Introduction
The utilization of mushrooms for their medicinal
properties is an established practice in Asian nations, though is
less common in European regions (1).
China is proposed to have 1,500–2,000 types of consumable
mushrooms, of which 981 species have been verified (2). There has been previous interest in the
use of mushrooms, not only as a healthy food sustenance containing
fundamental components such as proteins and polysaccharides, but
additionally as a source of bioactive secondary metabolites
(phenolic compounds, terpenes, steroids) with beneficial
properties, namely anti-inflammatory, antioxidant,
immunomodulatory, anticarcinogenic, antiviral, antibacterial,
antifungal, hepatoprotective, antineurodegenerative, antidiabetic,
antiangiogenic, and hypoglycemic properties, among others (3). Mushrooms have been utilized as dietary
supplements reciprocal to prescriptions for anticancer treatment.
In addition, the reported antiviral, hypocholesterolemic and
hepatoprotective properties of mushrooms (4) is concurrent with the identification of
seven partially-purified polysaccharides with cancer preventative
and immunomodulatory activities in consumable and therapeutic
mushroom species (Agaricus biosporus, Agaricus brasiliensis,
Phellinus linteus, Ganoderma lucidum, Ganoderma applanatum,
Lentinus edodes and Trametes versicolor), all of which
contained glucose as the prevalent monosaccharide, among varying
amounts of other α-glucans (3).
Coriolus versicolor (CV) is among the established species of
edible mushrooms (5). The significance
of CV has been acknowledged regarding it high content of biological
compounds including polysaccharides (starch, chitin), phenols
(gallic, protocatechuic acid, catechin), steroids (ergosterol),
vitamins (B1, B2, C and D) and minerals (calcium, selenium)
(3). Additionally, CV may have various
pharmacological properties, including antitumor, immunomodulatory,
cell reinforcement, antinociceptive, disease mitigative,
antimicrobial, hepatoprotective and hypolipidemic effects (6).
Cancer therapy may involve several modalities,
including surgery, radiotherapy, chemotherapy, immunotherapy,
hormone therapy, bone marrow transplantation and alternative
treatments, which may be used singly or in combination. The aim of
cancer treatment is to contain and remove the primary tumor and to
prevent its recurrence and/or metastasis (7). Therefore, to prevent health problems and
to reduce the cost of medical treatment and healthcare, the
development and use of effective natural drugs may aid to protect
the body against certain side effects of chemotherapy and
radiotherapy, and suppress the progression of many diseases
(8). The development of novel drugs
and health foods has thus become an important strategy in the
biotechnology, food and medicinal industries (9). Improving and prolonging human life and
resisting aging are important aims of both the general public and
research (10,11). These aims may be achievable through the
development of novel drugs and health foods that promote human
longevity and immunocompetence (12).
Glucans are considered to be the most well
established and potent derivatives of mushrooms that have antitumor
and immunomodulatory properties (13).
Among the diverse glucans present in mushrooms are α-glucans, which
have also been identified in parasitic and bacterial cell
communities (14). Notably, glucans
have been indicated to increase the secretion of a range of key
cytokines, including interleukins (ILs) and interferons (IFNs)
(15,16). Our group previously demonstrated the
anticancer and immunomodulatory activities of a glucan from CV with
potential as a therapeutic in cancer treatment (3). The present study aimed to determine the
effect of a CV glucan (CVG), namely (1→6)-α-D-glucopyranosyl
(Glcp), on the stimulation of cytokine production in a sarcoma-180
tumor-bearing mouse model.
Materials and methods
Materials
The fruiting body of CV used in the present study
was from the Changbai Mountain region (Changchun, China) and was
collected by Professor Yi Xin at the Department of Biotechnology,
Dalian Medical University (Dalian, China). Specific pathogen free
Kunming mice (18–20 g, 6–7-weeks-old, female, C57BL) were obtained
from the Animal Center of Dalian Medical University. Other
materials used included RPMI 1640 medium with improved nutrient
solution (01-100-1A, 01-100-1B; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), cyclophosphamide (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), sarcoma-180 cells (S-180; Nanjing KeyGen
Biotech. Co. Ltd., Nanjing, China), and ELISA kits for IFN-α
(411002; Thermo Fisher Scientific, Inc.) and -γ (KE00063) and IL-2
(KE00017), −4 (KE00016), −6 (KE00007), −10 (KE00012) and −17A
(KE00015; ProteinTech Group, Inc., Rosemount, IL, USA). All
reagents and chemicals used were of analytical grade.
