Introduction
It has been previously demonstrated that vitamin
D3 affects cell proliferation, differentiation and
apoptosis (1). The antitumor
activity of 1,25-dihydroxyvitamin D3
[1,25(OH)2D3] is observed only when it is
applied in supraphysiological doses, which may cause the
side-effect of hypercalcemia (2).
For this reason, the synthesis of analogs has been initiated in
order to dissociate the calcemic effect from the anticancer
activity of calcitriol. One strategy of improving the anticancer
effects is to combine vitamin D with other agents in order to
develop therapeutic interventions that allow dose reduction, the
alleviation of toxicity and the maintenance of the growth
inhibitory potential. According to the World Health Organization
International Agency for Research on Cancer (IARC), gastric cancer
is the second leading cause of cancer-related mortality worldwide
(3). Despite significant progress
in the treatment of patients with gastric cancer in recent years,
there is a constant need for new therapies (4). Due to the low responsiveness of some
patients suffering from gastric cancer to cisplatin therapy, there
is a need to explore new combined therapeutic methods.
The role of vitamin D in inhibiting cancer cell
growth, inducing cell differentiation and promoting cell apoptosis
has been a research hotspot for the prevention and therapy of
certain types of cancer, including gastric cancer (5). Although vitamin D exerts a less
progressive direct killing effect on cancer cells, it can enhance
the cytotoxicity of certain anticancer drugs, such as paclitaxel
and may synergistically suppress the proliferation of cancer cells.
The synergistic effects of vitamin D have been found in combination
chemotherapy in various malignant somatic cells in vitro and
in vivo (6–8).
Cisplatin is a major chemotherapeutic agent used in
the treatment of gastric cancer. The National Comprehensive Cancer
Network (NCCN) guideline suggested that cisplatin should be used as
a first-line anticancer drug in the treatment of gastric cancer.
Cisplatin exerts anticancer effects through various mechanisms;
however, its most prominent mode of action is through the
generation of DNA lesions followed by the activation of DNA damage
response and the induction of cell apoptosis (9). Resistance to cisplatin arises
through multiple mechanisms involving reduced drug uptake,
increased drug inactivation, increased DNA damage repair, and the
inhibition of transmission of DNA damage recognition signals to the
apoptotic pathway (10). However,
some patients with gastric cancer are not sensitive to cisplatin
treatment. In addition, high-dose chemotherapy often results in
several side-effects; a high concentration of vitamin D also causes
hypocalcemia; thus, this limits the single use of both drugs.
1,25(OH)2D3 has been shown to
synergistically or additively enhance the antitumor activities of a
number of chemotherapeutic agents, including carboplatin,
cisplatin, docetaxel and paclitaxel in prostate cancer (11), breast cancer (12), bladder cancer (13) and murine models of squamous cell
carcinoma (SCC) (14). In the
present study, we investigated the synergistic effects of
1,25(OH)2D3 and cisplatin on apoptosis and
cell cycle distribution, as well as their mechanisms of action in
BGC-823 gastric cancer cells in vitro. Our aim was to
examine the biological effects of combined treatment with
1,25(OH)2D3 and cisplatin against gastric
cancer cells. We also wished to to evaluate the effects of
co-treatment with 1,25(OH)2D3 and cisplatin
on the apoptosis and cell cycle distribution of gastric cancer
cells, and to explore the possible mechanisms responsible for the
the synergistic anticancer effects of
1,25(OH)2D3 and cisplatin.
Materials and methods
Drugs
1,25(OH)2D3 was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
1,25(OH)2D3 was dissolved in absolute ethanol
(ETOH) to the concentration of 10−3 M and stored in
solution at −20°C. 1,25(OH)2D3 was freshly
diluted in culture medium to reach the required concentrations
prior to each experiment. The ethanol concentration in each test
condition never exceeded 0.1%.
Cisplatin was purchased from Shanghai Haoran
Bio-Technology Co., Ltd. (Shanghai, China). Cisplatin was dissolved
in sterile 0.9% NaCl to the concentration of 50 μg/ml and stored in
solution at 4°C. Cisplatin was freshly diluted in culture medium to
reach the required concentrations prior to each experiment.
Cell culture
The BGC-823 gastric cancer cell line was purchased
from the Central Laboratory of Xiangya Medical College of Central
South University, Changsha, China. The cells were cultured
according to standard conditions. In brief, the BGC-823 gastric
cancer cells were grown in RPMI-1640, 10% heat-inactivated fetal
bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin
at 37°C in a humid environment with 5% CO2. Cell media
and reagents were obtained from Gibco-Invitrogen (Carlsbad, CA,
USA). The culture media were changed every 48 h, and the cells were
passaged every 2–3 days to produce new generations. The cells were
plated in 25-cm2 flasks (Costar Life Sciences, Tewksbury
MA, USA), and split every 48 h by washing with D-Hank’s solution
and detached using 0.05% trypsin-EDTA. Half of the cells were
plated in new flasks with fresh culture medium and the remaining
cells were used for the experiments. The cells which had undergone
3 passages were selected for the experiments.
Drug treatment
The cells in the 1,25(OH)2D3
treatment group were cultured in RPMI-1640 culture medium with 10
nM 1,25(OH)2D3 for 72 h. The cells in the
cisplatin treatment group were treated with 0.2 μg/ml cisplatin
solution for 2 h following normal culture for 24 h; the cells were
then cultured with fresh medium, followed by washing twice with
D-Hank’s solution. The cells in the group co-treated with
1,25(OH)2D3 and cisplatin were treated with
0.2 μg/ml cisplatin for 2 h following culture for 24 h with
1,25(OH)2D3 alone, and were then cultured
with fresh culture medium with 10 nM
1,25(OH)2D3. The control group was treated
with ETOH in RPMI-1640 culture medium. The total culture time was
72 h for each group.
Preparation of cell extracts
The BGC-823 cells were seeded in 6-well plates
(5×104 cells/well) and left overnight to attach. The
cells were then treated with 1,25(OH)2D3 or
cisplatin alone, or a combination of both. The cells treated with
an equivalent amount of ETOH were used as the vehicle control. The
cells were washed with D-Hank’s solution and replenished with fresh
medium every 24 h. Following treatment for 72 h, the cells were
harvested for immunoblot analysis using RIPA buffer (Thermo
Scientific, Waltham, MA, USA) supplemented with protease
inhibitors.
