Introduction
CCl4, often used to induce liver damage,
is a well-known chlorinated hydrocarbon utilized as a solvent in
various industries as well as a vermifuge in medicine to treat
hookworm disease (1). For this
reason, workers are often poisoned by inhalation, ingestion and
absorption of CCl4. CCl4 also induces
hepatotoxicity, nephrotoxicity and hematotoxicity (2). The liver is a major target of human
CCl4 poisoning, whereas the kidney and erythrocytes are
minor target organs (3). In the
liver, CCl4 induces hepatic damage, necrosis (4) and apoptosis (5), and exposure for long periods leads
to fibrosis, cirrhosis and hepatic carcinoma (6). The kidney, as a minor target, shows
increased organ weight, localized glomerulosclerosis, and a higher
urinary protein content in animals following exposure to a high
concentration of CCl4. To date, extensive research has
been carried out in Oriental medicine to develop a novel
therapeutic drug capable of preventing organ damage induced by
CCl4.
Many lignan compounds isolated from Schisandra
chinensis have been considered as candidate substances for
protection against CCl4-induced damage. Over the past 20
years, S. chinensis has been well reported in traditional
Chinese medicine (7), and it has
been shown to contain many active lignans, including gomisins A, B,
C, D, E, F, G, K3, N, J, Schisandrol B, Schisandrin and Schisandrin
C (7,8). Among these, gomisin A was first
reported as one of five new dibenzocyclooctadiene lignans isolated
from the petroleum ether extract of S. chinensis fruits
(9). Its structure and function
have been investigated using chemical and spectral techniques in
both in vitro and in vivo studies. Among the
functions of gomisin A, its protective and regenerative effects
against experimental liver damage induced by various factors have
been well confirmed. For instance, disappearance of plasma
indocyanine green (ICG) induced by CCl4,
d-galactosamine, and orotic acid was not delayed by gomisin A,
which possesses a liver function-facilitating property in normal
and liver-damaged rats (10).
Moreover, the development of acute hepatic failure induced by
intravenous administration of heat-killed Propionibacterium
acnes followed by a small amount of gram-negative
lipopolysaccharide (LPS) for seven days was significantly protected
against by ingestion of food containing 0.06% gomisin A for 4 weeks
(11). Pretreatment with gomisin
A was also found to attenuate the activation of caspase-3,
elevation of serum TNF-α, the number of apoptotic cells, and DNA
fragmentation during D-galactosamine (GalN) and LPS-induced hepatic
apoptosis and liver injury (12).
The correlation between gomisin A and hepatitis C virus (HCV)
infection has been investigated using an in vitro MOLT-4
cell model and an in vivo animal model of acute hepatic
injury. Treatment with gomisin A both short-term and long-term
effectively inhibited HCV infection and protected against
immunological hepatopathy (13).
In a study on liver regeneration, pretreatment with gomisin A
stimulated regeneration of liver damaged by partial hepatectomy by
increasing ornithine decarboxylase activity, which regulates
important biochemical processes in the initial stage of liver
regeneration (14). Furthermore,
gomisin A was shown to be tightly correlated with
hepatocarcinogenesis. Finally, oral administration of gomisin A
significantly inhibited the increase in serum bile acids
(deoxycholic acid), occurrence of preneoplastic lesions, and the
number of GST-P-positive loci in the liver (15–17). Although hepatic and renal disease
induced by CCl4 has been studied extensively, the
mechanism by which these organs are protected against damage has
not been widely investigated. In addition, there are few studies on
whether or not gomisin A protects the liver and kidney against
damage induced by CCl4 exposure.
Therefore, the present study investigated the
protective effects of gomisin A against liver and kidney injury
induced by CCl4 exposure. Our results showed that
gomisin A significantly inhibited the increase in serum biochemical
markers indicative of liver and kidney toxicity, histological
damage, and caspase activation through differential regulation of
the MAPK signaling pathway.
Materials and methods
Preparation of gomisin A
Fruits of S. chinensis used in this study
were collected from Moonkyeng City, Korea in September, 2005. A
voucher specimen (accession no. SC-PNUNPRL-1) was deposited in the
Herbarium of Pusan National University. To purify gomisin A, dried
fruits of S. chinensis (2.5 kg) were ground into a fine
powder and successively extracted at room temperature with
n-hexane, EtOAc and MeOH. The hexane extract (308 g) was
evaporated under vacuum and chromatographed on a silica gel (40 μm;
J.T. Baker, Phillipsburg, NJ, USA) column (70×8.0 cm) with a step
gradient of 0, 5, 10, 20 and 30% EtOAc in hexane (each 1 liter)
(18). Of these extracts,
fraction 29 (1,992 mg) was separated on a silica gel column
(100×3.0 cm) with 15% CHCl3 in acetone to provide
gomisin A (973 mg). Pure gomisin A was identified by HPLC on a
Phenomenex Luna C18 column (150×4.6 mm ID; 5 μm particle size;
Phenomenex) (19). In addition,
the chemical structure of gomisin A used in this study was verified
by LC-MS (Bruker BioApex FT mass spectrometer) and NMR analysis
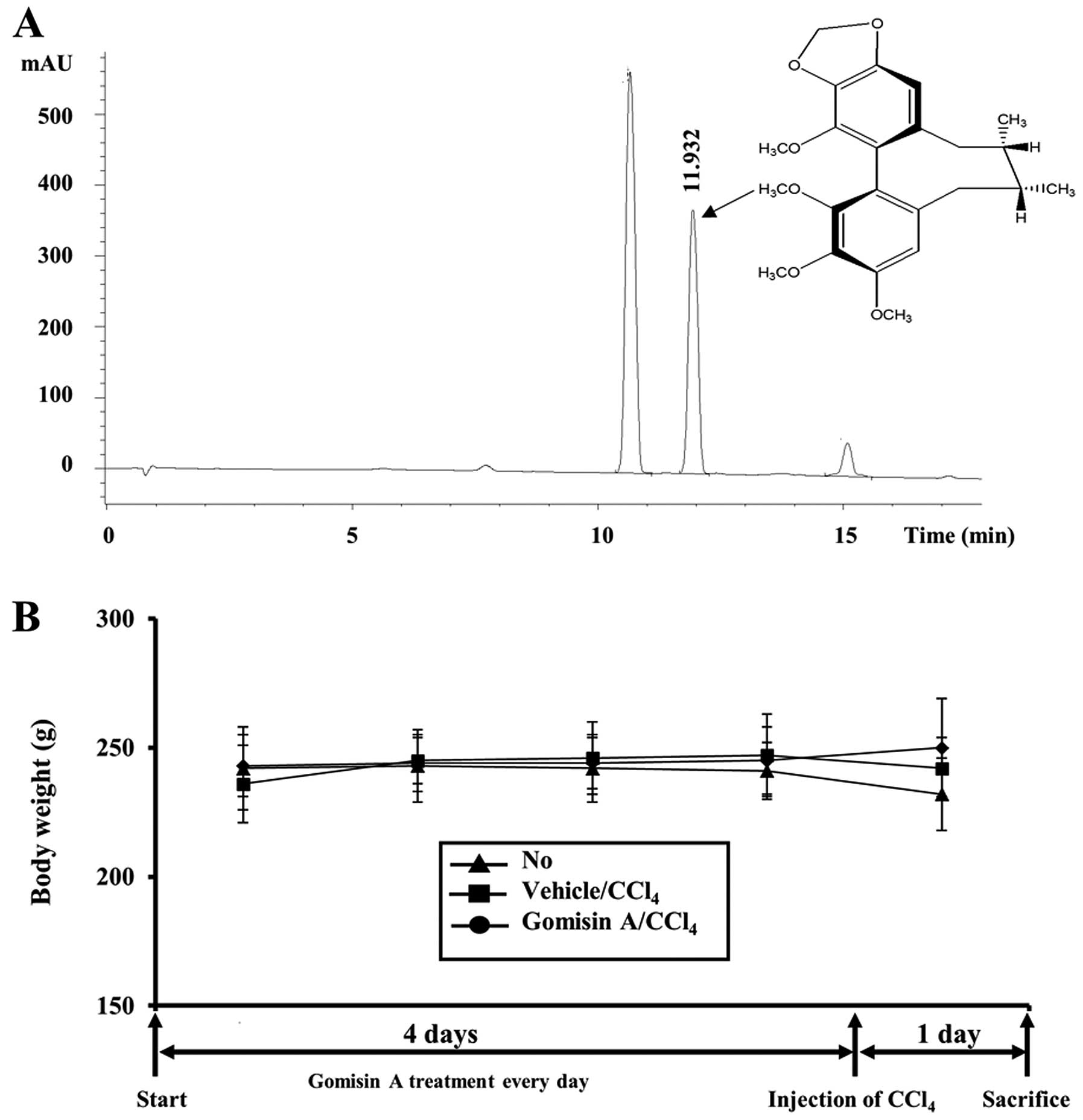
(Varian Inova 500 spectrometer) (20) (Fig.
