Introduction
Studies estimate that >30% of all malignancies
worldwide are initiated or exacerbated by inflammation, and
preclinical data also supports the concept that inflammation is a
pivotal component of tumor initiation (1). Hepatocellular carcinoma (HCC), the third
leading cause of cancer mortality worldwide, commonly develops in
an inflamed liver following a prolonged chronic hepatitis state
(2). Hepatic resection and liver
transplantation are the only treatments with curative intent for
HCC, where resection as a bridge to transplantation is also
emerging as a possible therapeutic strategy (3–6). For
patients with early HCC and decompensated cirrhosis, liver
transplantation is the treatment of choice, as the procedure
potentially cures the cirrhosis and the HCC, and the outcome is
accepted to be better than that of hepatic resection (7). HCC is most prevalent in areas with
endemic viral hepatitis B or C, which is typical in many African
and Asian countries (8). In these
endemic countries transplantation procedures are limited due to a
shortage of living donors, legal and economic issues and lack of
resources. Therefore, hepatic resection is considered to be the
practical curative treatment option for many patients with HCC
globally.
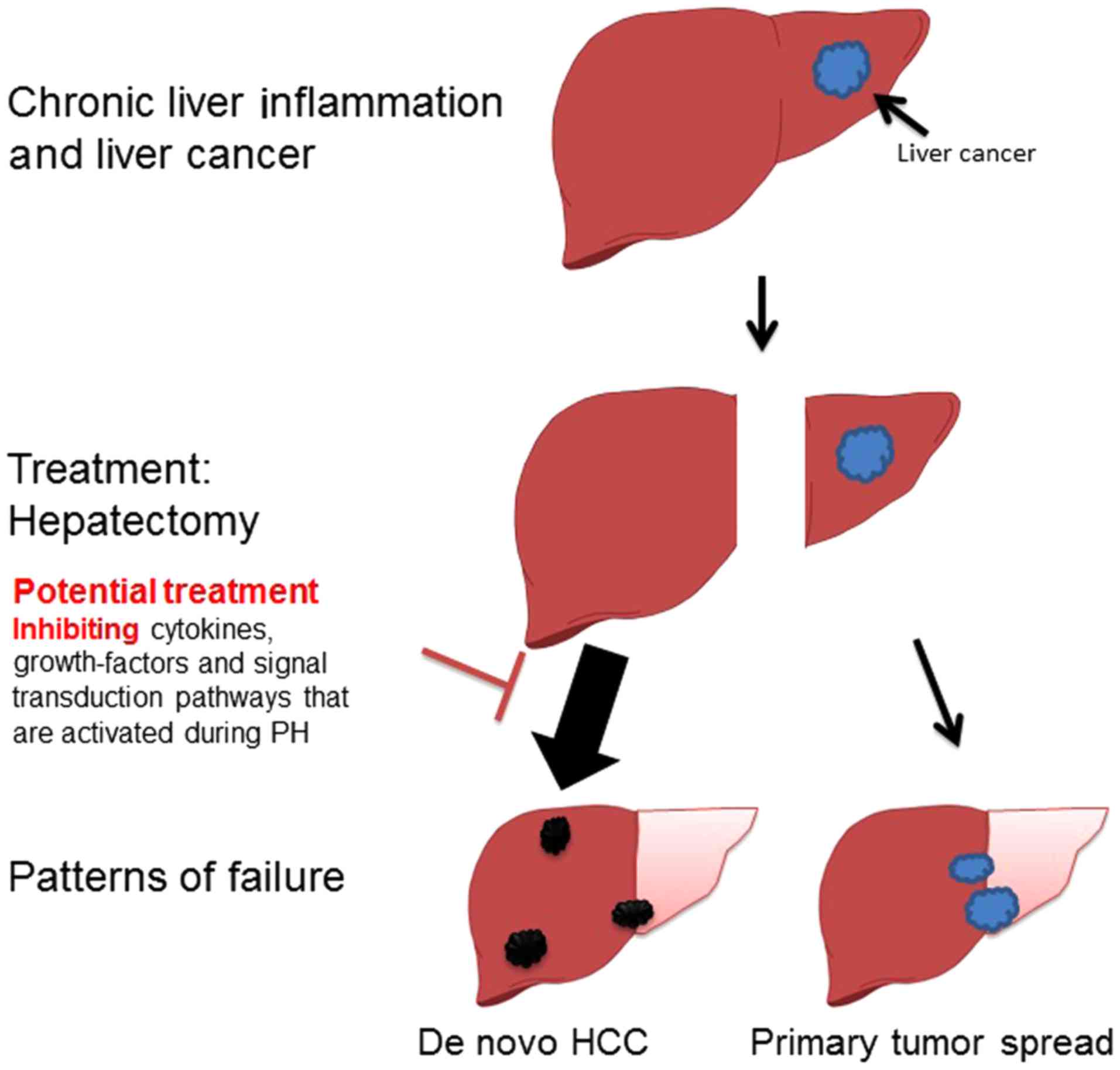
Different patterns of HCC recurrence
Despite improved resection techniques, and
subsequent decreased operative morbidity and mortality in hepatic
surgery, survival rates following partial hepatectomy (PH) are
suboptimal, predominantly due to tumor recurrence, which within
five years occurs in the range of 75 to 100% of cases (9,10). It was
estimated that 60–70% of recurrences were attributed to
intrahepatic lesions undetected at the time of resection, whereas
30–40% were de novo HCCs (Fig.
1) (11,12). The two different patterns of recurrence
are proposed to represent distinct carcinogenesis patterns that
have markedly different prognoses. Early recurrence usually
represents residual tumor spread from the primary main tumor and
remains in the remnant liver, which is an indicator of a poor
prognosis. Significant risk factors for early recurrence include
preoperative tumor rupture, venous invasion and non-anatomic
resection (13,14). Late recurrence usually results from
multicentric hepatocarcinogenesis. Possible risk factors for late
recurrence include cirrhosis, higher grade of hepatitis activity
and multiple tumors (11,14,15). In
certain studies from areas with endemic viral hepatitis, the
