Introduction
Ischaemic stroke is a common disease in the central
nervous system (CNS), and it occurs with high incidence, mortality
and disability rates (1–3). Neuronal apoptosis following cerebral
ischaemia-reperfusion leads to brain injury and neurological
impairment (4). In recent years,
studies have found that anoikis is a substantial path for neuron
apoptosis (5,6). Anoikis is programmed cell death that
occurs due to cell detachment from the extracellular matrix (ECM)
(7). The ECM provides an external
environment and support for cell survival (8,9). Enzymes
degrade the ECM during the cerebral ischaemia-reperfusion process,
leading to maladjustments in signal transmission between cells,
neuronal injury and apoptosis (10,11).
In Traditional Chinese Medicine (TCM), it is
believed that the spleen is the foundation of life and the source
of Qi and blood generation. Sijunzi decoction (SJZD) is one of the
most famous invigorating spleen therapy recipes in TCM. This
Chinese decoction has been used to treat gastrointestinal
disorders, including chronic gastritis and gastroduodenal ulcer,
for hundreds of years, and it is effective for nausea, vomiting and
diarrhoea (12,13). SJZD was not created for the treatment
of stroke initially, but an increasing number of studies have found
that SJZD is beneficial for stroke, For example, a study found that
SJZD could protect cerebral neurons via up-regulating p-ERK1/2 and
p-Akt, and inhibiting the expression of Bax protein (14). Another study demonstrated that it
significantly increased the expression of superoxide dismutase and
decreased malondialdehyde in rat brains, and thus protected the
cerebral ischemia-damaged neurons (15). One of the most commonly used models
for studying stroke in rats is the middle cerebral artery occlusion
(MCAO) model (16). MCAO is one of
the most severe types of stroke in clinic and the most common type
of ischemia (17). During the
process of model establishment in the current study, the MCA was
occluded with a filament, resulting in focal infarct to the
ipsilateral hemisphere.
The present study examined the anti-apoptotic effect
of SJZD in rats, following cerebral ischaemia-reperfusion.
Nimodipine is an effective medicine for the treatment of stroke,
and therefore was used as positive control treatment in the current
study. Tissue inhibitor of metalloproteinase 1 (TIMP-1), matrix
metalloproteinase 9 (MMP-9), collagen IV (COL IV), cell apoptotic
rate and the blood-brain barrier (BBB) were examined. The results
of the present study may highlight a neuroprotective effect of SJZD
in cerebral ischaemia-reperfusion.
Materials and methods
Animals and experimental design
The present study was a randomized, controlled
animal experiment, performed at the Hunan Provincial Key Laboratory
of Diagnostics in Chinese Medicine (Changsha, China). A total of
150 male Sprague-Dawley rats (weight, 250–280 g; age, 10–11 weeks)
were supplied by the Laboratory Animal Centre of Hunan University
of Chinese Medicine [license no. SYXK (Xiang) 2013–0005; Changsha,
China]. The rats were housed separately in plastic cages at 22°C
and 60% humidity under a light-dark cycle (12 h for each) and
allowed free access to food and water. The animals were randomly
divided into the following six groups (n=25 per group): Sham group
and untreated MCAO model group, nimodipine MCAO model group (10.8
mg/kg), and SJZD low dose (3 g/kg), SJZD medium dose (6 g/kg) and
SJZD high dose (9 g/kg) MCAO model groups. The nimodipine and SJZD
groups received corresponding treatment administrated via gastric
gavage once daily for 14 days after model establishment. The
animals in the sham and untreated model groups were administered
0.9% saline solution only via gastric gavage once daily for 14 days
after model establishment. The present study was approved by the
Brains Hospital of Hunan Province Ethics Committee (permit number,
Z2015003; Changsha, China). All procedures were performed in
accordance with the Guidance Suggestions for the Care and Use of
Laboratory Animals, formulated by the Ministry of Science and
Technology of China.
Chemicals and reagents
SJZD is composed of Radix et Rhizoma Ginseng,
Rhizome Atractylodis macrocephala, Wolfiporia extensa and
Radix et Rhizoma Glycyrrhizae (the ratio was 3:3:3:2). All herbs
(from Good Agricultural Practice planting base) were purchased from
LBX Pharmacy Chain Co., Ltd. All ingredients were identified by
experts from the School of Pharmacy, Hunan University of Chinese
Medicine to fulfil the quality requirements of the Pharmacopoeia of
the People's Republic of China (18). The mixture was decocted in boiling
water for 45 min and vacuum-dried to yield a final concentration of
2 g crude drug/ml. The crude drug was dissolved in distilled water
and administered at 3, 6 and 9 g/kg in accordance with a
preliminary pharmacology experiment performed in Provincial Key
Laboratory of TCM Diagnostics (Hunan University of Chinese
Medicine, Changsha, China).
Nimodipine tablets (20 mg; cat. no. BG28956; Bayer
AG) were dissolved in distilled water and 10.8 mg/kg was
administered in accordance with pharmacology experiments performed
in Provincial Key Laboratory of TCM Diagnostics.
Polyclonal rabbit antibodies against TIMP-1 (cat.
no. 10753-1-AP), MMP-9 (cat. no., 10375-2-AP) and COL IV (cat. no.
24460-1-AP) were purchased from ProteinTech Group, Inc. The PV-9000
2-step plus poly-HRP anti-mouse/rabbit IgG Detection system (cat.
no. WP140316) and DAB kit (cat. no. K145619C) were purchased from
Beijing Zhongshan Golden Bridge Co., Ltd. Evans blue (EB; cat. no.