1A).
Care and use of laboratory animals
Sprague-Dawley (SD) rats used in this study were
purchased from Samtaco BioKorea (Osan, Korea). All animal
experimental procedures were approved by the Institutional Animal
Care and Use Committee (IACUC) at Pusan National University
(approval no. PNU-2009-0007). Animals were handled at the Pusan
National University-Laboratory Animal Resources Center accredited
by the Korea FDA in accordance with USA NIH guidelines (accredited
unit no. 000996). All rats were housed under specific pathogen-free
(SPF) conditions with a strict light cycle (lights on at 06:00 h
and off at 18:00 h) and were fed a standard irradiated chow diet
(Purina Mills, Inc.) ad libitum.
Gomisin A treatment and measurement of
organ weight
Eight-week-old SD rats were randomly divided into
three subgroups with six rats/group. The first group of SD rats was
not treated with any compounds (non-treated group). The second
group received a comparable volume of olive oil via oral gavage
(vehicle/CCl4-treated group) daily, whereas the third
group received 100 mg/kg body weight per day of gomisin A via oral
injection for four days (gomisin A/CCl4-treated group).
On the fifth day, the second and third groups received 0.1 ml of
CCl4 solution via intraperitoneal injection. At 24 h
after CCl4 injection, the animals were immediately
euthanized using CO2 gas. Body weights as well as
weights of internal organs, including the liver, kidney, heart,
lung, spleen and thymus, were measured using a chemical balance.
Subsequently, liver and kidney tissues as target organs were
collected and stored in Eppendorf tubes at -70°C until being
assayed. For histological analysis, these tissues were fixed in 10%
formalin solution for 24 h.
Serum biochemical analysis
After the final administration of CCl4,
all rats were fasted for 24 h, and blood was collected from
abdominal veins. Serum was obtained by centrifugation of blood
incubated for 30 min at room temperature. Serum biochemical
components were assayed using an automatic serum analyzer (Hitachi
747; Hitachi, Japan). All assays were assessed using fresh serum
and conducted in duplicate.
Histological analysis
Liver and kidney tissues collected from rats were
fixed with 10% formalin for 24 h, embedded in paraffin wax, and
then sectioned into 5-μm slices. The liver and kidney sections were
then stained with hematoxylin and eosin (H&E; Sigma-Aldrich,
St. Louis, MO, USA). The stained liver and kidney tissue sections
were observed by light microscopy, and morphological features of
hepatocytes and kidney cells were assessed with Leica Application
Suite (Leica Microsystems, Switzerland).
Western blot analyses
Proteins prepared from tissues of the
vehicle/CCl4- and gomisin A/CCl4-treated SD
rats were separated by electrophoresis on a 4–20% SDS-PAGE gel for
3 h and then transferred to nitrocellulose membranes for 2 h at 40
V. Each membrane was incubated separately with the primary
antibody: anti-caspase-3 (#9662; Cell Signaling Technology, Boston,
MA, USA), anti-Bax (ab7977), anti-Bcl-2 (ab7973; both were from
Abcam, Cambridge, UK), anti-ERK (sc-94), anti-p-ERK (sc-7383; both
were from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
anti-JNK (#9252), anti-p-JNK (#9251), anti-p38 (#9212), anti-p-p38
(#9211; all were from Cell Signaling Technology), or anti-actin
(A5316; Sigma-Aldrich, St. Louis, MO, USA) overnight at 4°C. The
membranes were then washed with washing buffer (137 mM NaCl, 2.7 mM
KCl, 10 mM Na2HPO4, 2 mM
KH2PO4 and 0.05% Tween-20) and incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG (Zymed
Laboratories, Inc., South San Francisco, CA, USA) diluted 1:1,000
at room temperature for 2 h. The membrane blots were developed
using a Chemiluminescence Reagent Plus kit (ECL; Pfizer and
Pharmacia, New York, NY, USA).
Statistical analysis
Tests for significance between the vehicle- and
gomisin A-treated SD rats were performed using a one-way ANOVA test
of variance (SPSS for Windows, release 10.10, standard version;
SPSS, Chicago, IL, USA). Tests for significance between the
non-treated and CCl4-treated groups
(vehicle/CCl4 and gomisin A/CCl4) were
performed using a post-hoc test (SPSS for Windows, release 10.10,
standard version) of variance, and significance levels are provided
in the text. All values are reported as the means ± standard
deviation. A P-value <0.05 was considered to indicate a
statistically significant result.
Results
Protective effects of gomisin A on body
and organ weights
Generally, toxic effects on animal and human bodies
are confirmed by alterations in body and organ weights (21). In order to investigate the
protective effects of gomisin A against CCl4-induced
toxicity, we first measured the body weights of non-treated
vehicle- as well as gomisin A-pretreated rats for five days,
including one day following CCl4 exposure. No
significant differences in body weight were detected among the
three groups (Fig. 1B). However,
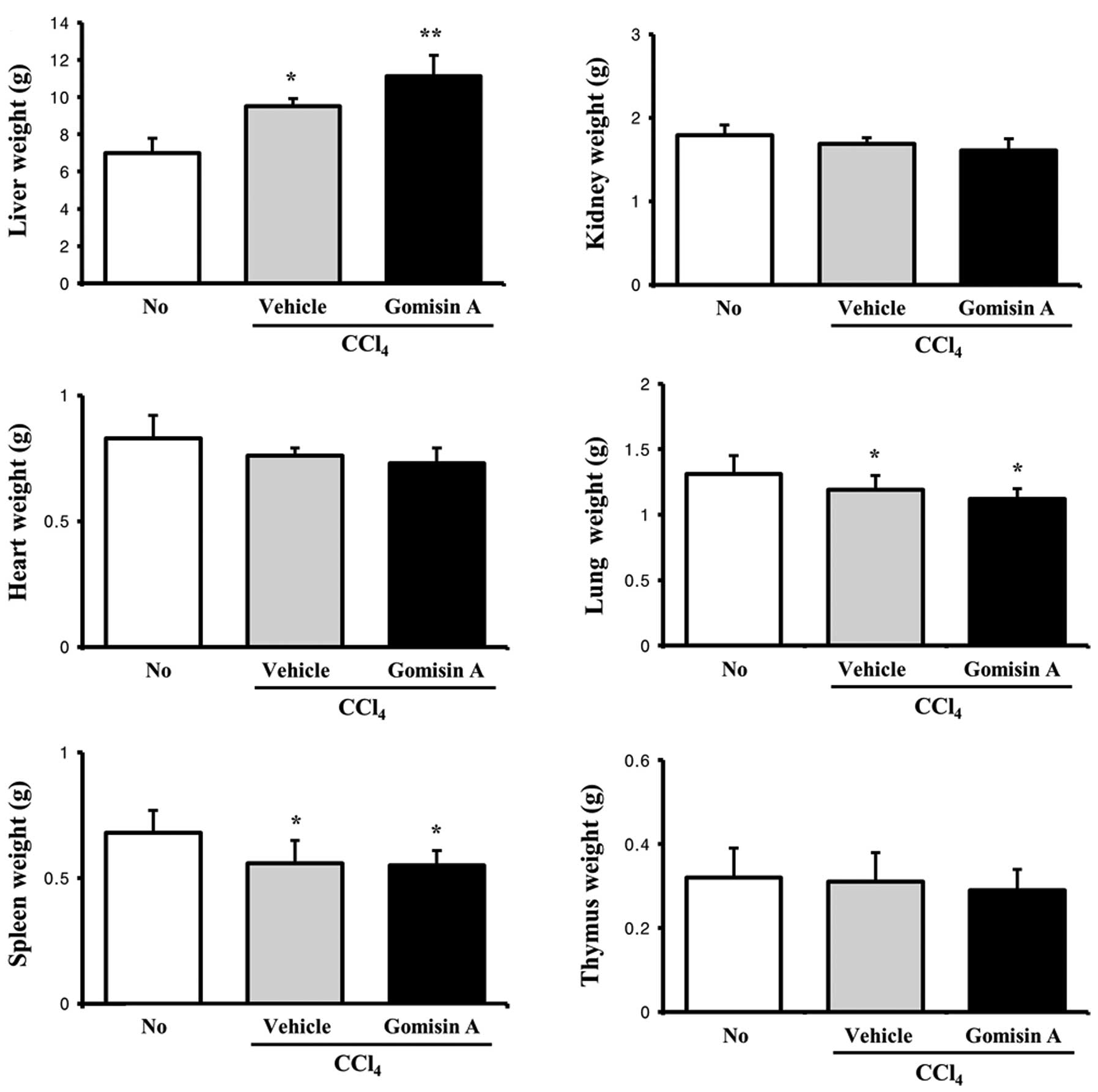
results of the organ weight analysis were different from those of
the body weight analysis. Of the six organs, five organs, including
the kidney, heart, lung, spleen and thymus, showed slightly lower
weights in the gomisin A/CCl4-treated group compared to
these values in the vehicle/CCl4-treated group, although
these results were not statistically significant. In contrast, the
liver weight was significantly higher in the gomisin
A/CCl4-treated group compared to the
vehicle/CCl4-treated group. In addition, the weights of
the liver, lung and spleen in the CCl4-treated group
were significantly lower than those in the non-treated group
(Fig. 2). Therefore, the present
results suggest that gomisin A did not affect body and organ
weights, apart from that of the liver.
