Introduction
Due to numerous enzymes and receptors, the
endothelium is an active platform for the interaction between blood
(including signaling substances carried in the blood) and the
vessel wall, playing a fundamental role in regulating vasomotor
activity, hemostasis and angiogenesis, as well as in controlling
inflammatory and immune processes. Key characteristics allowing the
endothelium to perform these functions include speed in the
stimulation of its cells and their response to stimuli.
Efficient vasoconstriction and vasodilatation, as
well as the resulting influence on the arterial pressure and blood
supply to tissues depend on a number of substances secreted by the
endothelium in response to stimulation by shear stress, hypoxia and
acetylcholine, bradykinin or serotonin action. Vasoactive
substances of endothelial origin include the group with
vasoconstrictive properties [such as endothelin, angiotensin II,
platelet-activating factor, thromboxane A2
(TXA2), leukotriene A4 and B4, ATP
and ADP] and the group, which stimulates dilation of the vessels
[such as nitric oxide (NO), prostacyclin (PGI2),
vasodilator-stimulated phosphoprotein, endothelium-derived
hyperpolarizing factor and adenosine as a product of the membranous
ectonucleases degrading ATP and ADP] (1). Due to secretion of NO, the endothelium
is also responsible for paracrine antioxidative action (limiting
oxidation of low-density lipoproteins on the subendothelial space)
and antiproliferative action (inhibiting mitogenesis of the smooth
muscle cells). PGI2 diffusing into the vessel lumen
determines the antiaggregant action of the endothelium on the
platelets.
Mitogenic and vasoconstrictive properties are
attributed to endothelin-1 (ET-1) in the vascular system. The serum
concentrations of ET-1 increase in a number of pathological
conditions, particularly in those associated with blood vessel
constriction. ET-1 is also associated with the underlying
pathomechanisms of primary pulmonary hypertension, arterial
hypertension and eclampsia.
Cyclooxygenase (prostaglandin G/H synthase) is an
enzyme transforming arachidonic acid to cyclic peroxides
(prostaglandin G2 and H2), which are unstable
reaction intermediates and precursors of TXA2,
PGI2 and other prostaglandins (PGE2,
PGF2 and PGD2). Two forms of cyclooxygenase
were isolated, cyclooxygenase-1 (COX-1) and COX-2. COX-1 is mainly
a constitutive isoform, which is present in the majority of cells
and tissues; however, certain cytokines, growth factors, mitogens,
ischemia and several other chemical and physical damaging factors
induce COX-2 synthesis. COX-1 may maintain functions, such as
cytoprotection of the gastric epithelium and COX-2 is the main
source of prostanoids during inflammation or the development of
neoplasms. However, in certain tissues, COX-2 is also a
constitutive enzyme (in the brain and kidney tissues) and is
produced in the endothelium as a result of laminar friction force
constituting a crucial link in the process of vascular tone
adaptation. Therefore, the assumption that COX-2 is the enzyme
independently responsible for inflammatory processes currently
appears to be incorrect. Previous studies on COX-2 knockout mice
reported that COX-1 was able to produce a sufficient amount of
prostaglandins to induce a full inflammatory response (2). In the heterological system of
expression, COX-1 preferentially binds with TXA2
synthase and PGF2 synthase, whereas COX-2 preferentially
binds with PGI2 synthase (3).
All eicosanoid receptors belong to a group of
receptors bound to G protein known as G protein-coupled receptors
(GPCRs). Activation of GPCRs results in the modulation of adenylyl
cyclase and phospholipase C activity due to the well-documented
role of cyclooxygenases in inflammatory processes, amplification
processes of pain sensations and in body temperature regulation.
These enzymes are an attractive target for pharmacological
symptomatic treatment. Non-steroidal anti-inflammatory drugs
(NSAIDs), acetylsalicylic acid and acetaminophen interact with
COX-1 and COX-2.
The hypothesis that anti-inflammatory effects may be
separated from an ulcerogenic effect encouraged investigators to
assess agents with a higher selectivity to COX-2 than to COX-1. A
group of compounds referred to as coxibs, which currently include
celecoxib, rofecoxib, etoricoxib, valdecoxib, lumiracoxib and
several others were identified. Further studies reported that their
selectivity with regards to COX-2 is similar to the selectivity of
previously identified NSAIDs, such as meloxicam, nimesulide and
diclofenac. In vivo, selective COX-2 inhibitors reduce the
production of PGI2 by endothelial cells without
simultaneous inhibition of the platelet thromboxane. Thus,
selective COX-2 inhibitors may increase the risk of a thrombus
(4–7). Furthermore, animal studies conducted
on mice and extensive epidemiological data suggest that the
probability of the occurrence of hypertension caused by NSAIDs
reflects a degree of COX-2 inhibition and selectivity of this
process. Constitutive activity of COX-2 was reported in the
vascular endothelial cells in patients with sclerosis or diabetes
mellitus (2) and increased concerns
as regards the safety of using this group of agents in humans
(8).
Celecoxib, which has an affinity to COX-2 that is
~375-fold higher compared to its affinity to COX-1, was registered
for the symptomatic treatment of inflammation and pain in patients
with osteoarthritis, rheumatoid arthritis and ankylosing
spondylitis. It is also used to reduce the number of adenomatous
polyps in patients with familial adenomatous polyposis, as a
therapy for supporting surgical treatment and for further
endoscopic control (9).
