Introduction
Computed tomography (CT) is an important method for
the diagnosis of cerebral cavernous malformation (CCM) prior to the
clinical application of magnetic resonance (MR). However, only
large CCM lesions complicated with hemorrhage and calcification are
visible in a CT scan. In addition, diagnoses of microangioma or CCM
in the posterior cranial fossa and brainstem have been missed
(1,2). Magnetic resonance imaging (MRI) has
gradually improved the sensitivity of the diagnosis of CCM
following the widespread use of MR, but certain sequences have
conspicuous limitations. The definitive diagnosis rate of CCM
almost equals that obtained from autopsy results when
T2*-weighted gradient echo imaging (GRE
T2*-WI) is used (3,4). GRE
T2*-WI has been considered to be the most
important diagnostic tool for CCM. Twelve patients, of which 2 were
diagnosed with familial cerebral cavernous malformation (FCCM) from
August, 2005 to June, 2007, were easily diagnosed with CCM by GRE
T2*-WI following brain CT and conventional
MRI examination.
Materials and methods
Patients
Twelve of 26 members in two families were diagnosed
with FCCM (8 male, 4 female, aged 8 to 74 years, mean age 36.5).
All patients were subjected to brain CT and conventional MR scans
prior to GRE T2*-WI. Various symptoms were
recorded, including repeated history of headache and dizziness (4
patients), hemiparalysis (4 patients), hemianesthesia (3 patients),
speech disfluency (2 patients), hydroposia bucking and dysphagia (2
patients), seizure (1 patient), hemiablepsia (1 patient), skin
angioma (6 patients) and asymptomatic manifestations (6 patients).
Fourteen individuals presented with no clinical symptoms and no
foci on MR scans. Each family member exhibited no vascular
malformations upon examination of the fundus of the eye. All
participants provided written informed consent before entering the
study. The present study was approved by the ethics committee of
Qilu Hospital of Shandong University (Jinan city, Shandong,
China).
MRI
All patients underwent conventional MRI using a 3.0T
scanner (GE Signa EXCITE II; General Electric, Waukesha, WI, USA),
including transverse and axial T1-weighted imaging
(T1WI), T2-weighted imaging
(T2WI), T2-fluid-attenuated inversion
recovery (T2Flair), diffusion weighted imaging (DWI),
magnetic resonance angiography (MRA) and spin-echo imaging (SE)
sequences. GRE T2*-WI was carried out
simultaneously [repeat time/echo time (TR/TE), 520/20 ms; flip
angle 20°; section thickness, 6 mm; field of view (FOV), 24x18;
matrix, 512x256]. The patients underwent a CT scan prior to
MRI.
MRI analysis
Two independent neuroradiologists evaluated the CT
and MR images. First, they ascertained whether pathological changes
were present in the brain. Second, they decided whether the changes
were CCM and identified the different CCM foci shown on various MR
sequences. Finally, they determined the location, size and quantity
of vascular malformation based on the locations of CCM
(cortex-subcortex, basal ganglia, thalamencephalon, brainstem and
cerebellum).
Statistical analysis
Data were expressed as the means ± standard
deviation. One-way ANOVA and the Newman-Keuls test were used for
statistical analysis of the results as appropriate. P<0.05 was
considered to indicate a statistically significant result.
Results
Features of CCM in MRI and CT images
Only 3 patients had larger vascular malformations
visible in CT scans. The foci were irregular hypodense and
hyperdense on the CT scans, usually complicated with hemorrhage and
calcification, and were surrounded by a distinct boundary without
edema or occupied effect (Fig. 1).