The complete purification, characterization and
biological activities of CVG have been reported in a previous study
by our group (3).
The study was completed in strict accordance with
the Guidelines for the Care and Use of Laboratory Animals of the
National Institutes of Health (Bethesda, MA, USA), and was approved
by the Committee for the Ethics of Animal Experiments of Dalian
Medical University. After the treatment period, surgical procedures
were performed following 250 mg/kg (intraperitoneal) sodium
pentobarbital euthanasia (Abbott Pharmaceutical Co., Ltd., Lake
Bluff, IL, USA) with efforts to minimize suffering.
Animals and treatment
A total of 60 mice were used in the present study,
which were housed in conventional polycarbonate cages containing
sawdust bedding under standard research facility conditions (room
temperature, atmospheric oxygen, 12 h light-dark cycle and free
access to standard mouse diet and water ad libitum). The mice were
randomly divided into six groups (n=10), and S-180 sarcoma cells
(0.2 ml, 2×106 cells) were injected subcutaneously into
the right axilla of the mice in five groups, while the remaining
served as a normal control. Thus, the groups were as follows:
Normal control (treated with 0.2 ml normal saline only); model
control (inoculated with sarcoma-180 cells and treated with 0.2 ml
normal saline); positive control (inoculated with sarcoma-180 cells
and treated with cyclophosphamide, 20 mg/kg body weight); and three
groups injected with 40, 100, 200 mg/kg body weight CVG,
respectively, following sarcoma-180 cells inoculation. The CVG was
dissolved in normal saline, and intraperitoneally injected in a
volume of 0.2 ml daily for 9 days beginning 24 h after tumor cell
transplantation. The formula of the CVG was [→6)-α-D-
Glcp-(1→]n, as determined previously (3).
Measurement of IL-2, −4, −6, −10, −17A
and IFN-α and -γ
The serum levels of IL-2, −4, −6, −10, −17A and
IFN-α and -γ were measured by ELISA as previously described
(17). Briefly, following the
treatment period, mice were euthanized and blood (1.5 ml) was
sampled from the eyeballs of the mice into a 2 ml eppendorf tube
and centrifuged for 15 min at 1,372 × g at 4°C. The upper fraction
of clear serum was collected and the levels of IL-2, −4, −6, −10,
−17A and IFN-α and -γ in the serum were measured by ELISA according
to kit instructions.
Statistical analysis
All experiments were conducted in triplicate, and
data were presented as the mean ± standard deviation. Statistical
analysis was performed with GraphPad Prism version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). One-way analysis of variance
followed by Fisher's least significant difference tests were used
for statistical comparisons between the treatment and control
groups, and P<0.05 was considered to indicate a statistically
significant difference.
