oncol. 14:2252016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
LiverTox, . Clinical and Research
Information on Drug-Induced Liver Injury. National Institute of
Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012
|
|
118
|
Ou A, Yung WKA and Majd N: Molecular
mechanisms of treatment resistance in glioblastoma. Int J Mol Sci.
22:3512020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Dymova MA, Kuligina EV and Richter VA:
Molecular mechanisms of drug resistance in glioblastoma. Int J Mol
Sci. 22:63852021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yu Y, Wang A, Wang S, Sun Y, Chu L, Zhou
L, Yang X, Liu X, Sha C, Sun K and Xu L: Efficacy of
Temozolomide-conjugated gold nanoparticle photothermal therapy of
Drug-resistant glioblastoma and its mechanism study. Mol Pharm.
19:1219–1229. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sherman JH, Bobak A, Arsiwala T, Lockman P
and Aulakh S: Targeting drug resistance in glioblastoma (Review).
Int J Oncol. 65:802024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Asija S, Chatterjee A, Yadav S, Chekuri G,
Karulkar A, Jaiswal AK, Goda JS and Purwar R: Combinatorial
approaches to effective therapy in glioblastoma (GBM): Current
status and what the future holds. Int Rev Immunol. 41:582–605.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lv W, Yang F, Ge Z, Xin L, Zhang L, Zhai
Y, Liu X, Guo Q, Mao X, Luo P, et al: Aberrant overexpression of
myosin 1b in glioblastoma promotes angiogenesis via VEGF-myc-myosin
1b-Piezo1 axis. J Biol Chem. 300:1078072024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yuan Z: From origin to present:
Establishment, mechanism, evolutions and biomedical applications of
CRISPR/Cas-based Macromolecular System in Brief. Biol Biotechnol.
2024.doi: 10.20944/preprints202408.1715.v2.
|
|
125
|
Fang Y, Li X and Tian R: Unlocking
glioblastoma vulnerabilities with CRISPR-Based genetic screening.
Int J Mol Sci. 25:57022024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chow RD, Guzman CD, Wang G, Schmidt F,
Youngblood MW, Ye L, Errami Y, Dong MB, Martinez MA, Zhang S, et
al: AAV-mediated direct in vivo CRISPR screen identifies functional
suppressors in glioblastoma. Nat Neurosci. 20:1329–1341. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tu KJ, Stewart CE, Hendrickson PG, Regal
JA, Kim SY, Ashley DM, Waitkus MS and Reitman ZJ: Pooled genetic
screens to identify vulnerabilities in TERT-promoter-mutant
glioblastoma. Oncogene. 42:3274–3286. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lin P, Chen W, Long Z, Yu J, Yang J, Xia
Z, Wu Q, Min X, Tang J, Cui Y, et al: RBBP6 maintains glioblastoma
stem cells through CPSF3-dependent alternative polyadenylation.
Cell Discov. 10:322024. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Waldman AD, Fritz JM and Lenardo MJ: A
guide to cancer immunotherapy: From T cell basic science to
clinical practice. Nat Rev Immunol. 20:651–668. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Farahzadi R, Fathi E, Vandghanooni S and
Valipour B: Hydrogel encapsulation of mesenchymal stem
cells-derived extracellular vesicles as a novel therapeutic
approach in cancer therapy. Biochim Biophys Acta Rev Cancer.
1879:1891772024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Guo Y, Hu D, Lian L, Zhao L, Li M, Bao H
and Xu S: Stem Cell-derived extracellular vesicles: A promising
nano delivery platform to the brain? Stem Cell Rev Rep. 19:285–308.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Luksik AS, Yazigi E, Shah P and Jackson
CM: CAR T cell therapy in glioblastoma: Overcoming challenges
related to antigen expression. Cancers (Basel). 15:14142023.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Xie HY, Mao M, Lei Y and Ma X: Challenges
and emerging strategies of immunotherapy for glioblastoma.
