Introduction
Spinal anesthesia provides fast, profound, and
high-quality block characteristics, and remains the preferred
choice for cesarean deliveries worldwide (1,2). Hypotension
is the most common serious adverse effect of spinal anesthesia for
cesarean deliveries, and the incidence of hypotension after spinal
anesthesia is >80% (3). Hypotension
is the physiological consequence of spinal anesthesia and can have
a potentially deleterious maternal and fetal impact (4). Therefore, prevention and treatment of the
negative effects, with special medical agents for the optimal
sustaining of the mother's blood pressure and fetal's circulation
are an important issue (5,6). Although studies have compared different
measures to elevate the hypotension induced by regional anesthesia,
vasopressors are nevertheless required to treat any significant
hypotension (7). Previous findings
revealed the development of hypotension after spinal block in
subjects undergoing cesarean section was not prevented despite
low-dose bupivacaine; however, the severity of maternal
hypotension, the number of ephedrine treatments, and the total dose
of ephedrine were decreased (8).
Previous results indicated that phenylephrine and ephedrine are
acceptable choices to combat maternal hypotension related to spinal
anesthesia in elective cesarean section, but the incidences of
bradycardia were significantly higher in the phenylephrine group
(9).
Ephedrine is the most commonly used drug among the
vasopressors (1). Since ephedrine
systemic absorption and peak effect are difficult to predict, the
prophylactic administration of ephedrine by the intramuscular route
is very controversial (10,11). The intravenous route may be more
effective and controllable, but ephedrine has been involved in
lower umbilical pH levels, especially when used in dosages high
enough to stem nausea and vomiting related to hypotension (9,12,13). We hypothesized that ephedrine combined
with low-dose bupivacaine intrathecal injection would be conductive
to maintaining stability in maternal hemodynamic. Even at the large
amount of 30 mg, the intrathecal injection of ephedrine is
considered safe (14).
The aim of the present study was to investigate the
effectiveness and the side effects of intravenous phenylephrine and
ephedrine in combating maternal hypotension resulting from spinal
anesthesia in patients undergoing elective cesarean section.
Materials and methods
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Shihezi University
School of Medicine in China, and is registered in the Chinese
Clinical Trial Register (no. ChiCTR-IPR-14005477). The present
study was registered prior to enrolling subjects in the study. All
the patients provided written informed consent prior to
inclusion.
This prospective, double-blind, randomized,
controlled and single-center study was designed in 2013 and
performed at the First Affiliated Hospital of Shihezi University
School of Medicine, a teaching hospital of Capital Medical
University, China. Included in the present study were 107 patients
who underwent elective cesarean section between June, 2014 and
April, 2015. The inclusion criteria were: Healthy and nulliparous
women with a signal, vertex presentation fetus at term (38–42
weeks), ASA physical status I or II, and undergoing elective
cesarean delivery under combined spinal-epidural anesthesia. The
exclusion criteria included: Age <18 or >40 years of age,
height <150 or >180 cm, weight <50 or >100 kg, the
presence of systemic disease (e.g., preeclampsia, hypertension,
diabetes mellitus), contraindications to an epidural technique, an
allergy or idiosyncratic reaction to local anesthetic or opioid
medications, an increased risk of cesarean delivery (e.g., trial of
labor after cesarean delivery, history of uterine rupture),
evidence of anticipated fetal anomalies, clinical signs or symptoms
of infection, and signs of labor onset.
The patients underwent preoperative fasting for 8 h
and water deprivation for >4 h. The patients were taken to the
operating room, and administered 8 ml/kg of lactated Ringer's
injection, within 20 min, via a 20-gauge cannula placed in a
forearm vein. The infusion speed was then adjusted to 6 ml/kg/h.
Each patient was randomly assigned to one of the two double-blind
study groups. The patients were in a supine position for ≥30 min
before the collection of their blood. Patients were positioned in
the right lateral position with flexion of thigh and legs, hip and
knees and flexion at the head. Using aseptic precautions, lumbar
puncture was performed at L3-4 using midline approach with 23G
sterile Quinke's needle. After visualization of clear and free flow
of cerebrospinal fluid, Group E received 2 ml of 0.25% bupivacaine,
containing 15 mg ephedrine and Group C was given 2 ml of 0.25%
bupivacaine. After anesthesia, patients were assisted into a supine
position and care was taken regarding the block plane prior to
surgery, once again for maternal blood collection.
