Introduction
Ischemic heart disease represents one of the leading
causes of death worldwide and has increased in incidence and
prevalence in recent years (1).
The mitochondria in cardiomyocytes are damaged in response to
ischemia and hypoxia, leading to excessive apoptosis (2). The cardiomyocyte loss induced by
apoptosis contributes to the development of ischemic heart disease
(3,4). Currently, an effective method for
overcoming hypoxia-induced cardiomyocyte apoptosis is still
lacking, due to the elusiveness of the underlying mechanism.
Therefore, it is of great importance to gain a better understanding
of the molecular mechanism underlying hypoxia-induced cardiomyocyte
apoptosis, which may help to provide potential therapeutic
approaches.
MicroRNAs (miRNAs or miRs) are a subset of
endogenous, short and non-coding RNAs that negatively modulate gene
expression (5,6). miRNAs can impact the 3′-untranslated
region (3′-UTR) of target mRNAs, inducing mRNA degradation and
translation inhibition (5,6).
Through modulation of gene expression in a post-transcriptional
manner, miRNAs participate in various biological processes,
including cell proliferation and apoptosis (7). A growing body of evidence suggests
that miRNAs are involved in ischemic heart disease (4,8,9),
and they have been emerging as a new therapeutic strategy for this
disease (10,11). Targeting specific miRNAs has
produced promising effects in inhibiting hypoxia-induced
cardiomyocyte apoptosis (12–15). However, the precise effect of
miRNAs on hypoxia-induced cardiomyocyte apoptosis requires further
investigation.
GATA-4, a zinc-finger transcription factor, has been
found to be an important regulator in cardiac development (16,17). GATA-4 mediates cardiac hypertrophy
by activating various genes including α-myosin heavy chain,
β-myosin heavy chain, myosin light chains, troponin I, troponin C
and atrial natriuretic factor (18–20). Various hypertrophic stimuli, such
as endothelin-1 and α-adrenergic agonist, can activate the
expression of GATA-4 in cardiomyocytes (21,22). GATA-4 also plays a critical role
in regulating anti-apoptotic signaling in cardiomyocytes in
response to hypoxic injury and myocardial ischemia or reperfusion
injury (23,24). GATA-4 inhibits doxorubicin-induced
cardiomyocyte apoptosis by activating the anti-apoptotic gene,
Bcl-2, in vitro and in vivo (23). GATA-4 has been suggested as a
promising therapeutic target for the treatment of ischemic heart
disease (25,26).
miR-200c has been found to be an apoptosis-related
miRNA in various pathological processes (27,28) and increases in the gracilis muscle
following ischemic injury (29).
After ischemic preconditioning or focal cerebral ischemia, miR-200c
was upregulated in the ischemic cortex (30). Inhibition of miR-200c was found to
attenuate infarct volume and neurologic deficits in mice following
cerebral ischemia (31). However,
the role of miR-200c in ischemic heart disease is unclear. In this
study, we aimed to investigate the potential role of miR-200c in
ischemic heart disease using an in vitro model. We found
that miR-200c was highly upregulated in cardiomyocytes exposed to
hypoxia. Downregulation of miR-200c by transfection of an miR-200c
inhibitor significantly reduced hypoxia-induced cardiomyocyte
apoptosis and improved cell survival. Importantly, GATA-4 was
identified as the target gene of miR-200c in cardiomyocytes.
Downregulation of miR-200c increased the expression of GATA-4 and
Bcl-2 in cardiomyocytes in response to hypoxia. Taken together, our
results suggest that downregulation of miR-200c protects
cardiomyocytes from hypoxia-induced apoptosis by targeting GATA-4,
providing a potential therapeutic molecular target for the
treatment of ischemic heart disease.