46160) was purchased from Sigma-Aldrich; Merck KGaA. The TUNEL
detection kit for assessing cell apoptosis (cat. no. KGA7023-A) was
purchased from Nanjing KeyGen Biotech Co., Ltd.
MCAO model establishment
The MCAO model was established as described
previously (19). Rats were
anaesthetized by intraperitoneal (i.p.) injection of 2%
pentobarbital sodium (30 mg/kg), and placed in a supine position.
The left common carotid artery (CCA), external carotid artery (ECA)
and internal carotid artery (ICA) were exposed and isolated
carefully. The CCA and ICA were clipped using artery clamps. The
ECA was ligated and cut ~5 mm from the CCA bifurcation. A
0.26-mm-diameter nylon filament (tip diameter 0.36±0.02 mm; Beijing
Sunbio Biotech Co., Ltd.) was inserted through the ECA stump and
gently advanced 10 mm to occlude the origin of the middle cerebral
artery. The filament was removed to restore blood flow after 1.5 h
of MCAO (reperfusion). Rats in the sham group underwent the same
surgical procedure, however the filament was not inserted as far as
the CCA bifurcation. The rats were housed individually and allowed
to recover. When they woke up, Horner's syndrome was observed. One
of the characteristics of Horner's syndrome is partial ptosis that
occurs on the same side (ipsilateral) (20). Neurobehavioural scores were assessed
using a five-grade scale (21) 2 h
after model establishment and 14 d after administration of
treatment, and the severity of neurological symptoms was graded on
a scale of 0–4 with slight modifications: 0, no functional
impairment; 1, cannot extend right forepaw; 2, whirls to the right;
3, leans to the right while walking; and 4, no autonomic activities
in combination with being unconscious. Rats with scores <2 were
excluded. The behavioural observations were performed in a blinded
manner.
Evaluation of BBB integrity
BBB integrity was assessed by determining EB
extravasation in 8 rats per group. EB dye (2% in saline, 4 ml/kg)
was injected into the right femoral vein 30 min before reperfusion.
Rats were deeply anaesthetized with 2% pentobarbital sodium (30
mg/kg; i.p.), perfused with PBS through the left ventricle to
remove the intravascular dye until a colourless perfusion fluid was
observed from the right atrium. Rats were executed by cervical
dislocation. The hippocampus of the ischaemic side was removed
quickly, weighed and homogenized in 2.5 ml PBS. The homogenized
solution was mixed with 2.5 ml trichloroacetic acid (60%) and
centrifuged at 1,640 × g for 20 min at 4°C. The supernatant was
used to determine EB absorbance at 620 nm using a
spectrophotometer. EB concentrations were calculated against a
standard curve, and the obtained results were expressed as µg/g
brain tissue.
Brain slice preparation
Rats were anaesthetized as aforementioned after 14
days of treatment administration, perfused through the heart with
0.9% saline solution (100 ml) followed by 4% paraformaldehyde in
0.1 M phosphate buffered saline, pH 7.4 at 4°C (150 ml). The brain
was removed immediately and cut coronally at the levels of the
optic chiasma and the posterior pole of the cerebrum. The area
between these two coronal sections was kept for
immunohistochemistry and TUNEL staining. A total of 8 samples from
each group were used for paraffin sectioning and detection of COL
IV expression. The section thickness was 5 µm. For TUNEL staining
and detection of TIMP-1 and MMP-9 expression, 9 samples from each
group were cut with a Leica VT1200S vibrating-blade microtome
(Leica Microsystems GmbH). Tissue blocks were adhered vertically to
the specimen holder of the vibrating-blade microtome. The clearance
angle was adjusted to 18°, the blade holder sectioning speed was
controlled with a motor at 0.5 mm/s, the amplitude was 2 mm, and
the thickness of each slice was 30 µm. Slices were collected in
24-well plates for TUNEL staining and for detecting the expression
of TIMP-1 and MMP-9.
Immunohistochemistry
TIMP-1, MMP-9 and COL IV expression was measured
using immunohistochemistry. Sections were immersed in hydrogen
peroxide (0.3% in PBS) for 10 min and incubated overnight at 4°C
with the following polyclonal rabbit anti-rat antibodies: TIMP-1
(1:100), MMP-9 (1:100) and COL IV (1:150). Slices were incubated
for 30 min at 37°C with ready-to-use poly-HRP anti-mouse/rabbit IgG
and detected using the DAB kit for 15 min at 37°C. Brownish yellow
particles in the nucleus or cytoplasm of cells in the hippocampus
indicated positive expression for TIMP-1 and MMP-9 proteins, and
the presence of these particles in the ECM indicated positive
staining for COL IV. A total of four sections from each specimen
were imaged, and six visual fields were selected randomly in each
section. Slices were observed under an optical microscope (Motic
B5; Motic China Group Co., Ltd.) at a magnification of ×400. The
average optical density (AOD) was calculated with Image-Pro
software (version 6.0.0.260; Media Cybernetics Inc.).
