Introduction
The importance of thyroid function during pregnancy
had been recognized long ago (1,2). Thyroid
function tests, most commonly including free triiodothyronine
(FT3), free thyroxine (FT4) and thyroid stimulating hormone (TSH),
are widely used to diagnose thyroid disorders (3). Previously, increasing numbers of studies
have demonstrated that maternal thyroid hormones are essential for
fetal development during pregnancy, and their deficiency can
influence the pregnancy outcome and developing fetus (4,5). Monen et
al (6) reported that increased TSH
and decreased FT4 are associated with more operative vaginal
deliveries and caesarean sections. León et al (7) reported that high maternal FT4 levels
during the first half of pregnancy were related to lower birth
weight and increased risk of small gestational age newborns.
Avoiding maternal thyroid disorders is of major importance because
of potential damage to fetal brain development, an increased
incidence of miscarriage, preterm delivery or fetal mortality
(8,9).
Therefore, it is very important to evaluate thyroid function
accurately during pregnancy.
The regulation of maternal thyroid function is
complex and varies with each stage of pregnancy. During pregnancy,
several physiological changes in pregnant women induce complex
endocrine and immune responses that, conversely, may affect the
course of pregnancy (10). The body
automatically adjusts the thyroid hormone concentrations changes
through concentrated iodine and achieves the new equilibrium state.
This equilibrium is difficult to achieve when iodine
supplementation is deficient (11).
However, excessive levels of iodine intake may potentially cause
more disease (12). Normally, maternal
thyroid function tends to adjust itself and these adjustments may
range from physiological adaptation to pathological derangement.
Previously, researchers recommended the use of pregnancy-specific
reference intervals to evaluate maternal thyroid function, which
alleviate the misinterpretation of thyroid function in pregnancy.
If a non-pregnant reference range is used, many maternal thyroid
diseases could be potentially misclassified (13). As a result, maternal thyroid hormones
assessment should be performed using pregnancy-specific reference
ranges ideally based on locally pregnant women data.
There is a significant difference in the biochemical
marker distribution in each stage of pregnancy. Therefore, the aim
of the present study was to investigate thyroid hormones levels
(FT3, FT4 and TSH) during pregnancy and compare with those in women
of reproductive age. Initially, the authors analyzed thyroid
hormone parameters including mean, median, interquartile range
(IQR), 2.5 th percentile (P2.5) and 97.5 th percentile (P97.5)
during pregnancy. Then, the authors established a series of
reference intervals of thyroid hormones during pregnancy according
to their gestational ages. In addition, parameters of thyroid
function between pregnancy women and control women of reproductive
age were comparatively analyzed.
Materials and methods
Pregnant women
The present study was conducted in the Fourth
People's Hospital of Wuxi, (Wuxi, China) between February 2010 and
June 2014. The study consisted of 4,903 pregnant women who were
regularly checked into the hospital during pregnancy, and 1,298
non-pregnant women who underwent routine health examination. Serum
samples of pregnant women were divided into four groups according
to gestational age: i) A total of 849 in the 8–14 week group, ii)
1,655 in the 15–20 week group, iii) 1,247 in the 21–36 week group
and iv) 1,152 in the ≥37 week group. Non-pregnant women were
divided into three groups according to their age: 20–40, 41–55 and
56–80 years, and women of reproductive age (20–40 years) as a
contrast. To compare the reference range between pregnant women and
women of reproductive age, age and body mass index (BMI) was
matched between the two groups. The estimated gestational ages were
determined by ultrasonic B-mode measurement of the fetal crown-rump
length. All cases were followed by a survey of hospital medical
record and telephone interview. Cases with thyroid or other
systemic disorders, such as positive thyroid antibodies or thyroid
nodules were excluded. The study protocol was approved by the
Ethics Committee of the Affiliated Hospital of Jiangnan University
(Wuxi, China) and all participants provided informed consent.
Sampling and analysis
Peripheral venous blood samples (3 ml) were
collected from each participant. The samples were centrifuged for 5
min at 1,000 × g. All serum obtained were immediately analyzed for
FT3, FT4 and TSH at the magnetic microparticle chemiluminescence
immunoassay (Unicel DXI800; Beckman Coulter, Inc., Brea, CA, USA).
The inter- and intra-assay precision were all <5.0% for FT3, FT4
and TSH.
