Introduction
The application of tissue engineering methods to
construct biologically active and functional teeth has been the
focus of oral medical research. Although the findings are currently
insufficient to support their application in tooth regeneration,
the intensive research on seed cells and scaffold materials has
provided a prospective method for tooth tissue engineering.
Duailibi et al (1) seeded tooth
bud cells from 4-day-old rats on polyglycolic acid
(PGA)-poly-L-lactate-co-glycolate (PLGA) scaffold material and
implanted the tooth bud cells in the omenta of adult rat hosts.
Dentin-, endodontium- and enamel-like structures were observed to
have formed 12 weeks after implantation. Young et al
(2) inoculated tooth bud cells from
pigs after proliferation onto tooth-shaped bio-scaffold material
PGA-PLGA and implanted these in the omenta of athymic rats, and
found that tissue-engineered tooth crowns containing dentin and
endodontium were formed.
A selection of scaffold materials, in addition to
seed cells, are important in the construction of tissue-engineered
teeth. Polylactic acid (PLA) is a typical biodegradable synthetic
polymer (3). As it has reliable
biosafety and is biodegradable and environmentally friendly, PLA
has been widely applied as a medical polymer material (4–6) and is a
commonly used biological scaffold material in tissue engineering
(7,8). In
the current study, extracts of PLA were combined with stem cells
from human exfoliated deciduous teeth (SHED) to evaluate the
biocompatibility, with the aim of providing an experimental basis
for subsequent tooth tissue engineering research.
Materials and methods
SHED culture
The present study was reviewed and approved by the
Institutional Review Board and the Ethics Committee at Zhengzhou
University (Zhengzhou, China) and was conducted following written
consent being obtained from the guardians of all participants.
Deciduous teeth that needed to be removed due to retention were
collected and surface sterilized. Their crowns were carefully split
in a sterile bench and their endodontium was removed, rinsed with
sterile 0.01 mol/l phosphate-buffered saline (PBS), sliced into
small pieces of ~1.0×1.0×1.0 mm and digested with 0.3% type I
collagenase (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C
for 40 min. The cell clumps were then prepared into a single cell
suspension, passed through a sieve (pore size, 70 µm) and
centrifuged at 120 × g for 5 min at room temperature. The cells
were washed again with sterile 0.01 mol/l PBS, resuspended into
Gibco α-minimum essential medium (α-MEM; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing Gibco 20% fetal bovine serum
(FBS; Thermo Fisher Scientific, Inc.) and cultured in 5-ml flasks
in an incubator supplemented with 5% CO2 at 37°C. The
culture medium was refreshed every 3 days. Upon reaching 80%
confluence, the cells were digested with 2.5 g/l trypsin
(Sigma-Aldrich; Merck KGaA) and passaged.
SHED phenotype identification
The P3 SHED at logarithmic phase were prepared as a
single cell suspension. Subsequent to three washes with PBS, cells
were resuspended in l ml PBS containing 3% FBS, counted and
aliquoted into 1.5-ml tubes with 100 µl cell suspension in each
tube to ensure that each tube had no less than 105
cells. These cells were then incubated with 5 µl pre-prepared,
light-protected, fluorescein isothiocyanate- and
phycoerythrin-labeled antibodies against human CD29 (1:500, cat.
no. ab52971), CD105 (1:500, cat. no. ab44967), CD146 (1:200, cat.
no. ab24577) and STRO-1 (1:100, cat. no. ab214086), all obtained
from Abcam (Cambridge, MA, USA). The cells were then incubated for
1 h at room temperature in the dark, washed with PBS three times,
resuspended and analyzed using flow cytometry (Beckman Coulter,
Inc., Fullerton, CA, USA) according to manufacturer's instructions.
Samples incubated with PBS served as the negative controls.
Preparation of PLA
The PLA (~2×1 mm size) was sterilized with ethylene
oxide, mixed with α-MEM containing 5% FBS in a sterile tube to 100
g/l, incubated at 37°C for 72 h and stored at 4°C for future
use.