Immunoblot analysis
The cell protein concentration was measured using a
BCA protein assay kit (Thermo Scientific) according to the
manufacturer’s instructions. An immunoblot analysis was performed
using a Bio-Rad wet electroblotting system (Bio-Rad, Hercules, CA,
USA) according to the manufacturer’s instructions. The results from
immunoblot analysis were quantified by measuring the optical
density of the immunoreactive bands using ImageJ software.
Mouse and rabbit antibodies against Bax, Bcl-2,
caspase-3, caspase-8, poly(ADP-ribose) polymerase (PARP), cleaved
PARP, phosphorylated (p-)ERK1/2, ERK1/2, p-AKT, AKT, p21, p27 and
β-actin were purchased from Cell Signaling Technology (Danvers, MA,
USA). β-actin was used as the loading control to ensure equal
protein loading among all wells in immunoblot analysis.
TUNEL assay
The In Situ Cell Death Detection Fluorescein kit
(Roche Applied Science, Indianapolis, IN, USA) was used to detect
cell apoptosis. Cell apoptosis was analyzed by TUNEL assay. In
brief, the procedure was as follows: BGC-823 gastric cancer cell
suspension was prepared following scheduled experiment treatment,
the test sample was washed 3 times in phosphate-baffered saline
(PBS) and adjusted to 2×107 cells/ml. Subsequently, 100
μl/tube suspension were transferred into a V-bottomed EP tube, and
then 100 μl/tube of a freshly prepared fixation solution were added
(4% paraformaldehyde in PBS, pH 7.4) to the cell suspension. The
cells were then resuspended and incubated for 60 min at 22°C. The
EP tubes were centrifuged at 300 × g for 10 min and the fixative
was removed by flicking off or suction. The cells were then washed
once with PBS, the EP tubes were centrifuged at 300 × g for 10 min
again, and finally, the cells were resuspended in 100 μl/tube
permeabilization solution (0.1% Triton X-100 in 0.1% sodium
citrate) for 5 min on ice. The TUNEL reaction mixture was prepared
immediately according to the kit’s instructions prior to use in the
experiments and kept on ice until use. The cells were washed twice
with PBS, then resuspended in 50 μl/tube TUNEL reaction mixture and
incubated for 60 min at 37°C in a humidified atmosphere in the
dark. The cells were then transferred to a tube to a final volume
of 500 μl in PBS. The well-prepared samples were tested in a
Beckman flow cytometry (Beckman Coulter, Miami, FL, USA) for the
analysis of cell apoptosis.
Cell cycle analysis
The BGC-823 gastric cancer cells were treated with
the vehicle control (ETOH), 1,25(OH)2D3
alone, cisplatin alone, or combined treatment with
1,25(OH)2D3 and cisplatin for 24 h. Following
treatment, the BGC-823 cells (1×106/sample) were
collected by trypsin digestion, then washed twice in cold PBS and
fixed for 24 h in 70% ETOH at −20°C. The cells were then washed
twice in PBS and incubated with RNAse (8 μg/ml; Fermentas, St.
Leon-Rot, Germany) at 37°C for 1 h. The cells were stained with
propidium iodide (0.5 mg/ml; Sigma-Aldrich) for 30 min at 37°C in
the dark. The cellular DNA content was determined using a Beckman
flow cytometry (Beckman Coulter) and ModFit LT 3.0 software (Verity
Software House Inc., Topsham, ME, USA). The experiment was repeated