Statistical analysis
Statistically analyzed was performed using the SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). Data were
described as mean ± standard deviation. One-way analysis of
variance was used to compare the levels of FT3, FT4 and TSH.
Results of parameters were also expressed as median, IQR, P2.5 and
P97.5. Post hoc analyses involved LSD and SNK tests. The limits of
the reference intervals were calculated as P2.5 to P97.5.
Comparisons among groups with different gestational ages were
performed with Kruskal-Wallis test (H test) and comparisons between
two different groups were compared with Wilcoxon test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Serum thyroid hormone levels in
non-pregnant women and normal pregnant women
A total of 4,903 pregnant women and 1,348
non-pregnant women fulfilled the criteria for selection and
volunteered to participate in the study and 513 non-pregnant women
(20–40 years) as controls and match their age and BMI with pregnant
women. The parameters were described as mean ± standard deviation
for pregnant women according to gestational weeks and according to
age for non-pregnant women. The mean age of 4,903 pregnant women
was 27.5 years old (range, 20–40 years). The mean weight of
pregnancy women was 58.1 kg (range, 41–85 kg) and 58.0 kg in
controls group (range, 40–79 kg).
Table I
presents the parameters of serum FT3, FT4 and TSH in non-pregnant
women
A significant difference in concentration of FT3
levels (3.20±0.38 vs. 3.11±0.41, P=0.014) was observed in the 56–85
year group compared with the 41–55 year group, but there was no
significant difference between the other groups. A significant
difference in FT4 levels (0.83±0.12 vs. 0.85±0.13 and 0.86±0.12,
P=0.008 and P=0.002, respectively) was observed in the 41–55 year
group compared with the 20–40 or 56–85 groups. TSH levels were
significantly higher in the 56–85 year group than that of other
groups (P=0.001 and P=0.021, respectively).
Table II
demonstrates the mean values of thyroid hormone levels in normal
pregnant women
From 8–14 to ≥37 weeks, the concentrations of
maternal serum FT3 and FT4 were continuous decline, and significant
differences were identified between each group (P<0.05 for all),
except for the FT4 levels in 21–36 weeks were equivalent to that of
the ≥37 week groups (P>0.05). While TSH was increased and
significant differences were identified between each group
(P<0.05 for all). Compared with the control groups (20–40 years,