Effect of PLA extract on SHED
morphology
The P2 SHED were seeded onto 6-well plates and
randomly divided into treatment and control groups with three
repeats per group. Cells in the treatment and control groups were
incubated with 100% PLA extract or with α-MEM containing 5% FBS,
respectively, at 37°C in a humidified incubator supplemented with
5% CO2 for 3 days. The cells were observed under an
inverted phase contrast microscope (Olympus Corporation, Tokyo,
Japan) for changes in cell adhesion and morphology.
Influence of PLA extract on the
proliferation activity of SHED
The P3 SHED at logarithmic phase were resuspended in
a medium of 100% PLA extract, a medium of 50% PLA extract plus 50%
α-MEM supplemented with 5% FBS, and a medium of 100% α-MEM
supplemented with 5% FBS, seeded into 96-well plates with 100 μl
per well with eight duplicates for each and cultured at 37°C in a
humidified incubator supplemented with 5% CO2. At 1, 3,
5 and 7 days after culture, 20 µl tetrazolium dye (MTT;
Sigma-Aldrich; Merck KGaA) was added to each well and the cells
were further cultured at 37°C for 4 h. Subsequently, 50 µl dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA) was added to each well. The
plate was oscillated at 600 rpm for 10 min and the optical density
value of each well was measured on a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a wavelength of 570
nm.
The P3 SHED at logarithmic growth phase were
prepared as a single cell suspension, seeded into each well of a
24-well plate with 4×103 cells per well and cultured to
normal growth stage (~24 h) at 37°C in a humidified incubator
supplemented with 5% CO2. After starving in serum-free
α-MEM for 12 h, the cells were cultured in α-MEM containing 10% FBS
overnight, stained according to the EdU staining kit (Guangzhou
RiboBio Co., Ltd., Guangzhou, China) and photographed with a
digital camera mounted on the microscope.
Effect of PLA extract on
mineralization ability of SHED
The P3 SHED at logarithmic growth phase were seeded
in 12-well plates with 2×104 cells per well and randomly
divided into treatment and control groups. Cells in the treatment
and control groups were incubated with 100% PLA extract or with
α-MEM containing 5% FBS, respectively, and cultured to >80%
confluency at 37°C in a humidified incubator supplemented with 5%
CO2. The medium was replaced with osteogenic medium,
which was α-MEM supplemented with 10% FBS, 100 nM dexamethasone
(Sigma-Aldrich; Merck KGaA), 50 µg/ml of ascorbic acid
(Sigma-Aldrich; Merck KGaA) and 5 mM β-glycerophosphate
(Sigma-Aldrich; Merck KGaA), half of the medium was replaced with
osteogenic medium 3 days later and fully refreshed with osteogenic
medium every 3 days subsequently. After 14 days of culture with the
osteogenic supplements, the cells were fixed in 4%
paraformaldehyde, washed twice in PBS and incubated in 0.1%
Alizarin Red S solution (Sigma-Aldrich; Merck KGaA) in Tris-HCl (pH
8.3) at 37°C for 30 min. After washing in PBS, the cells were
observed and imaged under an inverted microscope. The nodule area
per well was measured quantitatively using an image analysis
system, Image-Pro plus 5.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
RT-qPCR
The cultured SHED (14 days of osteogenic induction)
were washed three times with PBS and sufficiently dried. The cells
were cooled on ice and lysed with TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Total RNA was extracted using
TRIzol reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. These RNA were immediately reverse
transcribed into cDNA. RT-qPCR was performed in a final reaction
volume of 20 μl containing SYBR-Green PCR Master Mix (cat. no.
4309155; Thermo Fisher Scientific, Inc.), DNase-free water, primers
and cDNA. The target genes runt-related transcription factor 2
(Runx2) and osterix (Osx) and the internal control, β-actin under
the following conditions: Denaturation at 95°C for 3 min;
amplification (40 cycles) at 95°C for 15 sec and 60°C for 30 sec.
The PCR primer sequences are presented in Table I. Expression of each gene was monitored
using an Applied Biosystems® StepOne™ Real-Time PCR
system (Thermo Fisher Scientific, Inc.). Differences in gene
expression levels were calculated using the 2−ΔΔCq
method (9) for relative
quantification, and expressed as the fold change relative to the
untreated control.
 | Table I.Polymerase chain reaction primer
sequences. |
Table I.