The baseline SAP and heart rate (HR), lowest and
highest SBP and HR, nausea, vomiting, dizziness, and chest symptoms
were recorded every minute. The upper sensory level of anesthesia
was measured by assessing loss of pinprick discrimination at 10
min. The blocks extended to T6 or above, prior to the initiation of
surgery. Once hypotension occurred, defined as decrease in systolic
blood pressure of <90 mm Hg and/or 30% less than the basal
Systolic blood pressure, patients were given ephedrine
intravenously. The first rescue ephedrine time, total doses of
rescue ephedrine, and total dose of used ephedrine, duration of
surgery, and time of neonate extraction were all recorded as
minutes after the beginning of surgery. Incidences of bradycardia
(HR <60 bpm) were treated with 0.5 mg atropine i.v.; and any
tachycardia (HR >30% above the basal HR) was noted. After
delivery, Apgar score was assessed at 1 and 5 min by the attending
pediatrician. Arterial blood samples were taken from the umbilical
cord for blood-gas analysis within 2 min. After baby extraction,
all the patients received 20 units oxytocin by infusion through a
separate line. Maternal plasma noradrenaline was measured by
high-performance liquid chromatography.
Statistical analysis
The primary outcome was the incidence of
hypotension. The sample size was calculated according to our
preliminary data. A minimum of 50 patients in each group provided