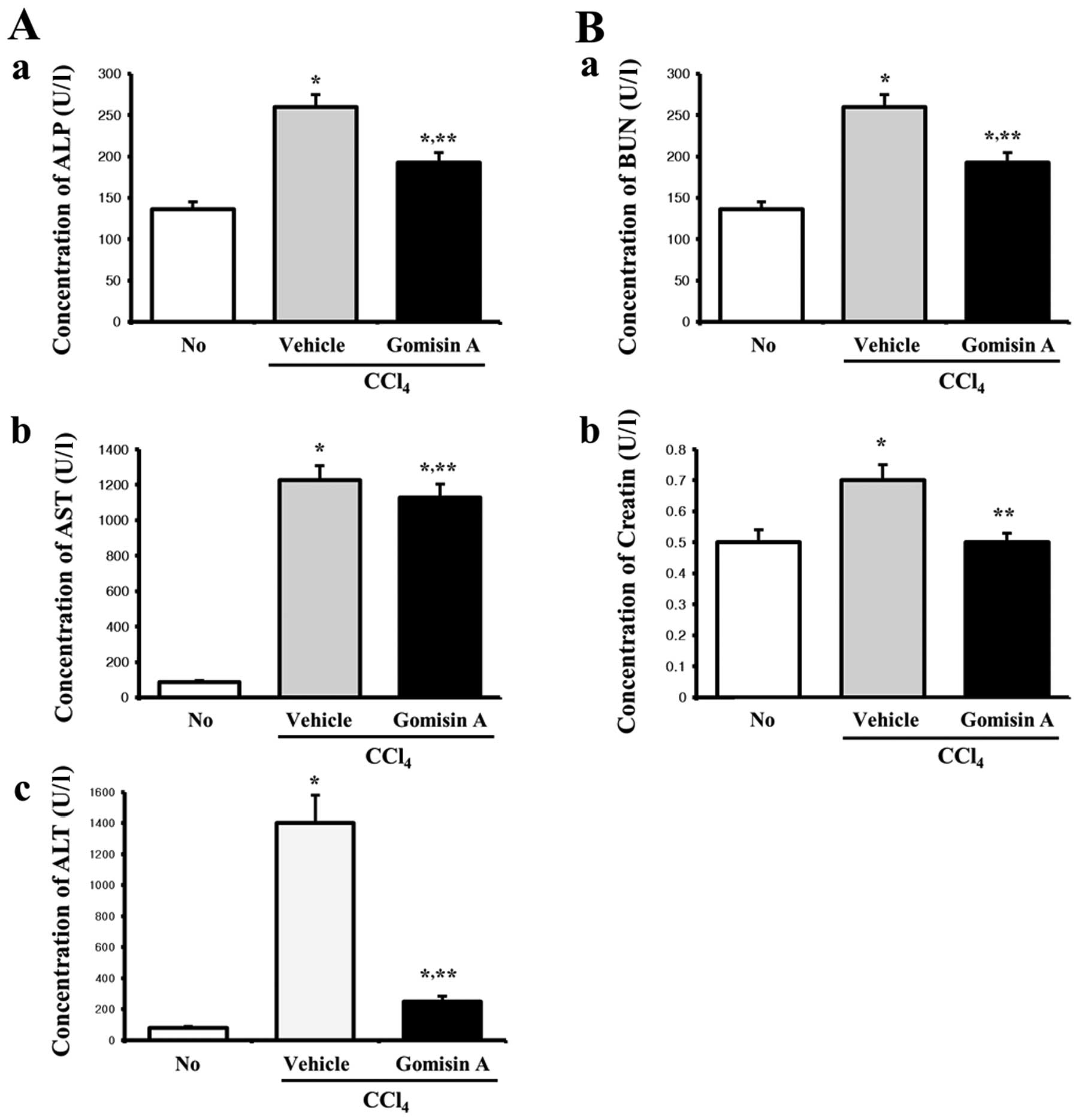
Effects of gomisin A on serum biochemical
analysis
Increased concentrations of alkaline phosphatase
(ALP), alanine transaminase (ALT) and aspartate transaminase (AST)
in serum are well-known factors indicating liver toxicity, whereas
blood urea nitrogen (BUN) and creatinine (CRE) are evidence of
kidney toxicity (21,22). To investigate the protective
effects of gomisin A against CCl4-induced toxicity in
terms of serum biochemical indicators, the levels of five
indicators, including ALP, AST, ALT, BUN and CRE, were measured in
the vehicle/CCl4- and gomisin A/CCl4-treated
rats. In regards to the liver toxicity factors, the levels of ALP,
AST and ALT were higher in the vehicle/CCl4-treated rats
than these levels in the non-treated rats. However, these levels
were significantly decreased in the gomisin
A/CCl4-treated rats when compared with the
vehicle/CCl4-treated rats, although the rate of decrease
varied for each factor (Fig. 3A).
Furthermore, the factors representing kidney toxicity showed
similar patterns as those representing liver toxicity.
Specifically, serum levels of BUN and CRE were significantly
increased upon CCl4 exposure. However, the
concentrations of these two factors were restored by gomisin A
pretreatment to similar levels as those in the non-treated group
(Fig. 3B). Therefore, these
results demonstrated that pretreatment with gomisin A conferred
protective effects against liver and kidney damage, although the
protective effects varied according to each factor.