3 times.
Data analysis
Statistical analysis was performed by employing
GraphPad Prism 5.0 software (GraphPad Software, CA, USA). All data
are presented as the means ± standard error of the mean (SEM). Each
experiment was repeated 3 times. The difference between the mean
values of 2 groups was evaluated using the Student’s t-test. A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
1,25(OH)2D3
enhances the anticancer and apoptotic effects of cisplatin and in
BGC-823 gastric cancer cells
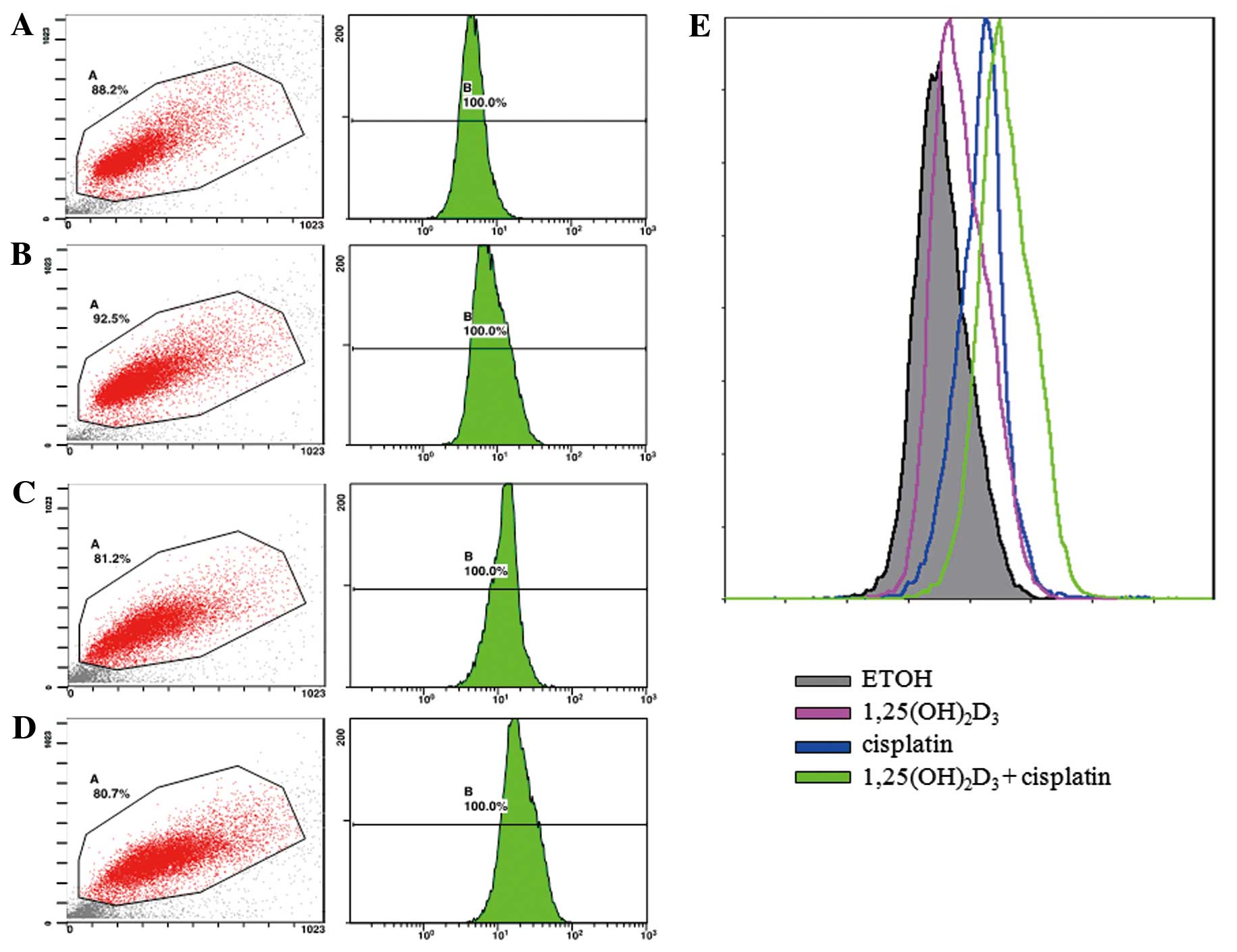
The apoptosis of BGC-823 gastric cancer cells was
evaluated by TUNEL assay. The apoptosis of the treated cancer cells
was expressed using a fluorescent signal determined by flow
cytometry. The density plots obtained by flow cytometry are shown
as Fig. 1. The peaks shown in
different colors represent the intensity of cell apoptosis
following treatment with ETOH (vehicle control),
1,25(OH)2D3 or cisplatin alone, as well as
co-treatment with 1,25(OH)2D3 and
cisplatin.
Treatment with 10 nM
1,25(OH)2D3 or 0.2 μg/ml cisplatin alone
significantly enhanced cell apoptosis compared with the control, as
indicated by the increased fluorescence intensity of the DNA
fragments in apoptotic cells (P<0.05). Co-treatment with 10 nM
1,25(OH)2D3 and 0.2 μg/ml cisplatin led to a
significantly (P<0.05) greater number of apoptotic BGC-823 cells
compared to treatment with cisplatin or
1,25(OH)2D3 alone (Table I).
 | Table IEffects of treatment with
1,25(OH)2D3 alone or in combination with
cisplatin on the apoptosis of BGC-823 cells. |
Table I
Effects of treatment with
1,25(OH)2D3 alone or in combination with
cisplatin on the apoptosis of BGC-823 cells.
| Group | Fluorescence
intensity (means ± SEM) |
|---|
| Control (ETOH) | 4.73±0.55 |
|
1,25(OH)2D3 | 9.2±1.14a |
| Cisplatin | 14.17±4.01a |
|
1,25(OH)2D3 +
cisplatin | 23.07±3.00a,b |
Effects of co-treatment with
1,25(OH)2D3 and cisplatin on the expression
of apoptosis-related proteins in BGC-823 gastric cancer cells
Following treatment with
1,25(OH)2D3 alone or in combination with
cisplatin for 72 h, the cells were harvested for immunoblot
analysis. The expression of a series of apoptosis-related proteins
was then determined. The cleavage of PARP was significantly higher
in the group co-treated with 1,25(OH)2D3 and
cisplatin (P<0.01) compared with the group treated with
cisplatin or 1,25(OH)2D3 alone (Fig. 2). In addition, the expression of
caspase-3, a key member of the caspase family, was significantly
reduced in the cells co-treated with
1,25(OH)2D3 and cisplatin (P<0.01)
compared with the cells treated with cisplatin or
1,25(OH)2D3 alone. However, no significant
change was observed in caspase-8 expression in all the treatment
groups [1,25(OH)2D3 or cisplatin treatment
alone or combined treatment (P>0.05)]. Bax expression was
significantly upregulated by 1,25(OH)2D3
treatment alone (P<0.05) or the combined treatment with
1,25(OH)2D3 and cisplatin (P<0.01).
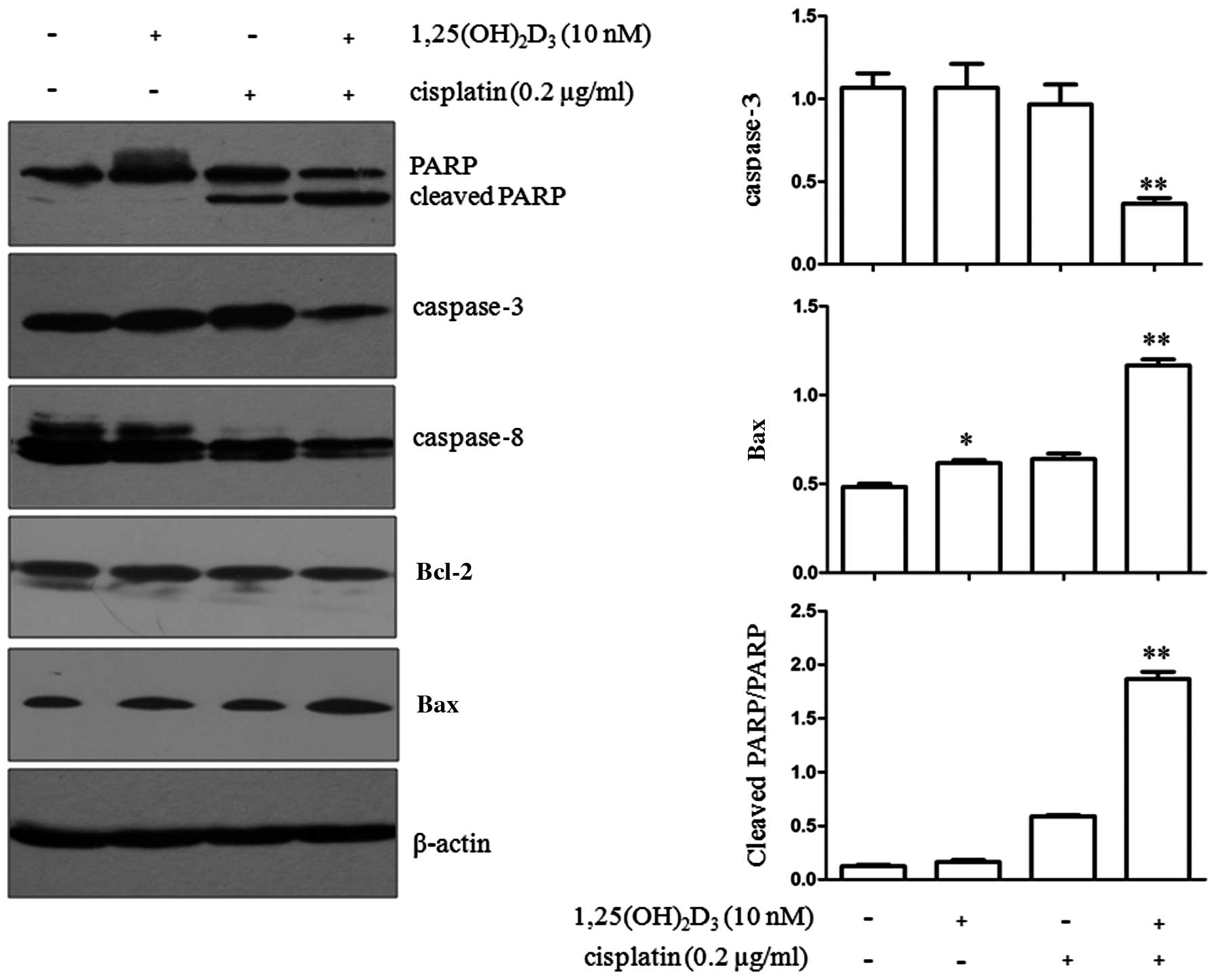 | Figure 2Effects of co-treatment with
1,25-dihydroxyvitamin D3
[1,25(OH)2D3] and cisplatin on the expression