non-pregnant women), the mean values of FT3, FT4 and TSH were lower
in pregnant women for all weeks (P<0.05 for all), except for the
TSH levels in the ≥37 week group (P>0.05; Fig. 1).
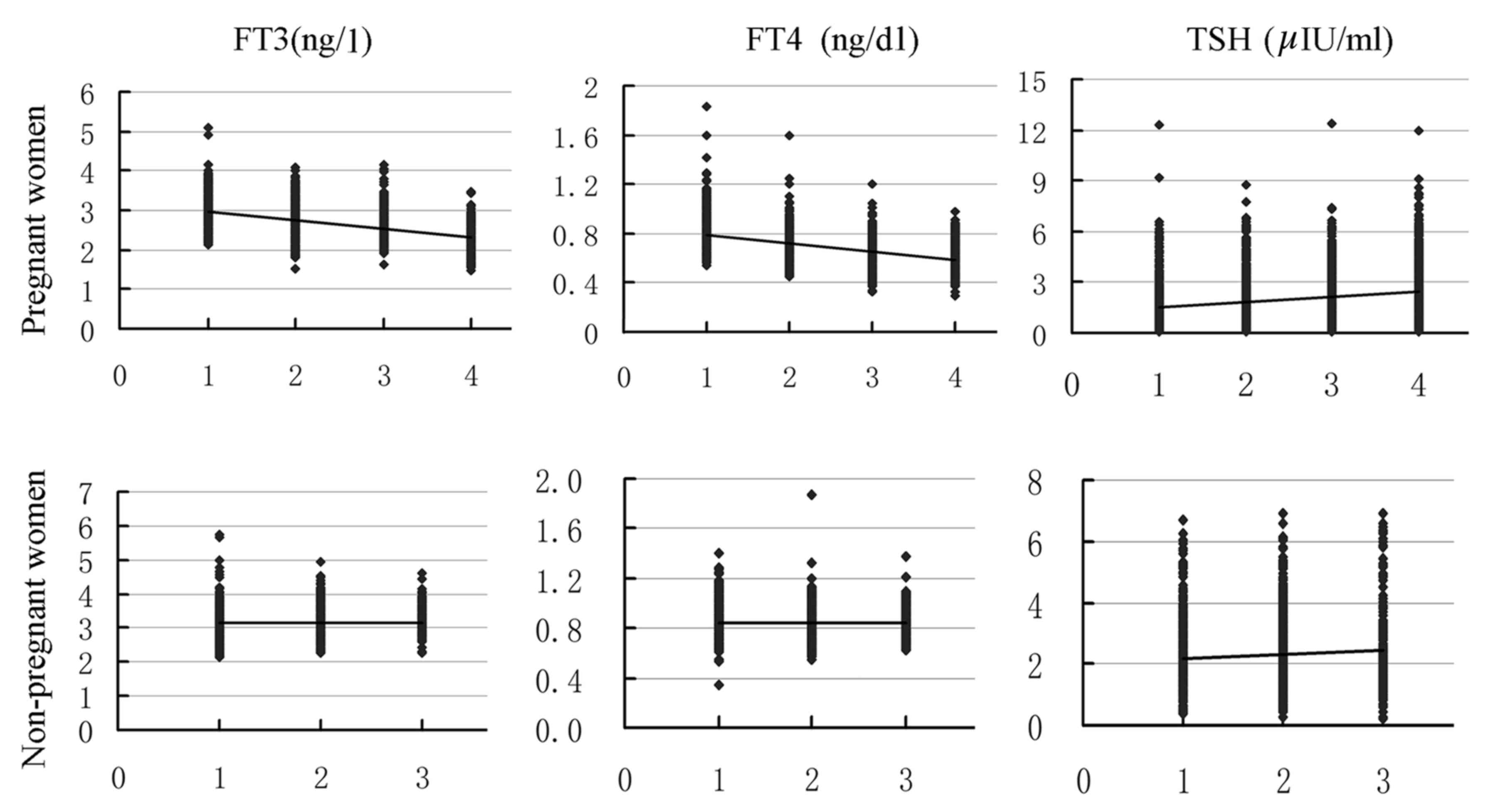 | Figure 1.Fluctuations of thyroid hormone levels
in normal pregnancies and non-pregnant women. From 8–14 to ≥37
weeks, the concentrations of maternal serum FT3 and FT4 continuous
declined, while TSH was increased in normal pregnant women. From
the ages of 20–40 to 56–85, the TSH concentration in non-pregnant
women increased, but no trend for FT3 and FT4 was observed. 1, 8–14
weeks; 2, 15–20 weeks; 3, 21–36 weeks; 4, ≥37 weeks in pregnant
women and 1, age 20–40; 2, age 41–55; 3, age 56–85 years in
non-pregnant women. |
Reference intervals of thyroid
function in non-pregnant women and normal pregnant women
Tables III and
IV present the median, P2.5-P97.5 and
IQR of serum FT3, FT4 and TSH. The median of TSH levels
demonstrated an overall increased trend from the 20–40 group to the
56–85 group in non-pregnant women and from the 8–14 to the ≥37
group in pregnant women. FT3 and FT4 levels demonstrated an overall
decreased trend from the 8–14 to ≥37 weeks group in pregnant women
except for the FT4 in ≥37 weeks. While in non-pregnant women, FT3
and FT4 levels were lower in the 41–55 age group than that of the
other groups (P<0.05 for all). The reference intervals of FT3,
FT4 and TSH in 20–40 ages were 2.44–4.04 ng/l, 0.63–1.13 ng/dl and
0.74–5.13 uIU/ml, respectively based on the 2.5–97.5 th
percentiles, which were significantly higher than all normal
pregnant women groups.
 | Table III.Reference intervals of thyroid
function in non-pregnant women. |
Table III.
Reference intervals of thyroid
function in non-pregnant women.
|
|
| FT3 (2.5–3.9
ng/l) | FT4 (0.58–1.64
ng/dl) | TSH (0.34–5.6
uIU/ml) |
|---|
|
|
|
|
|---|
| Groups (age
range) | n | M (P2.5-P97.5) | IQR | M (P2.5-P97.5) | IQR | M (P2.5-P97.5) | IQR |
|---|
| 20–40 | 513 | 3.10 (2.44–4.04) | 0.52 | 0.84 (0.63–1.13) | 0.17 | 1.98 (0.74–5.13) | 1.29 |
| 41–55 | 592 | 3.07 (2.46–4.07) | 0.5 | 0.82 (0.63–1.07) | 0.17 | 2.03 (0.65–5.28) | 1.55 |
| 56–85 | 193 | 3.15 (2.63–4.05) | 0.48 | 0.87 (0.65–1.17) | 0.19 | 2.08 (0.65–6.33) | 1.57 |
| Total | 1,298 | 3.09 (2.45–4.06) | 0.51 | 0.83 (0.63–1.09) | 0.16 | 2.01 (0.66–5.46) | 1.44 |
 | Table IV.Reference intervals of thyroid
function in normal pregnant women. |
Table IV.