TUNEL staining
TUNEL staining was performed to quantify cell
apoptosis using a TUNEL Detection kit, according to the
manufacturer's instructions. Briefly, sections were fixed with 4%
paraformaldehyde at 37°C for 20 min, immersed in the 3%
H2O2 at 37°C for 10 min, and dipped into 0.1%
TritonX-100 on ice for 2 min. Biotin-dUTP (50 µl) from the kit was
added to each section and then incubated at 37°C for 60 min,
followed by incubation with 50 µl streptavidin-HRP from the kit at
37°C for 30 min. After detection with the DAB kit for 10 min at
37°C, sections were sealed with neutral gum. Six images of randomly
selected visual fields were taken for each section with a Motic B5
optical microscope at ×400 magnification. TUNEL-positive cells in
the ipsilateral hippocampus were observed and quantified with
Image-Pro software (version 6.0.0.260). Brownish yellow particles
in the nuclei of the cells in the hippocampal sections indicated
TUNEL-positive, apoptotic cells. The apoptotic rate was calculated
quantified with Image-Pro software (version 6.0.0.260).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 24.0; IBM Corp.). Data were analysed using
one-way ANOVA followed by the least significant difference test or
Dunnett's T3 test. The results are expressed as the mean ± SEM.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Status of rats following MCAO model
establishment
In the untreated MCAO model group, 2 rats died on
days 2 and 5 after surgery due to subarachnoid haemorrhage and
cerebral oedema induced by ischaemia, respectively. In the
nimodipine group, 1 rat died on day 6 after surgery due to
subarachnoid haemorrhage. In the SJZD low dose group, 2 rats died
on days 3 and 4 after surgery due to subarachnoid haemorrhage and
cerebral oedema, respectively. In the SJZD medium dose group, 2
rats died of subarachnoid haemorrhage on days 2 and 4 after
surgery. No neurobehavioural scores of the surviving rats were
<2, and no rats were excluded.
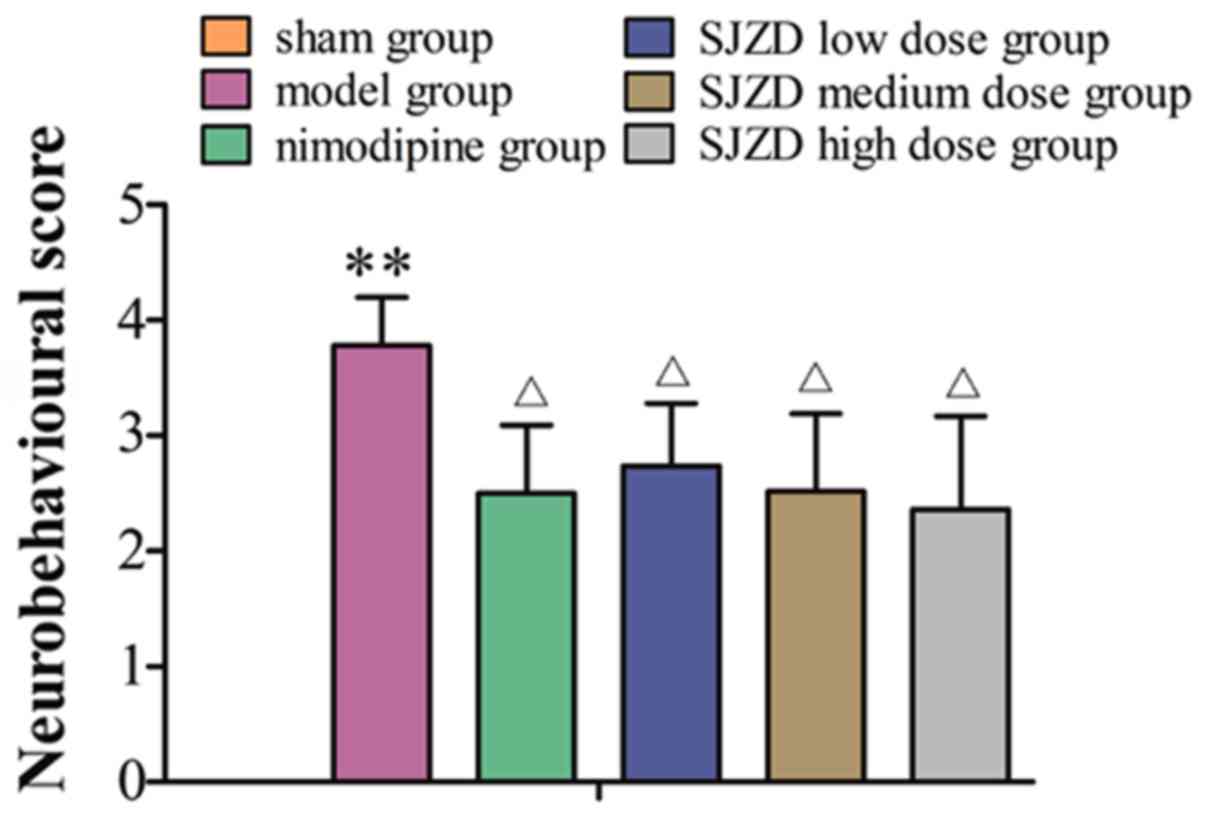
SJZD treatment improves
neurobehavioural scores following MCAO
No neurobehavioural abnormalities of the
contralateral limb were observed in the sham group. Horner's
syndrome could be observed in rats in the model groups. These rats
exhibited paralysis of the contralateral limb, turned or rotated to
the contralateral side, limped, and appeared lethargic and
inactive. Rats failed to abduct their contralateral forelimb when
their tails were held or were not able to independently move their
contralateral limbs and demonstrated spontaneous rotation to the
contralateral side. The sham group had neurobehavioural scores of
0. The neurobehavioural scores were markedly higher in the model
group compared with the sham group (P<0.01), and were
significantly decreased in the SJZD- and nimodipine-treated groups
compared with the untreated model group (P<0.05; Fig. 1).