The number of available studies on the role of
celecoxib in modulating receptors of intracellular signaling
pathways, which eliminate endothelin-dependant vascular
constriction, is currently limited. The present study compared
celecoxib and substances with known vasodilating properties
(considering the dependence of these properties on the presence of
the endothelium) in a series of experiments on mesenteric arteries
using ET-1 to induce vascular constriction.
Investigating the methods for antagonizing
constrictive properties of endothelin by modifying intracellular
signaling pathways of the vascular smooth muscles and studying
their dependence on paracrine activity of the endothelium may
contribute to more effective treatment of the aforementioned
diseases and aid in the search for novel methods to prevent their
complications.
This study aimed to compare the vasodilating
properties of selected phosphodiesterase (PDE) inhibitors and
celecoxib in human mesenteric arteries constricted with ET-1, as
well as investigate the role of the endothelium in relaxation.
Materials and methods
Materials and reagents
Human mesenteric arteries were obtained from
subjects whose organs were collected for transplantation and stored
under the same conditions. Human superior mesenteric arteries were
collected in compliance with binding legal regulations. The
Bioethics Committee at the Collegium Medicum, Nicolaus Copernicus
University (Bydgoszcz, Poland) provided consent for conducting this
experiment (KB/344/2005 and KB/252/2008). The patient or the
patients’ family gave informed consent. All the reagents used in
this study were purchased from Sigma-Aldrich (Poznan, Poland).
Treatment of perfused human mesenteric
arteries with endothelium
In the first series of experiments, the mesenteric
arteries with a maintained endothelium, constricted by the addition
of ET-1 (6.5×10−9 mol/L) were treated with increased
concentrations of the following: sildenafil (PDE5 inhibitor;
0.1×10−9 to 1×10−6 M), zaprinast (PDE5 and 6
inhibitor; 3×10−8 to 3×10−4 M), rolipram
(PDE4 inhibitor; 3×10−7 to 3×10−3 M) and
celecoxib (COX-2 inhibitor; 3×10−9 to 3×10−5
M). Based on the observed changes in perfusion pressure,
concentration response curves (CRCs) were prepared for the
respective inhibitors and the following were calculated:
EC50 (concentration causing an effect equal to half of