Chembiochem. e2024008482025.doi: 10.1002/cbic.202400848 (Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Quan Q, Guo L, Huang L, Liu Z, Guo T, Shen
Y, Ding S, Liu C and Cao L: Expression and clinical significance of
PD-L1 and infiltrated immune cells in the gastric adenocarcinoma
microenvironment. Medicine (Baltimore). 102:e363232023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Mamdani H, Matosevic S, Khalid AB, Durm G
and Jalal SI: Immunotherapy in lung cancer: Current landscape and
future directions. Front Immunol. 13:8236182022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ribatti D, Cazzato G, Tamma R, Annese T,
Ingravallo G and Specchia G: Immune checkpoint inhibitors targeting
PD-1/PD-L1 in the treatment of human lymphomas. Front Oncol.
14:14209202024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Maghrouni A, Givari M, Jalili-Nik M,
Mollazadeh H, Bibak B, Sadeghi MM, Afshari AR, Johnston TP and
Sahebkar A: Targeting the PD-1/PD-L1 pathway in glioblastoma
multiforme: Preclinical evidence and clinical interventions. Int
Immunopharmacol. 93:1074032021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Sarkar S, Greer J, Marlowe NJ, Medvid A,
Ivan ME, Kolishetti N and Dhar S: Stemness, invasion, and
immunosuppression modulation in recurrent glioblastoma using
nanotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol.
16:e19762024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sousa F: Emerging cytokine delivery with
nanomedicine for brain cancer treatment. Expert Opin Drug Deliv.
21:513–516. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Hameedat F, Mendes BB, Conniot J, Di
Filippo LD, Chorilli M, Schroeder A, Conde J and Sousa F:
Engineering nanomaterials for glioblastoma nanovaccination. Nat Rev
Materials. 9:628–642. 2024. View Article : Google Scholar
|
|
141
|
Lim SH, Yee GT and Khang D:
Nanoparticle-based combinational strategies for overcoming the
Blood-brain barrier and Blood-tumor barrier. Int J Nanomedicine.
19:2529–2552. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Song M, Tian J, Wang L, Dong S, Fu K, Chen
S and Liu C: Efficient delivery of lomitapide using hybrid
Membrane-coated tetrahedral DNA nanostructures for glioblastoma
therapy. Adv Mater. 36:e23117602024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Song Z, Zhao L, Fang W, Guo S, Xu A, Zhan
Z, Cai Y, Xue SS, Chai P, Jiang Q, et al: Glioma cell membrane
camouflaged cinobufotalin delivery system for combinatorial
orthotopic glioblastoma therapy. Nano Res. 16:11164–11175. 2023.
View Article : Google Scholar
|
|
144
|
Ruan W, Xu S, An Y, Cui Y, Liu Y, Wang Y,
Ismail M, Liu Y and Zheng M: Brain-Targeted Cas12a
ribonucleoprotein nanocapsules enable synergetic gene Co-editing
leading to potent inhibition of orthotopic glioblastoma. Adv Sci
(Weinh). 11:e24021782024. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Rominiyi O, Vanderlinden A, Clenton SJ,
Bridgewater C, Al-Tamimi Y and Collis SJ: Tumour treating fields
therapy for glioblastoma: Current advances and future directions.
Br J Cancer. 124:697–709. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Khagi S, Kotecha R, Gatson NTN, Jeyapalan
S, Abdullah HI, Avgeropoulos NG, Batzianouli ET, Giladi M,
Lustgarten L and Goldlust SA: Recent advances in tumor treating
fields (TTFields) therapy for glioblastoma. Oncologist. Oct
14;oyae227, 2024 doi: 10.1093/oncolo/oyae227 (Epub ahead of print).
PubMed/NCBI
|
|
148
|
Chen D, Le SB, Hutchinson TE, Calinescu
AA, Sebastian M, Jin D, Liu T, Ghiaseddin A, Rahman M and Tran DD:
Tumor treating fields dually activate STING and AIM2 inflammasomes
to induce adjuvant immunity in glioblastoma. J Clin Invest.
132:e1492582022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Hendricks-Wenger A, Hutchison R,
Vlaisavljevich E and Allen IC: Immunological effects of histotripsy
for cancer therapy. Front Oncol. 11:6816292021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Sørensen BS and Horsman MR: Tumor hypoxia:
Impact on radiation therapy and molecular pathways. Front Oncol.