Reference intervals of thyroid
function in normal pregnant women.
|
|
| FT3 (2.5–3.9
ng/l) | FT4 (0.58–1.64
ng/dl) | TSH (0.34–5.6
uIU/ml) |
|---|
|
|
|
|
|---|
| Gestational
weeks | n | M (P2.5-P97.5) | IQR | M (P2.5-P97.5) | IQR | M (P2.5-P97.5) | IQR |
|---|
| 8–14 |
849 | 2.90
(2.33–3.65) | 0.45 | 0.81
(0.59–1.11) | 0.16 | 1.28
(0.17–4.59) | 1.22 |
| 15–20 | 1,655 |
2.72(2.30–3.45) | 0.45 | 0.70
(0.58–0.91) | 0.11 | 1.68
(0.32–4.68) | 1.1 |
| 21–36 | 1,247 | 2.62
(2.07–3.24) | 0.38 | 0.60
(0.44–0.80) | 0.12 | 1.88
(0.56–4.95) | 1.24 |
| ≥37 | 1,152 | 2.26
(1.73–2.84) | 0.39 | 0.61
(0.44–0.82) | 0.14 | 2.3
(0.53–5.10) | 1.67 |
| Total | 4,903 | 2.63
(1.90–3.43) | 0.52 | 0.68
(0.46–0.97) | 0.16 | 1.78
(0.31–5.06) | 1.37 |
Discussion
A previous study described thyroid diseases as the
second most frequent endocrine disorder that affects women of
reproductive age (8). In the present
study, the authors investigated thyroid hormone changes in pregnant
women and compared with control women of reproductive age in
Jiangsu, China. Results of the study revealed that the levels of
FT3 and FT4 declined and TSH increased continuously from 8–14 to
≥37 weeks in normal pregnant women. Compared with controls, matched
in their age and BMI, the mean values of FT3, FT4 and TSH were
significantly lower in pregnant women in each group except for the
TSH level in the ≥37 weeks group.
Gestational thyroid dysfunction is a common and
associated with maternal and child disease. When thyroid disorders
remains untreated in a pregnant woman some disease can appear. Such
examples include risk of miscarriage, hypertension, growth
restriction and placental abruption (14–16). The
basic premise for clinical management of thyroid disease in
pregnancy is an accurate laboratory measurement of maternal serum
thyroid hormones. Due to complicated physiological changes during
pregnancy, thyroid hormones reference values are different from
those in non-pregnant state (17).
Therefore, calculating pregnancy-specific reference intervals for
thyroid hormone according to local region normal pregnant women
database is required.
Previously, several studies have already described
the role of thyroid function during pregnancy. Karmon et al
(18) reported that thyroid function
impacts the likelihood of pregnancy at the level of the oocyte.
However, Moncayo et al (19)
reported that thyroid function parameters in normal pregnancies do
not differ from those in non-pregnant women in Austria. Elahi et
al (20) reported that the mean
FT4 level was decreased, and FT3 and TSH increased significantly in
pregnant women compared to non-pregnant women in Pakistan.
Therefore, the changes of thyroid hormone during pregnancy were
substantially varied in different regions and remain
controversial.
Investigation into thyroid function in pregnancy
remains a matter of interest. The aim of the present study was to
describe thyroid hormone changes during pregnancy and compare with
control women of reproductive age. The current study indicated that
the parameters of serum FT3, FT4 and TSH in non-pregnant women were
close to that of the manufacturer's instructions, but significantly
higher in old women (56–85 years) than 41–55 year-old-women.