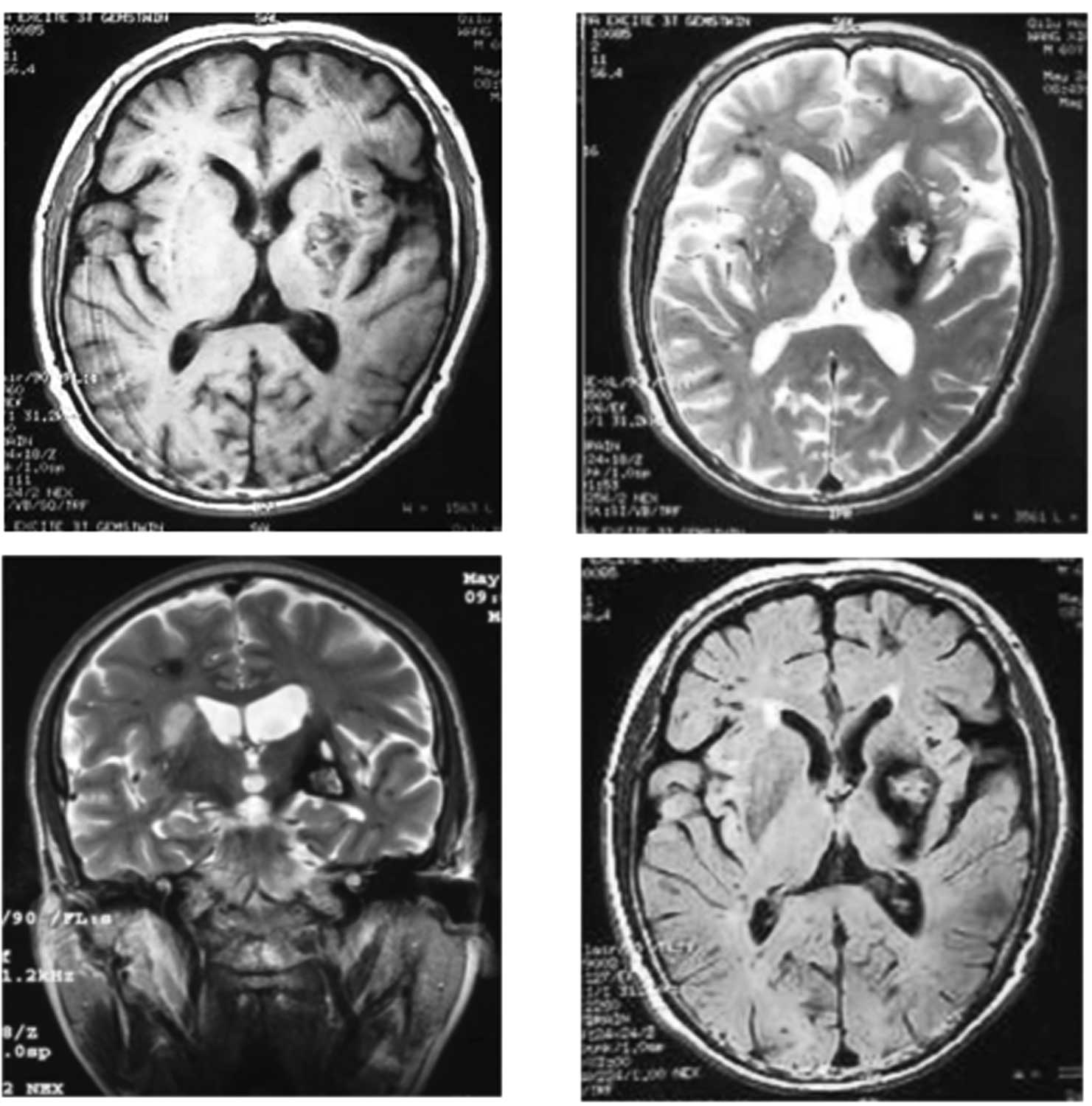
With T1WI, T2WI, T2Flair and DWI
sequences, the foci showed long T1 and long
T2 signal intensities with distinct boundaries. These
lesions demonstrated high signal intensities on T2Flair
images, high and low mixed signals on T1WI scans, and a
core of mixed signal intensity with a surrounding rim of decreased
signal intensity on T2WI scans with a distinct boundary
and no evident edema. A low signal black rim that completely
surrounded the larger lesion with a mixed signal intensity core of
high and low signal intensity was typical of CCM; the foci
presented as clump-like or ‘popcorn-like’ with a distinct boundary
surrounding the foci without edema or occupied effect (Fig. 2).