90% statistical power to detect a 30% difference (from 50 to 20%)
in the incidence of hypotension, at a two-sided P<0.05.
Considering a presumed dropout rate of 20%, 70 patients were
initially enrolled in each of the two groups.
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses. Data were analyzed using the
Shapiro-Wilk test to determine the distribution. If normally
distributed, the data were presented as mean ± standard deviation
and two independent groups were compared using the Student's
t-test. Data not distributed normally were presented as median
(min-max), and were analyzed using a Mann-Whitney U test.
Categorical variables were analyzed by using a Chi-square test, or
Fisher's exact test if the number of subjects in any contingency
table cell was expected to be <5. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient data
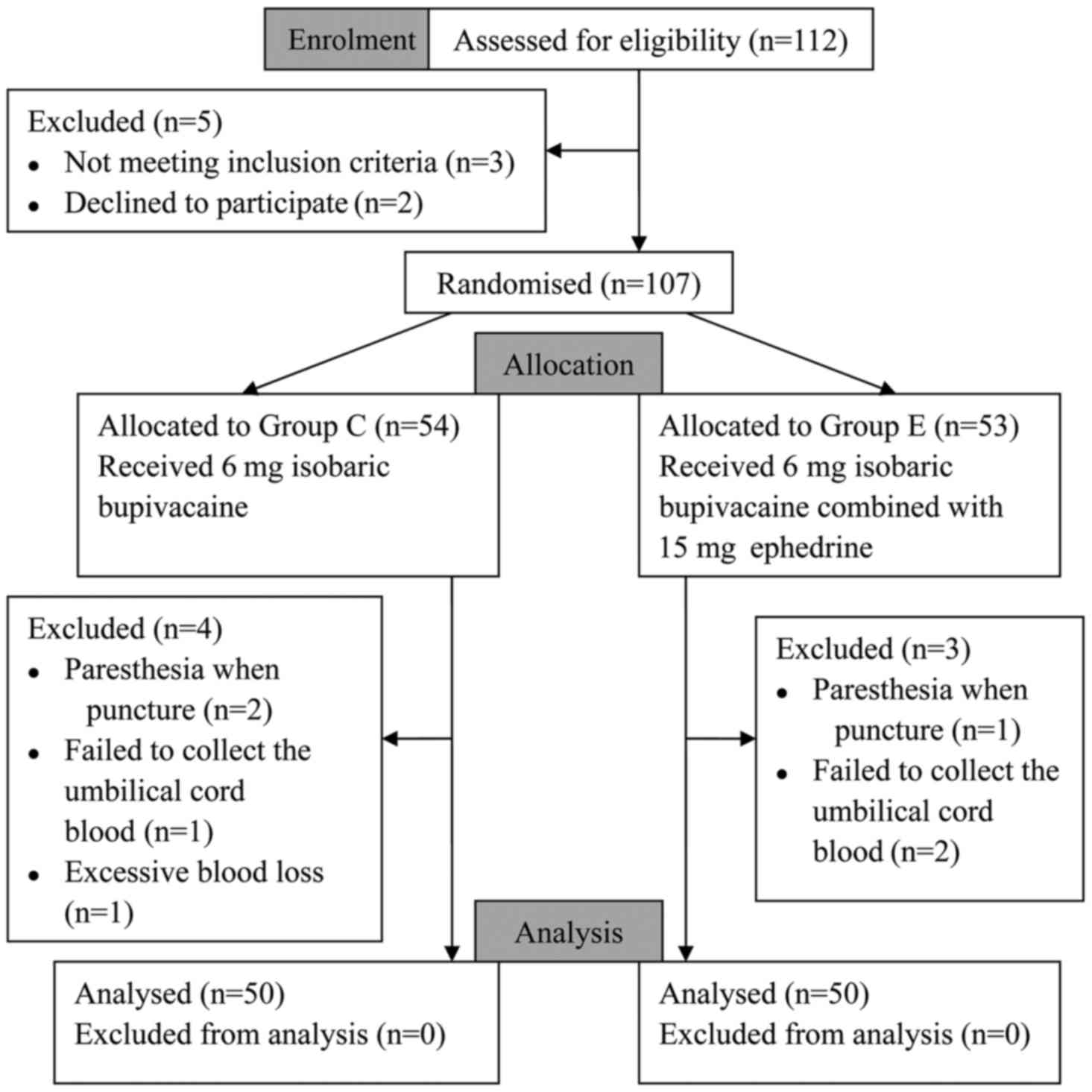
The present study was conducted between June, 2014
and April, 2015. Initially, 112 patients were assessed for study
eligibility. Five subjects were excluded from the study, of whom 3
did not meet the inclusion criteria and 2 declined to participate,
and the remaining 107 subjects were randomized. Group C included 54
subjects and group E 53 subjects. Subsequently, 4 subjects were
excluded from group C and 3 from group E. Finally, there were 50
patients analyzed in each group (Fig.
1).
The demographic characteristics of the two groups
