Introduction
Autoimmune thyroiditis (AIT), which is also known as
chronic lymphocytic thyroiditis or Hashimoto's thyroiditis, is an
organ-specific T-cell-mediated disease with a prevalence of 5–10%
in the Chinese population (1).
Patients with early-stage AIT may present transient hyperthyrea;
however in the advanced stage, hypothyroidism may occur (2,3). The
pathogenesis of AIT is considered to be associated with various
factors, including genetic and environmental factors, age, and sex
hormone levels (4); however, its
exact mechanism remains to be elucidated. In clinical practice, the
treatment of AIT is predominantly dependent on thyroid hormone
replacement therapy. However, patients who undergo such therapies
typically require additional treatment, potentially for the rest of
their lives, to repair potential injuries to the thyroid gland
(5).
Mesenchymal stem cells (MSCs) are prototypical adult
stem cells with the capacity to self-renew and differentiate in
vivo. Recently, Cipriani et al (6) demonstrated that MSCs exhibit
immunomodulatory and immunosuppressive properties, as they are able
to regulate the immune reactions of the host via various signaling
pathways. It was also demonstrated that MSCs were able to
contribute to the regeneration of cells in injured organs via
cellular migration. Therefore, these cells have been extensively
used in tissue engineering due to their extensive distribution
in vivo and their capacity for easy isolation and
proliferation (7,8). Immunomodulatory properties have
previously been reported to be a typical feature of MSCs due to a
lack of expression of immuno-co-stimulatory factors such as the
major histocompatibility complex class II antigen, cluster of
differentiation (CD)80, CD40 and CD86, which supports that MSCs
have a low immunogenicity (9).
Extensive studies have been performed to investigate the
transplantation of cells, tissues or organs in tissue engineering
(10), which have also reported that
intercellular cell adhesion molecule-1 (ICAM-1) is able to increase
the immunosuppressive capacity of MSCs and contribute to the
migration of MSCs in vitro (11). Furthermore, ICAM-1 may affect the
differentiation of MSCs through modulating the mitogen-activated
protein kinase (MAPK)-signaling pathway (12). The aim of the present study was to
investigate the effects of ICAM-1-expressing MSCs on the repair of
experimental AIT (EAT) mice.
Materials and methods
Animals and cell lines
A total of 60 female C57BL/6 mice (age, 6 weeks)
with a body weight of 20±2 g were purchased from Vital River
Laboratories Co., Ltd., (Beijing, China), provided with free access
to food and water and raised in sterilized conditions for 1 week at
24±2°C in a humidity of 40–50% and a 12 h dark/light cycle. A total
of 10 C57BL/6 mice (male, n=5; female, n=5; age, 1 week; weight,
4–5 g) used for the cultivation of primary MSCs were purchased from
the Animal Center of the Academy of Military Medical Sciences
(Beijing, China), and housed in the same conditions described
above. C3H10T1/2 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA). The C3H10T1/2-MIGR1/MSC and
C3H10T1/2-MIGR1-ICAM-1/MSC cell lines were constructed in the
authors' laboratory at Tianjin Medical University General Hospital
(Tianjin, China). Study protocols were approved by the Ethics
Committee of Tianjin Medical University General Hospital (Tianjin,
China).
Reagents
Porcine thyroglobulin, complete Freund's adjuvant
and incomplete Freund's adjuvant were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Commercial ELISA kits for total
triiodothyronine (TT3; cat. no. 80985; Crystal Chem, Inc., Downers
Grove, IL, USA), total thyroxine (TT4; cat. no. 80983; Crystal
Chem, Inc.), thyroid stimulating hormone (TSH; cat. no. RTC700R;
Xinbosheng Biotech Co., Ltd., Shenzhen, China) and anti-thyroid
peroxidase (TPOAb; cat. no. 05–50085; Zhuzhou Zeye Biotech Co.,
Ltd., Zhuzhou, China), anti-thyroid microsomal (TMAb; cat. no.
KB12639; Shanghai Jianglai Biotech Co., Ltd., Shanghai, China) and
anti-thyroglobulin antibodies (TGAb; cat. no. MA512408; Beinuo Life
Science, Shanghai, China) were used. TRIzol reagent was purchased
from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
TaqDNA polymerase, dNTP mixture, PrimeScript reverse transcription
kit (cat. no. 639505), RNase inhibitor and oligo d(T)15 primers
were purchased from Takara Bio, Inc., (Otsu, Japan). Rabbit
anti-mouse antibodies against total-mitogen-activated protein
kinase p38 (p38; cat. no. 9212), phosphorylated (p)-P38 (cat. no.
9216), total extracellular signal-regulated kinase (ERK) (cat. no.
9101) and p-ERK (cat. no. 4696), and goat anti-rabbit horseradish
peroxidase (HRP)-conjugated antibodies were purchased from Cell
Signaling Technology, Inc., (Danvers, MA, USA). Anti-mouse β-actin
antibodies (cat. no. 3700) were also purchased from Cell Signaling
Technology, Inc., and CM-DiI was purchased from Sigma-Aldrich
(Merck KGaA). Type II collagenase, α-MEM medium and fetal bovine
serum were purchased from Gibco (Thermo Fisher Scientific,
Inc.).
Experimental design
The EAT model was established in 6-week-old C57BL/6
mice according to a previously described method (13). Subsequently, mice were randomly
divided into the following groups (n=10 each): Normal control; EAT
model; primary MSC group, which was subjected to EAT induction,
followed by administration of MSCs (3×105) via the
caudal vein; C3H10T1/2/MSC group with similar function of MSCs,
which was subjected to EAT induction, followed by administration of
C3H10T1/2/MSCs (3×105) via the caudal vein;
C3H10T1/2-MIGR1/MSC group, which was subjected to EAT induction,
followed by administration of C3H10T1/2-MIGR1/MSCs
(3×105) via the caudal vein; and
C3H10T1/2-MIGR1-ICAM-1/MSC group, which was subjected to EAT
induction, followed by administration of
C3H10T1/2-MIGR1-ICAM-1/MSCs (3×105) via the caudal vein.