Materials and methods
Cell cultures
Rat cardiomyocyte cell line H9c2 and 293T cells were
both purchased from the American Type Cult ure Collection (ATCC;
Manassas, VA, USA). Cells were grown in Dulbecco's modified Eagle's
medium (DMEM) containing 10% fetal bovine serum (FBS) (both from
Gibco, Rockville, MD, USA) and 1% penicillin-streptomycin solution
(Sigma-Aldrich, St. Louis, MO, USA). Cells were routinely cultured
in a humidified incubator containing 5% CO2 at 37°C. For
the induction of hypoxia, H9c2 cells were grown in a hypoxia
chamber containing 94% N2, 5% CO2 and 1%
O2 at 37°C.
Cell transfection
The miR-200c mimics, miR-200c inhibitor and negative
controls (NCs) were all purchased from GenePharma (Shanghai, China)
and transfected into cells using Lipofectamine 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA), following the manufacturer's
instructions. The GATA-4 siRNA and NC siRNA were both purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and
transfected into cells according to the manufacturer's
instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies) and reverse transcribed into cDNA
using M-MLV Reverse Transcriptase (BioTeke Co., Ltd., Beijing,
China) or TaqMan MicroRNA Reverse Transcription kit (Applied
Biosystems, Foster City, CA, USA). The cDNA was used as the
template for RT-qPCR with SYBR-Green PCR Master Mix and appropriate
primers on 7900HT Fast Real-Time PCR system (both from Applied
Biosystems). U6 was used as the internal control for normalization
of miR-200c. β-actin was used as the internal control for
normalization of GATA-4 and Bcl-2. Relative gene expression was
calculated by the 2−ΔΔCq method. The primer sequences
used in the experiments were as follows: miR-200c forward,
5′-GTAATACTGCCGGGTAATGATGGA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
U6 forward, 5′-GCGCGTCGTGAAGCGTTC-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; GATA-4 forward, 5′-GTGCCAACTGCCAGACTACC-3′
and reverse, 5′-AGCCTTGTGGGGACAGCTTC-3′; Bcl-2 forward,
5′-AGTTCGGTGGGGTCATGTGTG-3′ and reverse,
5′-CCAGGTATGCACCCAGAGTG-3′; β-actin forward,
5′-TCAGGTCATCACTATCGGCAAT-3′ and reverse,
5′-AAAGAAAGGGTGTAAAACGCA-3′.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell viability was assessed using the MTT
colorimetric assay. In brief, the cells were seeded in 96-well
plates at 2×104 cells/well and cultured overnight. The
cells were transfected with the miR-200c inhibitor or NC inhibitor
for 24 h and then subjected to hypoxic conditions for 24 h.
Afterwards, the cells were treated with 20 µl of 5 mg/ml MTT
(Sigma-Aldrich) and cultured for 4 h. The purple-colored formazan
crystals in living cells were solubilized by 200 µl of
dimethyl sulfoxide (DMSO; Sigma-Aldrich). After 15 min, the
absorbance of the solution at 490 nm was detected by a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Lactate dehydrogenase (LDH) assay
Cell injury was measured using an LDH assay kit
(Roche Applied Science, Indianapolis, IN, USA) according to the
manufacturer's instructions. The cells were lysed by 0.2% Triton
X-100 (Sigma-Aldrich) and the supernatants were collected after
centrifugation at 10,000 × g for 10 min at 4°C. The supernatants
were then incubated with pyruvate and nicotinamide adenine
dinucleotide hydrogen for 30 min at 37°C. After the addition of 0.4
M NaOH, the absorbance at 530 nm was detected by a microplate
reader (Bio-Rad Laboratories, Inc.).
Annexin V/propidium iodide (PI) apoptosis
assay
Cell apoptosis was measured by using an Annexin V/PI
apoptosis detection kit (Beyotime Institute of Biotechnology,
Haimen, China) following the manufacturer's recommended
instructions. In conclusion, the cells were digested with 2.5 g/l
trypsin (Sigma-Aldrich) and then washed with phosphate-buffered
saline (PBS). The cells were then re-suspended in binding buffer
supplemented with 10 µl of Annexin V. After incubation for
30 min, 5 µl of PI solution was added and the cells were
incubated for a further 5 min. Cells were analyzed by flow
cytometry (BD Biosciences, San Jose, CA, USA).