were similar. There were no statistical differences between the
groups with respect to age, weight, height, gestational weeks,
delivery time and operative time (Table
I). Sensory blockade extended to T6 and above within 15 min in
all the patients. All the patients had adequate surgical
anesthesia.
 | Table I.Demographic data in the two groups
(data expressed as mean ± standard deviation). |
Table I.
Demographic data in the two groups
(data expressed as mean ± standard deviation).
| Variables | Group C (n=50) | Group E (n=50) | P-value |
|---|
| Age (years) | 26.86±5.23 | 28.12±4.87 | 0.22 |
| Weight (kg) | 73.66±10.61 | 77.62±12.11 | 0.09 |
| Height (cm) | 161.20±5.26 | 163.00±4.60 | 0.07 |
| Weeks of gestation
(week) | 37.20±1.03 | 37.64±1.33 | 0.07 |
| Time of baby
extraction (min) | 13.16±3.72 | 14.86±5.00 | 0.06 |
| Duration of surgery
(min) | 82.10±12.41 | 78.46±10.07 | 0.11 |
Blood pressure and HR
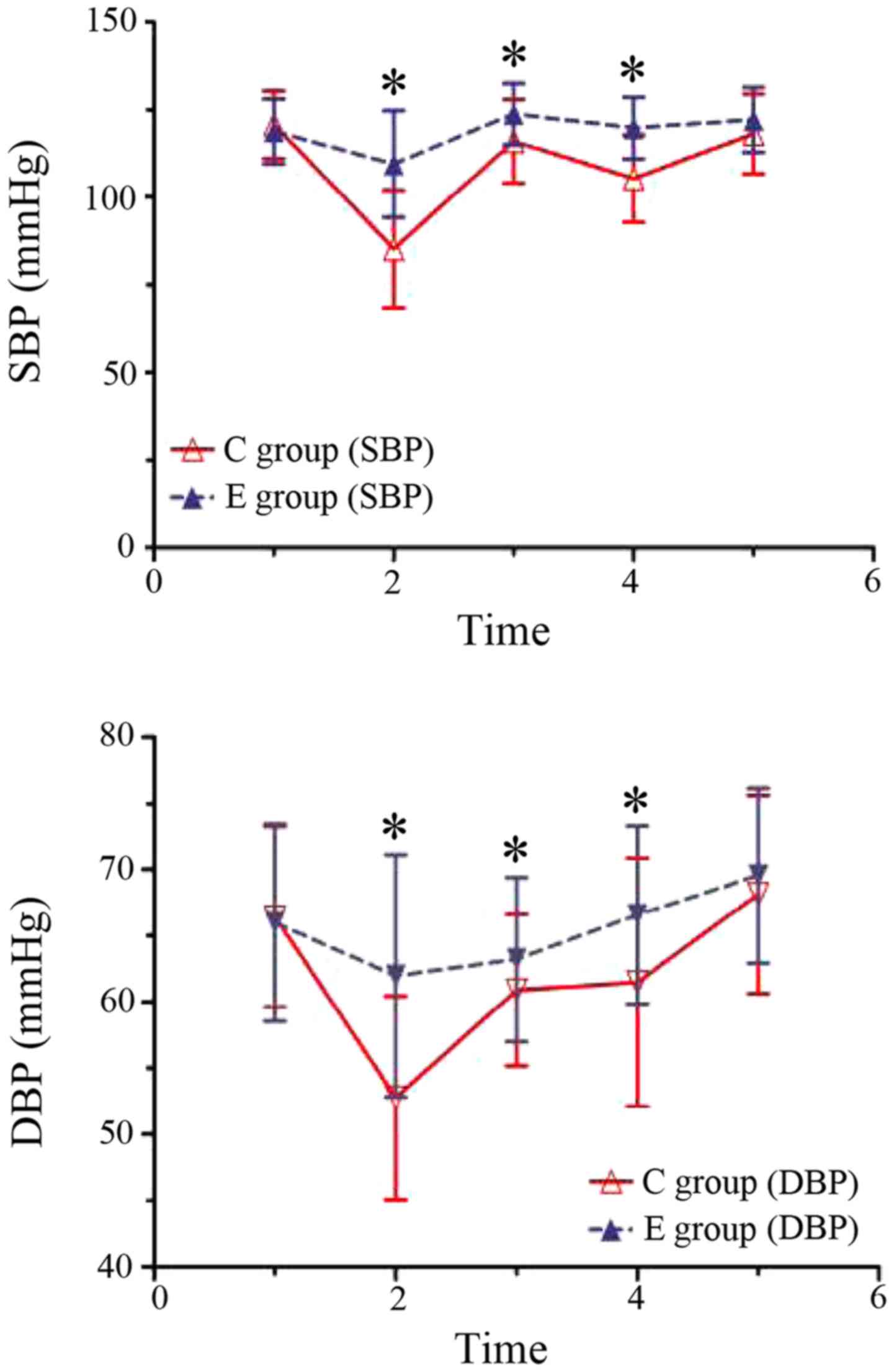
There was no significant difference in the SBP and
HR vaues at baseline between the study groups (P>0.05).
Post-anesthesia 3 min and 1 min after the delivery of the fetus,
the mean BPs in group C were significantly lower than those of
group E (P<0.05), and significant decreases of the mean BP were
observed in group C compared to the baseline (P<0.05). However,
no difference of the mean BP in group E was observed compared to
the the baseline (P>0.05) (Fig. 2).