High-dose SJZD treatment decreases EB
extravasation following MCAO
As shown in Fig. 2,
EB extravasation was significantly increased in the model group
compared with the sham group (P<0.01). Compared with the
untreated model group, EB extravasation decreased in the SJZD
high-dose and nimodipine groups (both P<0.01). EB extravasation
in the SJZD low and medium-dose groups was not significantly
different to that in the model group. Extravasation in the SJZD
high-dose group was lower than that in the SJZD low- and
medium-dose groups (P<0.01).
SJZD treatment impacts expression of
TIMP-1, MMP-9 and COL IV in the hippocampus following MCAO
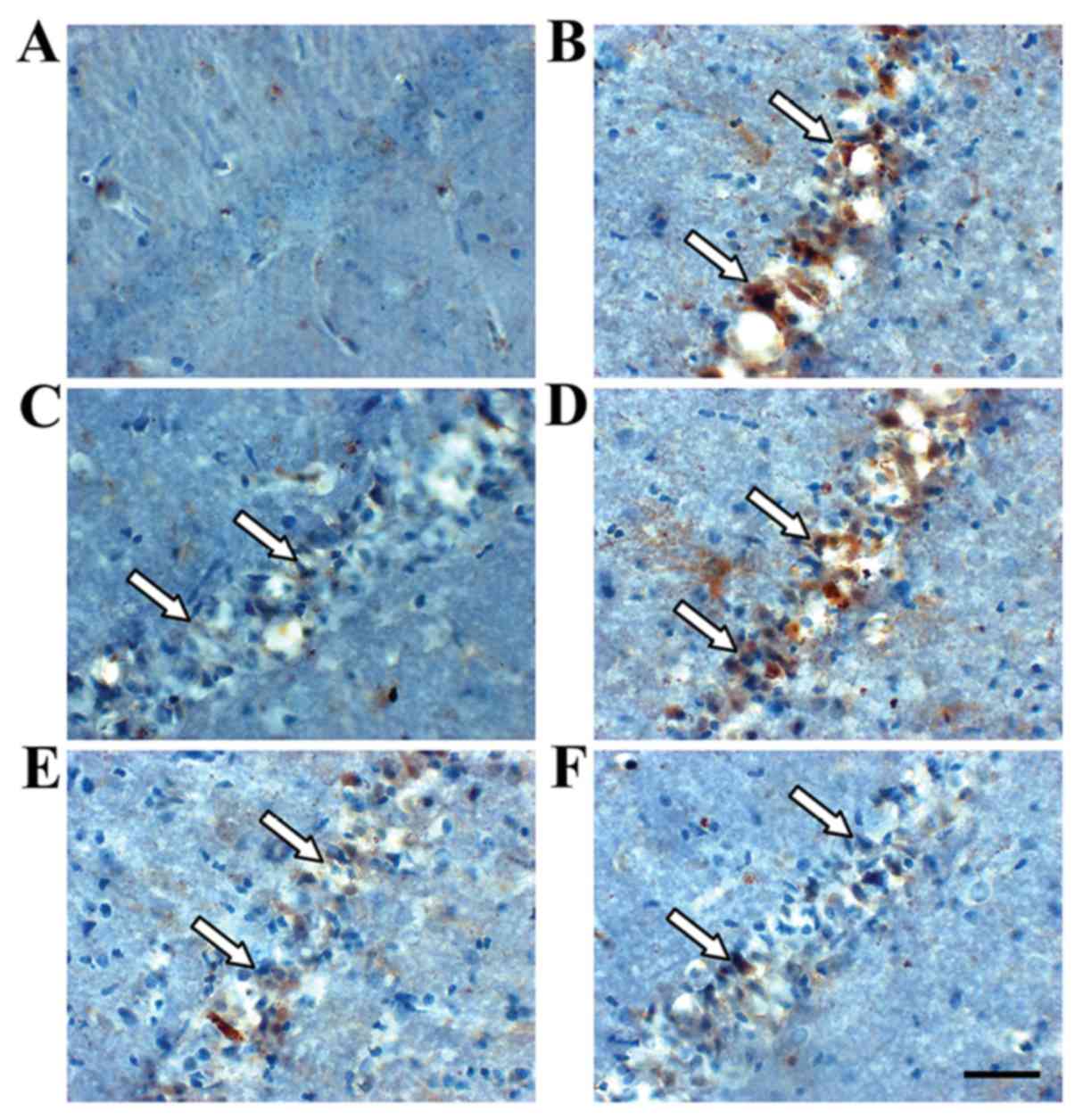
Immunohistochemistry revealed TIMP-1- and
MMP-9-positive cells in the hippocampus (Figs. 3 and 4). TIMP-1 and MMP-9 expression increased
significantly in the hippocampus of the model group compared with
the sham group (P<0.01; Fig. 6).
COL IV decreased in the hippocampus of the model group compared
with the sham group (P<0.01; Figs.
5 and 6). TIMP-1 and COL IV
expression increased significantly, and MMP-9 expression decreased
significantly in all SJZD and nimodipine groups compared with the
untreated model group (P<0.01). TIMP-1 and COL IV expression
decreased, and MMP-9 expression increased in the SJZD low- and
medium-dose groups compared with the SJZD high-dose group
(P<0.01; Fig. 6).