C3H10T1/2-MIGR1/MSCs were cells transfected with C3H10T1/2-MIGR1
plasmid, and C3H10T1/2-MIGR1-ICAM-1/MSC were cells transected with
C3H10T1/2-MIGR1-ICAM-1 plasmid.
ELISA
Blood samples were harvested from the angular vein
of each mouse at day 28 post-treatment to determine the serum TT3,
TT4, TSH, TPOAb, TMAb and TGAb according to the manufacturer's
protocol. All tests were performed a minimum of three times.
Hematoxylin and eosin (H&E)
staining
Following anesthesia with 3% pentobarbital
(Sigma-Aldrich; Merck KGaA), mice were sacrificed via cervical
dislocation. An incision was subsequently made on the skin along
the cervical median, and the muscles were separated to expose the
thyroid gland. The gland was removed using an ophthalmic scissor.
Thyroid gland tissues were fixed using 4% formalin and embedded in
wax, followed by preparation of 4 µm-thick sections. H&E
staining was subsequently performed to evaluate thyroid gland
injury by evaluating the size of the follicles, inflammatory
infiltration, colloidal substance retention and epithelial
injury.
Calculation of splenic index
Following sacrifice, an incision was made under the
inferior margin of the left rib to expose the spleen. The spleen
was obtained using an ophthalmic scissor. The width, thickness and
craniocaudal length of the spleen were determined and were used to
calculate the splenic index according to the previous description
(14).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from splenic cells using
TRIzol reagent according to the manufacturer's protocol. cDNA
synthesis was performed using ~2 µg RNA with PrimeScript reverse
transcription kit qPCR was performed using SYBR-Green on an Applied
Biosystems 7500 Software v2.0.6 system (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with the primers listed in Table I. PCR reactions were performed in a
total volume of 10 µl containing 5 µl 2X SYBR Premix, 0.2 µl each
specific primer to a final concentration of 200 nM, and 1 µl cDNA
template. The PCR conditions consisted of denaturation at 94°C for
3 min, followed by 40 cycles of 94°C for 30 sec, 60°C for 30 sec,
and 72°C for 60 sec. The mRNA level was normalized to that of
β-actin, and all reactions were performed at least in triplicate.
The amplification results for real-time PCR were calculated using
the 2−ΔΔCq method (15).
 | Table I.Primer sequences for the polymerase
chain reaction amplification of IL-4, IL-10, IL-17 and INF-γ
mRNA. |
Table I.
Primer sequences for the polymerase
chain reaction amplification of IL-4, IL-10, IL-17 and INF-γ
mRNA.
| Primer | Sequence (5′-3′) |
|---|
| IL-4 | Forward:
CGAGTTGACCGTAACAGACAT |
|
| Reverse:
CGTCTTTAGCCTTTCCAAGAAG |
| IL-10 | Forward:
CATTCATGGCCTTGTAGACACCTT |
|
| Reverse:
TCTCCCCTGTGAAAATAAGAGCAAG |
| Il-17 | Forward:
GTCGTTCAGATTGAGAGACAAGG |
|
| Reverse:
CTCATCCTTCAAAGACAGCCTCA |
| INF-γ | Forward:
CTGGTGGACCACTCGGATGA |
|
| Reverse:
TTACTACCTTCTTCAGCAACAGCAA |
| β-actin | Forward:
GGAGATTACTGCCCTGGCTCCTA |
|
| Reverse:
GACTCATCGTACTCCTGCTTGCTG |
Western blot analysis
Splenic tissues were homogenized at room temperature
for 2 min in radioimmunoprecipitation assay buffer containing
protease and phosphatase inhibitors (BB-3201-1; Bestbio, Shanghai,
China). Protein concentration was determined using a bicinchoninic
acid assay, and 40 µg protein per lane was separated by 10%
SDS-PAGE and transferred to a Hybond-P membrane. The membrane was
subsequently blocked with 5% non-fat milk and incubated with rabbit
anti-mouse p-p38 (1:200), total-p38 (1:500), p-ERK (1:1,000) and
total-ERK (1:200) antibodies overnight at 4°C. Subsequently, the
mixture was incubated with the HRP-conjugated goat anti-rabbit
antibody (1:1,000) for 1 h at room temperature. Following washing
with Tris-buffered saline Tween-20, the bound primary antibody was
visualized using enhanced chemiluminescence and exposed to X-ray
film. The relative density of each protein was analyzed using TL120
imaging software (version v2009; TotalLab, Ltd., Newcastle upon
Tyne, UK). The same membrane probed with anti-β-actin antibodies
(1:2,000) served as the loading control. All experiments were
performed a minimum of three times.
Determination of MSC migration in
vivo
To determine the migration of MSCs in vivo,
1-week-old C57BL/6 mice were randomly divided into the following
groups, subsequent to EAT induction (n=10 each): i) CM-DiI-labeled
primary MSCs; ii) CM-DiI-labeled C3H10T1/2/MSC; iii) CM-DiI-labeled
C3H10T1/2-MIGR1/MSC; and iv) CM-DiI-labeled
C3H10T1/2-MIGR1-ICAM-1/MSC, which were administered the relevant
cells via the caudal vein. For the CM-DiI labeling process, MSCs
(2×106) were resuspended with 1 ml PBS and labeled with
2 µl CM-DiI at 37°C for 5 min. Subsequently, the mixture was
incubated at 4°C for 5 min, and centrifuged at 179 × g for 10
min.
Following anesthesia with 3% pentobarbital
(Sigma-Aldrich; Merck KGaA) and sacrifice via cervical dislocation,
mice were fixed on an operating table to expose the chest, the
chest was shaved and the fascia removed, and the sternum and ribs
were removed to expose the heart. PBS was infused from the left
ventricle to expel circulated blood from the right atrium.
Subsequently, the lung and thyroid gland were washed using PBS, and
fixed with 10% paraformaldehyde. Following embedding, 5-µm sections
were cut and observed via fluorescence microscopy to determine the
positive staining.