the maximum effect), pD2 (negative common logarithm of
EC50 value) and relative potency (RP), i.e., quotient of
EC50 control value and EC50 value analyzed in
the experimental systems. This series of experiments facilitated
the comparison of the efficacy of selected PDE inhibitors and
celecoxib in the dilation of mesenteric arteries.
Treatment of perfused human mesenteric
arteries without endothelium
The next series of experiments were conducted in a
manner similar to the aforementioned one; however, prior to the
experiment, the endothelium was removed from the vessels using
compressed air in accordance with the methodology developed by
Koller et al (10).
Precision of endothelium removal was verified using a perfusate
containing acetylcholine chloride in a concentration of
1×10−5 M. The occurrence of constriction of the vessel
was recognized as confirmation that the endothelium was absent.
This series of experiments facilitated the comparative evaluation
of the efficacy of selected PDE inhibitors and celecoxib in the
dilation of mesenteric arteries and the influence of the
endothelium.
Statistical analysis
Statistical analysis was performed by calculating
the mean values and standard deviations. The results are presented
as the means of serial measurements with consideration of the
standard error of the mean. P<0.05 was considered to indicate a
statistically significant difference. Values of 0.05≤P<0.1
expressed a trend towards statistical significance, but values of
P≤0.1 were not significant.
Results
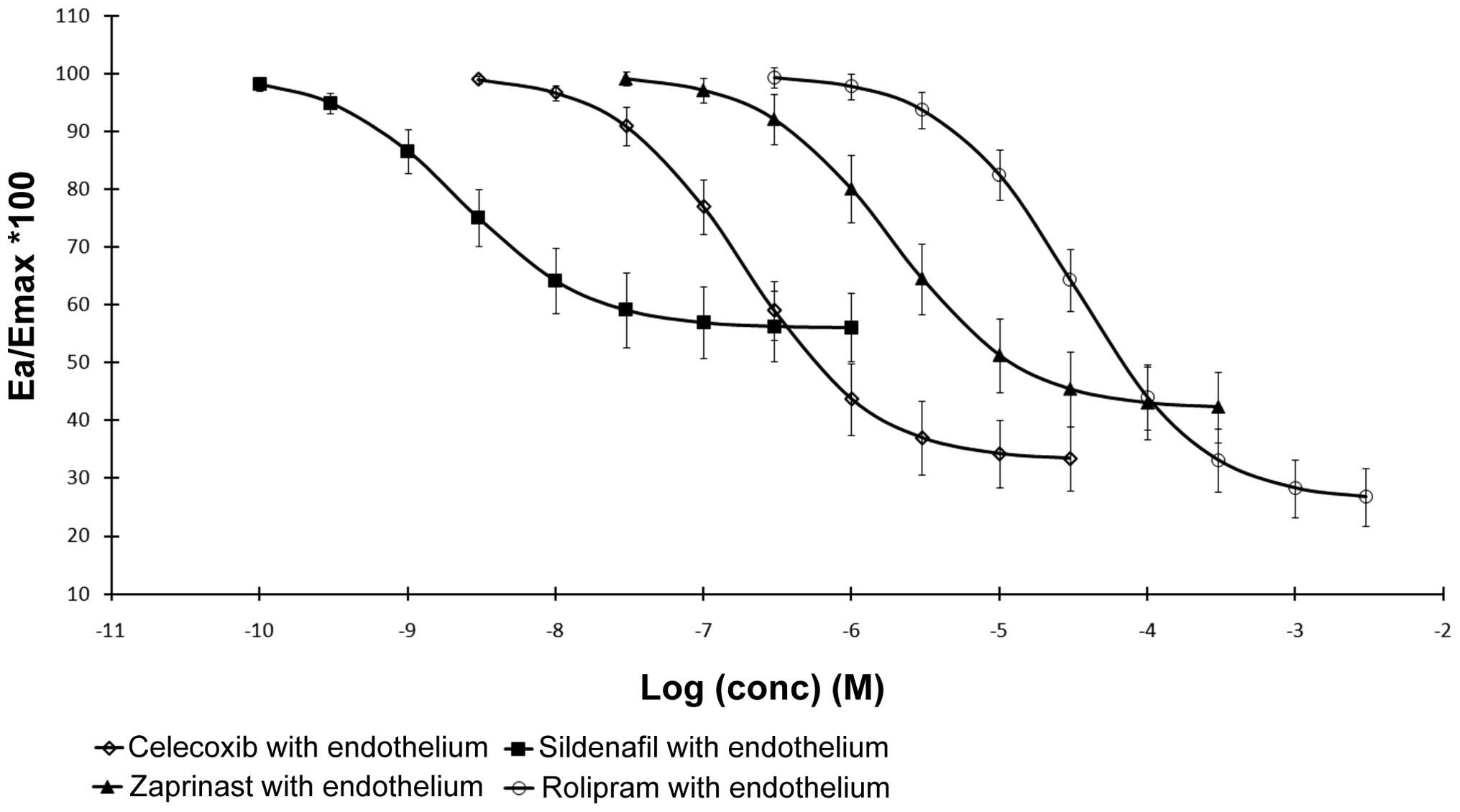
PDE inhibitors and celecoxib decreased
the perfusion pressure in human mesenteric arteries with
endothelium
The series of experiments conducted on perfused
human mesenteric arteries with a maintained endothelium revealed
that all the PDE inhibitors and celecoxib triggered a
concentration-dependent decrease in perfusion pressure in isolated
arteries constricted by ET-1 (Fig.
1). The PDE inhibitors and COX-2 inhibitor indicated
characteristics of non-competitive (functional) antagonists and did
not completely eliminate vascular constriction caused by ET-1
(Fig. 3). The basic pharmacometric
parameters of human mesenteric arteries (with and without
endothelium) treated with PDE inhibitors and celecoxib and
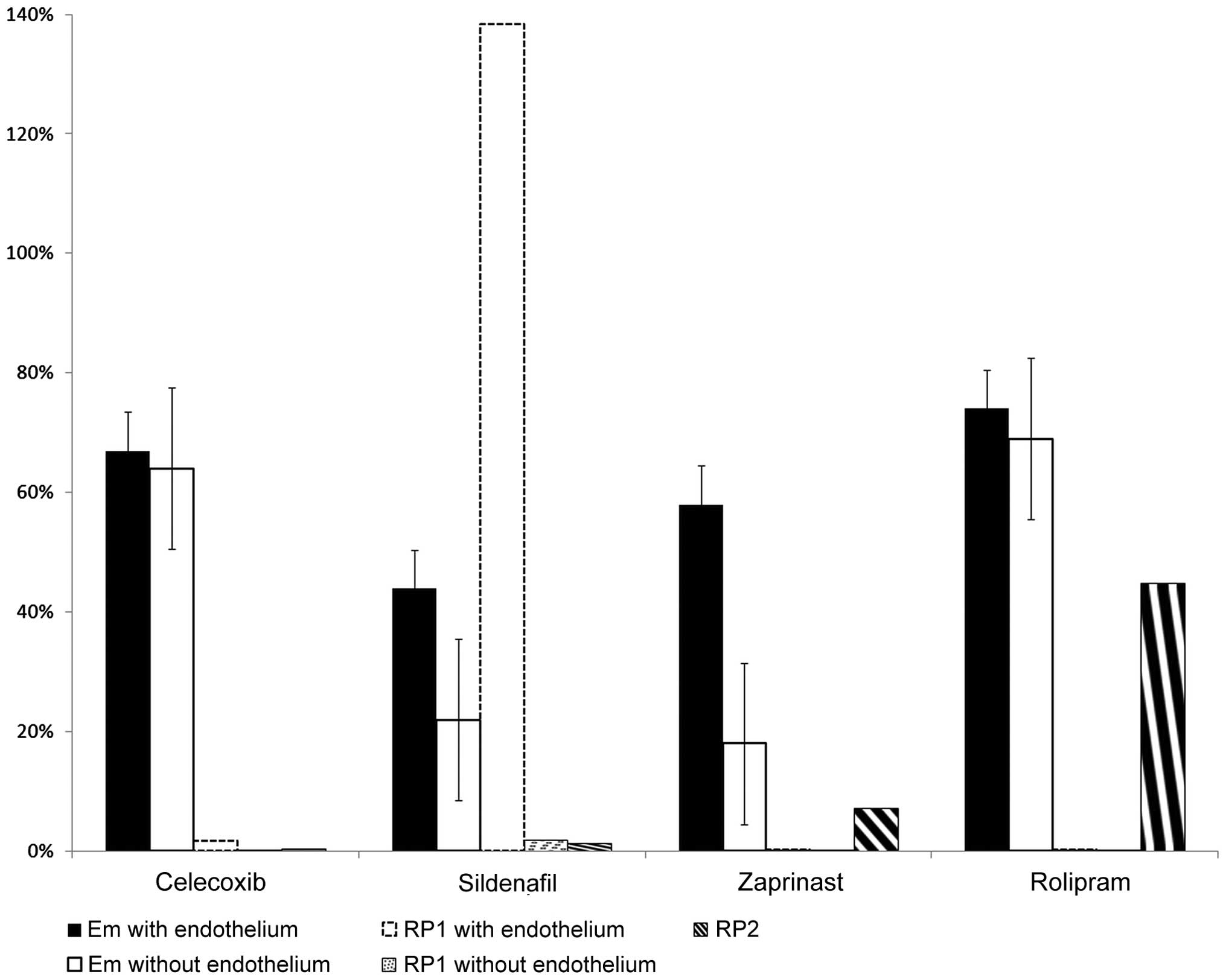
constricted by ET-1 are summarized in Table I.