majority of the recurrences were multicentric in location and
distant from the resection margin (16–18). In one
study it was found that there was no significant difference in the
recurrence rate that occurred between major resection and minor or
localized resection of the liver (16). This indicated that in these patients
the prominent pattern of failure was de novo HCCs. This
hypothesis was further confirmed in a study where it was
demonstrated that gene expression profiling from liver tissue
adjacent to the tumor was correlated with survival in contrast to
the genome signature of the tumor itself (19). Therefore, patients with de novo
HCC recurrence, post-curative PH, are the patients that may benefit
from inhibition of the accelerated carcinogenesis following liver
resection.
Animal models of HCC accelerated
carcinogenesis
HCC in rodents and humans share common features, and
various mouse models of this disease have been investigated to
establish the underlying molecular mechanisms of liver cancer
(20). Few animal studies
investigating the effects of liver regeneration on tumor
progression were performed using transplanted tumor cells
(subcutaneously or directly into the liver), or using chemically
induced tumors (21–23). In these animal models, PH was shown to
affect and enhance the initiation and promotion phases of
carcinogenesis when compared with sham surgery. However, these
models have no underlying liver inflammation, as is the case in the
majority of humans with HCC. Therefore, until recently, there was
insufficient information regarding the mechanisms by which the
inflammatory microenvironment affects liver regeneration, and the
effect of inflammation and regeneration on
hepatocarcinogenesis.
The Mdr2-knockout (KO) mouse is a model with
similarities to human HCC. These mice lack the Mdr2 P-glycoprotein,
which is responsible for phosphatidylcholine transport across the
canalicular membrane. The absence of phospholipids from bile leads
to portal inflammation and slowly developing HCC, which closely
mimics the human disease in this regard (24,25). In the
Mdr2-KO mice model Barash et al (26) demonstrated that PH, prior to the
development of HCC, led to enhanced hepatocarcinogenesis. It was
proposed that under the regenerative proliferative stress induced
by liver resection in these mice, the genomic unstable hepatocytes,
generated during chronic inflammation, escape senescence and
apoptosis, and reenter the cell cycle, triggering enhanced
tumorigenesis (26).
