Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal
necrolysis (TEN) have shared characteristics of erythematous
cutaneous reaction with blister formation accompanied by mucosal
involvement. Patients with SJS have desquamation of the skin
affecting <10% of their body surface area whereas patients with
TEN have >30% body surface area involvement. Patients with skin
lesions affecting between 10 and 30% of their body surface area are
considered to have SJS/TEN overlap (1). These epidermal detachments are
considered life-threatening (2) and
are most frequently manifested by adverse drug reaction. In
addition to occurring as a reaction to drugs, these skin eruptions
are also associated with underlying infectious diseases, and
include the cutaneous manifestations of disseminated candidiasis
(3,4), Mycoplasma pneumoniae (5), Chlamydia pneumoniae (6), cytomegalovirus infection (7) and human immunodeficiency virus
(8,9). The mortality rates of these skin
eruptions have been reported to range from 16 to 25% (10–13).
Treatment is based on symptoms and supportive fluid and electrolyte
replacement. Dermal coverage to prevent secondary infection and the
loss of fluid are also crucial aspects of treatment. Several
immunomodulative therapies have been suggested to treat SJS and/or
TEN, particularly glucocorticoids and immunoglobulin. Prognostic
factors and scoring systems have been used to define the mortality
risk in these patients, including the severity-of-illness score of
toxic epidermal necrolysis (SCORTEN) scale (14).
In this study, the clinical manifestations, drug
implications, treatment and outcomes of patients with SJS and/or
TEN who had been hospitalized over the past 5 years in a tertiary
referral care center were retrospectively reviewed and
analyzed.
Patients and methods
Patient data
The protocol was approved by the ethics committee of
the King Chulalongkorn University Hospital (Bangkok, Thailand) and
complies with the Declaration of Helsinki. The authors
retrospectively reviewed all patients who had been hospitalized
with a discharge diagnosis of severe skin eruption during the
previous 5 years. The medical records were evaluated and classified
according to patient history, pre-existing conditions, suspected
causes, degree of skin and mucosal involvement, diagnosis,
treatment and outcome. The patients were divided into three groups,
namely SJS, SJS/TEN overlap and TEN, based on the percentage of
body surface area involvement. These three groups of patients were
analyzed to determine the difference in clinical manifestations,
underlying diseases, clinical course, treatment and mortality.
Statistical analysis
Results are expressed as mean ± standard deviation,
unless otherwise indicated. Differences between groups were
compared by unpaired t-testing and one way analysis of variance.
The level of significance was set at 5%. All statistical analyses
were carried out with SPSS software (version 17.0; SPSS, Inc.,
Chicago, IL, USA).
Results
Patient clinical data
During the 5-year period, 43 of the 47 patients that
were hospitalized for SJS, TEN and SJS/TEN overlap had complete
medical records to review. The mean age of the subjects was 49.5
(range, 20–85) years. Twenty-four patients (55.8%) were diagnosed
with SJS, 9 (20.9%) were classified with SJS/TEN overlap and 10
(23.3%) were categorized as having TEN. The demographic data and
underlying diseases are shown in Table
I. Mucosal membrane involvement was observed in the oral cavity
in 97.7% of cases and eye involvement was observed in 88.4% of the
study population. The clinical characteristics of the patients are
shown in Table II.
 | Table I.Demographic and baseline clinical data
of the patients. |
Table I.
Demographic and baseline clinical data
of the patients.
| Baseline and clinical
variables | SJS | SJS/TEN overlap | TEN | Total |
|---|
| Cases, n (%) | 24 (55.8) | 9 (20.9) | 10 (23.3) | 43 |
| Mean age (range),
years | 46.5 (20–77) | 54.4 (25–85) | 52.4 (21.84) | 49.5 |
| Gender, n (%) |
|
|
|
|
| Male | 13 (54.2) | 7 (77.8) | 5 (50.0) | 25 |
|
Female | 11 (45.8) | 2 (22.2) | 5 (50.0) | 18 |
| Underlying disease
(%) |
|
|
|
|
|
Diabetes | 8.3 | – | 30.0 | 11.6 |
|
Hypertension | 16.7 | 33.3 | 40.0 | 25.6 |
| Gout | 12.5 | – | 30.0 | 13.9 |
| Chronic
kidney disease | 12.5 | 11.1 | 20.0 | 13.9 |
|
Epilepsy | 12.5 | – | – | 7.0 |
|
Cerebrovascular disease | 4.2 | 11.1 | 10.0 | 7.0 |
|
Cardiovascular disease | 4.2 | 22.2 | 10.0 | 9.3 |
| HIV
infection | 33.3 | 22.2 | 60.0 | 37.2 |
|
Tuberculosis | 4.2 | 11.1 | 20.0 | 9.3 |
| Viral
hepatitis B/C | – | – | 20.0 | 4.7 |
|
Other | 8.3 | – | – | 11.6 |
| Alcohol
drinking | 12.5 | 22.2 | 10.0 | 14.0 |
| History
of malignancy | 12.5 | – | 20.0 | 11.6 |
| History
of allergy | 12.5 | 11.1 | 10.0 | 11.6 |
 | Table II.Clinical presentation and
manifestation of the patients. |
Table II.