Polymerase chain reaction primer
sequences.
| Gene | Direction | Primers |
|---|
| Runx2 | Forward |
CACTGGCGCTGCAACAAGA |
|
| Reverse |
CATTCCGGAGCTCAGCAGAATAA |
| Osx | Forward |
TGGCGTCCTCCCTGCTTG |
|
| Reverse |
TGCTTTGCCCAGAGTTGTTG |
| β-actin | Forward |
TGGCACCCAGCACAATGAA |
|
| Reverse |
CTAAGTCATAGTCCGCCTAGAAGCA |
Western blot analysis
Whole cell lysates for western blotting were also
extracted with lysis buffer and the total protein concentration was
determined with Bradford protein assay kit (P0006; Beyotime
Institute of Biotechnology, Shanghai, China) following the
manufacturer's instructions. The samples of 50 µg protein were
loaded onto SDS gels and transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked in 5% non-fat dried milk in PBST (PBS+0.1% Tween-20). The
membranes were probed overnight with the following primary
antibodies: anti-RUNX2 (1:100, cat. no. ab54868), anti-Osx (1:400,
cat. no. ab57335) and anti-β-actin (1:800, cat. no. ab3280). All
antibodies were obtained from Abcam. The membranes were incubated
at 37°C for 30 min with a horseradish peroxidase-conjugated
secondary antibody (1:2,000; cat. no. BA1056; Boster Biological
Technology, Ltd., Wuhan, China). The membranes were developed using
enhanced chemiluminescence (ECL) or ECL plus (GE Healthcare Life
Sciences, Chalfont, UK) according to the manufacturer's