Caspase-3 activity assay
Caspase-3 activity was measured by a caspase-3
activity assay kit (Roche Applied Science), according to the
manufacturer's instructions. In brief, the cells were lysed and the
supernatants were collected after centrifugation at 16,000 × g for
15 min at 4°C. The supernatants were then incubated with 10
µl Ac-DEVD-pNA (2 mM) for 2 h at 37°C. The absorbance at 405
nm was determined using a microplate reader (Bio-Rad Laboratories,
Inc.).
Western blot analysis
Proteins were lysed in lysis buffer and protein
concentrations were measured using a bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology). Equivalent amounts of
proteins were loaded on 10% sodium dodecyl sulfate-polyacrylamide
gels for separation, followed by protein transfer to a
polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The
membrane was blocked with 5% non-fat dry milk for 1 h at 37°C,
followed by incubation with primary antibodies at 4°C overnight.
The membrane was washed with Tris-buffered saline containing 0.1%
Tween-20 (TBST) and then incubated with horseradish
peroxidase-conjugated secondary antibodies (1:2,000; sc-2004; Santa
Cruz Biotechnology, Inc.) for 1 h at 37°C. After being washed with
TBST, the protein bands were developed using a Pierce ECL Western
Blotting kit (Pierce, Rockford, IL, USA). Gray values of protein
bands were detected by Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). The primary antibodies
including anti-GATA-4 (1:500; sc-9053), anti-Bcl-2 (1:500; sc-783)