SJZD treatment decreases the apoptotic
rate in the hippocampus following MCAO
TUNEL staining identified apoptotic cells in the
hippocampus (Fig. 7). The apoptotic
rate increased in the hippocampus of the model group compared to
the sham group, and SJZD and nimodipine treatment decreased
apoptotic rate compared with that observed in the untreated model
group. The apoptotic rate was significantly lower in the SJZD
high-dose group than in the low- and medium-dose groups (Fig. 8).
Discussion
In TCM, most doctors choose classic methods to treat
stroke, including blood-activating stasis removal, replenishment of
Qi and blood, acupuncture, heat dissipation and detoxification
(22–26). Our previous studies found that
modified SJZD improved neurobehavioural function, decreased the
neuronal apoptosis index and MMP-2 expression, and increased
laminin expression in the ECM after cerebral ischaemia-reperfusion
(27–29). However, the mechanism underlying this
therapeutic effect is not known. Damage and degradation of the ECM
plays a key role during cerebral ischaemia-reperfusion (30–32).
The ECM is composed of a conglomerate assembly of
proteins and proteoglycans, and it provides mechanical support and
physical strength to the integrity of tissues, organs and the
entire body (33,34). The ECM is necessary for the CNS, as
it unites neurons and glial cells to form its basic structure
(35,36). The triggering of intracellular and
extracellular proteolytic processes during cerebral ischaemia often
results in ECM degradation, which leads to oedema, haemorrhage,
glial cell activation, inflammatory cell infiltration and neuronal
injury (37). The ECM is also a
constituent of the BBB (38,39). The BBB maintains the stability of the
internal environment in the CNS (40), and it is composed of vascular
endothelial cells, tight junctions of endothelial cells, basement
membrane and the end feet of surrounding astrocytes (41,42). COL
IV is a major component of the basement membrane (43,44). It
offers adequate adhesion for nerve growth as it guides the nerve
fibre to grow directionally along the matrix bridge (45). In the present study, it was
hypothesised that SJZD treatment may protect against damage due to
cerebral ischaemia by stabilising the ECM.
The primary components of the basement membrane are
substrates of MMP-9, which is involved in the regulation of nearly
all ECM components (46,47). A previous study demonstrated that
MMPs usually exist as proenzymes in the CNS (48). Activated MMPs destroy the tight
junctions of endothelial cells, the basement membrane of
capillaries and cerebrovascular integrity, which results in aseptic
inflammation and BBB destruction (49). These changes cause blood constituent
extravasation, vasogenic brain oedema and cerebral haemorrhage
(50). MMP-9 activates other MMPs,
which leads to a waterfall effect, and its increasing expression is
associated with cerebrovascular diseases (51). Decreasing expression of MMP-9
inhibits aseptic inflammation and BBB destruction to a certain
extent (52), and avoids neuronal
damage and apoptosis (53). The
results show that MMP-9 expression increased significantly,
indicating that it has a key role during MCAO.
TIMPs are specific endogenous inhibitors of MMPs
(54). Cysteine residues in the
active site of TIMPs combine with the zinc-ion active centre of
activated MMPs to inhibit MMP binding with substrates, preventing
ECM degradation (55). Therefore,
TIMPs are a potential target for the treatment of cerebral
ischaemia-reperfusion (56).
MMPs and TIMPs are key factors that regulate the BBB
integrity (57). Cerebrovascular
occlusion results in secondary haemorrhage and brain oedema due to
BBB destruction, plasma extravasation and leukocyte infiltration
(58–60). It was reported BBB destruction in the
present MCAO model increased the degree of neurological impairment
and cerebral infarction (61). In
the current study, the expression of MMP-9, TIMP-1 and COL IV
changed remarkably after SJZD treatment. These studies suggest that
TIMPs and MMPs may mediate the anti-anoikis mechanism of SJZD
treatment via ECM stabilization in cerebral
ischaemia-reperfusion.
The present study found that the permeability of the
BBB in the hippocampus, as well as MMP-9 expression and apoptotic
rate increased following MCAO, and COL IV expression significantly
decreased. The increased expression of TIMP-1 after MCAO was
consistent with previous reports (62,63).
SJZD treatment decreased BBB permeability, MMP-9 expression and
apoptotic rate, and increased COL IV and TIMP-1 expression. The
higher dose of SJZD was found to be as effective as nimodipine.
These results suggest that this invigorating spleen therapy may
protect neurons in cerebral ischaemia-reperfusion via stabilization
of the ECM, particularly when used at a higher dose. These data are
preliminary, and further studies are needed to confirm the
presented results.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (nos. 81373551,81473567 and
81603512), Key Science and Research Program of Hunan Department of
Science and Technology (no. 60010408), Project of Hunan Provincial
Education Department (no. 14B134) and China Postdoctoral Science
Foundation (nos. 2017T100604 and 2016M600632).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and HYH designed the study. PY, YMT and WXD
performed the experiments. WHL participated in the study design and
data collection. PY and XC performed statistical analyses. PY wrote
the manuscript, and LL revised the manuscript.
Ethics approval and consent to
participate
The current study was approved by the Brains
Hospital of Hunan Province Ethics Committee (grant no.