instructions.
Statistical analysis
Each experiment was repeated three times and data
were expressed as means ± standard deviations. Data were analyzed
using SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL,
USA). Differences between multiple groups were analyzed using
Student's t-test following homogeneity test of variance. P<0.05
was considered to indicate a statistically significant
difference.
Results
SHED culture and identification
Slow attachment was observed during the primary SHED
culture using an enzymatic digestion method; at 4 h of culture,
only 60% of cells were attached. At 12 h, cells had begun to
stretch and at 48 h, cells were substantially expanded. The passage
cells grew well, and were passaged every 3–4 days. Cells at the
center of a well exhibited unclear boundaries and were round or
irregular, forming a nodular shape, while the majority of cells at
the peripheral portion were small, spindle-shaped and
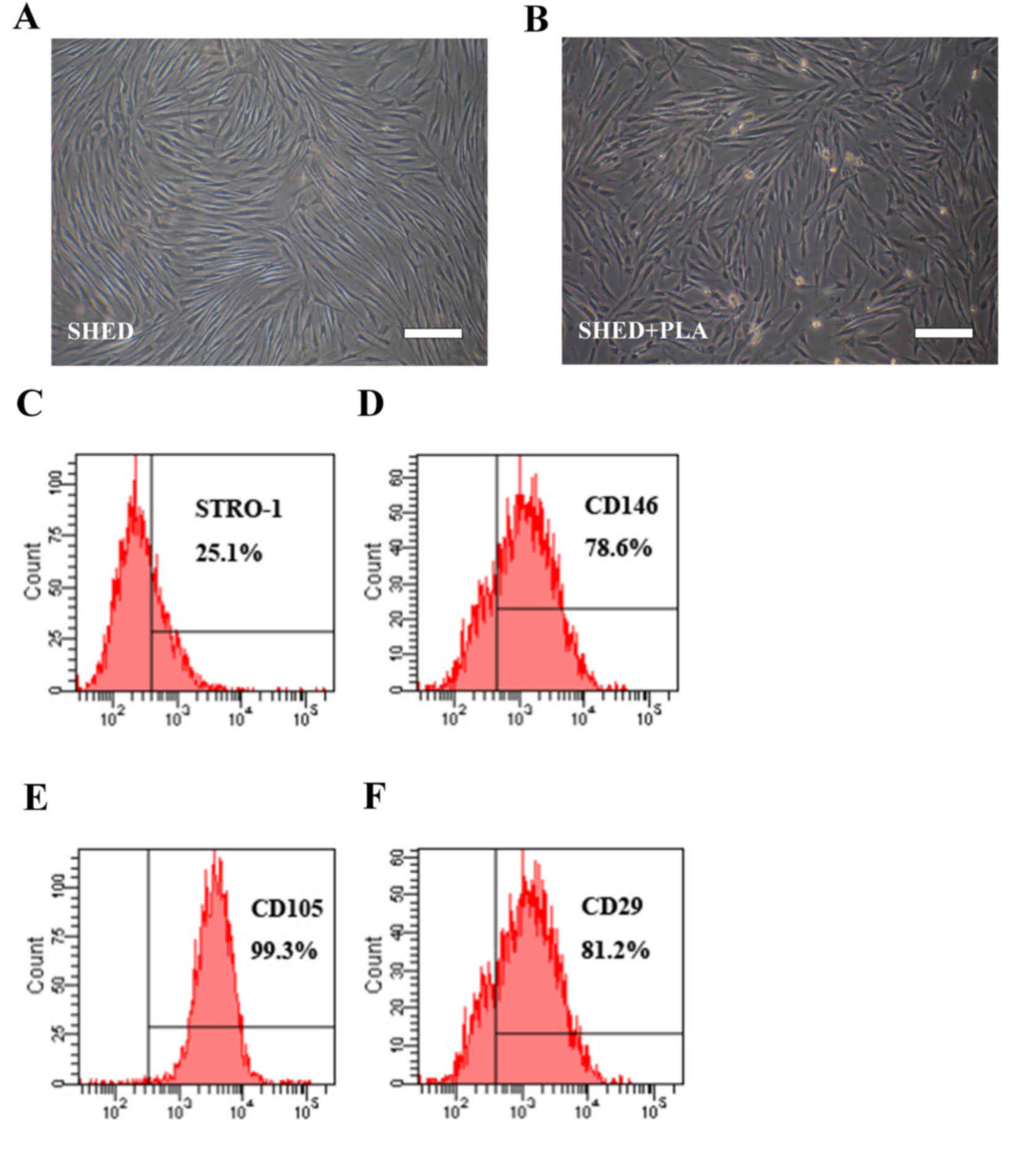
fibroblast-like, and had 1–2 protrusions (Fig. 1A and B). Flow cytometry indicated that
these cells were positive to mesenchymal stem cell surface markers,
STRO-1, CD146, CD29 and CD105 (Fig.
1C-F).
Effect of PLA extract on SHED
morphology
The morphology of cells cultured in PLA extract for
7 days was not significantly different when compared with that of