and anti-β-actin (1:600; sc-130656) were all purchased from Santa
Cruz Biotechnology, Inc.
Dual-luciferase reporter assay
The 3′-UTR of GATA-4 harboring either the miR-200c
binding site (GATA-4 3′-UTR-WT) or a mutant (GATA-4 3′-UTR-MT) was
cloned into the pmirGLO luciferase vector (Promega, Madison, WI,
USA). The constructed vectors were co-transfected into 293T cells
with the miR-200c inhibitor or NC inhibitor using Lipofectamine
2000 (Invitrogen Life Technologies,) and incubated for 48 h. The
cells were harvested and luciferase activities were measured using
a Dual-GLO Luciferase Assay system (Promega).
Statistical analysis
The results are presented as mean ± standard
deviation. Statistical analyses were performed using SPSS package
version 18.0 (SPSS, Inc., Chicago, IL, USA). Statistical
significance was determined by one-way analysis of variance (ANOVA)
followed by a Bonferroni correction. Differences were regarded as
statistically significant at values of p<0.05.
Results
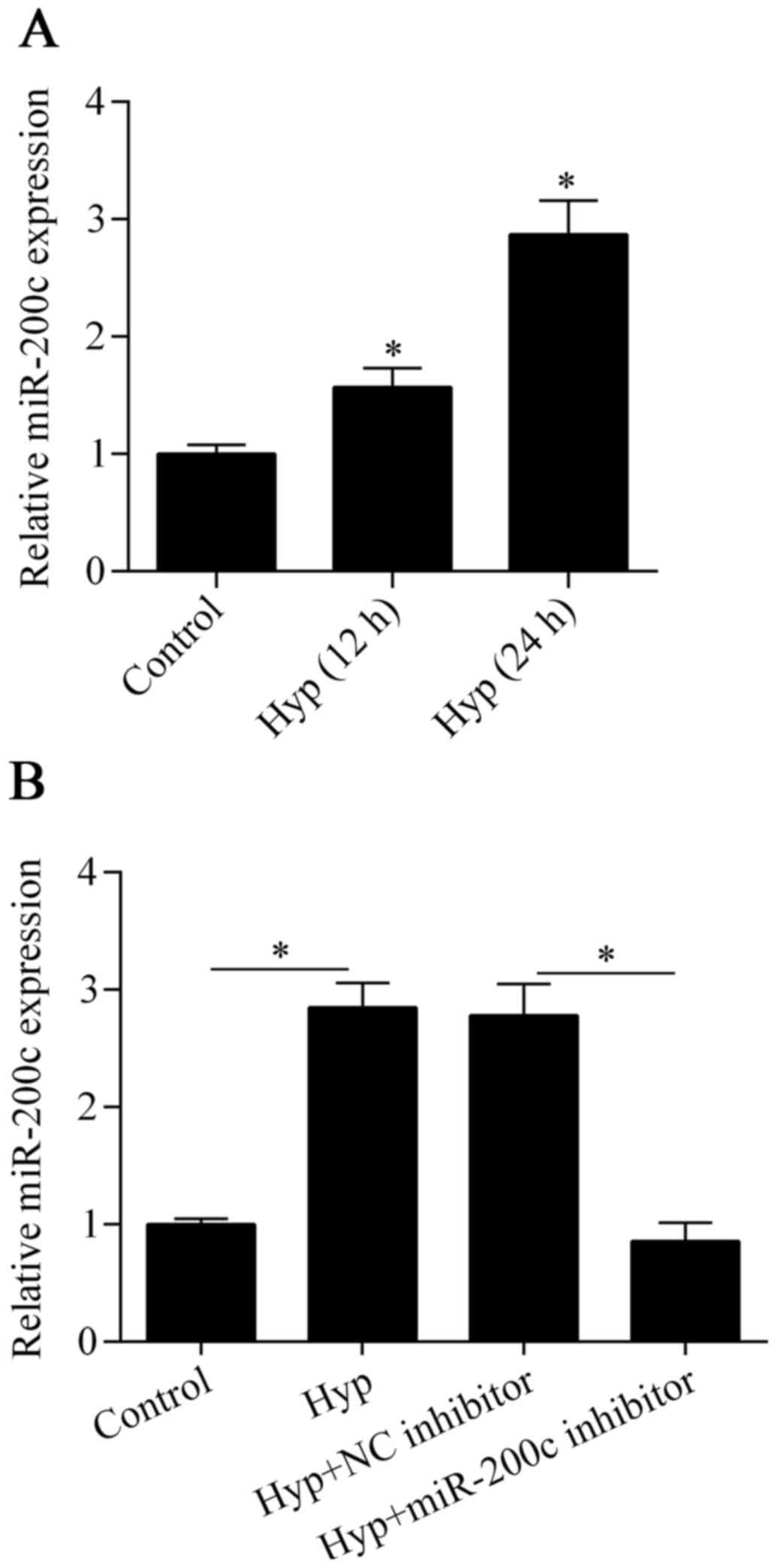
miR-200c is upregulated by hypoxia in
cardiomyocytes
To investigate the possible role of miR-200c in
ischemic heart disease, we detected the expression of miR-200c in
cardiomyocytes exposed to hypoxia using RT-qPCR. The results showed
that miR-200c was significantly upregulated after exposure to
hypoxia in comparison to the control (Fig. 1A), implying that miR-200c has an
important role in the hypoxic injury of cardiomyocytes.