Clinical presentation and
manifestation of the patients.
| Variables | SJS | SJS/TEN overlap | TEN | Total |
|---|
| Cases, n (%) | 24 (55.8) | 9 (20.9) | 10 (23.3) | 43 |
| Chief complaint
(%) |
|
|
|
|
| Rash | 37.5 | 66.7 | 80.0 | 53.5 |
|
Fever | 29.1 | 11.1 | 10.1 | 20.9 |
| Oral
ulcer | 16.7 | 11.1 | – | 11.6 |
|
Edema |
8.3 | – | 10.0 |
7.0 |
| Eye
symptom |
4.2 | 11.1 | – |
4.7 |
|
Myalgia |
4.2 | – | – |
2.3 |
| Duration prior to
admission (days) |
2.6 |
6.3 |
3.9 |
3.6 |
| Clinical
manifestation (%) |
|
|
|
|
| Oral
mucosa involvement | 100 | 100 | 90.0 | 97.7 |
| Eye
involvement | 83.3 | 88.9 | 100 | 88.4 |
| Genital
involvement | 41.7 | 55.6 | 90.0 | 55.8 |
|
Hepatitis | 41.7 | 66.7 | 50.0 | 48.8 |
|
Microscopic hematuria | 20.1 | 11.1 | 40.0 | 23.2 |
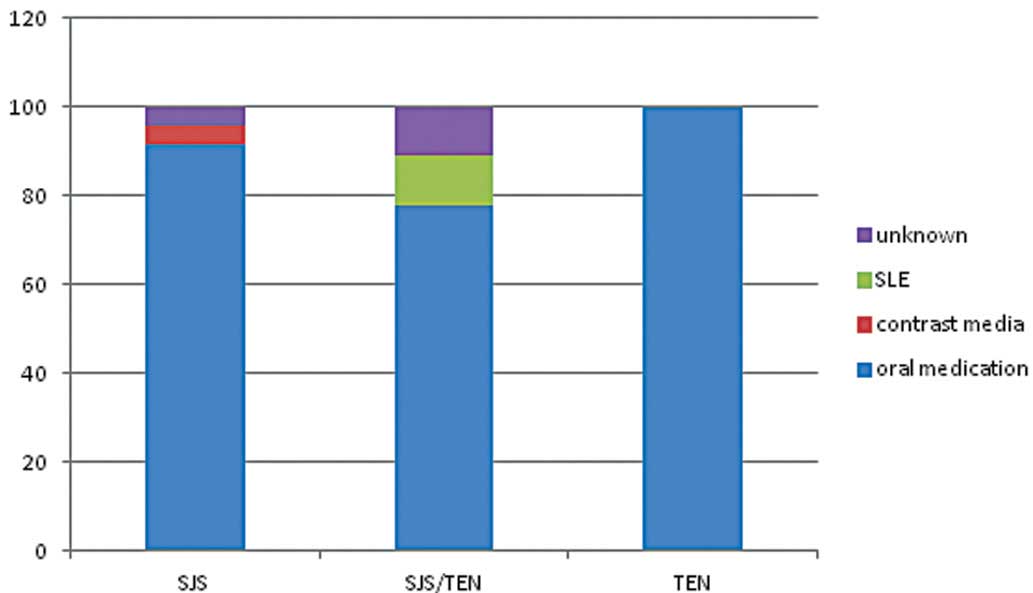
Causes of SJS, TEN and SJS/TEN
In 90.7% of patients, the mucocutaneous eruption was
associated with oral drug administration and 2.3% of patients
developed the lesions following treatment with contrast media.