Results
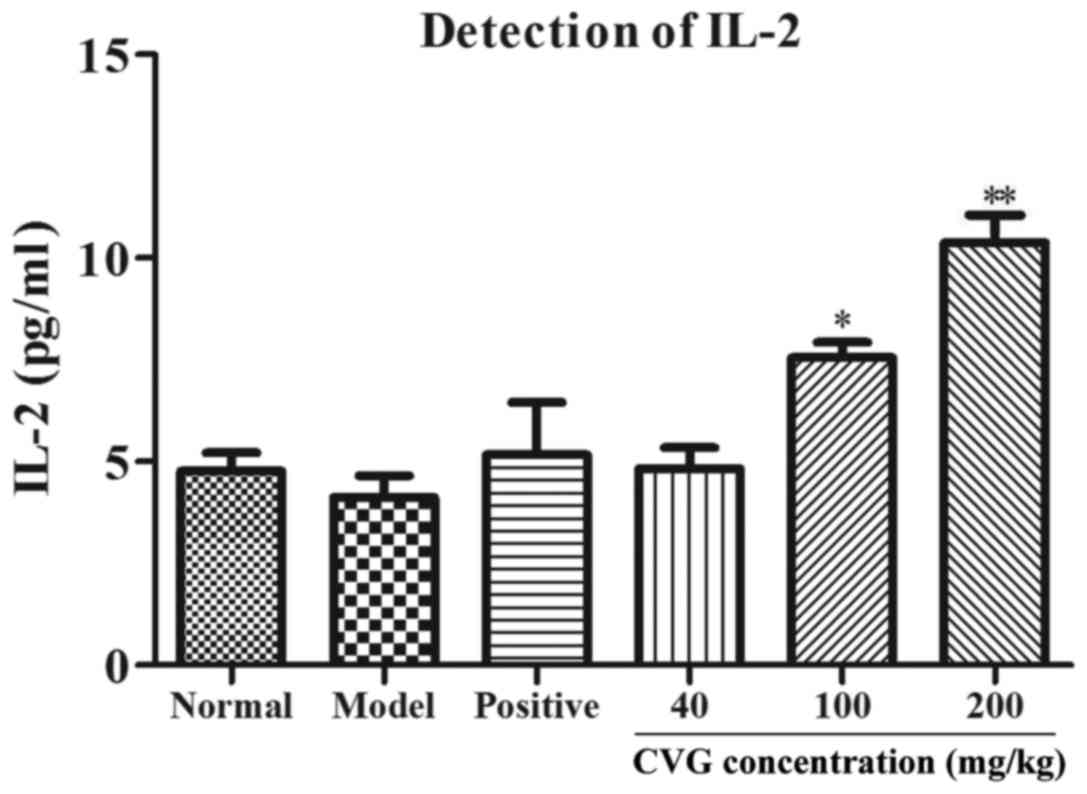
Effect of CVG on the stimulation of cytokine
production. The effect of CVG on the production of IL-2, −4, −6,
−10, −17A and IFN-α and -γ were determined in sarcoma-180-bearing
mice by ELISA (Figs. 1–7 and Tables
I–VII, respectively). The
administration of CVG resulted in significantly increased
production of IL-2, −4, −6, −10, −17A and IFN-α and -γ at doses of
100 (P<0.05) and 200 (P<0.01) mg/kg body weight when compared
with the cyclophosphamide positive control group. Meanwhile,
following stimulation with 40 mg/kg CVG, the levels of the
different cytokines did not differ significantly compared with the
positive control group. Following CVG stimulation, the
Th2-associated cytokines (IL-4, −6 and principally-10) (18,19) and Th17
cytokine (IL-17A) (19) were produced
at notably higher levels compared with the Th1-associated cytokines
(IL-2 and IFN-γ) (14). These results
indicate that CVG may promote the secretion of cytokines from T
cells, particularly of cytokines associated with Th2-mediated
humoral immunity and Th2 proliferation and differentiation such as
IL-4 and −6 (14). Thus, CVG may
enhance Th2-mediated humoral immunity, as an established
prerequisite for improved adaptive immunity (18).
 | Table I.Effect of CVG on IL-2 levels in the
serum of sarcoma-180-bearing mice. |
Table I.
Effect of CVG on IL-2 levels in the
serum of sarcoma-180-bearing mice.
| Groups | Dose (mg/kg) | IL-2 concentration
(pg/ml) |
|---|
| Normal | – | 4.77 |
| Model | – | 4.12 |
| Positive | 20 | 5.16 |
| CVG | 40 | 4.83 |
| CVG | 100 | 7.55a |
| CVG | 200 | 10.38b |
 | Table VII.Effect of CVG on IFN-γ levels in the
serum of sarcoma-180-bearing mice. |
Table VII.
Effect of CVG on IFN-γ levels in the
serum of sarcoma-180-bearing mice.
| Group | Dose (mg/kg) | IFN-γ concentration
(pg/ml) |
|---|
| Normal | – | 6.95 |
| Model | – | 5.46 |
| Positive | 20 | 7.59 |
| CVG | 40 | 7.32 |
| CVG | 100 | 7.92a |
| CVG | 200 | 11.41b |
Discussion
The present study aimed to identify the impact of
the glucan (1→6)-α-D-Glcp purified from CV on the stimulation of
cytokine production in a mouse model of sarcoma-180. CV is
considered as an important medicinal agent due to its provision of
essential nutrients and therapeutic applications, particularly
regarding its apparent pharmacological properties of antitumor,
immunomodulatory, cell reinforcement, antinociceptive, disease
mitigative, antimicrobial, hepatoprotective and hypolipidemic
activities, among others (5). A
control cyclophosphamide group was used in the present study, as
cyclophosphamide is frequently used as a chemotherapeutic for the
treatment of cancer, due to its targeting of rapidly dividing cells
(despite some interference with normal cell development) (20). Therefore, this enabled a comparison
between CVG and an official drug used for cancer treatment.