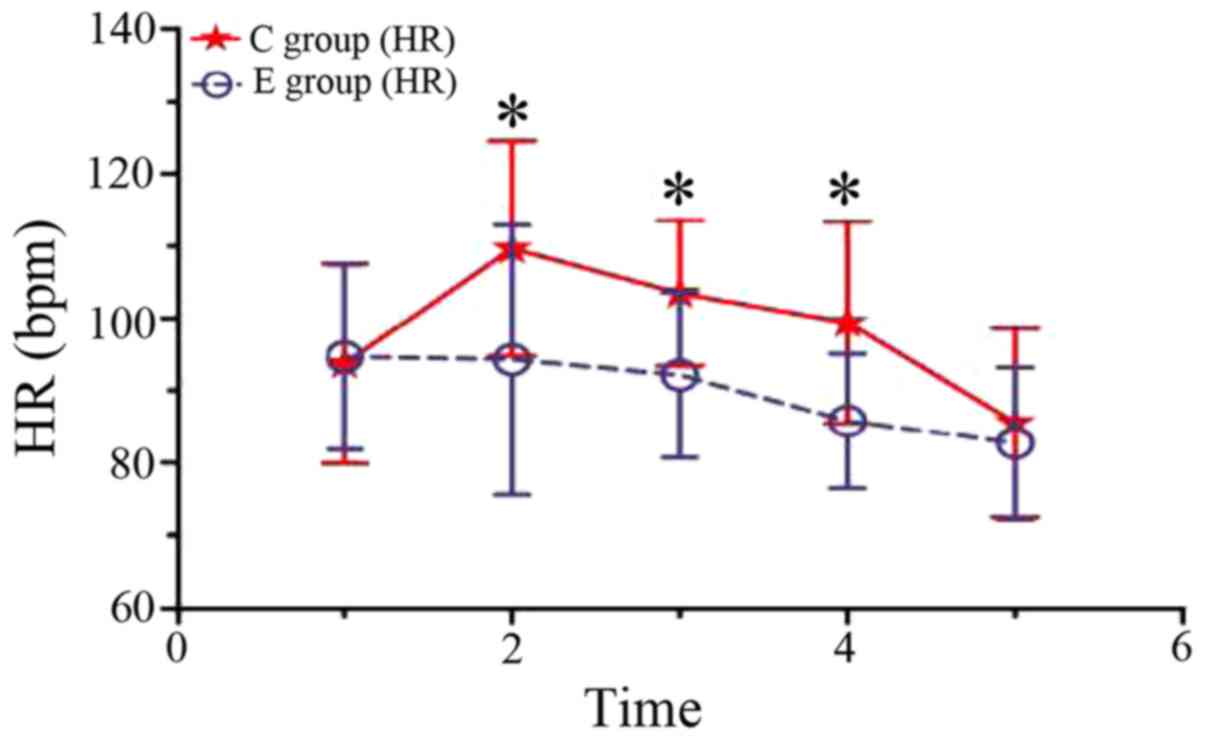
From post-anesthesia 3 min to the end of the surgery, the HR levels
in group C were significantly higher than those of group E
(P<0.05). Before the delivery of the fetus, significant
increases of the HR in group C were observed as compared with the
baseline (P<0.05), but no difference of the HR in group E was
observed as compared with the baseline (P>0.05). Following
delivery of the fetus, significant decreases of the HR in groups C
and E were observed as compared with the baseline (P<0.05)
(Fig. 3). The total doses of ephedrine
mean were 54.47, 46.53, P=0.139 in the two groups respectively, and
there were no obvious differences.
Complications
The incidences of hypotension, hypertension,
tachycardia, nausea or vomiting, and neonatal acidosis are
summarized in Table II. There was a
significant decrease in the incidence of hypotension in group E
compared with group C [6 (12%) vs. 40 (80%)] (P<0.01). There
were significantly lower incidences of tachycardia in group E
compared with group C [6 (12%) vs. 22 (44%)] (P<0.01). There
were significantly lower incidences of nausea and vomiting in group
E compared with group C [5 (10%) vs. 31 (62%)] (P<0.01). There
were significant lower incidences of neonatal acidosis in the E
group compared with the C group [3 (6%) vs. 17 (34%)] (P<0.01).
There was no difference in the ratio of hypertension between the
study groups (P>0.05).
 | Table II.Intraoperative complications in both
groups. |
Table II.