 | Table IPharmacometric parameters of human
mesenteric arteries (with and without endothelium) treated with PDE
inhibitors or celecoxib and constricted by ET-1. |
Table I
Pharmacometric parameters of human
mesenteric arteries (with and without endothelium) treated with PDE
inhibitors or celecoxib and constricted by ET-1.
| Treatments | No. | EC50
(M/L) | SE
(EC50) | P-value
(EC50) | pD2 | Em (%) | SE (Em %) | P-value (Em) | RP1 (%) | RP2 (%) |
|---|
| Mesenteric
arteries |
| with
endothelium |
| Celecoxib | 12 | 1.91E-07 | 0.08E-07 | - | −6.72 | 67 | 5.6 | - | 1.7 | 0.45 |
| Sildenafil | 12 | 2.29E-09 | 0.04E-09 | - | −8.64 | 44 | 6.3 | - | 138.4 | 1.35 |
| Zaprinast | 12 | 1.91E-06 | 0.05E-06 | - | −5.72 | 58 | 4.9 | - | 0.2 | 7.24 |
| Rolipram | 12 | 3.22E-05 | 0.04E-05 | - | −4.49 | 74 | 6.1 | - | 0.01 | 44.91 |
| without
endothelium |
| Celecoxib | 12 | 4.27E-05 | 0.04E-05 | <0.001 | −4.37 | 64 | 6.1 | NS | 0.01 | 0.45 |
| Sildenafil | 12 | 1.70E-07 | 0.07E-07 | <0.001 | −6.77 | 22 | 4.6 | <0.001 | 1.9 | 1.35 |
| Zaprinast | 12 | 2.63E-05 | 0.04E-05 | <0.001 | −4.58 | 18 | 4.4 | <0.001 | 0.01 | 7.24 |
| Rolipram | 12 | 7.17E-05 | 0.06E-05 | <0.001 | −4.14 | 69 | 5.8 | 0.052 | 0.00 | 44.91 |
Analysis of PDE inhibitors and celecoxib
in human mesenteric arteries without endothelium
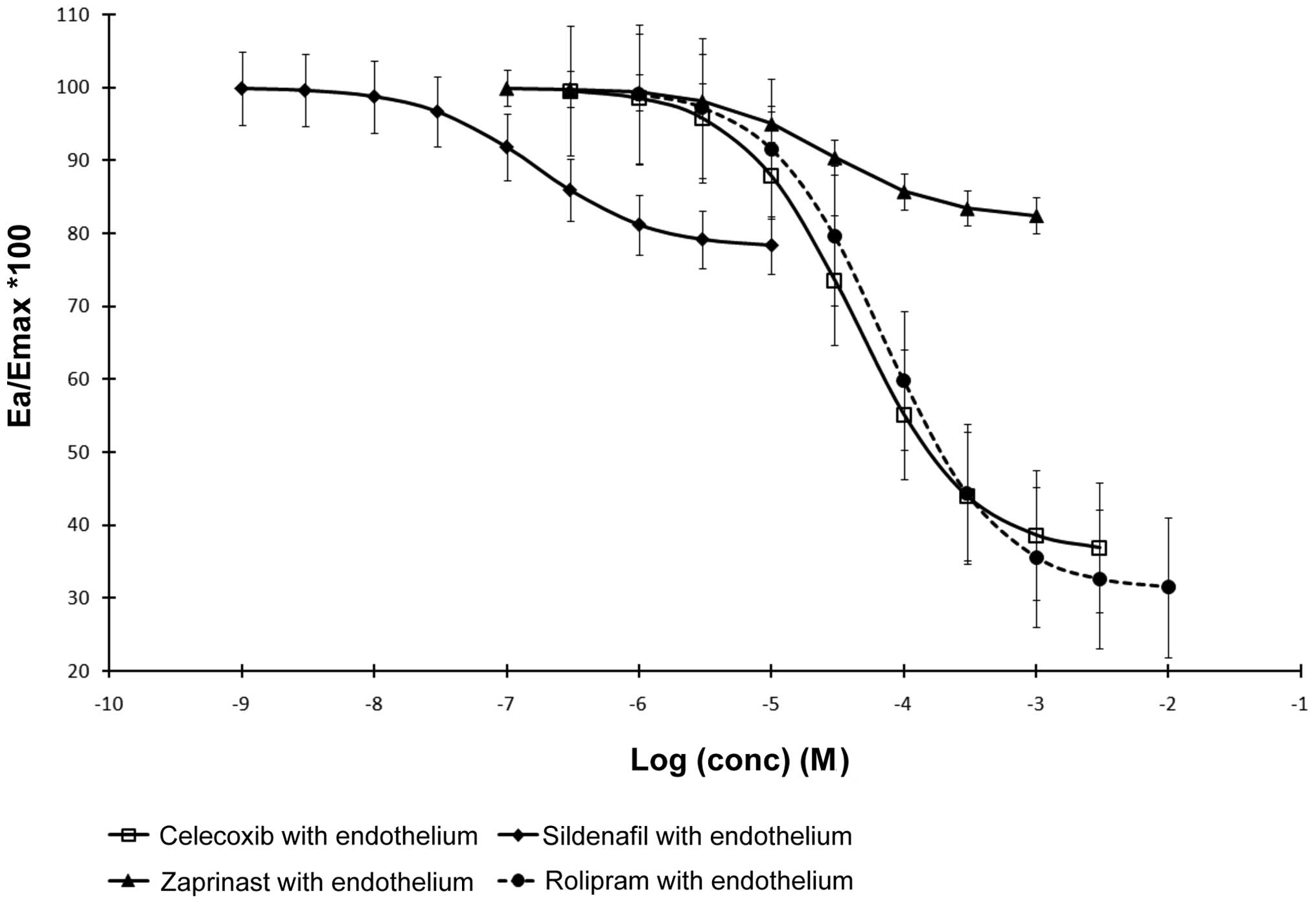
The series of experiments conducted on the arteries
with removed endothelium showed that CRCs for celecoxib and
rolipram were shifted to the right without a statistically
significant decrease in the maximum dilating effect of celecoxib
and the PDE4 inhibitor (Fig. 2).
Furthermore, CRCs for sildenafil and zaprinast were shifted to the
right, but with a simultaneous statistically significant decrease
in the maximum dilating effect of PDE5 and PDE5/6 inhibitors and
with an increased inclination angle in reference to the
concentration axis (Fig. 2). The
PDE inhibitors and COX-2 inhibitor demonstrated characteristics of
non-competitive (functional) antagonists in vessels without an
endothelium and did not completely eliminate vascular constriction
caused by ET-1.
Comparative analysis of CRCs for PDE
inhibitors and celecoxib in human mesenteric arteries with and
without endothelium
Comparative analysis of the CRCs for the PDE
inhibitors and the COX-2 inhibitor in mesenteric arteries with and
without the endothelium suggested that removal of the endothelium
corresponds to a model of competitive inhibition in reference to
the vasodilating effects of celecoxib and rolipram and
non-competitive inhibition in reference to the vasodilating effects
of zaprinast and sildenafil.
Discussion
A recognized method for limiting the use of
vasodilative factors is inhibiting the decomposition of cyclic
monoribonucleotides, cAMP and cGMP. Cyclic nucleotides, cAMP and