Targeting accelerated carcinogenesis during
hepatectomy
If PH potentially accelerates carcinogenesis, it may
be worthy to attempt to block potential signaling pathways
specifically during this procedure. Numerous cytokines, growth
factors and signal transduction pathways are activated during liver
regeneration, and some of these may present as potential targets
for prevention of accelerated carcinogenesis, although regeneration
may be compromised (Fig. 1) (27).
Sorafenib, a mulitkinase inhibitor, is the only
systemic therapeutic modality that significantly prolonged the
survival of HCC patients with advanced-stage disease and is
considered the standard of care for patients with metastatic or
locally advanced HCC (28,29). In a study that used an orthotropic HCC
model, sorafenib treatment over a short duration subsequent to PH
suppressed accelerated tumor growth (30). It was further demonstrated that
postoperative activation of the Raf-MEK-extracellular
signal-regulated kinase (ERK) signal transduction pathway
sensitizes HCC to sorafenib. Another preclinical study demonstrated
that sorafenib treatment around PH did not impact liver
regeneration when administered prior to surgery; however,
administration following PH reduced liver regeneration (31). Taken together, these studies indicate
that short duration sorafenib treatment surrounding PH for
early-stage HCC is a promising approach for preventing recurrence.
However, these studies do not capture the same scenario as in
humans, where it is speculated that, under the regenerative
proliferative stress induced by PH, the non-tumoral hepatocytes
escape senescence and apoptosis, triggering enhanced
tumorigenesis.
While sorafenib inhibits the
Raf-ERK/platelet-derived growth factor receptor (GFR)/vascular
endothelial GFR signaling pathways, other pathways that are
activated during liver regeneration and are crucial for HCC
development may be targetable. Signal transducer and activator of
transcription 3 (STAT3) and its activating cytokine, interleukin
(IL)-6 are key regulators of liver regeneration and act to prime
hepatocytes to transition from the G0 phase and progress to the
G1/S phase (32,33). Thus, the IL-6/STAT3 signaling pathway
may be of central importance to the development and progression of
HCC following PH. As many therapeutic agents that target the
IL-6/STAT3 pathway were recently developed (34) such an approach appears reasonable,
although it may be the case that inhibition of the IL-6/STAT3 axis
is detrimental to liver regeneration. In a recent study by Zahavi
et al (35) it was shown, using
the Mdr2-KO mice model, that sorafenib treatment during PH
inhibited various signal transduction pathways at the multikinase
levels, which did not result in increased morbidity or mortality.
In the early stages subsequent to PH, sorafenib treatment resulted
in decreased stellate cell activation and inflammatory response.
Finally, it was demonstrated that sorafenib treatment during PH at
3 months of age resulted in decreased fibrosis, and tumor formation
at age 8.5 months (35). The study
confirmed the hypothesis that short-term treatment during PH is
feasible and effective in inhibiting inflammation-associated
cancer, and is therefore a potential strategy for recurrence
prevention (35).
Future challenges
There are two major challenges that will be
encountered when evaluating the strategy of treatment using signal
transduction blockade during hepatic resection in patients with HCC
under inflamed conditions. The first issue includes the high
perioperative morbidity and the low survival rate with mortality up
to 20% surrounding surgery (36).
Using molecular inhibitors, which may inhibit recovery and
decelerate the regeneration of the liver, could be devastating for
certain patients and result in mortality; therefore, are not
accepted as legitimate in clinical trial design. The second issue
is the fact that major, sponsored, randomized clinical trials will
not be performed in this context, as the treatment duration is very
short (surrounding the surgery) and the potential benefit to the
pharmaceutical industry is limited. In order to overcome the lack
of incentive of the pharmaceutical companies, such studies require
sponsorship from academic institutions.
Conclusion
Oncologists have long recognized that in certain
cancers, surgical treatment demonstrates carcinogenic potential. In
the current review, the latest evidence of the potential
carcinogenesis of hepatectomy for the treatment of HCC is
described. In addition, potential treatment strategies for
decreasing this accelerated carcinogenesis were described.