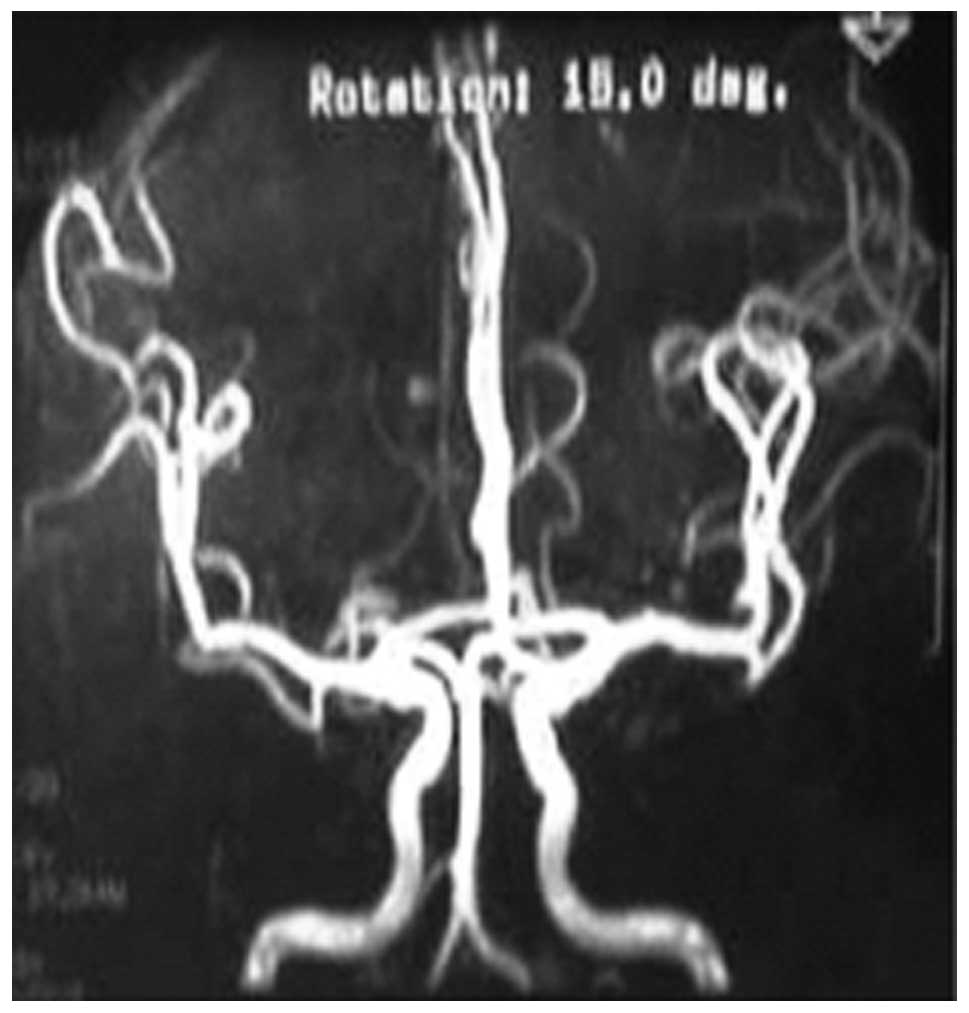
Appearance of CCM by brain MRA
All patients underwent MRA and revealed no
abnormality, with the exception of 2 elderly patients who suffered
cerebral atherosclerosis and minor angiostenosis (Fig. 3).