Intraoperative complications in both
groups.
| Variables | Group C (n=50,
%) | Group E (n=50,
%) | P-value |
|---|
| Hypotension | 40 (80) | 6 (12) | <0.01 |
| Hypertension | 4 (8) | 7 (14) | 0.34 |
| Tachycardia | 22 (44) | 6 (12) | <0.01 |
| Bradycardia | 3 (6) | 5 (10) | 0.46 |
| Nausea or
vomiting | 31 (62) | 5 (10) | <0.01 |
| Neonatal
acidosis | 17 (34) | 3 (6) | <0.01 |
Sensory block and plasma
norepinephrine levels
The effects of ephedrine intrathecal injection on
sensory blockade and maternal plasma noradrenaline are shown in
Table III. There were no differences
in the extent of sensory blockade or time-intervals. However, the
sensory blockade duration in the group E was significantly longer
than that of group C (P<0.01). Before and after anesthesia, the
maternal plasma noradrenaline content was altered, and significant
decreases of the maternal plasma noradrenaline content in group C
were observed as compared with baseline (P<0.05). However,
significant increases of the maternal plasma noradrenaline content
in group E were observed as compared with baseline (P<0.05).
There was no difference of the maternal plasma noradrenaline
content baseline in groups E and C (P>0.05). After anesthesia,
the maternal plasma noradrenaline content in group C was
significantly lower than that of group E (P<0.01).
 | Table III.Time of sensory block and plasma
norepinephrine levels in the two groups (data expressed as mean ±
standard deviation). |
Table III.
Time of sensory block and plasma
norepinephrine levels in the two groups (data expressed as mean ±
standard deviation).
| Variables | Group C (n=50) | Group E (n=50) | P-value |
|---|
| Time of sensory block
to T6, min | 7.65±1.27 |
8.06±1.76 | 0.18 |
| Duration of sensory
block to T6, min | 32.24±5.37 | 50.30±4.90 | <0.01 |
| Basal level of
maternal plasma norepinephrine, ng/l | 376.10±24.78 | 367.07±25.32 | 0.08 |
| Plasma norepinephrine
levels after anesthesia 8 min, ng/l | 325.45±26.88 | 402.66±23.20 | <0.01 |
Postoperative follow-up results showed that the two
groups had no neurological deficits performance (P>0.05).
Discussion
The current study is the first report, to the best
of our knowledge, to investigate the effects of ephedrine combined
with low-dose bupivacaine on maternal hemodynamics and spinal nerve
block in cesarean deliveries. Our findings demonstrated that
prophylactic subarachnoid injection of ephedrine during spinal
anesthesia for cesarean section can prevent hypotension without
significant maternal tachycardia or hypertension, decrease the
ratio of nausea and vomiting, improve the neonatal condition, and
decrease the ratio of neonatal acidosis. In addition, ephedrine
combined with low-dose bupivacaine subarachnoid injection prolonged
the sensory blockade duration of spinal anesthesia by
bupivacaine.
The incidence of hypotension during spinal
anesthesia for cesarean section is reported to be ≤80%, despite
fluid preload, lateral uterine displacement, low-dose bupivacaine
spinal anesthesia and use of vasopressor agents (15). In the anesthesia practice, prevention
and management of hypotension related to spinal anesthesia remains
a difficult problem and there has been no consensus on its optimal
management.
Ephedrine, an indirectly acting sympathomimetic
amine, is probably the vasopressor of choice in obstetric
anesthesia. Although ephedrine has mixed α- and β-adrenoreceptor
activity, it maintains arterial pressure mainly by increases in
cardiac output and heart rate as a result of its predominant
activity on β1-adrenoreceptors (16).
However, a meta-analysis of four randomized clinical trials by Lee
et al showed that ephedrine could not be used for
prophylaxis against hypotension, as it cannot prevent hypotension
in low doses, and in high doses, it may cause hypertension
(17). In addition, studies have shown
that a decrease in neonatal PH and BE is correlated with high-dose
ephedrine used in obstetrical anesthesia (12,18).