Z2015003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ECM
|
extracellular matrix
|
|
SJZD
|
Sijunzi decoction
|
|
TIMP-1
|
tissue inhibitor of metalloproteinase
1
|
|
MMP-9
|
matrix metalloproteinase 9
|
|
COL IV
|
collagen IV
|
|
MCAO
|
middle cerebral artery occlusion
|
|
EB
|
Evans blue
|
|
BBB
|
blood-brain barrier
|
|
TCM
|
Traditional Chinese Medicine
|
|
CNS
|
central nervous system
|
References
|
1
|
Arnberg F, Grafström J, Lundberg J,
Nikkhou-Aski S, Little P, Damberg P, Mitsios N, Mulder J, Lu L,
Söderman M, et al: Imaging of a clinically relevant stroke model:
Glucose hypermetabolism revisited. Stroke. 46:835–842. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jickling GC, Liu D, Ander BP, Stamova B,
Zhan X and Sharp FR: Targeting neutrophils in ischemic stroke:
Translational insights from experimental studies. J Cereb Blood
Flow Metab. 35:888–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meschia JF, Bushnell C, Boden-Albala B,
Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind
MS, Fornage M, et al: Guidelines for the primary prevention of
stroke: A statement for healthcare professionals from the American
Heart Association/American Stroke Association. Stroke.
45:3754–3832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lv Z, Liu C, Zhai M, Zhang Q, Li J, Zheng
F and Peng M: LPS Pretreatment attenuates cerebral
ischaemia/reperfusion injury by inhibiting inflammation and
apoptosis. Cell Physiol Biochem. 45:2246–2256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carneiro BR, Pernambuco Filho PC, Mesquita
AP, da Silva DS, Pinhal MA, Nader HB and Lopes CC: Acquisition of
anoikis resistance up-regulates syndecan-4 expression in
endothelial cells. PLoS One. 9:e1160012014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hill JW, Poddar R, Thompson JF, Rosenberg
GA and Yang Y: Intranuclear matrix metalloproteinases promote DNA
damage and apoptosis induced by oxygen-glucose deprivation in
neurons. Neuroscience. 220:277–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sater AP, Rael LT, Tanner AH, Lieser MJ,
Acuna DL, Mains CW and Bar-Or D: Cell death after traumatic brain
injury: Detrimental role of anoikis in healing. Clin Chim Acta.
482:149–154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo Z, Sun X, He Z, Jiang Y and Zhang X:
Role of matrix metalloproteinase-9 in apoptosis of hippocampal
neurons in rats during early brain injury after subarachnoid
hemorrhage. Neurol Sci. 31:143–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frisch SM, Schaller M and Cieply B:
Mechanisms that link the oncogenic epithelial-mesenchymal
transition to suppression of anoikis. J Cell Sci. 126:21–29. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mazumder MK, Bhattacharya P and Borah A:
Inhibition of matrix metalloproteinase-2 and 9 by Piroxicam confer
neuroprotection in cerebral ischemia: An in silico evaluation of
the hypothesis. Med Hypotheses. 83:697–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheema ZF, Santillano DR, Wade SB, Newman
JM and Miranda RC: The extracellular matrix, p53 and estrogen
compete to regulate cell-surface Fas/Apo-1 suicide receptor
expression in proliferating embryonic cerebral cortical precursors,
and reciprocally, Fas-ligand modifies estrogen control of
cell-cycle proteins. BMC Neurosci. 5:112004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian G, Wu C, Li J, Liang B, Zhang F, Fan
X, Li Z, Wang Y, Li Z, Liu D, et al: Network pharmacology based
investigation into the effect and mechanism of modified sijunzi
decoction against the subtypes of chronic atrophic gastritis.
Pharmacol Res. 144:158–166. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Qian J, Jia JG, Jin X, Yu DJ, Xie B,
Qian LY, Zhang LG and Guo CX: Effect of Sijunzi decoction on the
proliferation of side population cells of human gastric cancer cell
line. Zhongguo Zhong Xi Yi Jie He Za Zhi. 34:704–709. 2014.(In
Chinese). PubMed/NCBI
|
|
14
|
Hu KL, Li H, Liu WH, He Q, Zhong YL and
Peng ZY: Effect of modified Si Junzitang on expression of ERK1/2,
Akt, Bax in rats with cerebral ischemia/reperfusion injury. Chin J
Exp Traditional Med Formulae. 24:152–158. 2008.(In Chinese).
|
|
15
|
Liu S, Zhang YB, Cao WG, Zhang D and Liu
L: Comparision of the effects of three Chinese medicinal recipes to
cerebral ischemia. Pharmacol Clin Chin Materia Medica. 25:6–7.
2009.(In Chinese).
|
|
16
|
Liu F and McCullough LD: Middle cerebral
artery occlusion model in rodents: Methods and potential pitfalls.
J Biomed Biotechnol. 2011:4647012011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ng YS, Stein J, Ning M and Black-Schaffer
RM: Comparison of clinical characteristics and functional outcomes
of ischemic stroke in different vascular territories. Stroke.
38:2309–2314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chinese Pharmacopoeia Commission, .
Pharmacopoeia of the People's Republic of China. 1. 2015th. Chinese
Medical Science and Technology Press; Beijing: pp. 8–240. 2015
|
|
19
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Q, Zhou M, Wu X, Li Z, Liu B, Gao W,
Yue J and Liu T: Promoting therapeutic angiogenesis of focal
cerebral ischemia using thrombospondin-4 (TSP4) gene-modified bone
marrow stromal cells (BMSCs) in a rat model. J Transl Med.