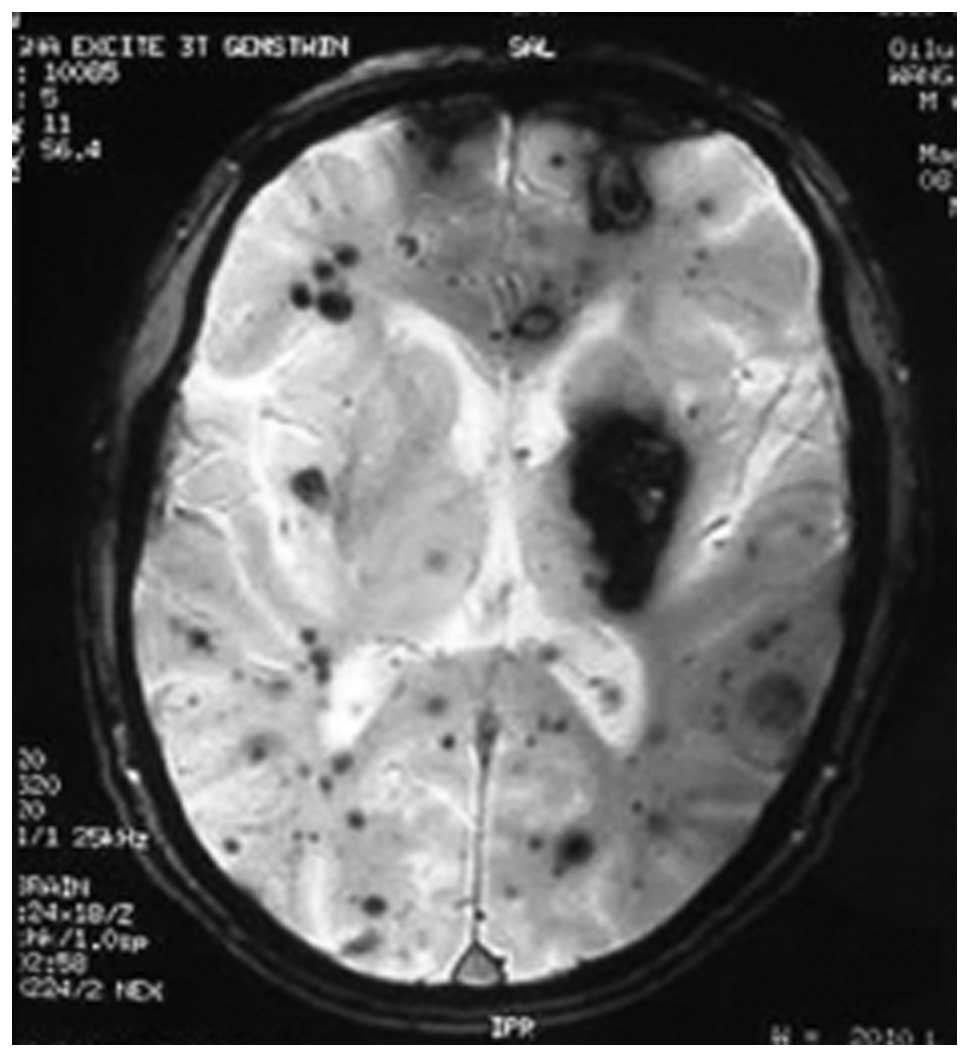
Appearance of CCM in SE and GRE
T2*-WI scans
The foci were characterized by specific mixed high-
and low-signal intensities surrounded by a black rim of decreased
signal intensity with a distinct boundary and no occupied effect.
Foci at various locations and of different sizes were seen clearly
by GRE T2*-WI and more distinctly than by SE
(Figs. 4 and 5).
Different appearance of CCM in CT,
conventional MRI and GRE T2*-WI scans
As shown in Table
I, 12 of the 26 members of the 2 families were diagnosed with
CCM by GRE T2*-WI. The patients were examined
by brain CT and conventional MRI prior to GRE
T2*-WI. Multiple foci were detected in all
CCM patients by GRE T2*-WI. The numbers of
foci observed ranged from 8 to 85 (mean 23) and the foci ranged in
size from 2 to 55 mm. Abnormal signal intensities were observed in
the images of 9 patients (9/12) by brain T1WI, 8
patients (8/12) by T2WI, 9 patients (9/12) by
T2Flair and 10 patients (10/12) by DWI, and 3, 3, 4 and
7 patients were diagnosed with CCM, respectively. The mean numbers
of foci detected were 5 (3–26) using T1WI, 5 (2–24)
using T2WI, 6 (3–29) using T2Flair and 7
using DWI (6–35). However, of the patients subjected to SE and GRE
T2*-WI, 11 patients (11/12) and 12 patients
(12/12), respectively, had abnormal signal intensities, and 9
patients (9/12) and 12 patients (12/12), respectively, were
diagnosed with CCM. The sensitivity of GRE
T2*-WI was much higher than that of CT
(P<0.05), but the difference between GRE
T2*-WI and other conventional MR sequences
had no statistical significance. The mean numbers of detected foci
were 17 (7–45) when SE was used and 23 (8–85) when GRE
T2*-WI was used. Three patients were observed
to have abnormal foci by CT and all were diagnosed with CCM. Three
very small CCM lesions were easily detected with GRE
T2*-WI but not diagnosed with SE. In
addition, multiple foci were detected with greater sensitivity by
GRE T2*-WI than by SE and other MR sequences
(P<0.05).