cGMP, are synthesized intracellularly by adenylyl and guanylyl
cyclases (11–13) in the presence of Mg2+
ions. They activate protein kinases, PKA and PKG, regulate the
intracellular Ca2+ concentration and influence the
function of ion channels (14–16).
An increased level of cyclic nucleotides results in a decreased
concentration of cytoplasmic Ca2+, decreased sensitivity
of the smooth muscles to calcium and consequently leads to dilation
(17,18).
The concentration of cGMP in vascular smooth muscle
cells is associated with the condition of the endothelium. A
stimulated endothelium produces NO, which diffuses into the
muscular layer and stimulates cGMP production by covalently binding
to a heme group of soluble guanylyl cyclases (sGC) (19,20).
It has been reported that aquaporin-1 transports NO through the
cell membranes (21) and the
synthesis of aquaporins is inhibited by ET-1 (22). However, Slupski et al (23) reported that sodium pump stimulation
by YC-1, as an additional mechanism of sGC activation independent
of cGMP, relaxed human mesenteric arteries, including blockade of
calcium ion influx.
The influence of the endothelium on reserves of cAMP
is much less explicit. Although locally produced PGE2
and PGI2 reduce the tone of the blood vessel walls by
GPCRs (IP and EP), the endothelium is also recognized as a
potential source of PGH2, a vasoconstricting prostanoid,
which acts by reducing the concentration of cAMP in the smooth
muscle cells. PGI2, the main metabolite of the
arachidonic acid released from the vascular endothelium, is mainly
produced in humans with the participation of COX-2 (24). PGH2 synthesis is
controlled by shear stress and autacoids, which constrict and
dilate vessels. A previous study suggested that the role of
PGI2 in the local regulation of vascular tone was not
significant, but studies analyzing the polymorphism of
PGI2 found an association between the risk of myocardial
infarction and severe hypertension (3). Simultaneously, it was reported that
PGI2 limits pulmonary hypertension induced by hypoxia
and general hypertension caused by angiotensin II (25). PGE2, produced in the
endothelium with the participation of COX-2 and through the
EP4 receptor, maintains the patent arterial duct until
birth, when decreased levels of PGE2 result in its
closing (26). Discrepant
observations in the case of PGE2 may result from the
multitude of receptors for this prostanoid.
The present study compared celecoxib, a COX-2
inhibitor with unique properties of inhibiting PDE5 and 4, with
classical PDE inhibitors occurring in the endothelium (PDE4, 5 and
6). Our results suggested that superior mesenteric arteries with a
maintained endothelium and constricted with ET-1 responded to the
vasodilative action of all the PDE inhibitors as well as celecoxib.