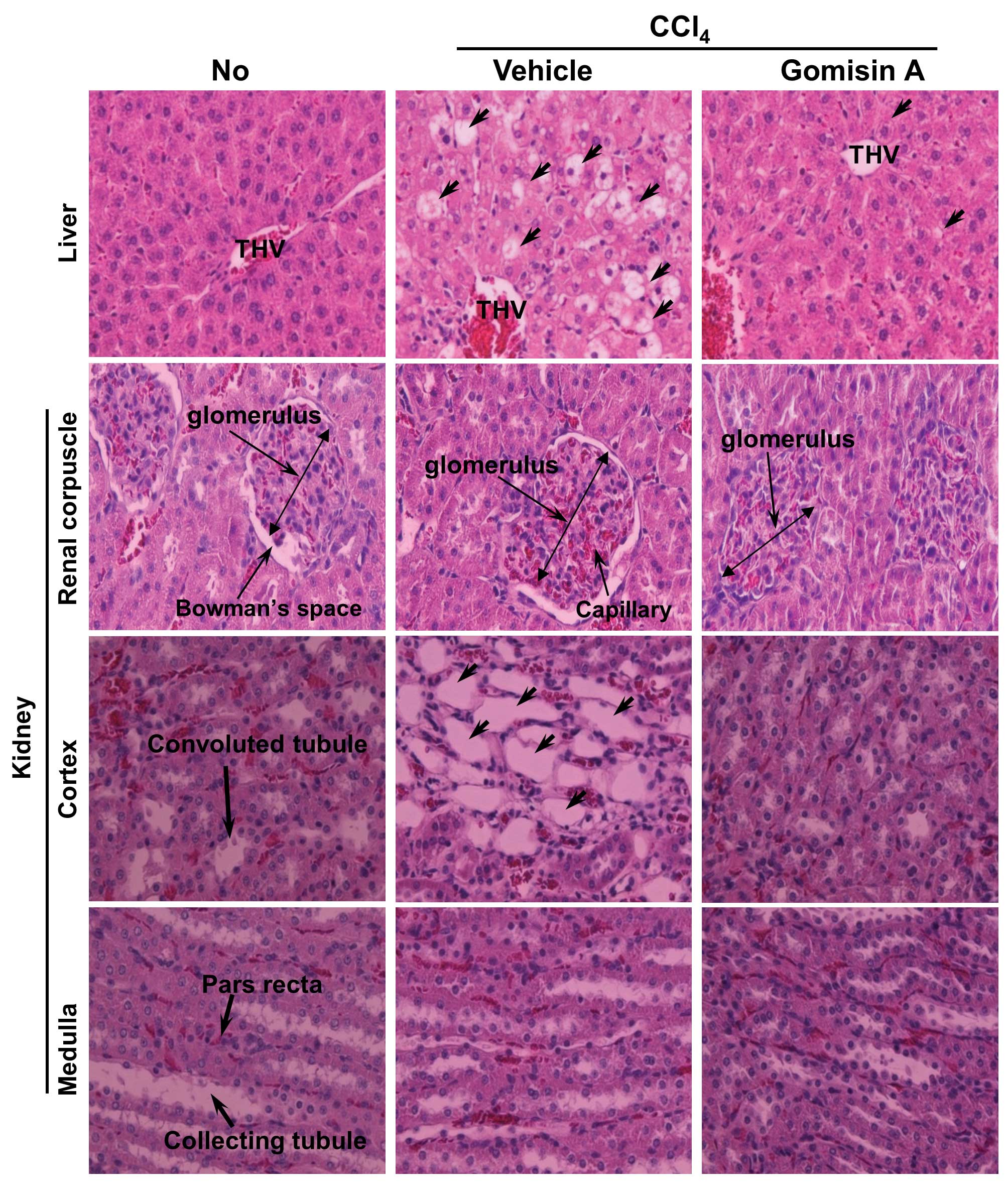
Protective effects of gomisin A against
tissue injury of the liver and kidney
Generally, histological staining is performed with
H&E to visualize the differences between tissue components
under normal and pathological conditions. Histological alterations
during hepatocellular damage along with the protective effects of
gomisin A were first identified by histological analysis of the
liver section. In the non-treated group, the histopathology of the
liver displayed a normal distribution of hepatocytes with clear
visible nuclei, a portal triad and central vein (Fig. 4). However, following
CCl4 treatment, extensive centrolobular necrosis was
observed in and around the terminal hepatic venule (THV) of the
liver. Furthermore, the central vein was significantly dilated in
the vehicle/CCl4-treated group compared with the
non-treated group (Fig. 4).
However, the liver section of the group pretreated with gomisin A
for four days displayed low hepatocellular necrosis, a poorly
dilated central vein, and regular arrangement of hepatocytes.
In the kidney, significant changes were detected
only in the cortex containing the Bowman’s capsule and convoluted
tubules, whereas the medulla region maintained its morphology.
Regarding the Bowman’s capsule, the vehicle/CCl4-treated
group showed an increased diameter of the glomerulus as well as a
higher number of capillaries in the glomerulus compared with the
non-treated group. In the gomisin A pretreatment group, the
diameter of the glomerulus, the number of capillaries, and Bowman’s
space were significantly decreased. In particular, Bowman’s space
completely disappeared in the gomisin A/CCl4-treated
group (Fig. 4). Regarding the
convoluted tubules, their diameters were dramatically increased in
the CCl4-exposed group compared with the non-treated
group. However, gomisin A pretreatment induced recovery of the
diameters of the convoluted tubules back to normal (Fig. 4). The above results suggest that
gomisin A pretreatment contributed to the reduction of hepatic
necrosis and dilation of the THV in livers of the SD rats after
CCl4 exposure. Furthermore, this lignan reduced the
occurrence of renal defects, including increased diameter of the
glomerulus, a higher number of capillaries and convoluted
tubules.
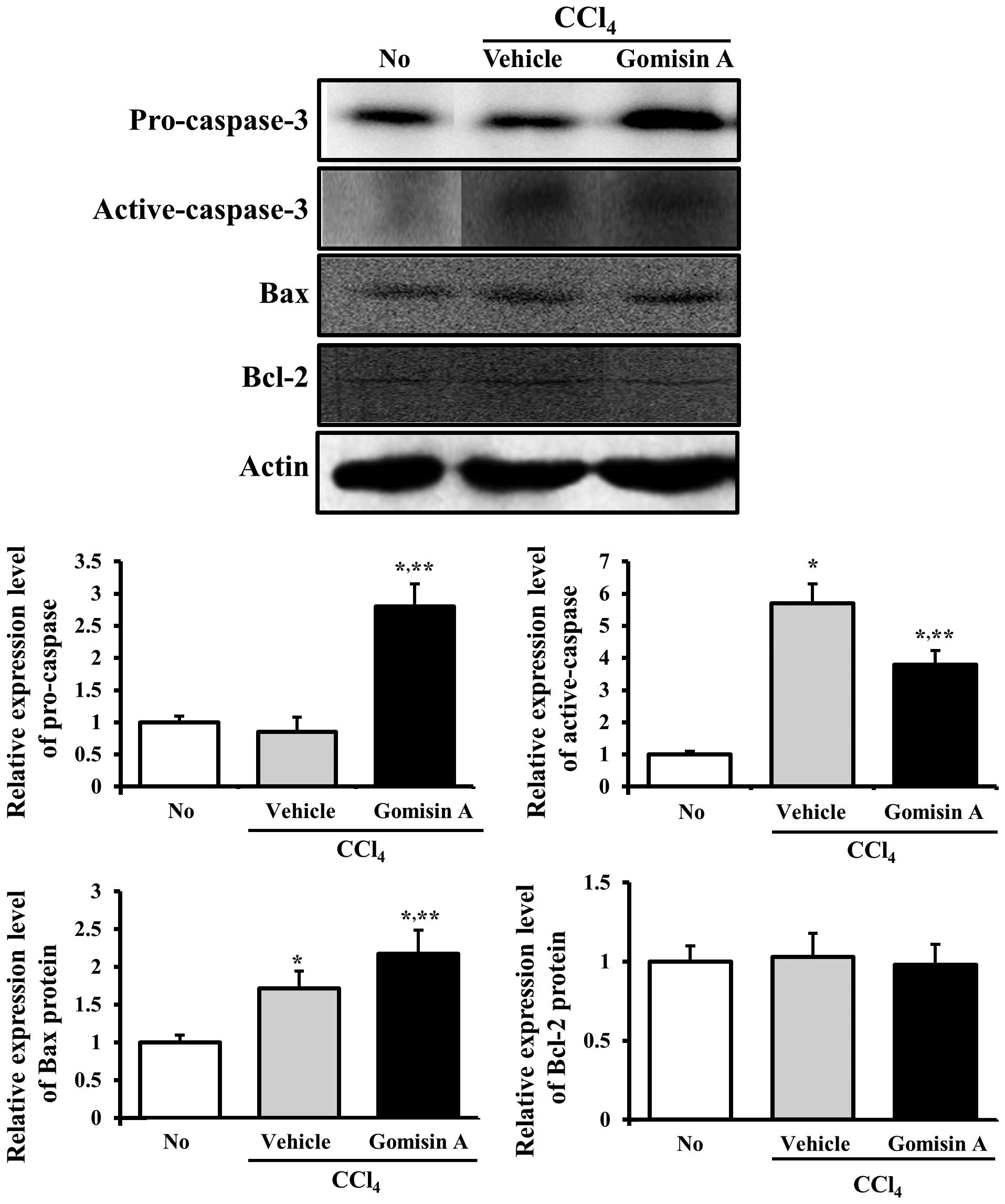
Effects of gomisin A on the apoptosis of
the liver and kidney
In order to investigate whether or not gomisin A
prevents the activation of apoptosis, alterations in
apoptosis-related proteins were examined in the liver and kidney
tissues of the rats pretreated with vehicle or gomisin A. First,
changes in the levels of these proteins were measured in liver
tissue. The pro-caspase-3 level was reduced significantly in the
vehicle/CCl4-treated group, whereas the level of active
caspase-3 increased. In the gomisin A/CCl4-treated
group, the levels of pro-caspase-3 and active caspase-3 were
recovered to the same levels as those of the non-treated group.