These mucocutaneous eruptions were considered to be associated with
underlying disease, such as the cutaneous manifestations of
systemic lupus erythematosus, in 2.3% of cases, as shown in
Fig. 1. Allopurinol was the most
common single drug causing the eruption (25.6%). The other
medications associated with these conditions were anticonvulsants
(23.1%) and antibiotics (23.1%). The duration of medication intake
prior to the skin eruption ranged from 1 to 60 days (mean, 14.9
days).
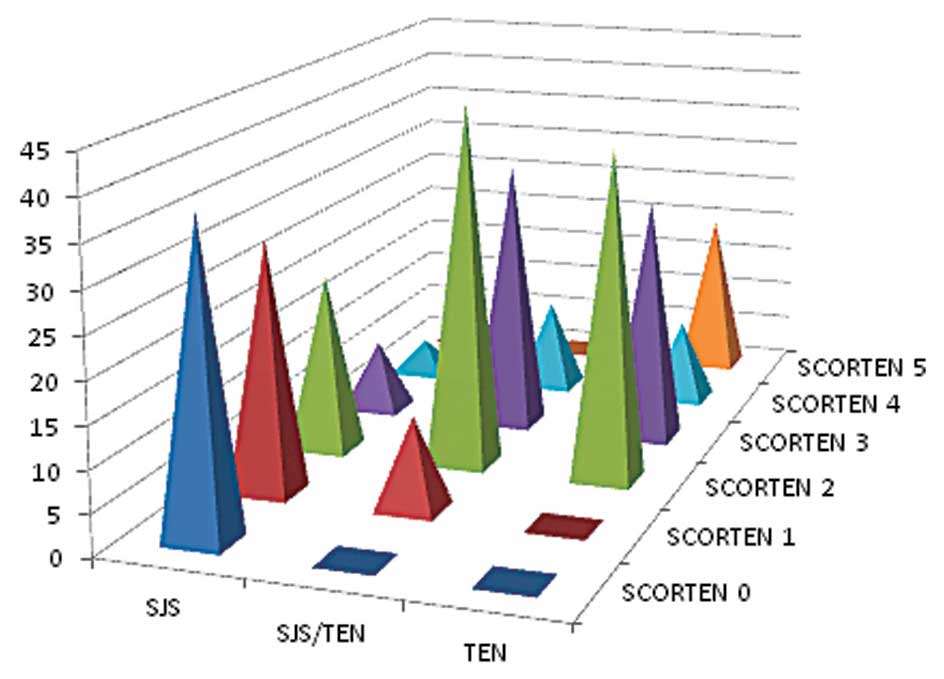
Disease severity
The SCORTEN scoring system was used to grade the
severity of these diseases. The majority of the patients in the SJS
(67.9%) group had a score of 0 or 1 while the majority of the
SJS/TEN overlap (44.4%) and TEN (40%) groups had a score of 2. In
the TEN group, 20% of the patients had a score of 5 as shown in
Fig. 2.
Treatment
A total of 28 patients (65.1%) were treated with
corticosteroids, as shown in Table
III. Intravenous dexamethasone was the most common agent used.
None of the patients received intravenous immunoglobulin treatment.
Antibiotics were used in 60% of patients with TEN.
 | Table III.Treatment regimens of the
patients. |
Table III.
Treatment regimens of the
patients.
| Treatment | SJS | SJS/TEN
overlap | TEN |
|---|
| Total steroid use,
n (%) | 14 (58.3) | 8 (88.8) | 6 (60) |
| Dexamethasone IV
(%) | 42.8 | 37.5 | 66.7 |
| Mean
dose (range), mg/day | 14.2 (8–20) | 20.6 (8–30) | 20 (8–40) |
| Prednisolone oral
(%) | 57.2 | 50.0 | 33.3 |
| Mean
dose (range), mg/day | 34.5 (20–60) | 57 (50–60) | 45 (30–60) |
| Total steroid
duration (range), days | 6.3 (3–8) | 5.7 (1–7) | 6.3 (3–10) |
| Antibiotics use
(%) | 29.1 | 22.2 | 60.0 |
| Fluid resuscitation
in first 24 h (range), ml | 3,081.2
(500–6,500) | 3,361.1
(1,200–4,850) | 3,973.0
(2,870–5,350) |
| LOS (days) | 9.7 (2–24) | 11.8 (2–41) | 15.9 (2–52) |
Survival
Three patients in the TEN group succumbed while
there was no mortality in the SJS and SJS/TEN overlap groups. The
causes of mortality were septicemia in 2 cases and arrhythmia
related to hyperkalemia in 1 case. Comparison between the survival
group and the non-survival group revealed that patient age >70
years of age (P=0.014) and body surface area involvement >20%
(P<0.01) were significant factors associated with mortality. The
use of systemic steroids was higher in the survival group in
comparison with the non-survival group (65.1 vs. 0%, respectively;
P=0.014). Table IV shows the
comparison analysis of the clinical characteristics between the
survival and non-survival groups.
 | Table IV.Results of univariate analysis of the
clinical characteristics of the survival and non-survival
groups. |
Table IV.