 | Table I.Comparison of 12 CCM diagnoses
obtained by brain CT, conventional MRI, SE and GRE
T2*-WI. |
Table I.
Comparison of 12 CCM diagnoses
obtained by brain CT, conventional MRI, SE and GRE
T2*-WI.
| Variables | T1WI | T2WI |
T2Flair | DWI | SE | GRE
T2*-WI | CT |
|---|
| Patients with
detected foci | 9 | 8 | 9 | 10 | 11 | 12 | 3 |
| Patients with
diagnosed CCM | 3 | 3 | 4 | 7 | 9 | 12 | 3 |
| Foci of CCMa | 5 (3–26) | 5 (2–24) | 6 (3–29) | 7 (6–35) | 17 (7–45) | 23 (8–85) | 1 (0–1) |
Distribution of foci by GRE
T2*-WI (mean)
The foci were distributed as follows:
cortex-subcortex, 4 (2–22); basal ganglia, 10 (3–36);
thalamencephalon, 4 (1–8); cerebellum, 3 (2–11); and brainstem, 2
(1–7).
Discussion
CCM is a common cerebral malformation and classified
as familial (50–67%) and sporadic (33–50%) forms based on patterns
of onset (5–10). Sporadic cases are frequently
reported while familial CCM is rare in China. CCM is composed of
numerous micrangium with dilated thin walls separated by nerve
fibers. However, normal brain tissue is not observed among blood
sinuses that lack elastic fibers and muscle tissues. Hemorrhage is
common. Progressive neurological deficits, seizures and headaches
are reported to be characteristic of episodes of rebleeding in the
brainstem and cerebral cavernomas (11–13).
The prevalence of CCM in the general population has been estimated
to be 0.4–0.8%, accounting for 10–20% of vascular malformations in
the central nervous system (10,14–16).
CCM was previously diagnosed post mortem but may be
diagnosed ante mortem with the widespread use of CT and MR
techniques. With brain MRI in particular, cases of FCCM and
multiple brain CCM may be identified and the natural history and
developmental process of CCM are well understood (9,17).
The features of FCCM on brain CT and MR images are associated with
the pathological structure and the development of cavernous
vascular malformations (18). Only
3 of the12 patients suffering from clinical symptoms were diagnosed
with CCM by brain CT, a positive diagnosis rate of 25%. The 3
patients presented with headache, movement and sensory problems
affecting the limbs or speech disfluency. Foci having an abnormally
high density or mixed high and low density were observed by brain
CT; calcification and a distinct boundary without a surrounding
zone of edema or occupied effect distinguished them from cerebral
hemorrhage. The foci were located in the subcortex or basal ganglia
region. The 3 patients were finally diagnosed as having multiple
CCM of the brain by MRI which revealed numerous microfoci in
addition to the larger foci identified by CT. The microfoci were
not shown in the brain CT images. Similarly, the 9 other patients
with brain multiple vascular malformations ascertained by MRI had
no or minor symptoms or signs due to the microfoci which were not
shown by CT. The foci identified in the brainstem and cerebellum by
MRI were also not shown by CT. Therefore, brain CT is only
sensitive to the larger vascular malformations with hemorrhage and
calcification. The foci which showed irregular mixed high and low
density without specificity and microvascular malformations were
not shown by CT. The diagnosis of FCCM by brain CT is clearly
limited.