With regards to sildenafil, a reduced perfusion pressure by ~44% of
the maximum effect of ET-1 was achieved. Zaprinast and rolipram
were found to be more effective in causing dilation. In the
mesenteric arteries with a maintained endothelium, celecoxib
triggered a concentration-dependent reduction in perfusion pressure
reaching 67% of the maximum effects of ET-1 and this result did not
statistically differ from those of rolipram and zaprinast.
Sildenafil showed the lowest EC50 value of 2.29 (±0.04)
× 10−9 M/L. In addition, low concentrations of PDE
inhibitors, celecoxib and other studied substances were not able to
reach dilation efficacy, which was equal to the efficacy of
sildenafil.
The reduced myorelaxing efficacy of the PDE5 and 6
inhibitors was notable while comparing the results obtained in
arteries with endothelium and those without endothelium. Our
results suggested that cAMP plays a crucial role in vasoreactivity
of the choke vessels, but it is mainly independent from the
endothelium. Celecoxib is a poor PDE4 inhibitor and showed its
ability to increase cAMP at high concentrations; however, at lower
concentrations it efficiently acts through PDE5 inhibition. Thus,
vasorelaxant pathways based on cGMP may be referred to as the
pathways controlled by the presence and condition of the
endothelium, and vasorelaxant pathways based on cAMP may be
referred to as the endothelium-independent pathways.
The vascular effects of celecoxib have been
approached carefully. Data provided by two large, randomized and
double-blinded clinical studies with the use of celecoxib
(Celecoxib Long-term Arthritis Safety Study) and rofecoxib
[Vioxx® Gastrointestinal Outcomes Research (VIGOR)] in
patients with chronic arthritis, despite the fact that fewer
adverse events associated with an alimentary tract in the group of
patients taking coxibs compared to subjects taking non-selective
NSAIDs was observed, detailed analysis of the results confirmed
doubts regarding the safety of coxibs in reference to their
influence, not only on the mucous membrane of the alimentary tract,
but also on the cardiovascular system (8,27–29).
VIGOR reported an increased risk in the occurrence of
cardiovascular events in the group of patients taking rofecoxib,
which led to its recall by the Food and Drug Administration in
December, 2004. In addition, previous studies have reported that
celecoxib increased the incidence of cardiovascular complications
compared to that of the placebo, but did not increase general
mortality (30,31). An explanation for the adverse events
caused by COX-2 inhibitors was ascribed to the action of these
drugs resulting in the decreased synthesis of PGI2,
which reveals vasodilating and anti-aggregating properties
previously known. In vivo, decreasing the synthesis of
PGI2 may have been associated with superior influence of
its functional antagonist, thromboxane (7,32).
While the safety of coxibs, which in the light of
previous data confirming increased cardiovascular risks may raise
doubts, the vasodilating action of celecoxib itself is
well-documented. Animal studies on guinea pigs and rats reported
that celecoxib dilated coronary arteries in guinea pigs and large
arteries in rats by intensifying the effects of NO/cGMP associated
with the inhibition of PDE5 (33).
In these studies, the vasodilating efficacy of celecoxib was found
to be reduced compared to that of sildenafil, but higher compared
to that of zaprinast. It was also reported that the vasodilating
efficacy of celecoxib was limited by the NO synthase inhibitor
(L-NAME) and guanylyl cyclase inhibitor. In direct measurements,
celecoxib increased the levels of cGMP in the smooth muscle treated
with sodium nitroprusside at a concentration of 5×10−7
M/L by ~5-fold. These unexpected actions of celecoxib were
explained with PDE5 block, which significantly compensated for the
decrease of cAMP associated with the assumed reduction in
PGI2 synthesis. EC50 of celecoxib was also
established in relation to human PDE5A1 at
1.6×10−5 M/L.
Previous studies have reported that the use of
celecoxib is associated with a reduced risk in developing or losing
control of hypertension, as well as with a reduced risk of acute
cardiovascular events, than it is with selective COX-2 inhibitors,
in particular, rofecoxib (31,34,35).
It was suggested that celecoxib may reduce endothelial dysfunction
(16,36,37).
Studies using recombinant human PDE have indicated that celecoxib
also inhibited the activity of PDE4
(EC50=1×10−5 M/L), but did not influence
PDE1, 2 and 3. It was emphasized that the effects of low
intracellular concentrations of PGI2-dependent cAMP
caused by celecoxib may be compensated by increased cGMP,
inhibition of PDE3 by cGMP and by the direct inhibition effects of
celecoxib on PDE4 (33). These
properties have not been confirmed on other selective COX-2
inhibitors except for valdecoxib, which may inhibit PDE5, but more
weakly compared to that of celecoxib (2). It is vital that the vasodilating
properties of celecoxib were also observed in vivo. An
increase in blood flow in the brachial artery was identified in
patients with hypertension following treatment with celecoxib in a
placebo-controlled trial (38).
This supports the hypothesis that celecoxib restores endogenous
vasorelaxing sensitivity.