Bcl-2 belongs to a family of proteins that includes both pro- and
anti-apoptotic members. Among these members, Bcl-2 proteins
stimulate anti-apoptosis while the Bax protein significantly
inhibits the anti-apoptotic actions of the Bcl-2 protein (23,24). To assess the effects of gomisin A
pretreatment on proteins associated with the apoptotic signaling
pathway, the expression levels of the Bcl-2 and Bax proteins were
determined in the vehicle/CCl4-treated and gomisin
A/CCl4-treated groups using western blot analysis. The
expression of the Bax protein was slightly increased in the
vehicle/CCl4-treated group compared to the non-treated
group, whereas it was further increased in the gomisin
A/CCl4-treated group. However, the expression level of
the Bcl-2 protein was maintained at a certain level regardless of
gomisin A pretreatment (Fig. 5).
Therefore, western blot analysis indicated that gomisin A reduced
the expression levels of proteins associated with anti-apoptosis in
the liver tissue.
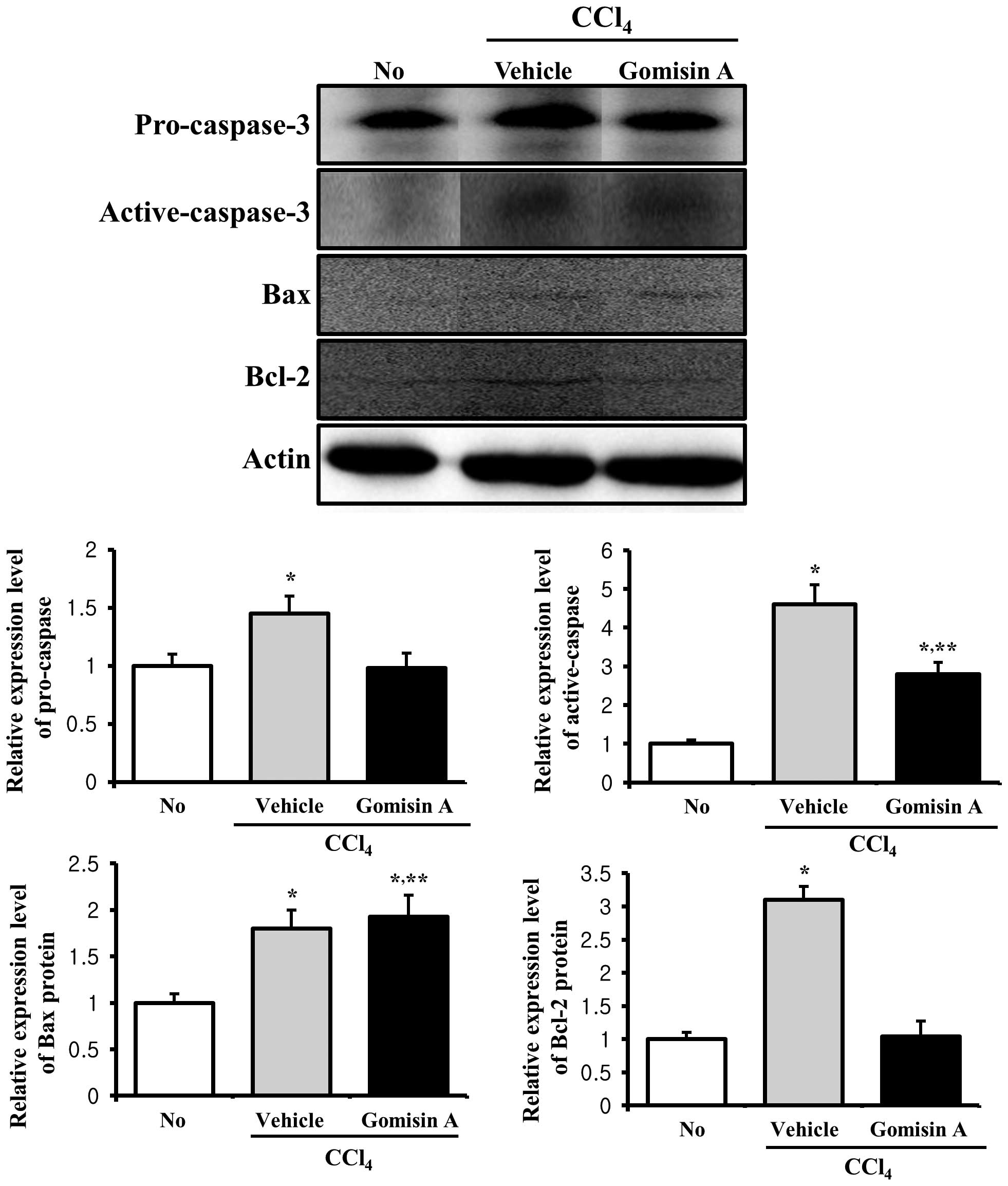
Additionally, alterations in the expression levels
of these proteins were detected in the kidney tissue. The
expression levels of pro-caspase-3 and active caspase-3 were
markedly increased in the vehicle/CCl4-treated group,
while their levels were decreased in the gomisin
A/CCl4-treated group (Fig.
6). The expression pattern of the Bax protein very much
resembled its pattern in liver tissue, whereas the pattern of Bcl-2
expression differed from that observed in the liver tissue.
Following CCl4 exposure, the expression of the Bcl-2
protein dramatically increased ~3-fold. However, gomisin A
pretreatment prevented an increase in the expression of this
protein (Fig. 6). Therefore,
these results indicate that gomisin A reduced the expression of
proteins associated with anti-apoptosis in the kidney tissue.