Statistical analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA)
was used to perform statistical analysis. Data are presented as the
mean ± standard deviation, and Student's t-test was performed for
inter-group comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
MSCs, especially
C3H10T1/2-MIGR1-ICAM-1/MSCs, reverse the expression of T4, TSH,
TPOAb, TMAb and TGAb in vivo
No statistical difference was observed in the TT3
levels between groups (Fig. 1A). For
the expression of TT4, a significant decrease was observed in the
EAT model group compared with that of normal controls (P<0.05).
However, following administration of MSCs, the level of TT4 was
markedly increased compared to the model group. Compared with the
C3H10T1/2-MIGR1/MSC group, the level of TT4 was significantly
increased in the C3H10T1/2-MIGR1-ICAM-1/MSC group (P<0.05;
Fig. 1B). The expression of TSH
significantly decreased in the C3H10T1/2-MIGR1-ICAM-1/MSC group
compared with the C3H10T1/2-MIGR1/MSC group (P<0.05; Fig. 1C). Levels of TPOAb, TGAb, TMAb and
TSH were significantly higher in the EAT model group compared with
those of normal controls (all P<0.05; Fig. 1C-F). Similarly, following
administration of MSCs, their expression was decreased,
particularly in the C3H10T1/2-MIGR1-ICAM-1/MSC and MSC groups.
Compared with the C3H10T1/2-MIGR1/MSC group, the levels of TPOAb,
TGAb, TMAb and TSH were significantly decreased in the
C3H10T1/2-MIGR1-ICAM-1/MSC group (all P<0.05).
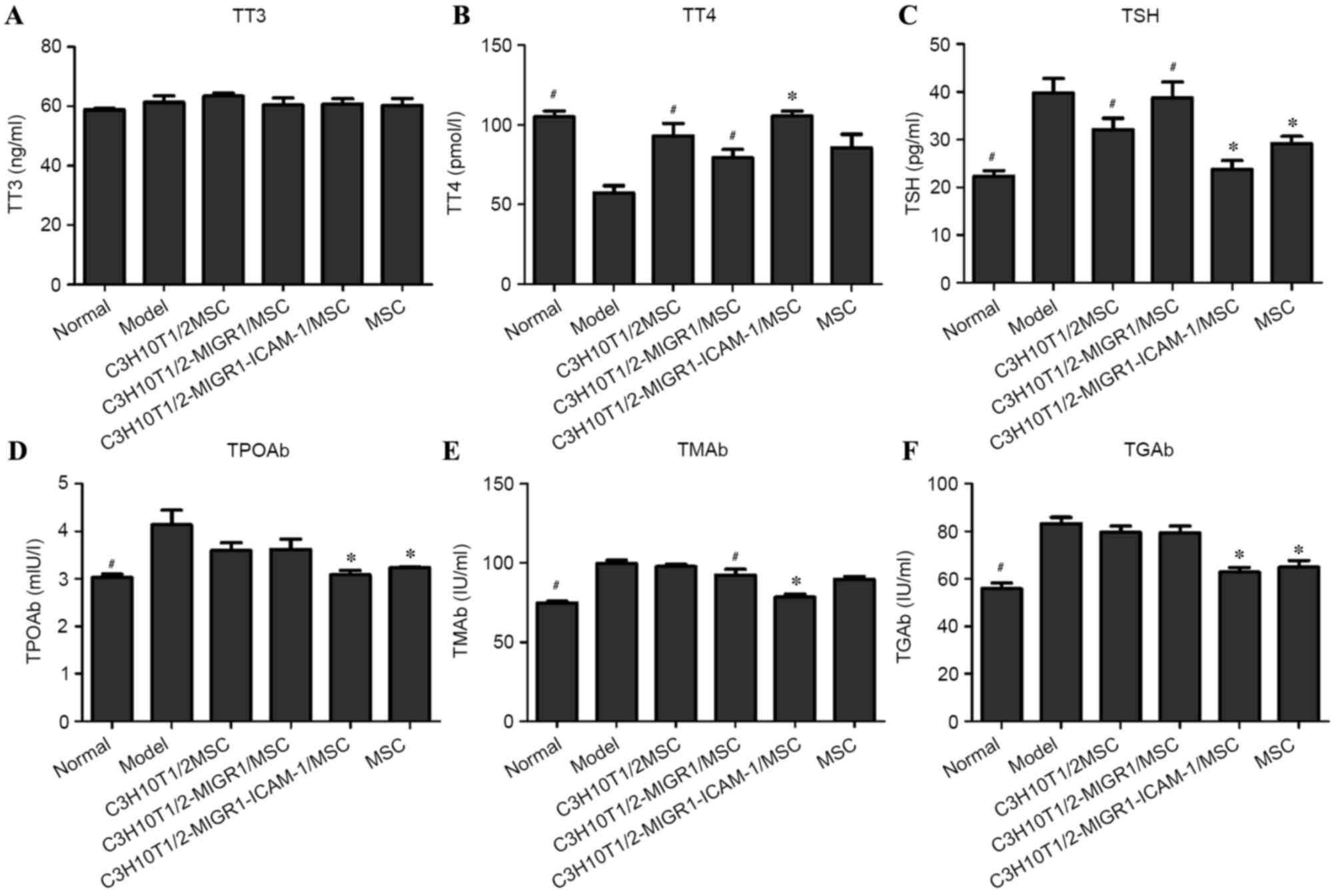 | Figure 1.Determination of (A) TT3, (B) TT4, (C)
TSH, (D) TPOAb, (E) TMAb and (F) TGAb levels by ELISA. All tests
were performed a minimum of three times, and data are presented as
the mean ± standard deviation. *P<0.05 vs.