Previous studies have found that intravenous ephedrine can pass
through the placenta into the fetus, thereby increasing fetal
metabolism, causing poor umbilical artery and vein blood
PCO2 increase, resulting in neonate academia (19). Our results have shown that, while both
groups used the ephedrine dosage, the incidence of neonatal
acidosis in the intrathecal group was significantly lower than that
of the intravenous group, which indicated that maternal
subarachnoid injected ephedrine does not have obvious adverse
effects on the fetus. This shows indirectly that ephedrine in
subarachnoid injection may be little or not absorbed into the
blood. Spinal anesthesia is prone to hypotension, and it occurs
early in sensory and motor block, due to spinal nerve roots within
the unmyelinated sympathetic nerve fibers and sensory fibers,
showing local anesthetics are more sensitive. Subarachnoid
injection in the present study on stabilizing hemodynamic effect of
ephedrine may be directly blocked by the local anesthetics on
sympathetic nerve fibers in the spinal nerve root block.
Clinical trials found that dexmedetomidine has a
dose-dependent effect on the onset and regression of sensory and
motor block when used as an adjuvant to bupivacaine in spinal
anesthesia (20). As a non-specific
adrenergic receptor agonist, ephedrine can be to combined directly
with α-2 receptors in the dorsal horn of the spinal cord,
inhibiting class C and class A afferent nerve fibers of pain
impulses, thereby inhibiting prominent former neurotransmitter
release and dorsal horn neuron hyperpolarization (21). By blocking the Na+ internal
flow and the generation and conduction of nerve impulses, local
anesthetic acts as a nerve block. The results of the present study
show that the sensory blockade duration in group E were
significantly longer than those of group C, suggesting that
ephedrine combined with bupivacaine subarachnoid injection prolongs
spinal anesthesia sensory block duration. This phenomenon may show
the synergistic effects of subarachnoid injection of the two drugs
on nerve fibers. This is consistent with the animal experimental
study of Djalali (22). Kroin et
al reported that clonidine prolonging the effect of peripheral
nerve block is achieved by adjusting the
hyperpolarization-activated cation current, and it is independent
of α receptors (23). In the present
study, while the mechanism of ephedrine prolonging the duration of
sensory block was the same, further investigation is needed.
In conclusion, the findings of the present study,
show that prophylactic use of phenylephrine infusion can prevent
mother's hypotension induced by spinal anesthesia in cesarean
delivery, without any significant adverse effect on the mother or
her fetus, improves neonatal condition and prolongs the sensory
blockade duration of spinal anesthesia by bupivacaine.
Acknowledgements
The present study was partially supported by the
First Affiliated Hospital of Shihezi University School of Medicine
Research Project (no. YL-2013-R009).
References
|
1
|
Kol IO, Kaygusuz K, Gursoy S, Cetin A,
Kahramanoglu Z, Ozkan F and Mimaroglu C: The effects of intravenous
ephedrine during spinal anesthesia for cesarean delivery: A
randomized controlled trial. J Korean Med Sci. 24:883–888. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moslemi F and Rasooli S: Comparison of
prophylactic infusion of phenylephrine with ephedrine for
prevention of hypotension in elective cesarean section under spinal
anesthesia: A randomized clinical trial. Iran J Med Sci. 40:19–26.
2015.PubMed/NCBI
|
|
3
|
Tamilselvan P, Fernando R, Bray J, Sodhi M
and Columb M: The effects of crystalloid and colloid preload on
cardiac output in the parturient undergoing planned cesarean
delivery under spinal anesthesia: A randomized trial. Anesth Analg.
109:1916–1921. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De-Giorgio F, Grassi VM, Vetrugno G,
d'Aloja E, Pascali VL and Arena V: Supine hypotensive syndrome as
the probable cause of both maternal and fetal death. J Forensic
Sci. 57:1646–1649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller R, Eriksson L, Fleisher L,
Wiener-Kronish J and Young W: Anaesthesia for Orthopaedic
SurgeryMiller's Anesthesia. 2. 7th. Churchill Livingstone
Publishers; Philadelphia (PA): pp. 22522010
|
|
6
|
Simin A, Zahra F, Pouya HM and Reza T:
Comparison of the effect of ephedrine and phenylephrine in
treatment of hypotension after spinal anesthesia during cesarean
section. Open J Obstet Gynecol. 2:192–196. 2012. View Article : Google Scholar
|
|
7
|
Loubert C: Fluid and vasopressor
management for Cesarean delivery under spinal anesthesia:
continuing professional development. Can J Anaesth. 59:604–619.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turhanoglu S, Kaya S and Erdogan H: Is
there an advantage in using low-dose intrathecal bupivacaine for
cesarean section? J Anesth. 23:353–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gunda CP, Malinowski J, Tegginmath A,
Suryanarayana VG and Chandra SB: Vasopressor choice for hypotension
in elective Cesarean section: Ephedrine or phenylephrine? Arch Med
Sci. 6:257–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhar D, Bharati S, Halder PS, Mondal S,
Sarkar M and Jana S: Efficacy of prophylactic intramuscular
ephedrine in prevention of hypotension during caesarean section
under spinal anaesthesia: A comparative study. J Indian Med Assoc.