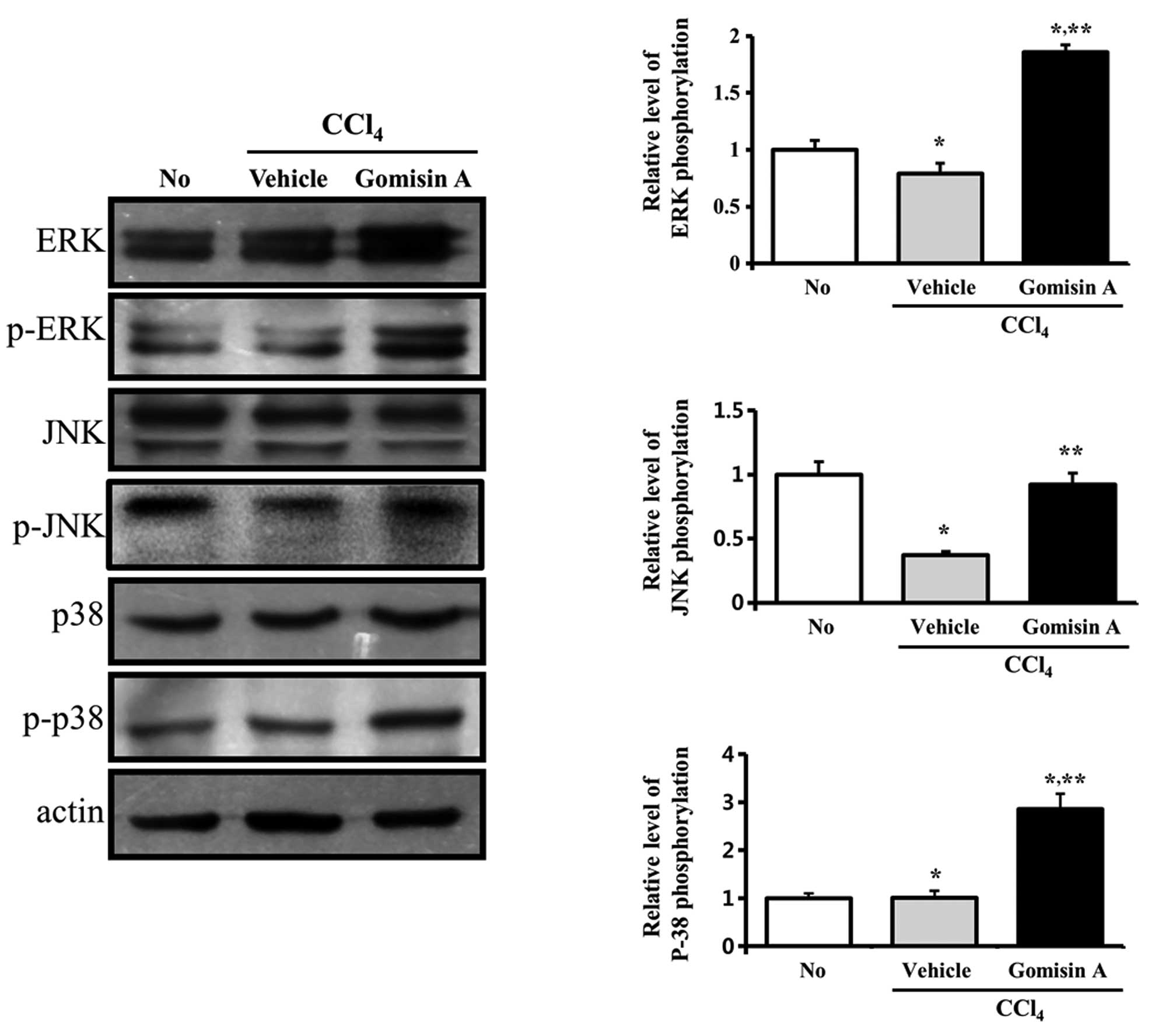
Effects of gomisin A on the MAPK
signaling pathway
We investigated the roles of different MAPK
signaling proteins on CCl4-induced liver and kidney
damage following gomisin A pretreatment. In regards to the liver,
the phosphorylation levels of ERK and JNK were decreased in the
vehicle/CCl4-treated group when compared with levels in
the non-treated group, whereas the phosphorylation level of p38 did
not significantly change. However, in the gomisin
A/CCl4-treated group, the phosphorylation levels of ERK
and p38 were significantly higher compared to those in the
vehicle/CCl4-treated group (Fig. 7).
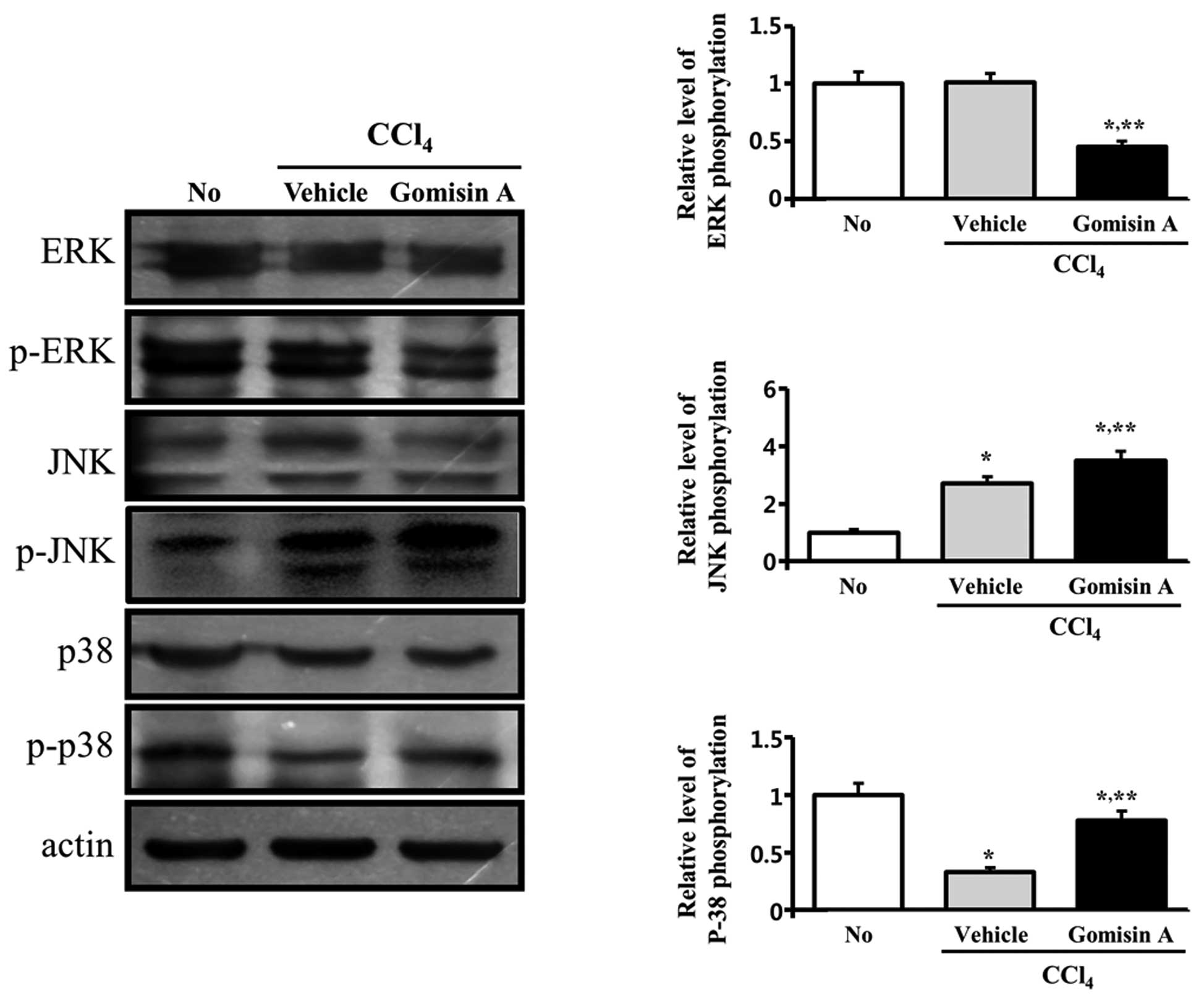
Furthermore, the MAPK signaling pathway in the
kidney showed a different response compared to the liver. In the
vehicle/CCl4-treated group, phosphorylation of JNK
increased when compared to its level in the non-treated group. In
contrast, phosphorylation of p38 was reduced by 50%, whereas the
phosphorylation level of ERK was maintained at a constant level.
However, in the gomisin A/CCl4-treatment group, the
phosphorylation level of ERK was significantly decreased when
compared to that in the vehicle/CCl4-treated group,
whereas phosphorylation of p38 and JNK was increased (Fig. 8). Therefore, these results showed
that gomisin A pretreatment had protective effects against liver
and kidney damage induced by CCl4 exposure through
differential regulation of the MAPK signaling pathway.