of apoptosis-related proteins in BGC-823 cells. BGC-823 gastric
cancer cells were treated with 1,25(OH)2D3,
or cisplatin alone or a combination of both agents. Each experiment
was repeated independently 3 times, and representative blots are
shown. The expression of poly(ADP-ribose) polymerase (PARP),
cleaved PARP, caspase-3, caspase-8, Bcl-2 and Bax is demonstrated.
β-actin was used as a loading control for total cellular proteins.
Values represent the means ± standard error of the mean (SEM) of
triplicate assays. *P<0.05 compared with ETOH
treatment alone; **P<0.01 compared with ETOH,
1,25(OH)2D3, or cisplatin treatment
alone. |
Regulation of AKT and ERK1/2
phosphorylation by co-treatment with
1,25(OH)2D3 and cisplatin
Cell apoptosis is regulated by multiple pathways.
AKT and ERK1/2 are two important kinases involved in cell
proliferation and apoptosis in gastric cancer (15,16). In the present study, we wished to
explore the effects of 1,25(OH)2D3 or
cisplatin treatment alone, as well as the effects of co-treatment
with both agents on the phosphorylation levels of AKT and ERK1/2.
As illustrated in Fig. 3,
treatment with 1,25(OH)2D3 (P<0.05) or
cisplatin (P<0.01) alone, as well as the combined treatment
(P<0.01) significantly reduced the phosphorylation level of AKT.
Furthermore, co-treatment with 1,25(OH)2D3
and cisplatin further reduced the phosphorylation level of AKT
compared to treatment with cisplatin alone (P<0.05).
ERK1/2 phosphorylation was also observed following
treatment with 1,25(OH)2D3 or cisplatin alone
or the combined treatment. Both agents decreased the
phosphorylation levels of ERK1/2 (P<0.01). Similarly,
co-treatment with 1,25(OH)2D3 and cisplatin
significantly reduced the phosphorylation levels of ERK1/2 compared
to treatment with cisplatin alone (P<0.05). These results
indicated that co-treatment with 1,25(OH)2D3
and cisplatin further enhanced the anti-proliferative effects of
cisplatin on BGC-823 gastric cancer cells.
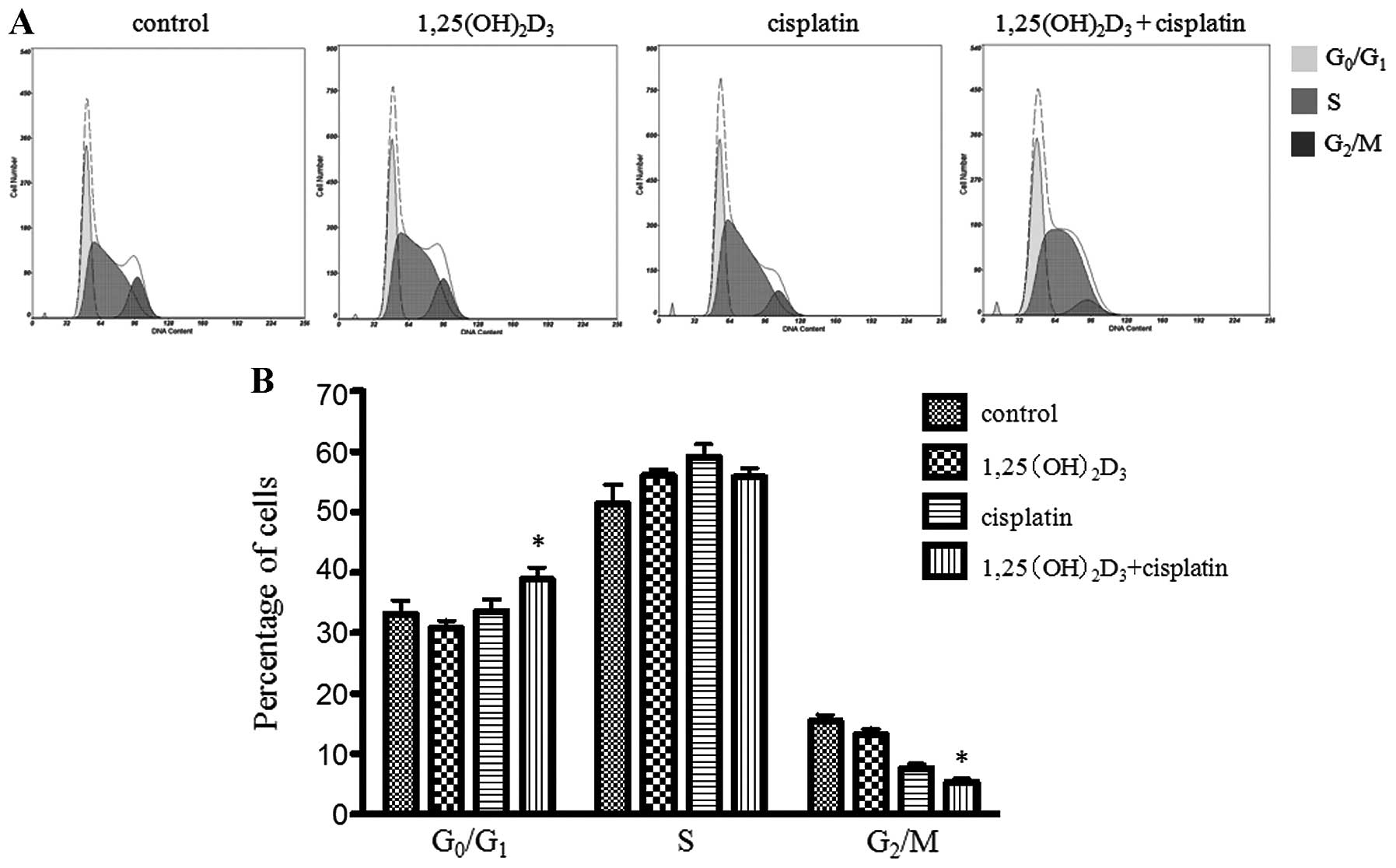
Effects of co-treatment with
1,25(OH)2D3 and cisplatin on cell cycle
distribution of BGC-823 gastric cancer cells
The evaluation of the cell cycle distribution was
carried out following treatment with
1,25(OH)2D3 or cisplatin alone or the comined
treatment. Co-treatment with 1,25(OH)2D3 and
cisplatin significantly increased the percentage of cells in the
G0/G1 phase when compared to the group
treated with ETOH (vehicle control),
1,25(OH)2D3 or cisplatin alone (P<0.05)
(Fig. 4). We observed that the
cells treated with both 1,25(OH)2D3 and
cisplatin had accumulated in the G0/G1 phase
and the number of cells was decreased in the G2/M phase,
when compared to the cells treated with cisplatin or
1,25(OH)2D3 alone. However, the percentage of
cells in the G2/M phase was not significantly affected
by treatment with 1,25(OH)2D3 alone. The
percentage of cells in the different cell cycle phases is shown in
Table II.
1,25(OH)2D3, in combination with cisplatin,
significantly increased the percentage of cells in the
G0/G1 phase and decreased the percentage of
cells in the G2/M phase, when compared to treatment with
cisplatin or 1,25(OH)2D3 alone
(P<0.05).
 | Table IIEffects of treatment with