cells cultured in α-MEM supplemented with 5% FBS, only certain
cells were short and spindle-shaped, and had a relatively smaller
volume (Fig. 1B).
Influence of PLA extract on the
proliferation activity of SHED
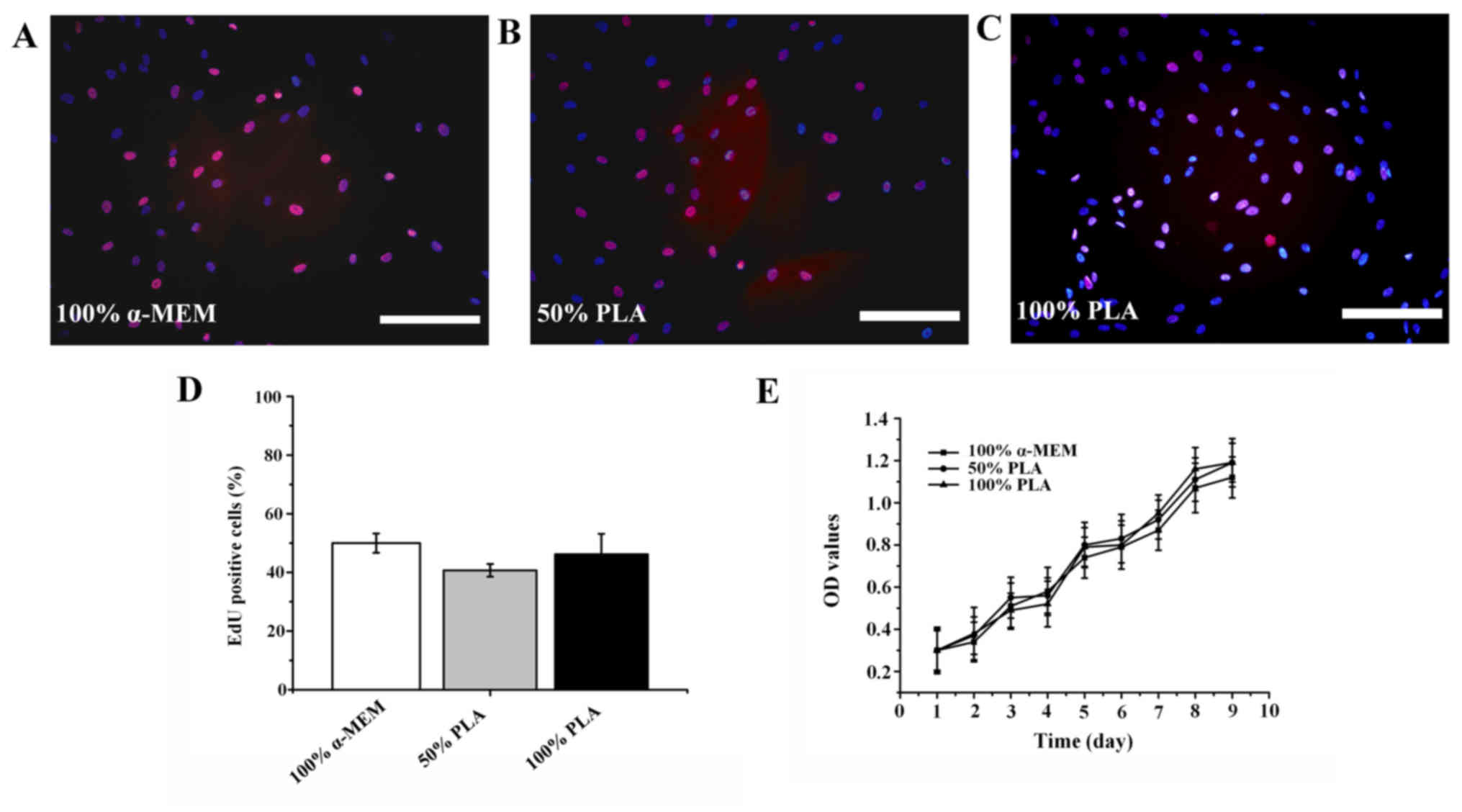
EdU staining demonstrated that the nuclei of
non-proliferating SHED were stained blue by Hoechst, while the
nuclei of proliferating SHED were stained red due to incorporation
of EdU. Thus, cells with red nuclei were positive, indicating that
they were proliferating cells. The experimental results
demonstrated that the number of proliferating cells was not
significantly different among the three groups (Fig. 2A-D; P>0.1), indicating that PLA
extract did not significantly affect the proliferation activity of
SHED. Furthermore, comparison of cell growth curves revealed that
the proliferation of SHED cultured in PLA extracts was not
significantly different from that of SHED cultured in α-MEM
supplemented with 5% FBS (Fig.
2E).
Effect of PLA extract on
mineralization ability of SHED
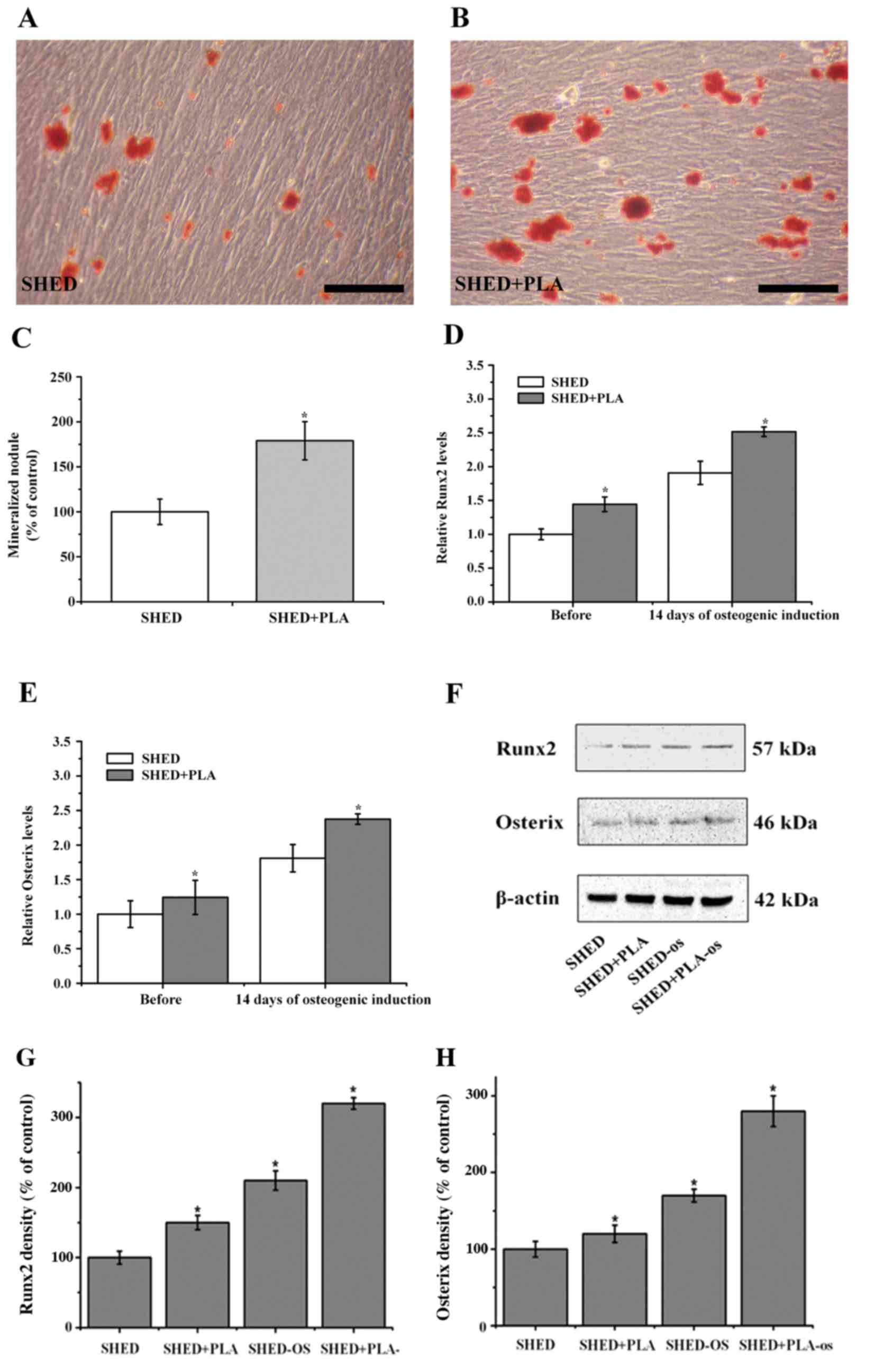
Alizarin Red S staining of cells after 14 days of
culture under osteogenic culture conditions resulted in red
mineralized nodules in irregular lumps or elongated shapes as
observed under an optical microscope. In SHED cultured in α-MEM
supplemented with 5% FBS, there were fewer mineralized nodules,
which were scattered and darker in color. By contrast, there were
significantly more mineralized nodules following culture in the
medium with PLA extract, the nodules were larger, more
concentrated, and lighter in color (Fig.
3A and B). Quantitative analysis of the Alizarin Red S staining
results indicated that the number of mineralized nodules of SHED
cultured in PLA extract was significantly higher than that of SHED
cultured in α-MEM supplemented with 5% FBS (Fig. 3C; P<0.05). The expression levels of
osteogenesis-associated genes at 14 days of osteogenic induction
were detected using RT-qPCR. The results demonstrated that
following osteogenic induction, the expression levels of RUNX2 and
Osx were increased in the treatment groups, although these
increases were significantly higher in SHED cultured in medium
containing PLA extract (Fig. 3D and E;
P<0.05). Similarly, western blot analysis also indicated that
the expression levels of these proteins were increased following
osteogenic induction, although these increases were also
significantly higher in cells cultured in medium containing PLA
extract (Fig. 3F; P<0.05). These
results indicated that the osteogenic differentiation ability was
significantly greater in SHED cultured in medium containing PLA
extract.