Discussion
The effects of several lignan compounds isolated
from S. chinensis on chronic liver injury induced by
CCl4 have been previously studied. The first attempt to
investigate their effects was performed using gomisin A (TJN-101)
(25). Oral administration of
gomisin A at a dose of 10 or 30 mg/kg/day for three or six weeks
was shown to increase serum biochemical parameters, to suppress
fibrosis proliferation, and to accelerate both the repair of liver
function and liver regeneration in rats subcutaneously injected
with CCl4. The results observed in this study closely
resemble those of the present study, although the treatment time
and concentration were quite different. Numerous novel effects of
gomisin A on hepatic and renal injury were observed in this study.
In particular, the effects of gomisin A on the apoptotic pathway
were investigated in terms of the expression levels of caspase-3,
Bax and Bcl-2 in the liver and kidney.
Schisandrin B (Sch B), a dibenzocyclooctadiene
derivative isolated from S. chinensis, was found to exhibit
protective effects against CCl4-induced hepatotoxicity.
Enhancement of the hepatic glutathione antioxidant system
constituted the first evidence of the protective effects of Sch B
against CCl4-induced toxicity in female Balb/c mice
(26). In a comparative
experiment, both Sch B and dimethyl diphenyl bicarbonate (DDB) at
the same dosage significantly suppressed an increase in plasma ALT
activity, although a decrease in plasma SDH activity was observed
only in the Sch B-pretreated group (27). In this study, the levels of three
indicators, including ALT, AST and ALP, were significantly reduced
by gomisin A, as in the Sch B administration study. However, only
the therapeutic effects of gomisin A on kidney toxicity were
determined in our study. Recently, the molecular mechanism
underlying the hepatoprotective effect of Sch B was elucidated. It
was shown that Sch B pretreatment induces hepatoprotection against
CCl4-induced liver injury by increasing hepatic
mitochondrial resistance to the Ca2+-stimulated
permeability transition (28). In
our study, gomisin A was shown to function via the apoptotic
mechanism and MAPK signaling pathway during chronic liver and
kidney injury, although the association between Ca2+
permeability and liver protection induced by gomisin A was not
investigated.
The effects of several S. chinensis extracts
on the antioxidant status have been investigated in animals
presenting with CCl4-induced liver injury. A
lignan-enriched extract of fruits of S. chinensis was shown
to facilitate the regeneration of the hepatic glutathione status
through a glutathione reductase-catalyzed and NADPH-mediated
reaction (29). In another study,
the lignan fraction of S. chinensis showed strong protective
effects against liver injury induced by CCl4 during
phase I oxidative metabolism (30). Furthermore, the combined herbal
extract of Ginkgo biloba, Panax ginseng and S.
chinensis was found to significantly improve hepatic
antioxidant capacity by increasing catalase activity and the
glutathione redox status (31).
As shown in the above studies, it is important to investigate the
alteration of the antioxidant status in liver tissue in order to
verify the protective effects of various lignans. Therefore, more
research is needed to further identify the effects of gomisin
A.
Reduction in pro-caspase-3 levels results in
increased levels of active caspase-3, since the apoptotic signal
induces cleavage of pro-caspase-3 (32 kDa) into two small fragments
(17 and 12 kDa) (32). In the
present study, high levels of active caspase-3 were detected in the
vehicle/CCl4-treated group, and their levels
significantly decreased upon gomisin A pretreatment (Figs. 5 and 6). Therefore, these results also confirm
that gomisin A may inhibit hepatic and renal apoptosis through
suppression of caspase-3 activity.
Apoptosis or programmed cell death plays critical
roles in a variety of physiological processes during fetal
development as well as in adult life. Defects in the apoptotic
process lead to the onset of many diseases involving progressive
cell accumulation as well as cancer in most cases. Furthermore,
apoptosis involves many families of proteins. Of these, Bcl-2
proteins are one of the key families that induce anti-apoptosis
(23). The Bax protein, another
member of the Bcl-2 family, inhibits the anti-apoptotic actions of
Bcl-2. In order to assess the effects of gomisin A pretreatment on
proteins associated with the apoptotic signaling pathway, the
expression levels of Bcl-2 and Bax were determined in the
vehicle/CCl4- and gomisin A/CCl4-treated
groups using western blot analysis. The expression of the Bcl-2
protein was markedly decreased only in the kidney of rats
pretreated with gomisin A, whereas its level was maintained in the
liver (Figs. 5 and 6). However, expression of the Bax
protein increased slightly in both organs pretreated with gomisin
A. These results indicate that gomisin A simultaneously reduces the
expression levels of proteins associated with anti-apoptosis while
increasing those of proteins associated with pro-apoptosis.
The MAPK family is involved in the control of growth
and differentiation, as well as in apoptotic signaling (33–35). Members of the MAPK pathway,
including ERK1/2, JNKs and p38 MAP kinase (p38), have been well
characterized in various studies. In particular, several studies
have shown that MAPK signaling proteins are activated by different
stimuli. For instance, p38 and JNK are activated in response to
many cytotoxic stresses such as hydrogen peroxide
(H2O2), UV radiation, tumor necrosis factor
(TNF-α), heat shock and X-rays (36–38). Furthermore, ERK is activated by
various growth factors and mitogens during the processes of cell
differentiation, growth and survival (36). The results of this study were
significantly different from those of the previous one (36). CCl4 as a cytotoxic
stressor induced an increase in JNK phosphorylation only in the
kidney, whereas its level was decreased in the liver (Figs. 7 and 8). However, significant decreases in the
phosphorylation level of ERK were detected in the liver and kidney
tissues of the vehicle/CCl4-treated rats. These results
suggest that the mechanism of action of CCl4 differs
from that of other cytotoxic stressors affecting the liver and
kidney. In particular, pretreatment with gomisin A dramatically
increased the phosphorylation of ERK and p38 in the liver, whereas
increased JNK and p38 phosphorylation was observed in the
kidney.
Taken together, our results revealed that gomisin A
is a potential therapeutic compound for the protection and
regeneration of the liver and kidney upon injury induced by
CCl4.
Acknowledgements
We would like to thank Jinhyang Hwang, an animal
technician, for directing the Laboratory Animal Resources
Center.
References
|
1
|
Das RK, Hossain SU and Bhattacharya S:
Protective effect of diphenylmethyl selenocyanate against
CCl4-induced hepatic injury. J Appl Toxicol. 27:527–537.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagano K, Umeda Y, Saito M, Nishizawa T,
Ikawa N, Arito H, Yamamoto S and Fukushima S: Thirteen-week
inhalation toxicity of carbon tetrachloride in rats and mice. J
Occup Health. 49:249–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomenson JA, Baron CE, O’Sullivan JJ,
Edwards JC, Stonard MD, Walker RJ and Fearnley DM: Hepatic function
in workers occupationally exposed to carbon tetrachloride. Occup
Environ Med. 52:508–514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siviková K, Piesová E and Dianovský J: The
protection of Vitamin E and selenium against carbon
tetrachloride-induced genotoxicity in ovine peripheral blood
lymphocytes. Mutat Res. 494:135–142. 2001.PubMed/NCBI
|
|
5
|
Jialan S, Kenichi A, Yoji I and Kenjiro W:
Evidence of hepatocyte apoptosis in rat liver after the
administration of carbon tetrachloride. Am J Pathol. 153:515–525.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wernke MJ and Schell JD: Solvents and
malignancy. Clin Occup Environ Med. 4:513–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail: an overview of Russian research
and uses in medicine. J Ethnopharmacol. 118:183–212. 2008.
|
|
8
|
Azzam HS, Goertz C, Fritts M and Jonas WB:
Natural products and chronic hepatitis C virus. Liver Int.