Results of univariate analysis of the
clinical characteristics of the survival and non-survival
groups.
| Variables | Survival group
(%) | Non-survival group
(%) | P-value |
|---|
| Age >70
years | 12.5 | 66.7 | 0.014 |
| Male gender | 55 | 100 | 0.128 |
| Eye
involvement | 87.5 | 100 | 0.515 |
| Oral mucosa
involvement | 97.5 | 100 | 0.782 |
| Genital
involvement | 52.5 | 100 | 0.110 |
| Involvement of 3
mucosal areas | 42.5 | 100 | 0.054 |
| Renal
involvement | 22.5 | 33.3 | 0.668 |
| Liver
involvement | 50.0 | 33.3 | 0.578 |
| Duration prior to
admission >72 h | 30.0 | 33.3 | 0.903 |
| Diabetes
mellitus | 10 | 33.3 | 0.224 |
| Hypertension | 25 | 33.3 | 0.750 |
| Chronic kidney
disease | 12.5 | 33.3 | 0.315 |
| HIV positive | 30.0 | 33.3 | 0.903 |
| Malignancy | 10 | 33.3 | 0.224 |
| Gout | 12.5 | 33.3 | 0.315 |
| Heart rate >100
bpm | 35 | 66.7 | 0.274 |
| BUN >20
mg/dl | 37.5 | 66.7 | 0.319 |
| Serum bicarbonate
<20 mEq/l | 27.5 | 66.7 | 0.154 |
| WBC >10,000 | 15 | 0 | 0.470 |
| BSA involvement
>20% | 22.5 | 100 | 0.004 |
| Steroid
treatment | 65.1 | 0 | 0.014 |
| Antibiotics
use | 30 | 100 | 0.014 |
| Fluid in 24 h
>2,500 ml | 70 | 100 | 0.206 |
| Infection | 30 | 100 | 0.014 |
| SCORTEN >2 | 27.5 | 66.7 | 0.154 |
Discussion
SJS, SJS/TEN overlap and TEN are rare but
life-threatening conditions. It is important to recognize the
clinical characteristics of the mucocutaneous eruption at early
stage due to the high mortality rate, which ranges from 16 to 25%
(1,10–12). The
most frequent cause of these conditions is medication (15,16). The
most common precipitating drug in this study was allopurinol.
Anticonvulsants and antibiotics were found to be the second common
causative agents in the present study, despite these two groups of
medication being reported as the most frequent etiology in a
previous study (17). The difference
between these results could be explained by the common medications
previously reported as having cutaneous adverse reactions now being
avoided. With advancements in the identification of specific human
leukocyte antigen (HLA) alleles that are associated with drug
reactions, screening to identify patients at risk for drug reaction
is becoming a part of standard clinical practice in certain
academic institutions (18–21). This could be one of the reasons why
the incidence of drug reactions toward commonly known medications
appears to have declined.
The use of systemic corticosteroid treatment in
patients with SJS/TEN is controversial, with concerns regarding an
increased rate of infections, the masking of septicemia, and delay
of epithelialization (22). By
contrast, the benefits of immunosuppressants (including
corticosteroids) have been reported as preventing ocular
complications (23–26). In the present study, 65% of patients
received systemic steroid treatment (range, 1–10 days). Notably,
there was no mortality in patients treated with systemic steroids.
By comparison, 3 cases of mortality were patients who did not
receive steroid treatment. The results of the present study suggest
the beneficial effect of systemic corticosteroid use in selected
groups of SJS and/or TEN patients.
The SCORTEN scoring system has been used to define
the severity of the disease and predict mortality for more than a
decade. The results of the present study revealed a lower number of
cases of mortality than predicted by SCORTEN score (14). There was no significant difference in
SCORTEN score between survival and non-survival groups. This
finding emphasizes the limitation of the SCORTEN as previously
mentioned in certain review articles (27–29).