17:1112019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao FL, Han XF, Wang XL, Zhao ZR, Guo AH,
Lu XJ and Zhao XF: The neurovascular protective effect of
alogliptin in murine MCAO model and brain endothelial cells. Biomed
Pharmacother. 109:181–187. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu JH, Huang YM, Ling W, Li Y, Wang M,
Chen XY, Sui Y and Zhao HL: Wen Dan Decoction for hemorrhagic
stroke and ischemic stroke. Complement Ther Med. 23:298–308. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu HX, Lin RH, Zhu XQ, Li ZF and Chen LD:
Anti-inflammatory effects of Gualou Guizhi decoction in transient
focal cerebral ischemic brains [Corrected]. Mol Med Rep.
12:1321–1327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo Q, Zhong M, Xu H, Mao X, Zhang Y and
Lin N: A systems biology perspective on the molecular mechanisms
underlying the therapeutic effects of buyang huanwu decoction on
ischemic stroke. Rejuvenation Res. 18:313–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang DS, Liu YL, Zhu DQ, Huang XJ and Luo
CH: Point application with angong niuhuang sticker protects
hippocampal and cortical neurons in rats with cerebral ischemia.
Neural Regen Res. 10:286–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang C, Wen Y, Fan XN, Tian G, Zhou XY,
Deng SZ and Meng ZH: Therapeutic effects of different durations of
acupuncture on rats with middle cerebral artery occlusion. Neural
Regen Res. 10:159–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong YL, Li H, Liu WH and Hu D: The
effect of spleen-strengthening therapy on the mRNA and protein
expression levels of INTβ3, ILK and FAK in Rats with cerebral
ischemia/reperfusion injury. Lishizhen Med Mat Med Res. 28:513–516.
2017.(In Chinese).
|
|
28
|
Li H, Liu WH, Zhou XQ and He Q: Effects of
spleen-strengthening therapy on MMP2 expression in brain tissue and
blood brain barrier permeability in rats with cerebral
ischemia/reperfusion injury. Hunan J TCM. 29:115–117. 2013.
|
|
29
|
Li H, Liu WH, Liao LY, Liu BY and Cai GX:
Effect of tonifying spleen therapy on the degradation of laminin in
rats with cerebral ischemia-reperfusion. Chin J Geriatr Heart Brain
Vessel Dis. 12:645–647. 2010.(In Chinese).
|
|
30
|
Adamczak JM, Schneider G, Nelles M, Que I,
Suidgeest E, van der Weerd L, Löwik C and Hoehn M: In vivo
bioluminescence imaging of vascular remodeling after stroke. Front
Cell Neurosci. 8:2742014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reinhard J, Renner M, Wiemann S, Shakoor
DA, Stute G, Dick HB, Faissner A and Joachim SC: Ischemic injury
leads to extracellular matrix alterations in retina and optic
nerve. Sci Rep. 7:434702017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ravi S, Caves JM, Martinez AW, Xiao J, Wen
J, Haller CA, Davis ME and Chaikof EL: Effect of bone
marrow-derived extracellular matrix on cardiac function after
ischemic injury. Biomaterials. 33:7736–7745. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma NK, Lim JK, Leong MF, Sandanaraj E, Ang
BT, Tang C and Wan AC: Collaboration of 3D context and
extracellular matrix in the development of glioma stemness in a 3D
model. Biomaterials. 78:62–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Klingberg F, Chow ML, Koehler A, Boo S,
Buscemi L, Quinn TM, Costell M, Alman BA, Genot E and Hinz B:
Prestress in the extracellular matrix sensitizes latent TGF-β1 for
activation. J Cell Biol. 207:283–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Faissner A and Reinhard J: The
extracellular matrix compartment of neural stem and glial
progenitor cells. Glia. 63:1330–1349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dzyubenko E, Gottschling C and Faissner A:
Neuron-glia interactions in neural plasticity: Contributions of
neural extracellular matrix and perineuronal nets. Neural Plast.
2016:52149612016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pandey AK, Bhattacharya P, Shukla SC, Paul
S and Patnaik R: Resveratrol inhibits matrix metalloproteinases to
attenuate neuronal damage in cerebral ischemia: A molecular docking
study exploring possible neuroprotection. Neural Regen Res.
10:568–575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neuwelt EA, Bauer B, Fahlke C, Fricker G,
Iadecola C, Janigro D, Leybaert L, Molnár Z, O'Donnell ME,
Povlishock JT, et al: Engaging neuroscience to advance
translational research in brain barrier biology. Nat Rev Neurosci.
12:169–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Limmer S, Weiler A, Volkenhoff A, Babatz F
and Klämbt C: The Drosophila blood-brain barrier: Development and
function of a glial endothelium. Front Neurosci. 8:3652014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ronaldson PT and Davis TP: Blood-brain
barrier integrity and glial support: Mechanisms that can be
targeted for novel therapeutic approaches in stroke. Curr Pharm
Des. 18:3624–3644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wong AD, Ye M, Levy AF, Rothstein JD,
Bergles DE and Searson PC: The blood-brain barrier: An engineering
perspective. Front Neuroeng. 6:72013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hladky SB and Barrand MA: Fluid and ion
transfer across the blood-brain and blood-cerebrospinal fluid
barriers; a comparative account of mechanisms and roles. Fluids
Barriers CNS. 13:192016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sazonova OV, Isenberg BC, Herrmann J, Lee
KL, Purwada A, Valentine AD, Buczek-Thomas JA, Wong JY and Nugent
MA: Extracellular matrix presentation modulates vascular smooth
muscle cell mechanotransduction. Matrix Biol. 41:36–43. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li S, Jin Z, Koirala S, Bu L, Xu L, Hynes
RO, Walsh CA, Corfas G and Piao X: GPR56 regulates pial basement
membrane integrity and cortical lamination. J Neurosci.