However, in normal pregnant women, the concentrations of maternal
serum FT3 and FT4 continuous decline from 8–14 to ≥37 weeks, and
significant difference were found between each group (P<0.05 for
all) except for FT4 in 21–36 weeks compared with ≥37 weeks
(P>0.05). While TSH were increased and significant difference
were found between each group (P<0.05 for all). Compared with
the control, the mean values of FT3, FT4 and TSH were lower in
pregnant women for all weeks (P<0.05 for all) except for TSH in
≥37 weeks (P>0.05). The present paper suggested a different
conclusion to Moncayo et al (19) and Elahi et al (20). Notably, there was an obvious variation
in different regions for thyroid hormone levels (21,22).
Therefore, it is necessary to establish the pregnancy-specific
reference values for a local population. If pregnancy-specific
reference values are used, thyroid hormone levels are more accurate
to reflect maternal thyroid function.
During pregnancy, profound changes in thyroid
physiology occur, resulting in different FT3, FT4 and TSH reference
intervals compared to the non-pregnant state. Researchers are
increasingly aware of the importance of evaluating maternal thyroid
function during pregnancy by pregnancy-specific reference
intervals, which reflected the changes of thyroid function in
pregnant women more realistically (23–25). The
previous study also gives the reference intervals of thyroid
hormones, which were calculated as P2.5 to P97.5. FT3, FT4 and TSH
reference intervals in control were 2.44–4.04 ng/l, 0.63–1.13 ng/dl
and 0.74–5.13 uIU/ml based on 2.5–97.5 percentiles; similar to that
of the manufacturer's instructions. In addition, this is
significantly higher than that of all groups in normal pregnant
women (Tables III and IV). If a non-pregnant reference interval is
used, a number of maternal thyroid diseases could be potentially
misclassified. Stricker et al (26) reported that 5.6–18.3% of
misclassification likely occurs in clinical practice due to the use
of the non-pregnant normal population reference values as a basis
for diagnosis.
Normal pregnant women are more likely to have lower
thyroid hormones, which maybe a normal physiology changes during
pregnancy. However, some studies have reported that thyroid
insufficiency may be associated with adverse obstetric outcome and
fetal neurodevelopment deficits. Therefore, it is important to know
whether pregnant women have thyroid hormone deficiency and whether
to supplement. Overall, the current reference intervals of thyroid
hormone for non-pregnant women were unavailable for pregnant women.
It may be advisable to establish pregnancy-specific reference
intervals for thyroid hormone according to a local Chinese pregnant
women database.
Acknowledgements
The present study was partially supported by grants
from the Medical Science Foundation of Wuxi, Jiangsu Province
(grant nos. YGZXZ1521 and YGZXH1402).
References
|
1
|
Allen EL and Miller EC: Thyroid function
in normal pregnancy, pathologic pregnancy, and in patients on oral
contraceptives. N C Med J. 28:417–429. 1967.PubMed/NCBI
|
|
2
|
Alemu A, Terefe B, Abebe M and Biadgo B:
Thyroid hormone dysfunction during pregnancy: A review. Int J
Reprod Biomed (Yazd). 14:677–686. 2016.PubMed/NCBI
|
|
3
|
Medici M, Korevaar TI, Visser WE, Visser
TJ and Peeters RP: Thyroid function in pregnancy: What is normal?
Clin Chem. 61:704–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vila L, Velasco I, González S, Morales F,
Sánchez E, Torrejón S, Soldevila B, Stagnaro-Green A and
Puig-Domingo M: Controversies in endocrinology: On the need for
universal thyroid screening in pregnant women. Eur J Endocrinol.
170:R17–R30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knight BA, Shields BM, Hattersley AT and
Vaidya B: Maternal hypothyroxinaemia in pregnancy is associated
with obesity and adverse maternal metabolic parameters. Eur J
Endocrinol. 174:51–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monen L, Pop VJ, Hasaart TH, Wijnen H, Oei
SG and Kuppens SM: Increased maternal TSH and decreased maternal
FT4 are associated with a higher operative delivery rate in
low-risk pregnancies: A prospective cohort study. BMC Pregnancy
Childbirth. 15:2672015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
León G, Murcia M, Rebagliato M,
Álvarez-Pedrerol M, Castilla AM, Basterrechea M, Iñiguez C,
Fernández-Somoano A, Blarduni E, Foradada CM, et al: Maternal
thyroid dysfunction during gestation, preterm delivery, and
birthweight. The Infancia y Medio Ambiente Cohort, Spain. Paediatr
Perinat Epidemiol. 29:113–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rovet JF: The role of thyroid hormones for
brain development and cognitive function. Endocr Dev. 26:26–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Teng W, Shan Z, Wang S, Li J, Zhu
L, Zhou J, Mao J, Yu X, Li J, et al: The prevalence of thyroid
disorders during early pregnancy in China: The benefits of
universal screening in the first trimester of pregnancy. Eur J
Endocrinol. 164:263–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Groot L, Abalovich M, Alexander EK,
Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D,
Mandel SJ, et al: Management of thyroid dysfunction during
pregnancy and postpartum: An Endocrine Society clinical practice
guideline. J Clin Endocrinol Metab. 97:2543–2565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niwattisaiwong S, Burman KD and Li-Ng M:
Iodine deficiency: Clinical implications. Cleve Clin J Med.