1,25(OH)2D3 alone or in combination with
cisplatin on cell cycle distribution of BGC-823 cells. |
Table II
Effects of treatment with
1,25(OH)2D3 alone or in combination with
cisplatin on cell cycle distribution of BGC-823 cells.
| Percentage of cells
in each cell cycle phase (means ± SEM) |
|---|
|
|
|---|
|
G0/G1 | S |
G2/M |
|---|
| Control (ETOH) | 33.17±1.27 | 51.37±1.82 | 14.27±0.73 |
|
1,25(OH)2D3 | 30.77±0.73 | 56.03±0.58 | 13.17±0.59 |
| Cisplatin | 33.57±1.17 | 59.14±1.21 | 7.60±0.44 |
|
1,25(OH)2D3 +
cisplatin | 38.87±1.14a | 55.83±0.79 | 5.28±0.35a |
Effects of co-treatment with
1,25(OH)2D3 and cisplatin on p21 and p27
protein expression in BGC-823 gastric cancer cells
As the anti-proliferative effects of
1,25(OH)2D3 commonly involve the upregulation
of p21 and/or p27 (17,18), we wished to determine the effects
of 1,25(OH)2D3 and cisplatin on the protein
expression of p21 and p27 in BGC-823 cells. p21 and p27 are
important cell cycle regulators (19) and they were also examined in our
study. As shown in Fig. 5,
co-treatment with 1,25(OH)2D3 and cisplatin
significantly increased the expression of p21 and p27 (P<0.01)
compared to treatment with ETOH, 1,25(OH)2D3
or cisplatin alone. The effects on p21 and p27 expression induced
by treatment with 1,25(OH)2D3 or cisplatin
alone differed. Treatment with cisplatin alone significantly
increased p27 expression (P<0.05), whereas
1,25(OH)2D3 had no such effect. Treatment
with 1,25(OH)2D3 or cisplatin alone did not
upregulate p21 expression. Co-treatment with
1,25(OH)2D3 and cisplatin further increased
the expression of p21 and p27 compared to treatment with cisplatin
alone (P<0.05).
Discussion
Gastric cancer remains the second most common cause
of cancer-related mortality worldwide (20). Surgery remains as the most common
curative treatment. However, the majority of patients with gastric
cancer develop local or distant recurrence (21). Meta-analyses have indicated that
certain patients treated with chemotherapy following surgery
benefit from this treatment strategy, while other patients have
undergone expensive and potentially toxic therapy without any
beneficial effects (22).
Cisplatin is widely used and has been demonstrated to be effective
in the palliative treatment of gastric cancer (23). Oxaliplatin, the third generation
platinum compound, plus 5-fluorouracil modulated with leucovorin
(FOLFOX) has been widely used as the first-line treatment in
advanced gastric cancer (24,25). However, resistance to oxaliplatin
and cisplatin remains a major obstacle to further increasing the
treatment response rate. In the present study, to the best of our
knowledge, we demonstrate for the first time the biological effects
of combined treatment with 1,25(OH)2D3 and
cisplatin against gastric cancer cell growth. We observed that
1,25(OH)2D3 induced the apoptosis of BGC-823
cells as shown by TUNEL assay. However, when used in combination
with cisplatin, the apoptotic signal significantly increased
compared to treatment with cisplatin alone. Previous studies have
indicated that the anticancer effects of vitamin D are limited in
certain types of cancer (8).