10:5622020. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
El Kaffas A, Gangeh MJ, Farhat G, Tran WT,
Hashim A, Giles A and Czarnota GJ: Tumour vascular shutdown and
cell death following Ultrasound-microbubble enhanced radiation
therapy. Theranostics. 8:314–327. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
D'Ammando A, Raspagliesi L, Gionso M,
Franzini A, Porto E, Di Meco F, Durando G, Pellegatta S and Prada
FJ: Sonodynamic therapy for the treatment of intracranial gliomas.
J Clin Med. 10:11012021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Verma J, Lal S and Van Noorden CJ:
Nanoparticles for hyperthermic therapy: Synthesis strategies and
applications in glioblastoma. Int J Nanomedicine. 9:2863–2877.
2014.PubMed/NCBI
|
|
154
|
Rego GNA, Nucci MP, Mamani JB, Oliveira
FA, Marti LC, Filgueiras IS, Ferreira JM, Real CC, Faria DP,
Espinha PL, et al: Therapeutic efficiency of multiple applications
of magnetic hyperthermia technique in glioblastoma using
aminosilane coated iron oxide nanoparticles: In vitro and in vivo
study. Int J Mol Sci. 21:9582020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tabatabaei SN, Tabatabaei MS, Girouard H
and Martel S: Hyperthermia of magnetic nanoparticles allows passage
of sodium fluorescein and Evans blue dye across the blood-retinal
barrier. Int J Hyperthermia. 32:657–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Qi G, Chen K, Guan W, Xie J, Chen X, Zhang
G, Yan R and Yang G: One-Pot synthesis of a pH-Sensitive MOF
integrated with glucose oxidase for amplified tumor
Photodynamic/Photothermal therapy. ACS Appl Mater Interfaces.
16:613952024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Lim KY, Won JK, Park CK, Kim SK, Choi SH,
Kim T, Yun H and Park SH: H3 G34-mutant high-grade glioma. Brain
Tumor Pathol. 38:4–13. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Goldman MJ, Baskin AM, Sharpe MA and
Baskin DS: Advances in gene therapy for high-grade glioma: A review
of the clinical evidence. Expert Rev Neurother. 24:879–895. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Xing Z, Jiang X, Chen Y, Wang T, Li X, Wei
X, Fan Q, Yang J, Wu H, Cheng J and Cai R: Glutamine deprivation in
glioblastoma stem cells triggers autophagic SIRT3 degradation to
epigenetically restrict CD133 expression and stemness. Apoptosis.
29:1619–1631. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Garros-Regulez L, Aldaz P, Arrizabalaga O,
Moncho-Amor V, Carrasco-Garcia E, Manterola L, Moreno-Cugnon L,
Barrena C, Villanua J, Ruiz I, et al: mTOR inhibition decreases
SOX2-SOX9 mediated glioma stem cell activity and temozolomide
resistance. Expert Opin Ther Targets. 20:393–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Bouchart C, Trépant AL, Hein M, Van Gestel
D and Demetter P: Prognostic impact of glioblastoma stem cell
markers OLIG2 and CCND2. Cancer Med. 9:1069–1078. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Anido J, Sáez-Borderías A, Gonzàlez-Juncà
A, Rodón L, Folch G, Carmona MA, Prieto-Sánchez RM, Barba I,
Martínez-Sáez E, Prudkin L, et al: TGF-β receptor inhibitors target
the CD44(high)/Id1(high) Glioma-initiating cell population in human
glioblastoma. Cancer Cell. 18:655–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Tchoghandjian A, Baeza N, Colin C, Cayre
M, Metellus P, Beclin C, Ouafik L and Figarella-Branger D: A2B5
cells from human glioblastoma have cancer stem cell properties.
Brain Pathol. 20:211–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Huang YK, Wang TM, Chen CY, Li CY, Wang
SC, Irshad K, Pan Y and Chang KC: The role of ALDH1A1 in
glioblastoma proliferation and invasion. Chem Biol Interact.
402:1112022024. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Kiefel H, Bondong S, Hazin J, Ridinger J,
Schirmer U, Riedle S and Altevogt P: L1CAM: A major driver for
tumor cell invasion and motility. Cell Adh Migr. 6:374–384. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Selvaraj S, Srinivas BH, Verma SK and Ms
G: Significance of Nestin and CD133 as cancer stem cell markers in
diffuse glioma and association with p53 expression and IDH status.
Int J Clin Exp Pathol. 17:208–218. 2024. View Article : Google Scholar : PubMed/NCBI
|