C3H10T1/2-MIGR1/MSC-treated group; #P<0.05 vs. model
group. TT3, total triiodothyronine; TT4, total thyroxine; TSH,
thyroid-stimulating hormone; Ab, antibody; TPO, thyroid peroxidase;
TM, thyroid microsomal; TG, thyroglobulin; model, experimental
autoimmune thyroiditis model; MSC, mesenchymal stem cell; ICAM,
intercellular adhesion molecule. |
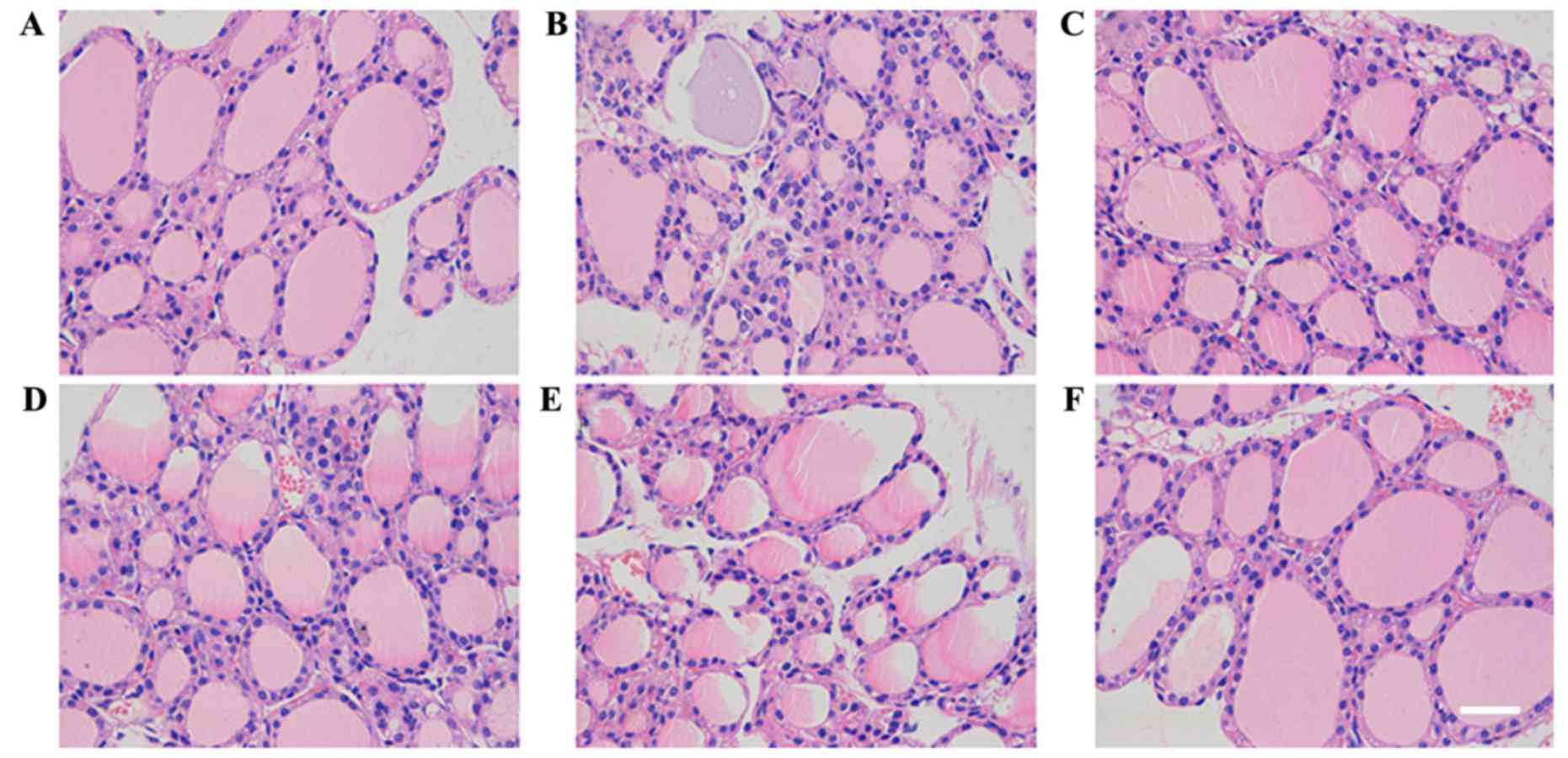
C3H10T1/2-MIGR1-ICAM-1/MSCs attenuate
thyroid follicle injury
Compared with the control group (Fig. 2A), injury of thyroid follicles was
determined visually by their uneven size and the presence of
epithelial hyperplasia and infiltration of inflammatory cells in
the EAT model group (Fig. 2B) as
revealed by H&E staining. In the C3H10T1/2-MIGR1-ICAM-1/MSC and
MSC groups thyroid gland injury, epithelial hyperplasia and
infiltration of inflammatory cells were attenuated;. however, in
the other groups, no marked difference was observed following the
administration of MSCs (Fig.
2C-F).
C3H10T1/2-MIGR1-ICAM-1/MSCs decrease
splenic index
The splenic index in the EAT model group was
significantly higher than that of the normal control group
(P<0.01). In the C3H10T1/2-MIGR1-ICAM-1/MSC group, the splenic
index was signifantly decreased compared with the EAT model group
(P<0.05). However, no significant improvement was observed in
the splenic index in the other treatment groups (Fig. 3).