The mortality rate in the presented study was 6.9%,
which was lower than the rates in previous studies (1,10–12).
This could be the result of early diagnosis, the immediate
discontinuation of causative medication, supportive medical care
and immunosuppressive treatment. The finding in the present study
concerning corticosteroid treatment is similar to that of the
EuroSCAR-and RegiSCAR studies (13,30),
which suggested the beneficial use of corticosteroid in selected
subgroups of patients with SJS and/or TEN. To confirm this finding,
a future prospective controlled study should be undertaken to
evaluate the benefit of corticosteroid treatment in SJS/TEN.
This study has several limitations. Firstly, in
terms of the small size of the study population selected from a
major tertiary care center. Secondly, the study was designed to
include only hospitalized patients. This may not provide the full
evaluation in both quality and quantity of management in general,
since the majority of the patients with mild forms of disease are
treated in primary local hospitals. Thirdly, genetic data was not
collected. With the advance and availability of genetic testing,
genetic screening may be warranted to assist in the selection of
treatment options and prevention. Lastly, this observational study
may suggest the beneficial effect of steroids in the treatment of
patients with SJS/TEN. However, a further double-blinded placebo
control study is required to confirm this suggestion.
The severe forms of mucocutaneous eruptions, SJS
and/or TEN, are mostly associated with adverse drug reactions. With
early recognition and selected treatments, the mortality rate could
be reduced. Improved understanding of clinical presentation and
risk factors should help physicians to improve the care of
high-risk individuals at an earlier stage. Patient age and the area
of mucocutaneous involvement have been identified as significant
factors associated with mortality.
Acknowledgements
The authors thank Stephen Pinder for proofreading
this manuscript.
References
|
1
|
Mockenhaupt M: The current understanding
of Stevens-Johnson syndrome and toxic epidermal necrolysis. Expert
Rev Clin Immunol. 7:803–813. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gerull R, Nelle M and Schaible T: Toxic
epidermal necrolysis and Stevens-Johnson syndrome: A review. Crit
Care Med. 39:1521–1532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mays SR, Bogle MA and Bodey GP: Cutaneous
fungal infections in the oncology patient: Recognition and
management. Am J Clin Dermatol. 7:31–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bodey GP: Dermatologic manifestations of
infections in neutropenic patients. Infect Dis Clin North Am.
8:655–675. 1994.PubMed/NCBI
|
|
5
|
Fournier S, Bastuji-Garin S, Mentec H,
Revuz J and Roujeau JC: Toxic epidermal necrolysis associated with
Mycoplasma pneumoniae infection. Eur J Clin Microbiol Infect
Dis. 14:558–559. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka A, Nakano M, Tani M, Kira M,
Katayama I, Nakagawa J, et al: Adult case of Stevens-Johnson
syndrome possibly induced by Chlamydophila pneumoniae
infection with severe involvement of bronchial epithelium resulting
in constructive respiratory disorder. J Dermatol. 40:492–494. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khalaf D, Toema B, Dabbour N and Jehani F:
Toxic epidermal necrolysis associated with severe cytomegalovirus
infection in a patient on regular hemodialysis. Mediterr J Hematol
Infect Dis. 3:e20110042011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kannenberg SM, Jordaan HF, Koegelenberg
CF, Von Groote-Bidlingmaier F and Visser WI: Toxic epidermal
necrolysis and Stevens-Johnson syndrome in South Africa: A 3-year
prospective study. QJM. 105:839–846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavlos R, Mallal S, Ostrov D, Pompeu Y and
Phillips E: Fever, rash and systemic symptoms: Understanding the
role of virus and HLA in severe cutaneous drug allergy. J Allergy
Clin Immunol Pract. 2:21–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Badia M, Trujillano J, Gascó E, Casanova
JM, Alvarez M and León M: Skin lesions in the ICU. Intensive Care
Med. 25:1271–1276. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neff P, Meuli-Simmen C, Kempf W, Gaspert
T, Meyer VE and Künzi W: Lyell syndrome revisited: Analysis of 18
cases of severe bullous skin disease in a burns unit. Br J Plast
Surg. 58:73–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rzany B, Mockenhaupt M, Baur S, Schröder
W, Stocker U, Mueller J, et al: Epidemiology of erythema
exsudativum multiforme majus, Stevens-Johnson syndrome and toxic