Brain MRI is much more sensitive to CCM than CT,
particularly when the focus is located in the posterior cranial
fossa or brainstem (19,20). The focus of CCM, particularly of
CCM with chronic repeated hemorrhage, is clearly seen by MRI due to
methemoglobin, hemosiderin deposition, thrombus, calcification and
surrounding reactive gliosis induced by repeated and multiple
hemorrhaging of the vascular malformation. The MRI signal
intensities of the foci vary according to the hemorrhagic period.
The foci show high T1-signal intensity and low
T2-signal intensity in the acute stage, and mixed high
and low signal intensity in chronic phase due to nomadic diluted
methemoglobin and hemosiderin deposition. The phenomenon of
hemosiderin deposition occurs 1 week after hemorrhage of the CCM
and shows first at the perimeter and extends to the core of focus.
The area of hemosiderin deposition shows lasting low T1
and T2 signal intensity, clearly by T2WI with
a surrounding black rim of hemosiderin deposition enhanced by
reactive gliosis which shows long T1 and T2
signal intensity. However, it was not possible to identify all CCM
patients and their respective foci using conventional MRI.
T1WI, T2WI, T2Flair and DWI
identified abnormal signal intensities in 9, 8, 9 and 10 of the 12
patients, respectively, and 3, 3, 4 and 7 of the patients,
respectively, were diagnosed with CCM. The mean numbers of foci
detected were 5, 5, 6 and 7, respectively. However, SE and GRE
T2*-WI detected abnormal signal intensities
in 11 and 12 patients, respectively, and diagnosed CCM in 9 and 12
patients, respectively; the mean numbers of foci detected were 17
and 23, respectively. Microvascular malformations from three
patients that were not observed by SE were easily identified using
GRE T2*-WI. GRE T2*-WI
was more sensitive to multiple foci than SE, and accurately and
reliably ascertained CCM of the brainstem and cerebellum.
Conventional MRI has more notably increased the detection of
abnormal foci than CT, but has a limitation in decision of focal
property, namely, although the focus was observed the etiological
diagnosis of the focus was confined. We suggest that the reason for
the difference between GRE T2*-WI and other
conventional MR sequences being not statistically significant is
that the number of patients studied is too few. However, GRE
T2*-WI not only accurately identified all
foci of each size but also provided a qualitative diagnosis based
on their features. Therefore, we consider that GRE
T2*-WI is a ‘gold’ standard for the diagnosis
of CCM and is almost as reliable as autopsy.
The 12 patients with FCCM all had multiple foci; the
foci ranged in number from 8 to 85 and in size from 2 to 55 mm. The
3 patients who had larger foci with repeated hemorrhage and
calcification were clearly identified with CT. Multiple foci were a
common feature of the FCCM cases studied. Non-familial CCM is
common in the clinic and usually comprises a single focus. The foci
of FCCM may be located in every region of the central nervous
system. The foci of our studied patients were almost all
supratentorial, while others were subtentorial. The majority were
located at the basal ganglia, with a mean of 11 foci, and the
larger foci of the 3 patients identified by CT were all located on
this region. The next most common locations was the
cortex-subcortex with a mean of 4 foci. Foci were also located in
the cerebral ganglion (4),
cerebellum (3) and brainstem
(2). Brainstem CCM foci were
mostly located in the pons, and were also located in the midbrain
but rarely in the medulla oblongata. Spinal cord MRI was not
performed due to the lack of symptoms relating to the spinal cord.
Therefore, the current study is not completely representative of
the distribution of CCM in the central nervous system in the
population.
In conclusion, GRE T2*-WI is
an available technique for detecting FCCM. Conventional brain MRI
should be performed to detect multiple foci of CCM, particularly in
the members of a family affected by FCCM. In addition, GRE
T2*-WI is significant in the early diagnosis
and treatment of FCCM.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (No. H0905) and the
National Natural Science Foundation of Shandong province (No.
ZR2010HM052)
References
|
1.
|
Hsu FPK, Rigamonti D and Huhn SL:
Epidemiology of cavernous malformations. Cavernous Malformations.