Preclinical experiments have suggested that vitamin D exerts minor
effects on the prevention or therapy of cancer in vivo,
although it inhibits cell proliferation and induces cell apoptosis
in vitro (26,27). However, vitamin D and its
analogues are still being focused on, as they exert synergistic
anticancer effects when used in combination with chemotherapeutic
drugs, such as platinum (28).
The mechanisms of cancer progression have been clarified and a
number of target proteins have been identifed to be important in
the treatment of cancer (29).
In the present study, we demonstrated the
differential effects of treatment with vitamin D3 alone
or in combination with cisplatin on the apoptosis of BGC-823
gastric cancer cells. Treatment with
1,25(OH)2D3 and cisplatin alone induced the
apoptosis of BGC-823 cells, as shown by TUNEL assay. Furthermore,
enhanced apoptosis was observed following co-treatment with both
agents, and the fluorescence intensity of apoptotic cells markedly
increased by 5-fold of the control, and by 1.6-fold that of
cisplatin (Table I). We also
observed the changes in protein expression following co-treatment
with 1,25(OH)2D3 and cisplatin. The caspase
pathway is involved in vitamin D-induced cell apoptosis (18,30). In the present study, caspase-3
expression was reduced following co-treatment with
1,25(OH)2D3 and cisplatin, which indicated a
greater apoptotic status in the BGC-823 cells. In addition, the
significantly increased cleavage of PARP and the expression of
pro-apoptotic Bax were also observed following co-treatment with
1,25(OH)2D3 and cisplatin when compared to
treatment with cisplatin alone.
Having demonstrated the synergism between
1,25(OH)2D3 and cisplatin, we sought to
explore the underlying mechanisms. Caspases play a crucial role in
apoptotic cell death induced by vitamin D3 (30). The apoptosis of gastric cancer
cells can be triggered by the extrinsic pathway activated by death
receptor and the intrinsic pathway regulated by Bcl-2 family
members and caspase cascades in the mitochondrion (31). Previous studies have indicated
that 1,25(OH)2D3-mediated apoptosis is
caspase-dependent and appears to act through the mitochondrial
pathway of cytochrome c release, caspase-9 activation, and
subsequent caspase-3 activation, finally the processing of PARP
(32). In our study, we found
that caspase-3 expression was significantly decreased following
treatment with 1,25(OH)2D3 and cisplatin,
while caspase-8 expression remained unaltered following the
combined treatment [1,25(OH)2D3 and
cisplatin] or treatment with 1,25(OH)2D3 and
cisplatin alone. This suggests that the mitochondrial pathway is
involved in vitamin D-mediated apoptosis, although the involvement
of other pathways cannot be ruled out.
We also found that the pro-apoptotic protein, Bax,
was upregulated following co-treatment with
1,25(OH)2D3 and cisplatin, while treatment
with cisplatin alone did not increase the expression of Bax. The
translocation of Bax to the mitochondria has been shown to be of
particular importance for the induction of vitamin D-mediated
apoptosis in certain cell types. The treatment of MCF-7 breast
cancer cells with 1,25(OH)2D3 has been shown
to result in the redistribution of Bax from the cytosol to the
mitochondria (33,34). Changes in the expression or
cellular distribution of Bcl-2 anti-apoptotic proteins are a
possible mechanism of 1,25(OH)2D3-mediated
apoptosis (35). However, Bcl-2
expression did not show a downregulation in the BGC-823 gastric
cancer cells treated with 1,25(OH)2D3 or
cisplatin. Cisplatin cytotoxicity results from the formation of
bifunctional, intrastrand DNA adducts (36). Cisplatin activates p53 and then
results in the increased transcription of p53 target genes,
including Bax and p21, as well as in cell cycle arrest and
apoptosis (37). In our study,
the combined use of cisplatin and 1,25(OH)2D3
enhanced the apoptosis of BGC-823 cells.
In our previous study, we reported that
1,25(OH)2D3-mediated apoptosis is associated
with the downregulation of the AKT and ERK survival signaling
pathways (18). Activated AKT
phosphorylates a host of proteins that affect cell growth, cell
cycle entry and cell survival. The decreased phosphorylation of AKT
may contribute to the anti-proliferative effects of
1,25(OH)2D3. siRNA-AKT has been shown to
promote 1,25(OH)2D3-induced apoptosis in SCC
cells through the caspase-10-caspase-3 pathway, whereas caspase-8
and caspase-9 are not involved (38). Akt may regulate apoptosis through
a number of different mechanisms depending on the apoptotic stimuli
and cell types, which involve the regulation of phosphorylation and
protein expression (39,40). In our study, as compared to
treatment with cisplatin alone, AKT phosphorylation was further
decreased in the cells that became apoptotic following combined
treatment with 1,25(OH)2D3 and cisplatin.
These data indicate that 1,25(OH)2D3 further
enhances the cisplatin-induced loss of survival signaling, and thus
further inhibits the proliferation of gastric cancer cells. The AKT
pathway presents an attractive target for anticancer therapies,
which may be applied in future anticancer chemotherapy.
It has been demonstrated that the MAPK-ERK pathway
is one of the most significant signal transduction pathways
(41), and several key growth
factors and genes promote tumor growth by activating this signaling
cascade. The downregulation of ERK phosphorylation is a
contributing factor to cellular apoptosis in gastric cancer
(42). The vitamin D analog,
Gemini, has been shown to suppress ErbB2-positive mammary tumor
growth through the inhibition of ErbB2/AKT/ERK signaling (43), and the knockdown of ZFX has been
shown to inhibit gastric cancer cell growth in vitro and
in vivo by downregulating the MAPK-ERK pathway (44). In our previous study, we observed
that the pERK/ERK ratio was decreased in
1,25(OH)2D3-treated BGC-823 cells (18), and we found a further reduction in
pERK/ERK in BGC-823 cells co-treated with
1,25(OH)2D3 and cisplatin. Therefore, the
promotion of gastric cancer cell apoptosis or inhibition of cell
growth due to the combined effects of vitamin D and cisplatin may
be explained, at least in part, by the inhibition of the ERK and
AKT pathway. However, the direct link between vitamin D and the ERK
or AKT pathway requires further investigation.