28:5817–5826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Johnson KM, Milner R and Crocker SJ:
Extracellular matrix composition determines astrocyte responses to
mechanical and inflammatory stimuli. Neurosci Lett. 600:104–109.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moore MC, Pandolfi V and McFetridge PS:
Novel human-derived extracellular matrix induces in vitro and in
vivo vascularization and inhibits fibrosis. Biomaterials. 49:37–46.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Z, Wang F, Wang BJ, Chu G, Cao Q,
Sun BG and Dai QY: Inhibition of leptin-induced vascular
extracellular matrix remodelling by adiponectin. J Mol Endocrinol.
53:145–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xie Y, Mustafa A, Yerzhan A, Merzhakupova
D, Yerlan P, N Orakov A, Wang X, Huang Y and Miao L: Nuclear matrix
metalloproteinases: Functions resemble the evolution from the
intracellular to the extracellular compartment. Cell Death Discov.
3:170362017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chopra K, Baveja A and Kuhad A: MMPs: A
novel drug target for schizophrenia. Expert Opin Ther Targets.
19:77–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fu S, Gu Y, Jiang JQ, Chen X, Xu M, Chen X
and Shen J: Calycosin-7-O-β-D-glucoside regulates nitric
oxide/caveolin-1/matrix metalloproteinases pathway and protects
blood-brain barrier integrity in experimental cerebral
ischemia-reperfusion injury. J Ethnopharmacol. 155:692–701. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang JJ, Huan SK, Hsieh KH, Chou HC, Hsiao
G, Jayakumar T and Sheu JR: Inhibitory effect of midazolam on
MMP-9, MMP-1 and MMP-13 expression in PMA-stimulated human
chondrocytes via recovery of NF-kB signaling. Arch Med Sci.
9:332–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
He ZJ, Huang ZT, Chen XT and Zou ZJ:
Effects of matrix metalloproteinase 9 inhibition on the blood brain
barrier and inflammation in rats following cardiopulmonary
resuscitation. Chin Med J (Engl). 122:2346–2351. 2009.PubMed/NCBI
|
|
53
|
Kwon M, Seo S, Chun H, Chung JM, Chung IK
and Hur KC: Dual effect of nerve growth factor on cell death of
PC12 cells Induced by serum deprivation. Mol Cells. 13:167–174.
2002.PubMed/NCBI
|
|
54
|
Kim YS and Joh TH: Matrix
metalloproteinases, new insights into the understanding of
neurodegenerative disorders. Biomol Ther (Seoul). 20:133–143. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li JS, Liu K, Liu JX, Wang MH, Zhao YW and
Liu ZG: Relationship between the changes in ischemia/reperfusion
cerebro-microvessel basement membrane injury and gelatinase system
in senile rat. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 20:656–659.
2008.(In Chinese). PubMed/NCBI
|
|
57
|
Michalski D, Hobohm C, Weise C, Pelz J,
Heindl M, Kamprad M, Kacza J and Härtig W: Interrelations between
blood-brain barrier permeability and matrix metalloproteinases are
differently affected by tissue plasminogen activator and hyperoxia
in a rat model of embolic stroke. Med Gas Res. 2:22012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu XR, Luo M, Yan F, Zhang CC, Li SJ,
Zhao HP, Ji XM and Luo YM: Ischemic postconditioning diminishes
matrix metalloproteinase 9 expression and attenuates loss of the
extracellular matrix proteins in rats following middle cerebral
artery occlusion and reperfusion. CNS Neurosci Ther. 18:855–863.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gu Y, Dee CM and Shen J: Interaction of
free radicals, matrix metalloproteinases and caveolin-1 impacts
blood-brain barrier permeability. Front Biosci (Schol Ed).
3:1216–1231. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhu M, Xing D, Lu Z, Fan Y, Hou W and Dong
H, Xiong L and Dong H: DDR1 may play a key role in destruction of
the blood-brain barrier after cerebral ischemia-reperfusion.
Neurosci Res. 96:14–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tan F, Fu W, Cheng N, Meng DI and Gu Y:
Ligustrazine reduces blood-brain barrier permeability in a rat
model of focal cerebral ischemia and reperfusion. Exp Ther Med.
9:1757–1762. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li DD, Song JN, Huang H, Guo XY, An JY,
Zhang M, Li Y, Sun P, Pang HG, Zhao YL and Wang JF: The roles of
MMP-9/TIMP-1 in cerebral edema following experimental acute
cerebral infarction in rats. Neurosci Lett. 550:168–172. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lenglet S, Montecucco F, Mach F, Schaller
K, Gasche Y and Copin JC: Analysis of the expression of nine
secreted matrix metalloproteinases and their endogenous inhibitors
in the brain of mice subjected to ischaemic stroke. Thromb Haemost.
112:363–378. 2014. View Article : Google Scholar : PubMed/NCBI
|