84:236–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bulmus N, Ustuner I, Seda Guvendag, Guven
E, Kir Sahin F, Senturk S and Baydur Sahin S: Thyroid diseases in
pregnancy: The importance of anamnesis. Pak J Med Sci.
29:1187–1192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lundqvist A, Johansson I, Wennberg A,
Hultdin J, Högberg U, Hamberg K and Sandström H: Reported dietary
intake in early pregnant compared to non-pregnant women - a
cross-sectional study. BMC Pregnancy Childbirth. 14:3732014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spencer L, Bubner T, Bain E and Middleton
P: Screening and subsequent management for thyroid dysfunction
pre-pregnancy and during pregnancy for improving maternal and
infant health. Cochrane Database Syst Rev. 9:CD0112632015.
|
|
15
|
Benhadi N, Wiersinga WM, Reitsma JB,
Vrijkotte TG and Bonsel GJ: Higher maternal TSH levels in pregnancy
are associated with increased risk for miscarriage, fetal or
neonatal death. Eur J Endocrinol. 160:985–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giacobbe AM, Grasso R, Triolo O, Tonni G
and Granese R: Thyroid diseases in pregnancy: A current and
controversial topic on diagnosis and treatment over the past 20
years. Arch Gynecol Obstet. 292:995–1002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carreto-Molina N, García-Solís P, Solís-S
JC, Robles-Osorio L, Hernández-Montiel HL and Vega-Malagón G:
Importance of iodine in pregnancy. Arch Latinoam Nutr. 62:213–219.
2012.PubMed/NCBI
|
|
18
|
Karmon AE, Batsis M, Chavarro JE and
Souter I: Preconceptional thyroid-stimulating hormone levels and
outcomes of intrauterine insemination among euthyroid infertile
women. Fertil Steril. 103:258–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moncayo R, Zanon B, Heim K, Ortner K and
Moncayo H: Thyroid function parameters in normal pregnancies in an
iodine sufficient population. BBA Clin. 3:90–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elahi S, Rizvi NB and Nagra SA: Iodine
deficiency in pregnant women of Lahore. J Pak Med Assoc.
59:741–743. 2009.PubMed/NCBI
|
|
21
|
Duan Y, Peng L, Cui Y and Jiang Y:
Reference intervals for thyroid function and the negative
correlation between FT4 and HbA1c in pregnant women of west China.
Clin Lab. 61:777–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leung AM: Thyroid function in pregnancy. J
Trace Elem Med Biol. 26:137–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang QW, Yu B, Huang RP, Cao F, Zhu ZQ,
Sun DC and Zhou H: Assessment of thyroid function during pregnancy:
The advantage of self-sequential longitudinal reference intervals.
Arch Med Sci. 7:679–684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cohen O, Pinhas-Hamiel O, Sivan E,
Dolitski M, Lipitz S and Achiron R: Serial in utero
ultrasonographic measurements of the fetal thyroid: A new
complementary tool in the management of maternal hyperthyroidism in
pregnancy. Prenat Diagn. 23:740–742. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krassas G, Karras SN and Pontikides N:
Thyroid diseases during pregnancy: A number of important issues.
Hormones (Athens). 14:59–69. 2015.PubMed/NCBI
|
|
26
|
Stricker R, Echenard M, Eberhart R,
Chevailler MC, Perez V, Quinn FA and Stricker R: Evaluation of
maternal thyroid function during pregnancy: the importance of using
gestational age-specific reference intervals. Eur J Endocrinol.
157:509–514. 2007. View Article : Google Scholar : PubMed/NCBI
|