Recently, it was reported that selective COX-2
inhibitors restored, not only the vasodilating response of
sclerotically degenerated arteries, but also the vasoconstricting
response. Experimental data provided by studies evaluating
sclerotically degenerated arteries in rabbits suggested an
increased sensitivity to noradrenalin to the level comparable to
the sensitivity of normal arteries following the use of
indomethacin. Lowered PGI2 synthesis was observed under
the influence of the COX-2 inhibitor, but this decrease was only
associated with sclerotically degenerated arteries (39). These data confirm previous in
vivo observations regarding COX-2 inhibitors, which may clearly
influence the vascular system not only by limiting the synthesis of
PGI2 and TXA2 (which appears the most
distinct), but also by increasing the sensitivity to vasodilating
as well as vasoconstricting factors. In conclusion, the present
study identified high vasorelaxing efficacy of celecoxib at the
background of the PDE inhibitors, which was observed not only in
the presence, but also in the absence of the endothelium and may be
evidence for relaxation caused by this COX-2 inhibitor in the cAMP-
and cGMP-dependent pathways.
References
|
1
|
Grześk G, Koziński M, Navarese EP,
Krzyżanowski M, Grześk E, Kubica A, Siller-Matula JM, Castriota F
and Kubica J: Ticagrelor, but not clopidogrel and prasugrel,
prevents ADP-induced vascular smooth muscle cell contraction: a
placebo-controlled study in rats. Thrombos Res. 130:65–69.
2012.PubMed/NCBI
|
|
2
|
Rajakariar R, Yaqoob MM and Gilroy WD:
COX-2 in inflammation and resolution. Mol Interv. 6:199–207. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smyth EM and FitzGerald GA: Prostaglandin
mediators. Handbook of Cell Signaling. Bradshaw RA and Dennis EA:
Second Edition. Academic Press; San Diego: pp. 265–273. 2003,
View Article : Google Scholar
|
|
4
|
Bresalier RS, Sandler RS, Quan H,
Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D,
Lanas A, Konstam MA and Baron JA: Cardiovascular events associated
with rofecoxib in a colorectal adenoma chemoprevention trial. N
Engl J Med. 352:1092–1102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
FitzGerald GA: Coxibs and cardiovascular
disease. N Engl J Med. 351:1709–1711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nussmeier NA, Whelton AA and Brown NT:
Complications of the COX-2 inhibitors parecoxib and waldecoxib
after cardiac surgery. N Engl J Med. 352:1081–1091. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solomon SD, McMurray JJV, Pfeffer MA,
Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E and
Bertagnolli M; Adenoma prevention with celecoxib (APC) study
investigators. Cardiovascular risk associated with celecoxib in a
clinical trial for colorectal adenoma prevention. N Engl J Med.
352:1071–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bavry AA, Khaliq A, Gong Y, Handberg EM,
Cooper-Dehoff RM and Pepine CJ: Harmful effects of NSAIDs among
patients with hypertension and coronary artery disease. Am J Med.
124:614–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bertagnolli MM, Hsu M, Hawk ET, Eagle CJ
and Zauber AG: Statin use and colorectal adenoma risk: results from
the adenoma prevention with celecoxib trial. Cancer Prev Res.
3:588–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koller A, Sun D, Huang A and Kaley G:
Corelease of nitric oxide and prostaglandins mediates
flow-dependent dilation of rat gracilis muscle arterioles. Am J
Physiol. 267:H326–H332. 1994.PubMed/NCBI
|
|
11
|
Ganesan AN, Maack C, Johns DC, Sidor A and
O’Rourke B: Beta-adrenergic stimulation of L-type
Ca2+channels in cardiac myocytes requires the distal
carboxyl terminus of α1Cbut not serine 1928. Circ Res.
98:e11–e18. 2006.PubMed/NCBI
|
|
12
|
Keef KD, Hume JR and Zhong J: Regulation
of cardiac and smooth muscle Ca2+channels (CaV1.2a,b) by
protein kinases. Am J Physiol Cell Physiol. 281:C1743–C1756.
2001.PubMed/NCBI
|
|
13
|
Kilic A, Bubikat A, Gassner B, Baba HA and
Kuhn M: Local actions of atrial natriuretic peptide counteract
angiotensin II stimulated cardiac remodeling. Endocrinology.
148:4162–4169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Münzel T, Feil R, Mülsch A, Lohmann SM,
Hofmann F and Walter U: Physiology and pathophysiology of vascular
signaling controlled by cyclic guanosine 3′,5′-cyclic
monophosphate-dependent protein kinase. Circulation. 108:2172–2183.
2003.
|
|
15
|
Rybalkin SD, Yan C, Bornfeldt KE and Beavo
JA: Cyclic GMP phosphodiesterases and regulation of smooth muscle
function. Circ Res. 93:280–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zaccolo M and Movsesian MA: cAMP and cGMP
signaling cross-talk: role of phosphodiesterases and implications
for cardiac pathophysiology. Circ Res. 100:1569–1578. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bender AT and Beavo JA: Cyclic nucleotide
phosphodiesterases: molecular regulation to clinical use. Pharmacol
Rev. 58:488–520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Somlyo AP and Somlyo AV:
Ca2+sensitivity of smooth muscle and nonmuscle myosin
II: modulated by G proteins, kinases, and myosin phosphatase.
Physiol Rev. 83:1325–1358. 2003.
|
|
19
|
Oberwittler H, Hirschfeld-Warneken A,
Wesch R, Willerich H, Teichert L, Heinz KH, Lehr E, Ding R, Haefeli
WE and Mikus G: Significant pharmacokinetic and pharmacodynamic
interaction of warfarin with the NO-independent sGC activator
HMR1766. J Clin Pharmacol. 47:70–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yetik-Anacak G, Xia T, Dimitropoulou C,
Venema RC and Catravas JD: Effects of hsp90 binding inhibitors on
sGC-mediated vascular relaxation. Am J Physiol Heart Circ Physiol.