Cytokines are cell signaling molecules that are
expressed in peptide, protein and glycoprotein forms (21). ILs and IFNs are two specialist types of
cytokine required in the management of immune system responses to
disease and viral invasion (22). In
the present study, serum cytokine production was investigated using
ELISA. Specifically, the cytokines IL-2, −4, −6, −10, −17A and
IFN-α, and -γ; associated with Th1 (IFN-γ and IL-2), Th2 (IL-10, −4
and −6 and IFN-α) and Th17 (IL-17A) cells, were investigated to
elucidate the fundamental systems by which CVG may exert anticancer
effects in a tumor-bearing mouse model.
IL-2 is a T cell-differentiating cytokine and
improves the cytolytic activities of cytotoxic T lymphocytes and
natural killer (NK) cells (23).
Meanwhile, IL-4 serves a key role by stimulating the
differentiation of naive T cells (Th0 cells) to Th2 cells (24). IL-6 is secreted by T cells and
macrophages to enhance the immune reaction (25), to aid the differentiation and
proliferation of T and B cells, and to advance the development of
plasma cells from B cells (26).
IL-10, also known as a human cytokine synthesis inhibitory factor,
is an inhibitory cytokine that may hinder pathogen invasion and
improve immunopathology (27).
Conversely, IL-17A is involved in initiating and mediating
proinflammatory reactions generally associated with unfavorably
susceptible responses (28). IFN-α may
activate resistant cells including cytotoxic T cells and
macrophages (29). IFN-γ is a primary
member of the class II IFNs that may specifically prevent viral
replication and exert immunostimulatory and immunomodulatory
impacts (20).
CVG significantly stimultated 1, 2 and Th17 cytokine
production at the doses of 100 and 200 mg/kg, but not at 40 mg/kg,
compared with the control cyclophosphamide group. Additionally, 2-
and Th17-dependent cytokines were observed at higher levels
compared with Th1 cytokines; most notably, IL-10 concentration
markedly exceeded that of the other cytokines.
Th1 and 2 cytokines serve essential roles in immune
regulation, including in antitumor resistance (30). Despite results suggesting that Th1 is
dominant over Th2 immunity in the induction of antitumor responses
(30), the current data suggested that
CVG served as a Th2 immune-inducer through IL-10-dependent
pathways. Similarly, gene therapy utilizing Th2 cytokines,
including IL-4, −6 and −10, has been reported to be effective in
tumor immunotherapy (19,30,31). Thus,
despite increased levels of IL-10 in numerous cancers and its
association with poor prognosis (30),
CVG may exert its apparent anticancer effects through the
beneficial immunomodulatory functions of IL-10, among other
mechanistic pathways. The beneficial effect of IL-10, as a potent
anti-inflammatory cytokine produced by monocytes, mast cells,
macrophages and dendritic cells, has been reported in a number of
cancers, including melanoma and breast and ovarian cancers, and may
occur through its suppression of major histocompatibility complex-I
activity and induction of NK-mediated tumor lysis (14,18,19). Furthermore, IL-10 has been identified
to suppress the growth and metastasis of tumors by inhibiting the
production of angiogenic factors (19,31).
Inflammation is a hallmark of cancer and the
suppression of inflammatory pathways has been targeted in cancer
therapy (32,33). IL-10 inhibits nuclear factor-κB
signaling, a key inflammatory pathway, and thus downregulates
proinflammatory cytokine expression in its role as an antitumor
cytokine (14). Furthermore, in
previous studies, IL-10 therapy inhibited colon inflammation and
carcinoma, while its deficiency in an experimental murine animal
model resulted in bacteria-induced carcinogenesis (30,31).