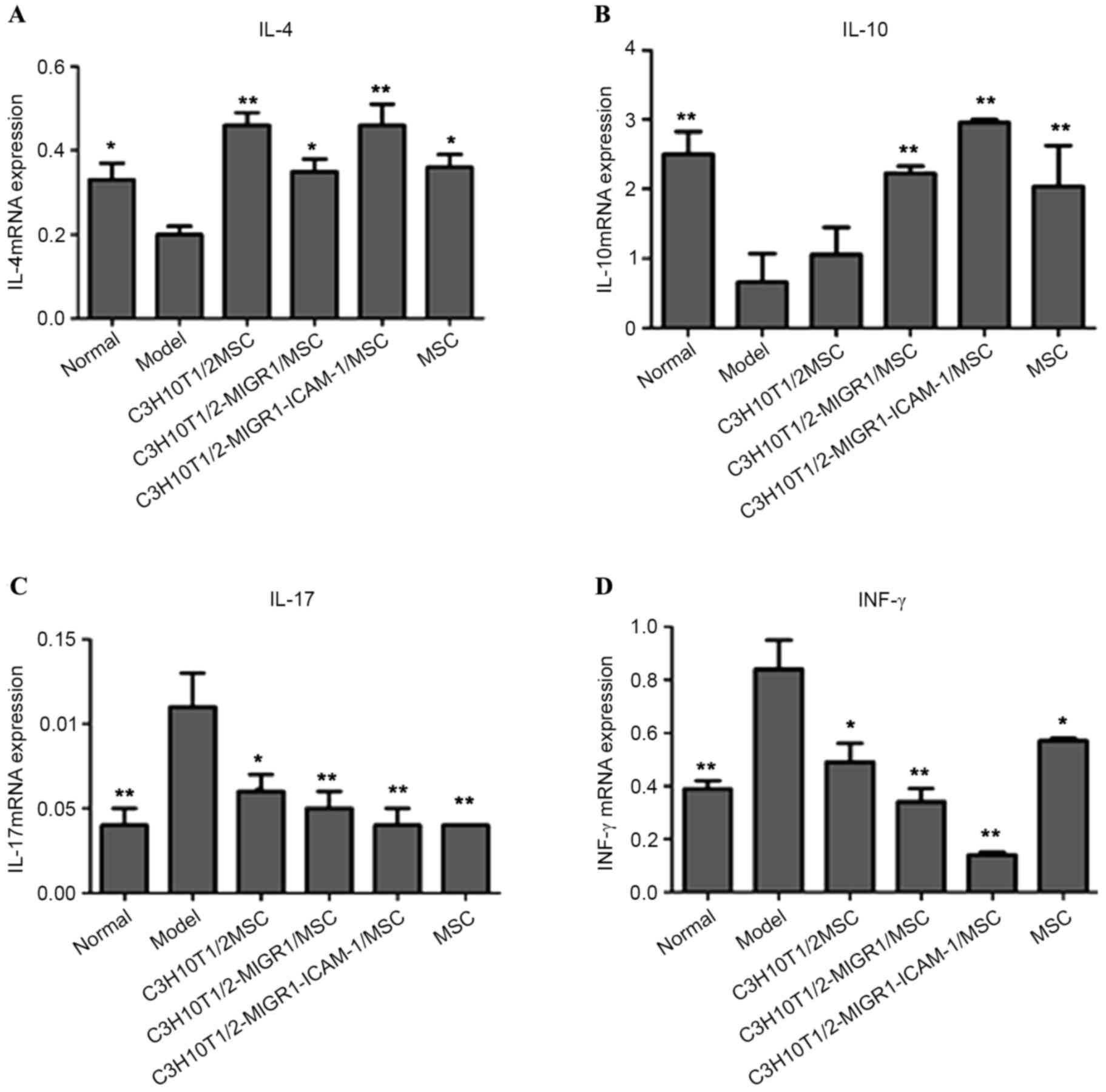
C3H10T1/2-MIGR1-ICAM-1/MSCs reverse
the mRNA expression of IL-4, IL-10, IL-17 and INF-γ
RT-qPCR indicated that IL-4 and IL-10 mRNA
expression was downregulated in the EAT model group compared with
that of normal controls (P<0.05; Fig.
4A and B). However, this downregulation was reversed in the
C3H10T1/2/MSC (IL-4, P<0.01), C3H10T1/2-MIGR1/MSC (IL-4,
P<0.05; IL-10, P<0.01), C3H10T1/2-MIGR1-ICAM-1/MSC (IL-4 and
IL-10, P<0.01) and MSC groups (IL-4, P<0.05; IL-10,
P<0.01) compared with the model group. The mRNA expression of
IL-17 and INF-γ was significantly increased in the EAT model group
compared with normal controls (both P<0.01; Fig. 4C and D). This upregulation was
significantly attenuated following treatment with MSCs (P<0.05).
The upregulation of IL-4 and IL-10 mRNA and downregulation of IL-17
and INF-γ mRNA were most apparent in the C3H10T1/2-MIGR1-ICAM-1/MSC
group compared with the other MSC groups.
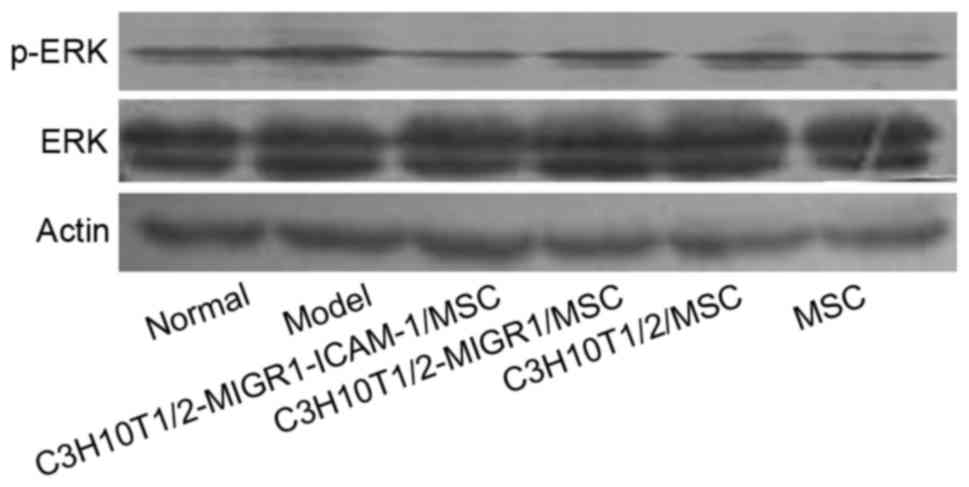
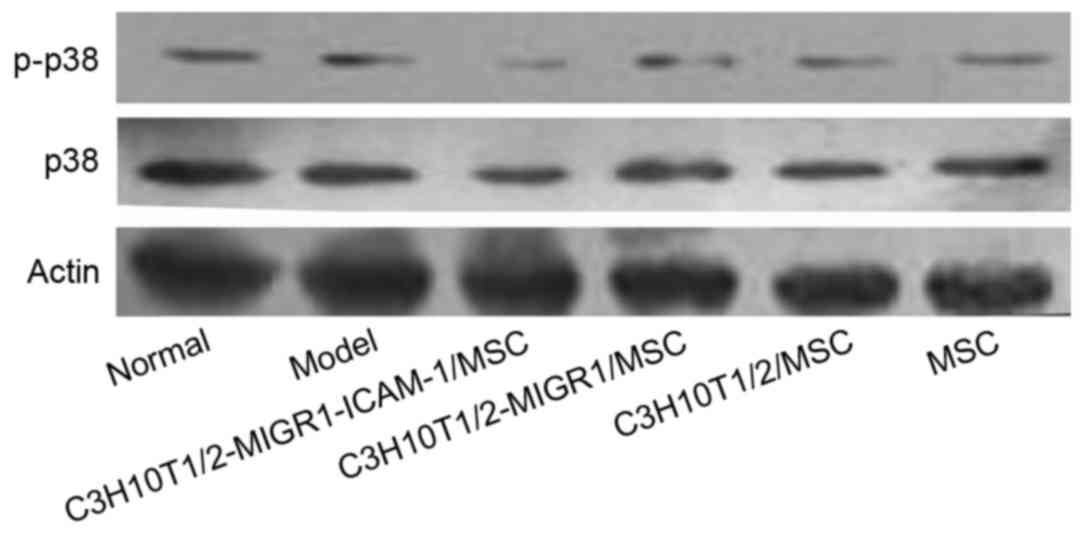
C3H10T1/2-MIGR1-ICAM-1/MSCs are