Different signal transduction pathway inhibitors are already
available and are currently used in many clinical trials for
different indications, such as metastatic cancers. Despite the risk
to fragile patients with HCC and liver inflammation, and despite
the low potential for financial gain, further clinical research
using different relevant signaling pathway inhibitors during
hepatectomy is encouraged.
Acknowledgements
The present study was supported by the Morasha
program of the Israel Science foundation (grant no. 1728/11) and
the Israel cancer association (grant no. 2014-0001).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
KO
|
knockout
|
|
PH
|
partial hepatectomy
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
References
|
1
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berasain C, Castillo J, Perugorria MJ,
Latasa MU, Prieto J and Avila MA: Inflammation and liver cancer:
New molecular links. Ann N Y Acad Sci. 1155:206–221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Fuster J and Bruix J; Barcelona
Clinic Liver Cancer (BCLC) Group, : Intention-to-treat analysis of
surgical treatment for early hepatocellular carcinoma: Resection
versus transplantation. Hepatology. 30:1434–1440. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adam R, Azoulay D, Castaing D, Eshkenazy
R, Pascal G, Hashizume K, Samuel D and Bismuth H: Liver resection
as a bridge to transplantation for hepatocellular carcinoma on
cirrhosis: A reasonable strategy? Ann Surg. 238:508–518; discussion
518-519. 2003.PubMed/NCBI
|
|
5
|
Marrero JA: Multidisciplinary management
of hepatocellular carcinoma: Where are we today? Semin Liver Dis.
33 Suppl 1:S3–S10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livraghi T, Mäkisalo H and Line PD:
Treatment options in hepatocellular carcinoma today. Scand J Surg.
100:22–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mazzaferro V, Bhoori S, Sposito C, Bongini
M, Langer M, Miceli R and Mariani L: Milan criteria in liver
transplantation for hepatocellular carcinoma: An evidence-based
analysis of 15 years of experience. Liver Transpl. 17 Suppl
2:S44–S57. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwartz M, Roayaie S and Konstadoulakis
M: Strategies for the management of hepatocellular carcinoma. Nat
Clin Pract Oncol. 4:424–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Llovet JM: Updated treatment approach to
hepatocellular carcinoma. J Gastroenterol. 40:225–235. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poon RT-P, Fan ST, Lo CM, Liu CL and Wong
J: Long-term survival and pattern of recurrence after resection of
small hepatocellular carcinoma in patients with preserved liver
function: Implications for a strategy of salvage transplantation.
Ann Surg. 235:373–382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto J, Kosuge T, Takayama T, Shimada
K, Yamasaki S, Ozaki H, Yamaguchi N and Makuuchi M: Recurrence of
hepatocellular carcinoma after surgery. Br J Surg. 83:1219–1222.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Imamura H, Matsuyama Y, Tanaka E, Ohkubo
T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T,
Kawasaki S, et al: Risk factors contributing to early and late
phase intrahepatic recurrence of hepatocellular carcinoma after
hepatectomy. J Hepatol. 38:200–207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poon RT, Fan ST, Ng IO, Lo CM, Liu CL and
Wong J: Different risk factors and prognosis for early and late
intrahepatic recurrence after resection of hepatocellular
carcinoma. Cancer. 89:500–507. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ercolani G, Grazi GL, Ravaioli M, Del
Gaudio M, Gardini A, Cescon M, Varotti G, Cetta F and Cavallari A:
Liver resection for hepatocellular carcinoma on cirrhosis:
Univariate and multivariate analysis of risk factors for
intrahepatic recurrence. Ann Surg. 237:536–543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wahab M Abdel, Sultan A, el-Ghawalby N,
Fathy O, Abu Zeid M, Abu el-Enin A, Abdallah T, Foad A, Kandeel T,
el-Shobari M, et al: Hepatic resection in cirrhotic liver for
treatment of hepatocellular carcinoma in Egyptian patients.
Experience with 140 cases in a single center.
Hepatogastroenterology. 51:559–563. 2004.PubMed/NCBI
|
|
17
|
Kumada T, Nakano S, Takeda I, Sugiyama K,
Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M, et
al: Patterns of recurrence after initial treatment in patients with
small hepatocellular carcinoma. Hepatology. 25:87–92. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adachi E, Maeda T, Matsumata T, Shirabe K,
Kinukawa N, Sugimachi K and Tsuneyoshi M: Risk factors for
intrahepatic recurrence in human small hepatocellular carcinoma.