The anti-proliferative effects of
1,25(OH)2D3 commonly involve the cell cycle
arrest of different cancer cells. 1,25(OH)2D3
inhibits cell proliferation, induces cell cycle arrest and promotes
the accumulation of cells in the G0/G1 phase
in multipotent mesenchymal cells (MMCs) (45). The vitamin D analogue, EB1089, has
been shown to significantly reduce cell growth in human hepatoma
cells (Hep-G2) and block Hep-G2 cell-associated tumor formation in
nude mice through cell cycle G1 phase arrest by the
accumulation of p27 (46). In
this study, we demonstrated that 1,25(OH)2D3
alone did not induce cell cycle G0/G1 arrest
or G2/M cell cycle change. However,
1,25(OH)2D3, in conjunction with cisplatin,
induced G0/G1 cell cycle arrest or a decrease
in the number of cells in the G2/M phase in BGC-823
cells, and we observed that the effects of this combined treatment
were more potent compared to the effects induced by cisplatin
alone.
Although a number of
1,25(OH)2D3 responsive genes are known, the
exact mechanisms of growth regulation by
1,25(OH)2D3 have not been completely defined.
However, an increase in p21 and/or p27 expression is an almost
universal feature (47). In our
study, p21 protein expression increased significantly following
co-treatment with 1,25(OH)2D3 and cisplatin,
and p27 was upregulated to a much higher degree following the
combined treatment compared to treatment with cisplatin alone. This
indicates that 1,25(OH)2D3 promotes the
effects of cisplatin, inducing cell cycle arrest in the
G0/G1 phase.
In conclusion, to the best of our knowledge, the
present study demonstrates for the first time that
1,25(OH)2D3 plays a synergistic role in
cisplatin-mediated growth inhibition and apoptosis in gastric
cancer cells. The combined use of 1,25(OH)2D3
and cisplatin may be used as a strategy to overcome resistance to
cisplatin and dose limitations, and to improve the anticancer
effects of chemotherapy.
Acknowledgements
This study was supported by grants from the
National Clinical Key Specialty Construction Project.
References
|
1
|
Deeb KK, Trump DL and Johnson CS: Vitamin
D signalling pathways in cancer: potential for anticancer
therapeutics. Nat Rev Cancer. 7:684–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eelen G, Verlinden L, De Clercq P,
Vandewalle M, Bouillon R and Verstuyf A: Vitamin D analogs and
coactivators. Anticancer Res. 26:2717–2721. 2006.PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abnet CC, Chen Y, Chow WH, et al:
Circulating 25-hydroxyvitamin D and risk of esophageal and gastric
cancer: Cohort Consortium Vitamin D Pooling Project of Rarer
Cancers. Am J Epidemiol. 172:94–106. 2010. View Article : Google Scholar
|
|
6
|
Fleet JC: Molecular actions of vitamin D
contributing to cancer prevention. Mol Aspects Med. 29:388–396.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feldman DR, Bosl GJ, Sheinfeld J and
Motzer RJ: Medical treatment of advanced testicular cancer. JAMA.
299:672–684. 2008. View Article : Google Scholar
|
|
8
|
Krishnan AV, Trump DL, Johnson CS and
Feldman D: The role of vitamin D in cancer prevention and
treatment. Rheum Dis Clin North Am. 38:161–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krege S, Beyer J, Souchon R, et al:
European consensus conference on diagnosis and treatment of germ
cell cancer: a report of the second meeting of the European Germ
Cell Cancer Consensus group (EGCCCG): part I. Eur Urol. 53:478–496.
2008. View Article : Google Scholar
|
|
10
|
Tanida S, Mizoshita T, Ozeki K, et al:
Mechanisms of cisplatin-induced apoptosis and of cisplatin
sensitivity: potential of BIN1 to act as a potent predictor of
cisplatin sensitivity in gastric cancer treatment. Int J Surg
Oncol. 2012:8628792012.
|
|
11
|
Moffatt KA, Johannes WU and Miller GJ:
1Alpha, 25dihydroxyvitamin D3 and platinum drugs act
synergistically to inhibit the growth of prostate cancer cell
lines. Clin Cancer Res. 5:695–703. 1999.PubMed/NCBI
|
|
12
|
Cho YL, Christensen C, Saunders DE, et al:
Combined effects of 1,25-dihydroxyvitamin D3 and
platinum drugs on the growth of MCF-7 cells. Cancer Res.
51:2848–2853. 1991.PubMed/NCBI
|
|
13
|
Ma Y, Yu WD, Trump DL and Johnson CS:
1,25D3 enhances antitumor activity of gemcitabine and
cisplatin in human bladder cancer models. Cancer. 116:3294–3303.
2010.PubMed/NCBI
|
|
14
|
Light BW, Yu WD, McElwain MC, Russell DM,
Trump DL and Johnson CS: Potentiation of cisplatin antitumor
activity using a vitamin D analogue in a murine squamous cell
carcinoma model system. Cancer Res. 57:3759–3764. 1997.
|
|
15
|
Almhanna K, Strosberg J and Malafa M:
Targeting AKT protein kinase in gastric cancer. Anticancer Res.
31:4387–4392. 2011.PubMed/NCBI
|
|
16
|
Yao J, Qian CJ, Ye B, Zhang X and Liang Y:
ERK inhibition enhances TSA-induced gastric cancer cell apoptosis
via NF-κB-dependent and Notch-independent mechanism. Life Sci.
91:186–193. 2012.PubMed/NCBI
|
|
17
|
Chen S, Law CS and Gardner DG: Vitamin
D-dependent suppression of endothelin-induced vascular smooth
muscle cell proliferation through inhibition of CDK2 activity. J
Steroid Biochem Mol Biol. 118:135–141. 2010. View Article : Google Scholar
|
|
18
|
Bao A, Li Y, Tong Y, Zheng H, Wu W and Wei
C: Tumor-suppressive effects of 1,25-dihydroxyvitamin D3
in gastric cancer cells. Hepatogastroenterology. 60:943–948.