The antitumor effect of CVG may partly occur through
the cellular immune response, particularly through spleen
lymphocyte proliferation, and CVG may serve as an immunomodulator;
thus indicating its potential to regulate the immune system to
control infections and other adverse health effects, with these
suppressive and/or potentiating functions likely benefiting overall
health (3). IL-10 drives the immune
system to aid B cells secrete protective antibodies, and in effect
suppresses the secretion of IL-2 and IFN-γ from Th1 cells (30,31), as
reflected in the present study, and thus may account for the
antitumor activity of CVG. Our group previously reported that
incubation of tumor cells with glucans suppressed the growth of the
tumors both in vitro and in vivo, by arresting the
cell cycle and promoting apoptosis (3). However, only 0.2 ml CVG was injected into
the mice per day for nine days (3) and
thus for CV-derived glucans to be applied in the medicinal and food
industries, more detailed studies are required. Nevertheless, the
present article provides insight into the activity of CVG, and may
provide a basis for future interpretation of the bioactivities of
CV extracts. Additionally, the present study may aid in the
selection of appropriate α-glucans for future clinical
investigations.
In conclusion, the glucan (1→6)-α-D-Glcp isolated
from CV stimulated the production of cytokines, and its potential
immunomodulatory and anticancer effects were implicated to be
IL-10/Th2-dependent. These results indicate the potential of CVG to
enhance human health as a natural supplement when used in the food
industry and in cancer therapies.
Acknowledgements
The present work was supported by the Science and
Technology Department Program of Liaoning Province, China (grant
no. 2,011,225,013).
References
|
1
|
Dai X, Stanilka JM, Rowe CA, Esteves EA,
Nieves C Jr, Spaiser SJ, Christman MC, Langkamp-Henken B and
Percival SS: Consuming Lentinula edodes (Shiitake) mushrooms
daily improves human immunity: A randomized dietary intervention in
healthy young adults. J Am Coll Nutr. 34:478–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Esposito P, La Porta E, Calatroni M,
Bianzina S, Libetta C, Gregorini M, Rampino T and Dal Canton A:
Renal involvement in mushroom poisoning: The case of Orellanus
syndrome. Hemodial Int. 19:E1–E5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Awadasseid AHJ, Gamallat Y, Xueqi S,
Eugene KD, Musa Hago A, et al: Purification, characterization, and
antitumor activity of a novel glucan from the fruiting bodies of
Coriolus Versicolor. PloS one. 12:e01712702017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dimitrijevic MV, Mitic VD, Cvetkovic JS,
Stankov VPJ, Mutic JJ, Snezana D and Mandic N: Update on element
content profiles in eleven wild edible mushrooms from family
Boletaceae. Eur Food Res Technol. 242:1–10. 2016. View Article : Google Scholar
|
|
5
|
Ekwemalor K, Asiamah E and Worku M: Effect
of a mushroom (Coriolus versicolor) based probiotic on the
expression of toll-like receptors and signal transduction in goat
neutrophils. J Mol Biol Res. 6:71–79. 2016. View Article : Google Scholar
|
|
6
|
Wahyuningsih SPA, Pramudya M and Sugiharto
S: Influence of polysaccharide krestin from Coriolus
versicolor extract on nitrite and malondialdehyde
concencentrations of Mus musculus serum exposed by
Mycobacterium tuberculosis. Biosaintifika. 8:12–17. 2016.
View Article : Google Scholar
|
|
7
|
Qin SY, Zhang AQ, Cheng SX, Rong L and
Zhang XZ: Drug self-delivery systems for cancer therapy.
Biomaterials. 112:234–247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Yu J, Lu X and He X: Nanoparticle
systems reduce systemic toxicity in cancer treatment. Nanomedicine
(Lond). 11:103–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu YL, Zhou L and Lu Y: Intrathecal
chemotherapy as a treatment for leptomeningeal metastasis of
non-small cell lung cancer: A pooled analysis. Oncol Lett.