Awad IA and Barrow DL: American Association of Neurological
Surgeons; Park Ridge, IL: pp. 13–23. 1993
|
|
2.
|
Ohue S, Fukushima T, Friedman AH, et al:
Retrosigmoid suprafloccular transhorizontal fissure approach for
resection of brainstem cavernous malformation. Neurosurgery.
66(Suppl 6): S306–S312. 2010. View Article : Google Scholar
|
|
3.
|
Labauge P, Brunereau L, Lévy C, et al: The
natural history of familial cerebral cavernomas: a retrospective
MRI study of 40 patients. Neuroradiology. 42:327–332. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wurm G and Fellner FA: Implementation of
T2*-weighted MR for multimodal image guidance
in cerebral cavernomas. Neuroimage. 22:841–846. 2004.
|
|
5.
|
Denier C, Goutagny S, Labauge P, et al:
Mutations within the MGC4607 gene cause cerebral cavernous
malformations. Am J Hum Genet. 74:326–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kim DS, Park YG, Choi JU, et al: An
analysis of the natural history of cavernous malformations. Surg
Neurol. 48:9–17. 1997. View Article : Google Scholar
|
|
7.
|
Moriarity JL, Wetzel M, Clatterbuck RE, et
al: The natural history of cavernous malformations: a prospective
study of 68 patients. Neurosurgery. 44:1166–1173. 1999.PubMed/NCBI
|
|
8.
|
Otten P, Pizzolato GP, Rilliet B and
Berney J: 131 cases of cavernous angioma (cavernomas) of the CNS,
discovered by retrospective analysis of 24,535 autopsies.
Neurochirurgie. 35:82–83. 1989.(In French).
|
|
9.
|
Rigamonti D, Hadley MN, Drayer BP, et al:
Cerebral cavernous malformations. Incidence and familial
occurrence. N Engl J Med. 319:343–347. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Robinson JR, Awad IA and Little JR:
Natural history of the cavernous angioma. J Neurosurg. 75:709–714.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Fritschi JA, Reulen HJ, Spetzler RF and
Zabramski JM: Cavernous malformations of the brain stem: a review
of 139 cases. Acta Neurochir. 130:35–46. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kondziolka D, Lunsford LD and Kestle JR:
The natural history of cerebral cavernous malformations. J
Neurosurg. 83:820–824. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Krraemer DL and Awad IA: Vascular
malformations and epilepsy: clinical considerations and basic
mechanisms. Epilepsia. 35(Suppl 6): S30–S43. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Gunel M, Awad IA, Finberg K, et al: A
founder mutation as a cause of cerebral cavernous malformation in
Hispanic Americans. N Engl J Med. 334:946–951. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Laberge-le Couteulx S, Jung HH, Labauge P,
et al: Truncating mutations in CCM1, encoding KRIT1, cause
hereditary cavernous angiomas. Nat Genet. 23:189–193.
1999.PubMed/NCBI
|
|
16.
|
Del Curling O Jr, Kelly DL Jr, Elster AD
and Craven TE: An analysis of the natural history of cavernous
angiomas. J Neurosurg. 75:702–708. 1991.PubMed/NCBI
|
|
17.
|
Moriarity JL, Clatterbuck RE and Rigamonti
D: The natural history of cavernous malformations. Neurosurg Clin N
Am. 10:411–417. 1999.
|
|
18.
|
Kattapong VJ, Hart BL and Davis LE:
Familial cerebral cavernous angiomas: clinical and radiologic
studies. Neurology. 45:492–497. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Chi LY, Wang SH, Liu XW, et al: Familial
cerebral cavernous malformation: features of clinical
manifestation, pathology and imaging in a Chinese family.
Cerebrovasc Dis. 26:206–208. 2008. View Article : Google Scholar
|
|
20.
|
Zabramski JM, Wascher TM, Spetzler RF, et
al: The natural history of familial cerebral cavernomas: results of
an ongoing study. J Neurosurg. 80:422–432. 1994. View Article : Google Scholar
|