2013.PubMed/NCBI
|
|
19
|
Mitrea DM, Yoon MK, Ou L and Kriwacki RW:
Disorder-function relationships for the cell cycle regulatory
proteins p21 and p27. Biol Chem. 393:259–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leung WK, Wu MS, Kakugawa Y, et al:
Screening for gastric cancer in Asia: current evidence and
practice. Lancet Oncol. 9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Macdonald JS: Treatment of localized
gastric cancer. Semin Oncol. 31:566–573. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carrato A, Gallego-Plazas J and
Guillen-Ponce C: Adjuvant therapy of resected gastric cancer is
necessary. Semin Oncol. 32(Suppl 9): S105–S108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Topuz E, Basaran M, Saip P, et al:
Adjuvant intraperitoneal chemotherapy with cisplatinum,
mitoxantrone, 5-fluorouracil, and calcium folinate in patients with
gastric cancer: a phase II study. Am J Clin Oncol. 25:619–624.
2002. View Article : Google Scholar
|
|
24
|
Cavanna L, Artioli F, Codignola C, et al:
Oxaliplatin in combination with 5-fluorouracil (5-FU) and
leucovorin (LV) in patients with metastatic gastric cancer (MGC).
Am J Clin Oncol. 29:371–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Louvet C, Andre T, Tigaud JM, et al: Phase
II study of oxaliplatin, fluorouracil, and folinic acid in locally
advanced or metastatic gastric cancer patients. J Clin Oncol.
20:4543–4548. 2002. View Article : Google Scholar
|
|
26
|
Mocellin S: Vitamin D and cancer:
deciphering the truth. Biochim Biophys Actas. 1816:172–178.
2011.PubMed/NCBI
|
|
27
|
Picotto G, Liaudat AC, Bohl L and Tolosa
de Talamoni N: Molecular aspects of vitamin D anticancer activity.
Cancer Invest. 30:604–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reichrath J, Friedrich M and Vogt T:
Vitamin D and its analogs in cancer prevention and therapy.
Anticancer Res. 32:209–210. 2012.PubMed/NCBI
|
|
29
|
Rahman N: Realizing the promise of cancer
predisposition genes. Nature. 505:302–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fingas CD, Altinbas A, Schlattjan M, et
al: Expression of apoptosis- and vitamin D pathway-related genes in
hepatocellular carcinoma. Digestion. 87:176–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang W, Zhao CH, Zhang N and Wang J:
Vitamin D analog EB1089 induces apoptosis in a subpopulation of
SGC-7901 gastric cancer cells through a mitochondrial-dependent
apoptotic pathway. Nutr Cancer. 65:1067–1075. 2013. View Article : Google Scholar
|
|
33
|
Narvaez CJ and Welsh J: Role of
mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7
breast cancer cells. J Biol Chem. 276:9101–9107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koshizuka K, Koike M, Kubota T, Said J,
Binderup L and Koeffler HP: Novel vitamin D3 analog
(CB1093) when combined with paclitaxel and cisplatin inhibit growth
of MCF-7 human breast cancer cells in vivo. Int J Oncol.
13:421–428. 1998.
|
|
35
|
Wagner N, Wagner KD, Schley G, Badiali L,
Theres H and Scholz H: 1,25-dihydroxyvitamin D3-induced
apoptosis of retinoblastoma cells is associated with reciprocal
changes of Bcl-2 and bax. Exp Eye Res. 77:1–9. 2003.
|
|
36
|
Basu A and Krishnamurthy S: Cellular
responses to cisplatin-induced DNA damage. J Nucleic Acids.
2010:2013672010. View Article : Google Scholar
|
|
37
|
Wang S, Li W, Xue Z, et al: Molecular
imaging of p53 signal pathway in lung cancer cell cycle arrest
induced by cisplatin. Mol Carcinog. 52:900–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma Y, Yu WD, Kong RX, Trump DL and Johnson
CS: Role of nongenomic activation of phosphatidylinositol
3-kinase/Akt and mitogen-activated protein kinase/extracellular
signal-regulated kinase kinase/extracellular signal-regulated
kinase 1/2 pathways in 1,25D3-mediated apoptosis in squamous cell
carcinoma cells. Cancer Res. 66:8131–8138. 2006.
|
|
39
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao H, Shi W, Liu S, et al:
1,25-Dihydroxyvitamin D(3) prevents puromycin
aminonucleoside-induced apoptosis of glomerular podocytes by
activating the phosphatidylinositol 3-kinase/Akt-signaling pathway.
Am J Nephrol. 30:34–43. 2009. View Article : Google Scholar
|
|
41
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen XJ, Wang HB, Ma XQ and Chen JH:
β,β-Dimethylacrylshikonin induces mitochondria dependent apoptosis
through ERK pathway in human gastric cancer SGC-7901 cells. PLoS
One. 7:e417732012.
|
|
43
|
Lee HJ, So JY, DeCastro A, et al: Gemini
vitamin D analog suppresses ErbB2-positive mammary tumor growth via
inhibition of ErbB2/AKT/ERK signaling. J Steroid Biochem Mol Biol.
121:408–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu S, Lao XY, Sun TT, et al: Knockdown of
ZFX inhibits gastric cancer cell growth in vitro and in vivo via
downregulating the ERK-MAPK pathway. Cancer Lett. 337:293–300.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Artaza JN, Sirad F, Ferrini MG and Norris
KC: 1,25(OH)2vitamin D3 inhibits cell
proliferation by promoting cell cycle arrest without inducing
apoptosis and modifies cell morphology of mesenchymal multipotent
cells. J Steroid Biochem Mol Biol. 119:73–83. 2010.
|
|
46
|
Luo W, Chen Y, Liu M, et al: EB1089
induces Skp2-dependent p27 accumulation, leading to cell growth
inhibition and cell cycle G1 phase arrest in human hepatoma cells.
Cancer Invest. 27:29–37. 2009. View Article : Google Scholar
|
|
47
|
Banerjee P and Chatterjee M:
Antiproliferative role of vitamin D and its analogs - a brief
overview. Mol Cell Biochem. 253:247–254. 2003. View Article : Google Scholar : PubMed/NCBI
|