Discussion
The key issues in tissue engineering are seed cells,
scaffold biomaterials and cytokines. For tissue engineering
research, it is particularly important to select the appropriate
seed cells and scaffold biomaterials (10). PLA is an excellent biocompatible and
biodegradable polymer. In an aqueous environment, PLA is degraded
by simply hydrolyzing the ester bond to form its final degradation
product, lactic acid, which is converted into pyruvate in the
tricarboxylic acid cycle and eventually excreted in the form of
CO2 and H2O (11,12).
Effective stem cell proliferation and culture on biodegradable
material PLA scaffolds have been reported (13,14). Using
PLA as a tissue-engineering scaffold provides a porous structure
inside the stent for cell growth and nutrient transport, as well as
the mechanical strength and geometric shape to support and guide
cell growth (15,16).
Adult stem cells refer to specific stem cells
present in adult tissues and organs. Under normal circumstances,
these cells are in a resting state, and used to regenerate cells
and maintain cellular homeostasis. When tissues are damaged, these
stem cells proliferate and differentiate to the cell types in the
tissue, participating in tissue repair and reconstruction. In 2000,
Gronthos et al (17) isolated
cells with characteristics of stem cells from the endodontium.
Later, Shi and Gronthos subcutaneously implanted a mix of single
cell suspension and hydroxyapatite/tricalcium phosphate (HA/TCP)
powder into immunodeficient mice, forming the dentin-endodontium
complex-like structures (18), and
first proposed the concept of dental pulp stem cells. In 2003,
Miura et al (19) found
pluripotent SHED, which revealed a novel field for dental pulp stem
cell research. Furthermore, studies have shown that SHED are
superior to the permanent dental pulp stem cells in terms of
proliferation and mineralization (20,21). SHED
provide an improved source of seed cells for tooth tissue
engineering. In the current study, SHED were isolated using an
in vitro enzymatic digestion method and flow cytometry
identified that these cells are positive to STRO-1, CD146, CD29 and
CD105, and possess the phenotypic characteristics of mesenchymal
stem cells.
Morphological observation results indicated that
SHED cultured in vitro in PLA extraction properly attach,
grow and reproduce, and demonstrated that biomaterial PLA has good
biocompatibility, and is not toxic and inhibitory to cells, as well
as being conducive to cell growth. In the present study, PLA
extract at 100 g/l displayed improved characteristics for cell
proliferation. MTT and EdU analyses demonstrated that SHED were
capable of normal growth and proliferation in PLA extract, further
proving that PLA is not toxic to cells and has good
biocompatibility. SHED secrete calcium matrix and aggregate
further, forming mineralized nodules, which is the foundation for
forming osteogenic/dentogenic dentin (22). Thus, the ability of forming mineralized
nodules is an important indicator for evaluating the function of
dental pulp stem cells. Alizarin Red S staining revealed that the
quantity of mineralized nodules was significantly greater in cells
cultured in PLA extract than in cells cultured in α-MEM
supplemented with 5% FBS, indicating that PLA has improved
mineralization induction ability for SHED. Furthermore, RT-qPCR and
western blot analyses indicated that the expression levels of
osteogenic marker genes were increased in SHED cultured in PLA
medium. Adhered stem cells with strong mineralization ability
significantly promote the formation of tissue-engineered teeth. As
obtaining SHED is relatively convenient and noninvasive, their
application is more simple and feasible compared with the other
adult stem cells.
In conclusion, the application of the biomaterial,
PLA in tooth tissue engineering is feasible due to its good
histocompatibility and ability to improve the mineralization rate
of SHED. However, its construction form requires improvement in
order to increase cell attachment rate and cell proliferation.
Since the simulation of complex biological environments has not yet
been fully achieved, human tooth tissue engineering remains in its
infancy. The present study may provide an experimental basis upon
which human tooth regeneration using tissue engineering techniques
may be achieved. As PLA is biodegradable and discharged to the
environment in the form of CO2 and H2O, the
current study hypothesizes that the material may be applied in the
restoration of deciduous teeth following root canal treatment.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant nos. 31400839 and 81402231).
References
|
1
|
Duailibi MT, Duailibi SE, Young CS,
Bartlett JD, Vacanti JP and Yelick PC: Bioengineered teeth from
cultured rat tooth bud cells. J Dent Res. 83:523–528. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young CS, Terada S, Vacanti JP, Honda M,
Bartlett JD and Yelick PC: Tissue engineering of complex tooth
structures on biodegradable polymer scaffolds. J Dent Res.