Gastroenterology. 108:768–775. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoshida Y, Villanueva A, Kobayashi M, Peix
J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, et
al: Gene expression in fixed tissues and outcome in hepatocellular
carcinoma. N Engl J Med. 359:1995–2004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: From genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Picardo A, Karpoff HM, Ng B, Lee J,
Brennan MF and Fong Y: Partial hepatectomy accelerates local tumor
growth: Potential roles of local cytokine activation. Surgery.
124:57–64. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Jong KP, Lont HE, Bijma AM, Brouwers
MA, de Vries EG, van Veen ML, Marquet RL, Slooff MJ and Terpstra
OT: The effect of partial hepatectomy on tumor growth in rats: In
vivo and in vitro studies. Hepatology. 22:1263–1272. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asaga T, Suzuki K, Umeda M, Sugimasa Y,
Takemiya S and Okamoto T: The enhancement of tumor growth after
partial hepatectomy and the effect of sera obtained from
hepatectomized rats on tumor cell growth. Jpn J Surg. 21:669–675.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lammert F, Wang DQ-H, Hillebrandt S, Geier
A, Fickert P, Trauner M, Matern S, Paigen B and Carey MC:
Spontaneous cholecysto- and hepatolithiasis in Mdr2-/− mice: A
model for low phospholipid-associated cholelithiasis. Hepatology.
39:117–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mauad TH, van Nieuwkerk CM, Dingemans KP,
Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA,
Verkruisen RP, Groen AK, Ou de Elferink RP, et al: Mice with
homozygous disruption of the mdr2 P-glycoprotein gene. A novel
animal model for studies of nonsuppurative inflammatory cholangitis
and hepatocarcinogenesis. Am J Pathol. 145:1237–1245.
1994.PubMed/NCBI
|
|
26
|
Barash HR, Gross E, Edrei Y, Ella E,
Israel A, Cohen I, Corchia N, Ben-Moshe T, Pappo O, Pikarsky E, et
al: Accelerated carcinogenesis following liver regeneration is
associated with chronic inflammation-induced double-strand DNA
breaks. Proc Natl Acad Sci USA. 107:2207–2212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taub R: Liver regeneration: From myth to
mechanism. Nat Rev Mol Cell Biol. 5:836–847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng A-L, Kang Y-K, Chen Z, Tsao CJ, Qin
S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety
of sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng Y-X, Wang T, Deng Y-Z, Yang P, Li JJ,
Guan DX, Yao F, Zhu YQ, Qin Y, Wang H, et al: Sorafenib suppresses
postsurgical recurrence and metastasis of hepatocellular carcinoma
in an orthotopic mouse model. Hepatology. 53:483–492. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hora C, Romanque P and Dufour JFF: Effect
of sorafenib on murine liver regeneration. Hepatology. 53:577–586.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cressman DE, Greenbaum LE, DeAngelis RA,
Ciliberto G, Furth EE, Poli V and Taub R: Liver failure and
defective hepatocyte regeneration in interleukin-6-deficient mice.
Science. 274:1379–1383. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cressman DE, Diamond RH and Taub R: Rapid
activation of the Stat3 transcription complex in liver
regeneration. Hepatology. 21:1443–1449. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sansone P and Bromberg J: Targeting the
interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol.
30:1005–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zahavi T, Lanton T, Divon MS, Salmon A,
Peretz T, Galun E, Axelrod JH and Sonnenblick A: Sorafenib
treatment during partial hepatectomy reduces tumorgenesis in an
inflammation-associated liver cancer model. Oncotarget.
7:4860–4870. 2016.PubMed/NCBI
|
|
36
|
Wei AC, Tung-Ping Poon R, Fan ST and Wong
J: Risk factors for perioperative morbidity and mortality after
extended hepatectomy for hepatocellular carcinoma. Br J Surg.
90:33–41. 2003. View
Article : Google Scholar : PubMed/NCBI
|