109:300–303, 307. 2011.PubMed/NCBI
|
|
11
|
Egger C, McCrackin M-A, Hofmeister E,
Touzot-Jourde G and Rohrbach B: Efficacy of preanesthetic
intramuscular administration of ephedrine for prevention of
anesthesia-induced hypotension in cats and dogs. Can Vet J.
50:179–184. 2009.PubMed/NCBI
|
|
12
|
Veeser M, Hofmann T, Roth R, Klöhr S,
Rossaint R and Heesen M: Vasopressors for the management of
hypotension after spinal anesthesia for elective caesarean section.
Systematic review and cumulative meta-analysis. Acta Anaesthesiol
Scand. 56:810–816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin FQ, Qiu MT, Ding XX, Fu SK and Li Q:
Ephedrine versus phenylephrine for the management of hypotension
during spinal anesthesia for cesarean section: An updated
meta-analysis. CNS Neurosci Ther. 18:591–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Q, Yao S, Wang G, et al: Clinical
anesthesiology. 4. 3rd. People's Medical Publishing Press; Beijing:
pp. 127–128. 2011
|
|
15
|
Rout CC and Rocke DA: Prevention of
hypotension following spinal anesthesia for cesarean section. Int
Anesthesiol Clin. 32:117–135. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Critchley LA, Stuart JC, Conway F and
Short TG: Hypotension during subarachnoid anaesthesia: Haemodynamic
effects of ephedrine. Br J Anaesth. 74:373–378. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee A, Kee WD Ngan and Gin T: A
dose-response meta-analysis of prophylactic intravenous ephedrine
for the prevention of hypotension during spinal anesthesia for
elective cesarean delivery. Anesth Analg. 98:483–490. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aragão FF, Aragão PW, Martins CA, Filho N
Salgado and Ede S Barroqueiro: Comparison of metaraminol,
phenylephrine and ephedrine in prophylaxis and treatment of
hypotension in cesarean section under spinal anesthesia. Rev Bras
Anestesiol. 64:299–306. 2014.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cooper DW, Carpenter M, Mowbray P, Desira
WR, Ryall DM and Kokri MS: Fetal and maternal effects of
phenylephrine and ephedrine during spinal anesthesia for cesarean
delivery. Anesthesiology. 97:1582–1590. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Al-Mustafa MM, Abu-Halaweh SA, Aloweidi
AS, Murshidi MM, Ammari BA, Awwad ZM, Al-Edwan GM and Ramsay MA:
Effect of dexmedetomidine added to spinal bupivacaine for
urological procedures. Saudi Med J. 30:365–370. 2009.PubMed/NCBI
|
|
21
|
Kawasaki Y, Kumamoto E, Furue H and
Yoshimura M: Alpha 2 adrenoceptor mediated presynaptic inhibition
of primary afferent glutamatergic transmission in rat substantia
gelatinosa neurons. Anesthesiology. 98:682–689. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Djalali AG, Wang JC-F, Perez-Valdivieso
JR, Danninger T, Fritsch G, Zurakowski D and Gerner P: Ephedrine
shows synergistic motor blockade when combined with bupivacaine or
lidocaine for spinal anesthesia in a rat model. Anesth Analg.
116:944–948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kroin JS, Buvanendran A, Beck DR, Topic
JE, Watts DE and Tuman KJ: Clonidine prolongation of lidocaine
analgesia after sciatic nerve block in rats Is mediated via the
hyperpolarization-activated cation current, not by
alpha-adrenoreceptors. Anesthesiology. 101:488–494. 2004.
View Article : Google Scholar : PubMed/NCBI
|