associated with the p38 and ERK signaling pathways in the mouse
spleen
Western blot analysis revealed the expression of
p-ERK and p-p38 was upregulated in the model group compared with
the normal control group. Compared with the model group, the
expression of p-ERK and p-p38 was decreased in the
C3H10T1/2-MIGR1-ICAM-1/MSC group. No statistical difference was
observed in the expression of p-ERK and p-p38 in the
C3H10T1/2-MIGR1/MSC, C3H10T1/2/MSC and primary MSC groups compared
with the model group (Figs. 5 and
6).
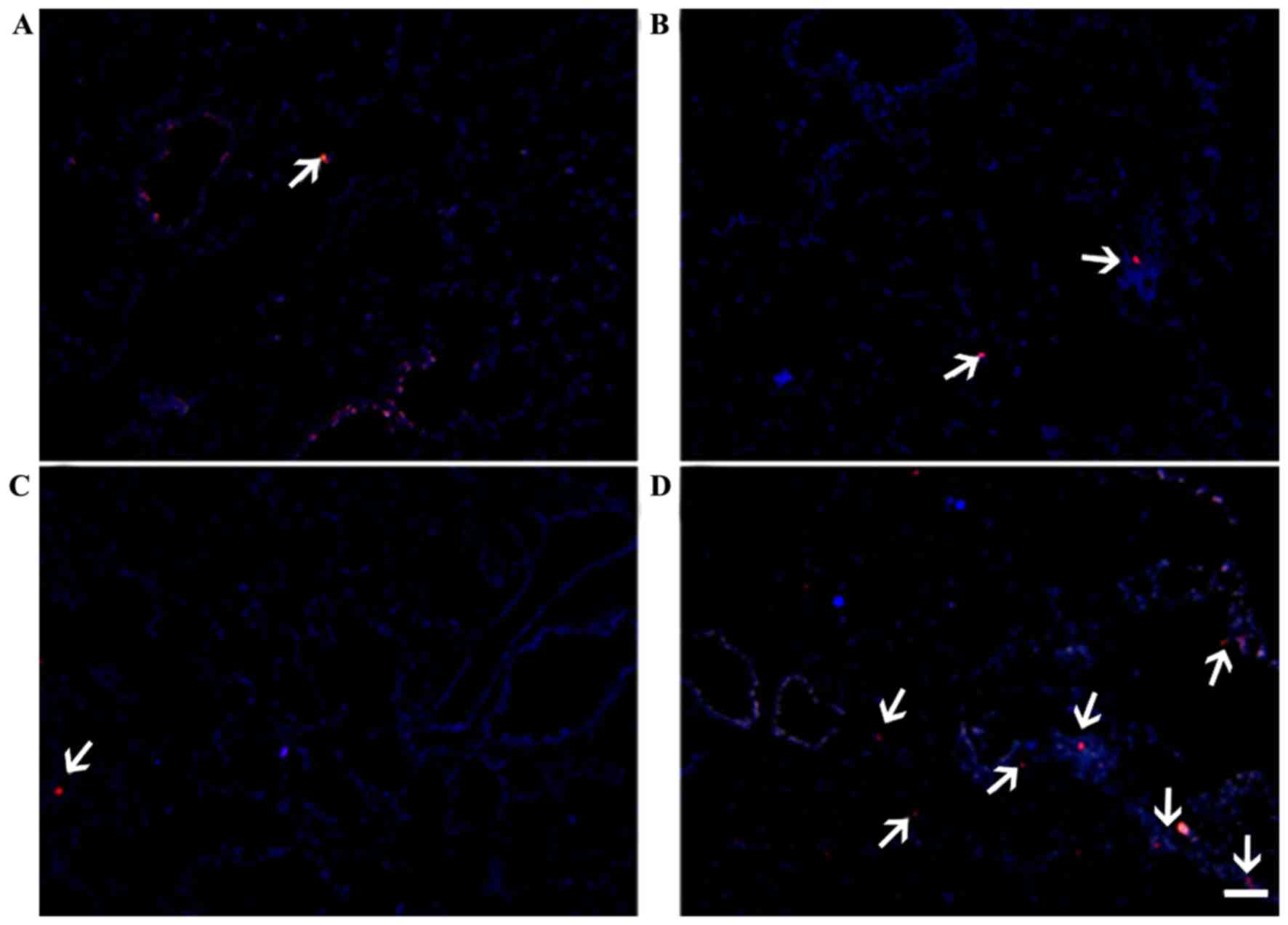
ICAM-1-overexpression modulates the
localization of MSCs in the thyroid gland and lung
Red fluorescence was observed in the thyroid gland
(Fig. 7) and lung (Fig. 8), indicating the presence of
CM-DiI-labeled MSCs. Localization in these tissues was markedly
increased in the C3H10T1/2-MIGR1-ICAM-1/MSCs group in the thyroid
gland compared with the other groups. The localization of primary
MSCs in the lung was superior to that in the thyroid gland in all
groups, which may be associated with the arrest of MSCs by the
extensive vascular network in the lung.