27:17–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ikeya Y, Taguchi H, Yosioka I and
Kobayashi H: The constituents of Schisandra chinensis Baill
I Isolation and structure determination of five new lignans,
gomisin A, B, C, F and G, and the absolute structure of
schizandrin. Chem Pharm Bull. 27:1383–1394. 1979.
|
|
10
|
Maeda S, Takeda S, Miyamoto Y, Aburada M
and Harada M: Effects of gomisin A on liver functions in
hepatotoxic chemical-treated rats. Jpn J Pharmacol. 38:347–353.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizoguchi Y, Kawada N, Ichikawa Y and
Tsutsui H: Effect of gomisin A in the prevention of acute hepatic
failure induction. Planta Med. 57:320–324. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SH, Kim YS, Kang SS, Bae K, Hung TM
and Lee SM: Anti-apoptotic and hepatoprotective effects of gomisin
A on fulminant hepatic failure induced by D-galactosamine and
lipopolysaccharide in mice. J Pharmacol Sci. 106:225–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cyong JC, Ki SM, Iijima K, Kobayashi T and
Furuya M: Clinical and pharmacological studies on liver diseases
treated with Kampo herbal medicine. Am J Chin Med. 28:351–360.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kubo S, Ohkura Y, Mizoguchi Y,
Matsui-Yuasa I, Otani S, Morisawa S, Kinoshita H, Takeda S, Aburada
M and Hosoya E: Effect of gomisin A (TJN-101) on liver
regeneration. Planta Med. 58:489–492. 1992. View Article : Google Scholar
|
|
15
|
Ohtaki Y, Hida T, Hiramatsu K, Kanitani M,
Ohshima T, Nomura M, Wakita H, Aburada M and Miyamoto KI:
Deoxycholic acid as an endogenous risk factor for
hepatocarcinogenesis and effects of gomisin A, a lignan component
of Schisandra fruits. Anticancer Res. 16:751–755.
1996.PubMed/NCBI
|
|
16
|
Miyamoto K, Hiramatsu K, Ohtaki Y,
Kanitani M, Nomura M and Aburada M: Effects of gomisin A on the
promotor action and serum bile acid concentration in
hepatocarcinogenesis induced by
3′-methyl-4-dimethylamino-azobenzene. Biol Pharm Bull.
18:1443–1445. 1995.PubMed/NCBI
|
|
17
|
Nomura M, Ohtaki Y, Hida T, Aizawa T,
Wakita H and Miyamoto K: Inhibition of early
3-methyl-4-dimethylaminoazobenzene-induced hepatocarcinogenesis by
gomisin A in rats. Anticancer Res. 14:1967–1971. 1994.PubMed/NCBI
|
|
18
|
Choi YW, Takamatsu S, Khan SI, Srinivas
PV, Ferreira D, Zhao J and Khan IA: Schisandrene, a
dibenzocyclooctadiene lignan from Schisandra chinensis:
structure-antioxidant activity relationships of
dibenzocyclooctadiene lignans. J Nat Prod. 69:356–359. 2006.
|
|
19
|
Avula B, Dentali S and Khan IA:
Simultaneous identification and quantification by liquid
chromatography of benzethonium chloride, methyl paraben and
triclosan in commercial products labeled as grapefruit seed
extract. Pharmazie. 62:593–596. 2007.
|
|
20
|
Yim SY, Lee YJ, Lee YK, Jung SE, Kim JH,
Kim HJ, Son BG, Park YH, Lee YG, Choi YW and Hwang DY: Gomisin N
isolated from Schisandra chinensis significantly induces
anti-proliferative and pro-apoptotic effects in hepatic carcinoma.
Mol Med Rep. 2:725–732. 2009.
|
|
21
|
Wasan KM, Najafi S, Wong J and Kwong M:
Assessing plasma lipid levels, body weight, and hepatic and renal
toxicity following chronic oral administration of a water soluble
phytostanol compound FMVP4, to gerbils. J Pharm Pharm Sci.
4:228–234. 2001.
|
|
22
|
Crook MA: Clinical Chemistry and Metabolic
Medicine. 7th edition. Hodder Arnold; London: pp. 4262006
|
|
23
|
Apakama I, Robinson MC, Walter NM,
Charlton RG, Royds JA, Fuller CE, Neal DE and Hamdy FC: Bcl-2
overexpression combined with p53 accumulation correlates with
hormone refractory prostate cancer. Br J Urol. 74:1258–1262.
1996.PubMed/NCBI
|
|
24
|
Joensuu H, Pylkkänen L and Toikkanen S:
Bcl-2 protein expression and long-term survival in breast cancer.
Am J Pathol. 145:1191–1198. 1994.PubMed/NCBI
|
|
25
|
Takeda S, Kase Y, Arai I, Ohkura Y,
Hasegawa M, Sekiguchi Y, Tatsugi A, Funo S, Aburada M and Hosoya E:
Effects of TJN-101, a lignan compound isolated from
Schisandra fruits, on liver fibrosis and on liver
regeneration after partial hepatectomy in rats with chronic liver
injury induced by CCl4. Nihon Yakurigaku Zasshi.
90:51–65. 1987.(In Japanese).
|
|
26
|
Ip SP, Poon MK, Che CT, Ng KH, Kong YC and
Ko KM: Schisandrin B protects against carbon tetrachloride toxicity
by enhancing the mitochondrial glutathione redox status in mouse
liver. Free Radic Biol Med. 21:709–712. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ip SP, Yiu HY and Ko KM: Differential
effect of schisandrin B and dimethyl diphenyl bicarboxylate (DDB)
on hepatic mitochondrial glutathione redox status in carbon
tetrachloride intoxicated mice. Mol Cell Biochem. 205:111–114.
2000. View Article : Google Scholar
|
|
28
|
Chiu PY, Leung HY, Siu AH, Poon MK and Ko
KM: Schisandrin B decreases the sensitivity of mitochondria to
calcium ion-induced permeability transition and protects against
carbon tetrachloride toxicity in mouse livers. Biol Pharm Bull.
30:1108–1112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ko KM, Ip SP, Poon MK, Wu SS, Che CT, Ng
KH and Kong YC: Effect of a lignan-enriched fructus schisandrae
extract on hepatic glutathione status in rats: protection against
carbon tetrachloride toxicity. Planta Med. 61:134–137. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu M, Lin KF, Yeung RY and Li RC:
Evaluation of the protective effects of Schisandra chinensis
on Phase I drug metabolism using a CCl4 intoxication
model. J Ethnopharmacol. 67:61–68. 1999.
|
|
31
|
Chang HF, Lin YH, Chu CC, Wu SJ, Tsai YH
and Chao JC: Protective effects of Ginkgo biloba, Panax
ginseng, and Schisandra chinensis extract on liver
injury in rats. Am J Chin Med. 35:995–1009. 2007.
|
|
32
|
Mazumder S, Plesca D and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Boil. 414:13–21.
2008.PubMed/NCBI
|
|
33
|
Marshall CJ: MAP kinase kinase kinase, MAP
kinase kinase and MAP kinase. Curr Opin Genet Dev. 4:82–89. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Waskiewicz AJ and Cooper JA: Mitogen and
stress response pathways: MAP kinase cascades and phosphatase
regulation in mammals and yeast. Curr Opin Cell Biol. 7:798–805.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fanger GR, Gerwins P, Widmann C, Jarpe MB
and Johnson GL: MEKKs, GCKs, MLKs, PAKs, TAKs, and tpls: upstream
regulators of the c-Jun amino-terminal kinases? Curr Opin Genet
Dev. 7:67–74. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huot J, Houle F, Marceau F and Landry J:
Oxidative stress-induced actin reorganization mediated by the p38
mitogen-activated protein kinase/heat shock protein 27 pathway in
vascular endothelial cells. Circ Res. 80:383–392. 1997. View Article : Google Scholar
|
|
37
|
Van Rij AM, Thomson CD, McKenzie JM and
Robinson MF: Selenium deficiency in total parenteral nutrition. Am
J Clin Nutr. 32:2085–2086. 1979.PubMed/NCBI
|
|
38
|
Scorziello A, Santillo M, Adornetto A,
Dell’Aversano C, Sirabella R, Damiano S, Canzoniero LMT, Di Renzo
GF and Annunziato L: NO-induced neuroprotection in ischemic
preconditioning stimulates mitochondrial Mn-SOD activity and
expression via RAS/ERK1/2 pathway. J Neurochem. 103:1472–1480.
2007. View Article : Google Scholar : PubMed/NCBI
|