12:1301–1314. 2016.PubMed/NCBI
|
|
10
|
Gong Y, Yang C, Yin X, Zhu M, Yang H, Wang
Y, Li Y, Liu L, Dong X, Cao S, et al: The effect of essential
medicines programme on rational use of medicines in China. Health
Policy Plan. 31:21–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang N, Awadasseid A, Yuan Y, Cui J, Guo
T, Gou D, Bai Y, Zhou Y and Gao J: Biotransformation of ginsenoside
Rb1 for ginsenoside Rd preparation by Lysinibacillus
massiliensis No 24. Indian J Biotechnol. 15:400–406. 2016.
|
|
12
|
Elbe S, Roemer-Mahler A and Long C:
Medical countermeasures for national security: A new government
role in the pharmaceuticalization of society. Soc Sci Med.
131:263–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prasad S, Rathore H, Sharma S and Yadav
AS: Medicinal mushrooms as a source of novel functional food. Int J
Food Sci Nutr Diet. 4:221–225. 2015.
|
|
14
|
Khan MS, Zhang X, You L, Fu X and Abbasi
AM: Structure and bioactivities of fungal polysaccharides.
Polysaccharides: Bioactivity and Biotechnology. 1–14. 2014.
View Article : Google Scholar
|
|
15
|
Habijanic J, Berovic M, Boh B, Plankl M
and Wraber B: Submerged cultivation of Ganoderma lucidum and
the effects of its polysaccharides on the production of human
cytokines TNF-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. N
Biotechnol. 32:85–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ali MF, Driscoll CB, Walters PR, Limper AH
and Carmona EM: β-glucan-activated human B lymphocytes participate
in innate immune responses by releasing proinflammatory cytokines
and stimulating neutrophil chemotaxis. J Immunol. 195:5318–5326.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wagener AH, de Nijs SB, Lutter R, Sousa
AR, Weersink EJ, Bel EH and Sterk PJ: External validation of blood
eosinophils, FE(NO) and serum periostin as surrogates for sputum
eosinophils in asthma. Thorax. 70:115–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szymczak I and Pawliczak R: The active
metabolite of vitamin D3 as a potential immunomodulator. Scand J
Immunol. 83:83–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1–19. 2014.
View Article : Google Scholar
|
|
20
|
Parker BS, Rautela J and Hertzog PJ:
Antitumour actions of interferons: Implications for cancer therapy.
Nat Rev Cancer. 16:131–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khodabandehloo H, Gorgani-Firuzjaee S,
Panahi G and Meshkani R: Molecular and cellular mechanisms linking
inflammation to insulin resistance and β-cell dysfunction. Transl
Res. 167:228–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tavakolpour S and Tavakolpour V:
Interleukin 4 inhibition as a potential therapeutic in pemphigus.
Cytokine. 77:189–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin NT and Martin MU: Interleukin 33 is
a guardian of barriers and a local alarmin. Nat Immunol.
17:122–131. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conti P and Kempuraj D: Important role of
mast cells in multiple sclerosis. Mult Scler Relat Disord. 5:77–80.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmidt-Arras D and Rose-John S: IL-6
pathway in the liver: From physiopathology to therapy. J Hepatol.
64:1403–1415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Raggi C, Mousa HS, Correnti M, Sica A and
Invernizzi P: Cancer stem cells and tumor-associated macrophages: A
roadmap for multitargeting strategies. Oncogene. 35:671–682. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang WS, Liao CH, Tsai CW, Hu PS, Wu HC,
Hsu SW, Ji HX, Hsiao CL and Bau DT: The role of IL-10 promoter
polymorphisms in renal cell carcinoma. Anticancer Res.
36:2205–2209. 2016.PubMed/NCBI
|
|
28
|
Cai S, Batra S, Langohr I, Iwakura Y and
Jeyaseelan S: IFN-γ induction by neutrophil-derived IL-17A
homodimer augments pulmonary antibacterial defense. Mucosal
Immunol. 9:718–729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyazaki Y and Niino M: Endogenous type I
interferons and their regulators in multiple sclerosis. Clin Exp
Neuroimmunol. 8:17–23. 2017. View Article : Google Scholar
|
|
30
|
Lippitz BE: Cytokine patterns in patients
with cancer: A systematic review. Lancet Oncol. 14:e218–e228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mannino MH, Zhu Z, Xiao H, Bai Q,
Wakefield MR and Fang Y: The paradoxical role of IL-10 in immunity
and cancer. Cancer Lett. 367:103–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Snaric J: Link between colorectal cancer
and bacteria by Tom Noerper. 2014.
|
|
33
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|