81:695–700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu X, Holzwarth JM and Ma PX:
Functionalized synthetic biodegradable polymer scaffolds for tissue
engineering. Macromol Biosci. 12:911–919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao W and Wang J: Synthetic
micro/nanomotors in drug delivery. Nanoscale. 6:10486–10494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wiebe J, Nef HM and Hamm CW: Current
status of bioresorbable scaffolds in the treatment of coronary
artery disease. J Am Coll Cardiol. 64:2541–2551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vanaman M and Fabi SG: Décolletage:
Regional Approaches with Injectable Fillers. Plast Reconstr Surg.
136:276S–281S. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gentile P, Chiono V, Carmagnola I and
Hatton PV: An overview of poly(lactic-co-glycolic) acid
(PLGA)-based biomaterials for bone tissue engineering. Int J Mol
Sci. 15:3640–3659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Venkatesan J and Kim SK:
Nano-hydroxyapatite composite biomaterials for bone tissue
engineering--a review. J Biomed Nanotechnol. 10:3124–3140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Langer R and Vacanti JP: Tissue
engineering. Science. 260:920–926. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong W, Shin EJ, Culkin DA, Hedrick JL
and Waymouth RM: Zwitterionic polymerization: A kinetic strategy
for the controlled synthesis of cyclic polylactide. J Am Chem Soc.
131:4884–4891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castro-Aguirre E, Iñiguez-Franco F,
Samsudin H, Fang X and Auras R: Poly(lactic acid)-Mass production,
processing, industrial applications, and end of life. Adv Drug
Deliv Rev. 107:333–366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sudwilai T, Ng JJ, Boonkrai C, Israsena N,
Chuangchote S and Supaphol P: Polypyrrole-coated electrospun
poly(lactic acid) fibrous scaffold: Effects of coating on
electrical conductivity and neural cell growth. J Biomater Sci
Polym Ed. 25:1240–1252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vuornos K, Björninen M, Talvitie E,
Paakinaho K, Kellomäki M, Huhtala H, Miettinen S,
Seppänen-Kaijansinkko R and Haimi S: Human Adipose Stem Cells
Differentiated on Braided Polylactide Scaffolds Is a Potential
Approach for Tendon Tissue Engineering. Tissue Eng Part A.
22:513–523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim IG, Hwang MP, Du P, Ko J, Ha CW, Do SH
and Park K: Bioactive cell-derived matrices combined with polymer
mesh scaffold for osteogenesis and bone healing. Biomaterials.
50:75–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salerno A, Guarino V, Oliviero O, Ambrosio
L and Domingo C: Bio-safe processing of polylactic-co-caprolactone
and polylactic acid blends to fabricate fibrous porous scaffolds
for in vitro mesenchymal stem cells adhesion and proliferation.
Mater Sci Eng C. 63:512–521. 2016. View Article : Google Scholar
|
|
17
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi S and Gronthos S: Perivascular niche
of postnatal mesenchymal stem cells in human bone marrow and dental
pulp. J Bone Miner Res. 18:696–704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miura M, Gronthos S, Zhao M, Lu B, Fisher
LW, Robey PG and Shi S: SHED: Stem cells from human exfoliated
deciduous teeth. Proc Natl Acad Sci USA. 100:5807–5812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galler KM, Cavender A, Yuwono V, Dong H,
Shi S, Schmalz G, Hartgerink JD and D'Souza RN: Self-assembling
peptide amphiphile nanofibers as a scaffold for dental stem cells.
Tissue Eng Part A. 14:2051–2058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cordeiro MM, Dong Z, Kaneko T, Zhang Z,
Miyazawa M, Shi S, Smith AJ and Nör JE: Dental pulp tissue
engineering with stem cells from exfoliated deciduous teeth. J
Endod. 34:962–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K,
Sonoyama W, Patel V, Gutkind S, Young M, Gronthos S, et al:
Pharmacologic stem cell based intervention as a new approach to
osteoporosis treatment in rodents. PLoS One. 3:e26152008.
View Article : Google Scholar : PubMed/NCBI
|