291:H260–H268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herrera M and Garvin JL: Novel role of
AQP-1 in NO-dependent vasorelaxation. Am J Physiol Renal Physiol.
292:F1443–F1451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanaka K and Koyama Y: Endothelins
decrease the expression of aquaporins and plasma membrane water
permeability in cultured rat astrocytes. J Neurosci Res.
89:320–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Slupski M, Szadujkis-Szadurski L, Grzesk
G, Szadujkis-Szadurski R, Szadujkis-Szadurska K, Wlodarczyk Z,
Masztalerz M, Piotrowiak I and Jasiński M: Guanylate cyclase
activators influence reactivity of human mesenteric superior
arteries retrieved and preserved in the same conditions as
transplanted kidneys. Transplant Proc. 39:1350–1353. 2007.
View Article : Google Scholar
|
|
24
|
Chenevard R, Hurlimann D, Bechir M,
Enseleit F, Spieker L, Hermann M and Riesen W: Selective COX-2
inhibition improves endothelial function in coronary artery
disease. Circulation. 107:405–409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simionescu M: Implications of early
structural-functional changes in the endothelium for vascular
disease. Arterioscler Thromb Vasc Biol. 27:266–274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Foudi N, Kotelevets L, Louedec L, Leseche
G, Henin D, Chastre E and Norel X: Vasorelaxation induced by
prostaglandin E(2) in human pulmonary vein: role of the EP(4)
receptor subtype. Br J Pharmacol. 154:1631–1639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grosser T, Fries S and FitzGerald GA:
Biological basis for the cardiovascular consequences of COX-2
inhibition: therapeutic challenges and opportunities. J Clin
Invest. 116:4–15. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mamdani M, Juurlink DN, Lee DS, Rochon PA,
Kopp A, Naglie G, Austin PC, Laupacis A and Stukel TA:
Cyclooxygenase-2 inhibitors versus non-selective non-steroidal
anti-inflammatory drugs and congestive heart failure outcomes in
elderly patients: a population-based cohort study. Lancet.
363:1751–1756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wolfe F, Zhao S and Pettitt D: Blood
pressure destabilization and edema among 8538 users of celecoxib,
rofecoxib, and nonselective nonsteroidal antiinflammatory drugs
(NSAID) and nonusers of NSAID receiving ordinary clinical care. J
Rheumatol. 31:1143–1151. 2004.
|
|
30
|
Aw TJ, Haas SJ, Liew D and Krum H:
Meta-analysis of cyclooxygenase-2 inhibitors and their effects on
blood pressure. Arch Intern Med. 165:490–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brinker A, Goldkind L, Bonnel R and Beitz
J: Spontaneous reports of hypertension leading to hospitalisation
in association with rofecoxib, celecoxib, nabumetone and oxaprozin.
Drugs Aging. 21:479–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng Y, Austin SC, Rocca B, Koller BH,
Coffman TM, Grosser T, Lawson JA and FitzGerald GA: Role of
prostacyclin in the cardiovascular response to thromboxane A2.
Science. 296:539–541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klein T, Eltze M, Grebe T, Hatzelmann A
and Kömhoff M: Celecoxib dilates guinea-pig coronaries and rat
aortic rings and amplifies NO/cGMP signaling by PDE5 inhibition.
Cardiovasc Res. 75:390–397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Francois H, Athirakul K, Howell D, Dash R,
Mao L, Kim HS, Rockman HA, Fitzgerald GA, Koller BH and Coffman TM:
Prostacyclin protects against elevated blood pressure and cardiac
fibrosis. Cell Metab. 2:201–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gudbjornsson B, Thorsteinsson SB,
Sigvaldason H, Einarsdottir R, Johannsson M, Zoega H, Halldorsson M
and Thorgeirsson G: Rofecoxib, but not celecoxib, increases the
risk of thromboembolic cardiovascular events in young adults - a
nationwide registry-based study. Eur J Clin Pharmacol. 66:619–625.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akiko H, Kazunao K, Kazuhiko T, Naoki I,
Kazuo U, Kyoichi O and Hiroshi W: Cyclooxygenase-dependent
vasoconstricting factor(s) in remodelled rat femoral arteries.
Cardiovasc Res. 79:161–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Flórez A, de Haro J, Martínez E, Varela C,
Bleda S and Acín F: Selective cyclooxygenase-2 inhibition reduces
endothelial dysfunction and improves inflammatory status in
patients with intermittent claudication. Rev Esp Cardiol.
62:851–857. 2009.PubMed/NCBI
|
|
38
|
Widlansky ME, Price DT, Gokce N, Eberhardt
RT, Duffy SJ, Holbrook M, Maxwell C, Palmisano J, Keaney JF Jr,
Morrow JD and Vita JA: Short- and long-term COX-2 inhibition
reverses endothelial dysfunction in patients with hypertension.
Hypertension. 42:310–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Foudi N, Norel X, Rienzo M, Louedec L,
Brink C, Michel JB and Back M: Altered reactivity to norepinephrine
through COX-2 induction by vascular injury in hypercholesterolemic
rabbits. Am J Physiol Heart Circ Physiol. 297:H1882–H1888. 2009.
View Article : Google Scholar : PubMed/NCBI
|