Discussion
The onset of AIT is associated with viral infection
and excessive uptake of iodides, which may result in autoimmune
disorders (16), potentially leading
to injury of the thyroid gland and infiltration of lymph nodes
(17). At present, there is no
specific treatment option that has been recommended for AIT.
Recently, MSCs with the capacity to self-renew and differentiate
in vivo, have been demonstrated to have immunomodulatory
properties and contribute to the regeneration of cells in injured
organs via cellular migration (18,19).
Previous studies have indicated that an imbalance of
T cell subtypes serves an important role in the pathogenesis of AIT
(20,21), as the imbalance may induce immune
dysfunction, expose thyroid antigens and stimulate the generation
of antigen-presenting cells. Furthermore, it may induce the
formation of precursor cells in the presence of various cytokines,
such as IL-10 and IL-4 (22). The
present study demonstrated that C3H10T1/2-ICAM-1/MSCs may
contribute to the expression of IL-4 and IL-10 in the immune cells,
and that they may downregulate the expression of IL-17 and INF-γ.
Among these factors, INF-γ and IL-17 are generated by Th1 cells,
whereas IL-4 and IL-10 are generated by Th2 cells. Therefore, these
findings suggest that C3H10T1/2-ICAM-1/MSCs were associated with
the regulation of the immune system via recruiting cytokines and
modulating the expression of T cell subtypes.
MAPKs are a highly conserved family of
serine/threonine protein kinases involved in a variety of
fundamental cellular processes (23). The major components of MAPKs are
ERKs, c-Jun N-terminal kinases and p38, which have been reported to
have crucial roles in the regulation of gene expression and
cytoplasm function (24). MAPKs may
also be associated with the proliferation, apoptosis,
differentiation, necrosis and expression of cellular adhesion
molecules (25). Previous studies
have demonstrated that p38 signaling was activated in MSCs under
the stimulation of inflammatory factors, which would subsequently
trigger the overexpression of adhesion molecules, as well as MSC
immune regulation (26,27).
In the present study, the expression of p-ERK and
p-p38 was upregulated in the EAT model group, whereas in the
C3H10T1/2-ICAM-1/MSC group, a marked increase was observed in the
dephosphorylation of ERK and p38 compared with other groups,
indicating that C3H10T1/2-ICAM-1/MSCs may modulate the ERK and p38
signaling pathways. These findings suggest that, under the
stimulation of exogenous thyroglobulin, immunocytes were stimulated
and the ERK and p38 signaling pathways were activated.
Additionally, phosphorylation was increased, which further
contributed to immunocyte activation. However, following the
administration of C3H10T1/2-ICAM-1/MSCs, the activation of these
pathways was reduced, and the expression of IL-4, IL-10, IL-17 and
IFN-γ was modulated, which suggests that inflammatory
factor-induced activation of the p38 signaling pathway may
contribute to the expression of ICAM-1 in MSCs and immunocytes, and
therefore inhibit the immune response. Furthermore, the level of
TMAb was decreased and injury of the thyroid gland and inflammatory
infiltration was attenuated.
Nesting of MSCs in the target organ is also of
importance for the immunosuppressive effects in vivo
(28). In the presence of
inflammatory factors, MSCs are able to migrate to injury sites and
are distributed in peripheral tissues. At the same time, MSCs may
secrete factors involved in the formation of neovessels, and
contribute to the regeneration of injury tissues (29). Yen et al (30) previously demonstrated that the
expression of adhesion molecules in MSCs and the surface of
vascular endothelial cells was upregulated in the presence of
inflammatory factors, which increased cellular adhesion between
MSCs and vascular endothelial cells and therefore contributed to
the migration of MSCs to injury tissues. In the present study, to
confirm the migration of MSCs in vivo, they were labeled
with CM-DiI. The results indicated that a more notable MSC
migration to the injured thyroid gland occurred in the
ICAM-1-expressing cells.
In conclusion, the present findings suggest that
C3H10T1/2-ICAM-1/MSCs were able to affect the differentiation,
proliferation and migration of immunocytes by modulating the p38
and ERK signaling pathways, which suggests that ICAM-1 may affect
the immunoregulatory effects of MSCs by modulating the migration of
MSCs in vivo. These findings may provide rationale for the
development of novel therapies for AIT using MSCs which express
ICAM-1.
Acknowledgements
The present study was supported by the Tianjin
Natural Science Fund (grant no. 12JCYBJC17100).
References
|
1
|
Chistiakov DA: Immunogenetics of
Hashimoto's thyroiditis. J Autoimmune Dis. 2:12005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Vries L, Bulvik S and Phillip M:
Chronic autoimmune thyroiditis in children and adolescents: At
presentation and during long-term follow-up. Arch Dis Child.