epidermal necrolysis in Germany (1990–1992): Structure and results
of a population-based registry. J Clin Epidemiol. 49:769–773. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schneck J, Fagot JP, Sekula P, Sassolas B,
Roujeau JC and Mockenhaupt M: Effects of treatments on the
mortality of Stevens-Johnson syndrome and toxic epidermal
necrolysis: A retrospective study on patients included in the
prospective EuroSCAR study. J Am Acad Dermatol. 58:33–40. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bastuji-Garin S, Fouchard N, Bertocchi M,
Roujeau JC, Revuz J and Wolkenstein P: SCORTEN: A
severity-of-illness score for toxic epidermal necrolysis. J Invest
Dermatol. 115:149–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chung WH and Hung SI: Recent advances in
the genetics and immunology of Stevens-Johnson syndrome and toxic
epidermal necrosis. J Dermatol Sci. 66:190–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perkins JR, Ayuso P, Cornejo-Garcia JA and
Ranea JA: The study of severe cutaneous drug hypersensitivity
reactions from a systems biology perspective. Curr Opin Allergy
Clin Immunol. 14:301–306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mockenhaupt M, Viboud C, Dunant A, Naldi
L, Halevy S, Bouwes Bavinck JN, et al: Stevens-Johnson syndrome and
toxic epidermal necrolysis: assessment of medication risks with
emphasis on recently marketed drugs. The EuroSCAR-study. J Invest
Dermatol. 128:35–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF,
Yang CC, et al: Carbamazepine-induced toxic effects and HLA-B*1502
screening in Taiwan. N Engl J Med. 364:1126–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mallal S, Phillips E, Carosi G, Molina JM,
Workman C, Tomazic J, et al: HLA-B*5701 screening for
hypersensitivity to abacavir. N Engl J Med. 358:568–579. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hershfield MS, Callaghan JT, Tassaneeyakul
W, Mushiroda T, Thorn CF, Klein TE, et al: Clinical
Pharmacogenetics Implementation Consortium guidelines for human
leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol
Ther. 93:153–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tassaneeyakul W, Jantararoungtong T, Chen
P, Lin PY, Tiamkao S, Khunarkornsiri U, et al: Strong association
between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome
and toxic epidermal necrolysis in a Thai population. Pharmacogenet
Genomics. 19:704–709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mockenhaupt M: Severe drug-induced skin
reactions: clinical pattern, diagnostics and therapy. J Dtsch
Dermatol Ges. 7:142–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Araki Y, Sotozono C, Inatomi T, Ueta M,
Yokoi N, Ueda E, et al: Successful treatment of Stevens-Johnson
syndrome with steroid pulse therapy at disease onset. Am J
Ophthalmol. 147:1004–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
López-Garcia JS, Rivas Jara L,
Garcia-Lozano CI, Conesa E, de Juan IE and Murube del Castillo J:
Ocular features and histopathologic changes during follow-up of
toxic epidermal necrolysis. Ophthalmology. 118:265–271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Rojas MV, Dart JK and Saw VP: The
natural history of Stevens Johnson syndrome: patterns of chronic
ocular disease and the role of systemic immunosuppressive therapy.
Br J Ophthalmol. 91:1048–1053. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prabhasawat P, Tesavibul N,
Karnchanachetanee C and Kasemson S: Efficacy of cyclosporine 0.05%
eye drops in Stevens Johnson syndrome with chronic dry eye. J Ocul
Pharmacol Ther. 29:372–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hague JS, Goulding JM, Long TM and Gee BC:
Respiratory involvement in toxic epidermal necrolysis portends a
poor prognosis that may not be reflected in SCORTEN. Br J Dermatol.
157:1294–1296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thong BY: Stevens-Johnson syndrome/toxic
epidermal necrolysis: An Asia-pacific perspective. Asia Pac
Allergy. 3:215–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
von Wild T, Stollwerck PL, Namdar T, Stang
FH, Mailänder P and Siemers F: Are multimorbidities underestimated
in scoring systems of Stevens-Johnson syndrome and toxic epidermal
necrolysis like in SCORTEN. Eplasty. 12:e352012.PubMed/NCBI
|
|
30
|
Lee HY, Dunant A, Sekula P, Mockenhaupt M,
Wolkenstein P, Valeyrie-Allanore L, et al: The role of prior
corticosteroid use on the clinical course of Stevens-Johnson
syndrome and toxic epidermal necrolysis: a case-control analysis of
patients selected from the multinational EuroSCAR and RegiSCAR
studies. Br J Dermatol. 167:555–562. 2012. View Article : Google Scholar : PubMed/NCBI
|