94:33–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeBoer MD and LaFranchi S: Differential
presentation for children with autoimmune thyroiditis discovered
because of symptomdevelopment or screening. J Pediatr Endocrinol
Metab. 21:753–761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lichiardopol C and Mota M: The thyroid and
autoimmunity. Rom J Intern Med. 47:207–215. 2009.PubMed/NCBI
|
|
5
|
Heymann WR: Chronic urticaria and
angioedema associated with thyroid autoimmunity: Review and
therapeutic implications. J Am Acad Dermatol. 40:229–232. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cipriani P, Carubbi F, Liakouli V,
Marrelli A, Perricone C, Perricone R, Alesse E and Giacomelli R:
Stem cells in autoimmune diseases: Implications for pathogenesis
and future trends in therapy. Autoimmun Rev. 12:709–716. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oreffo RO, Cooper C, Mason C and Clements
M: Mesenchymal stem cells: Lineage, plasticity, and skeletal
therapeutic potential. Stem Cell Rev. 1:169–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding DC, Shyu WC and Lin SZ: Mesenchymal
stem cells. Cell Transplant. 20:5–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu S, Yuan M, Hou K, Zhang L, Zheng X,
Zhao B, Sui X, Xu W, Lu S and Guo Q: Immune characterization of
mesenchymal stem cells in human umbilical cord Wharton's jelly and
derived cartilage cells. Cell Immunol. 278:35–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wada N, Gronthos S and Bartold PM:
Immunomodulatory effects of stem cells. Periodontol 2000.
63:198–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buschmann K, Koch L, Braach N, Mueller H,
Frommhold D, Poeschl J and Ruef P: CXCL1-triggered interaction of
LFA1 and ICAM1 control glucose-induced leukocyte recruitment during
inflammation in vivo. Mediators inflamm. 2012:7391762012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JD, Xu FF, Zhu H, Li XM, Tang B, Liu
YL and Zhang Y: ICAM-1 regulates differentiation of MSC to
adipocytes via activating MAPK pathway. Zhongguo Shi Yan Xue Ye Xue
Za Zhi. 22:160–165. 2014.(In Chinese). PubMed/NCBI
|
|
13
|
Kong YC: Experimental autoimmune
thyroiditis in the mouse. Curr Protoc Immunol. 15:Unit
15.72007.PubMed/NCBI
|
|
14
|
Prassopoulos P, Daskalogiannaki M,
Raissaki M, Hatjidakis A and Gourtsoyiannis N: Determination of
normal splenic volume on computed tomography in relation to age,
gender and body habitus. Eru Radiol. 7:246–248. 1997. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagayama Y, Horie I, Saitoh O, Nakahara M
and Abiru N: CD4+ CD25+ naturally occurring regulatory T cells and
not lymphopenia play a role in the pathogenesis of iodide-induced
autoimmune thyroiditis in NOD-H2 h4 mice. J Autoimmun. 29:195–202.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Armengol MP, Juan M, Lucas-Martín A,
Fernández-Figueras MT, Jaraquemada D, Gallart T and Pujol-Borrell
R: Thyroid autoimmune disease: Demonstration of thyroid
antigen-specific B cells and recombination-activating gene
expression in chemokine-containing active intrathyroidal germinal
centers. Am J Pathol. 159:861–873. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi EW, Shin IS, Lee HW, Park SY, Park
JH, Nam MH, Kim JS, Woo SK, Yoon EJ, Kang SK, et al:
Transplantation of CTLA4Ig gene-transduced adipose tissue-derived
mesenchymal stem cells reduces inflammatory immune response and
improves Th1/Th2 balance in experimental autoimmune thyroiditis. J
Gene Med. 13:3–16. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang SK, Shin IS, Ko MS, Jo JY and Ra JC:
Journey of mesenchymal stem cells for homing: Strategies to enhance
efficacy and safety of stem cell therapy. Stem Cells Int.
2012:3429682012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakaguchi S, Ono M, Setoguchi R, Yagi H,
Hori S, Fehervari Z, Shimizu J, Takahashi T and Nomura T: Foxp3+
CD25+ CD4+ natural regulatory T cells in dominant self-tolerance
and autoimmune disease. Immunol rev. 212:8–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wing K and Sakaguchi S: Regulatory T cells
exert checks and balances on self tolerance and autoimmunity. Nat
Immunol. 11:7–13. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yudoh K, Matsuno H, Nakazawa F, Yonezawa T
and Kimura T: Reduced expression of the regulatory CD4+ T cell
subset is related to Th1/Th2 balance and disease severity in
rheumatoid arthritis. Arthritis Rheum. 43:617–627. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arbabi S and Maier RV: Mitogen-activated
protein kinases. Crit Care Med. 30 Suppl 1:S74–S79. 2002.
View Article : Google Scholar
|
|
24
|
Schett G, Tohidast-Akrad M, Smolen JS,
Schmid BJ, Steiner CW, Bitzan P, Zenz P, Redlich K, Xu Q and
Steiner G: Activation, differential localization, and regulation of
the stress-activated protein kinases, extracellular
signal-regulated kinase, c-Jun N-terminal kinase and p38
mitogen-activated protein kinase, in synovial tissue and cells in
rheumatoid arthritis. Arthritis Rheum. 43:2501–2512. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao L, Liu X, Liang J, Han S, Wang Y, Yin
Y, Luo Y and Li J: Phosphorylation of p38 MAPK mediates hypoxic
preconditioning-induced neuroprotection against cerebral ischemic
injury via mitochondria translocation of Bcl-xL in mice. Brain Res.
1503:78–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren G, Zhao X, Zhang L, Zhang J,
L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C and Shi Y:
Inflammatory cytokine-induced intercellular adhesion molecule-1 and
vascular cell adhesion molecule-1 in mesenchymal stem cells are
critical for immunosuppression. J immunol. 184:2321–2328. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luz-Crawford P, Noël D, Fernandez X,
Khoury M, Figueroa F, Carrión F, Jorgensen C and Djouad F:
Mesenchymal stem cells repress Th17 molecular program through the
PD-1 pathway. PLoS One. 7:e452722012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng J, Zou ZM, Zhou TL, Su YP, Ai GP,
Wang JP, Xu H and Dong SW: Bone marrow mesenchymal stem cells can
be mobilized into peripheral blood by G-CSF in vivo and integrate
into traumatically injured cerebral tissue. Neurolo Sci.
32:641–651. 2011. View Article : Google Scholar
|
|
30
|
Yen BL, Huang HI, Chien CC, Jui HY, Ko BS,
Yao M, Shun CT, Yen ML, Lee MC and Chen YC: Isolation of
multipotent cells from human term placenta. Stem Cells. 23:3–9.
2005. View Article : Google Scholar : PubMed/NCBI
|