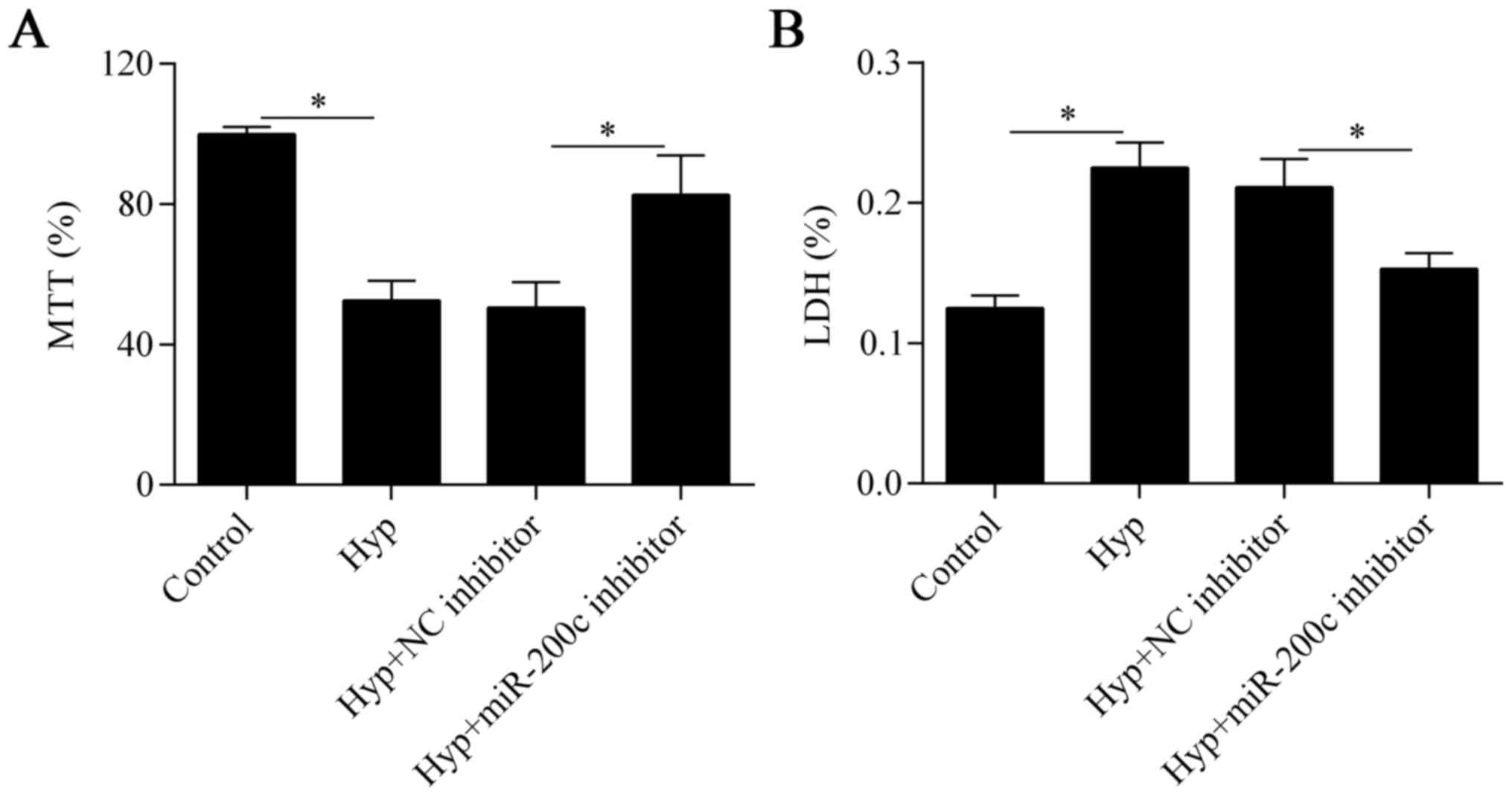
Downregulation of miR-200c attenuates
hypoxia-induced cell injury
To investigate the biological effect of miR-200c on
hypoxia-treated cardiomyocytes, we inhibited the expression of
miR-200c in cells by transfecting them with an miR-200c inhibitor
(Fig. 1B). We then detected the
effect of miR-200c suppression on cell viability with the MTT
assay. The results showed that cell viability was markedly impaired
by hypoxia but was partially improved by miR-200c inhibition
(Fig. 2A). We next evaluated the
effect of miR-200c inhibition on hypoxia-induced cell injury with
the LDH assay. We found that the hypoxia-induced cell injury was
also significantly reversed by the downregulation of miR-200c
(Fig. 2B). Taken together, these
data suggest that downregulation of miR-200c improves cell survival
under hypoxic conditions.
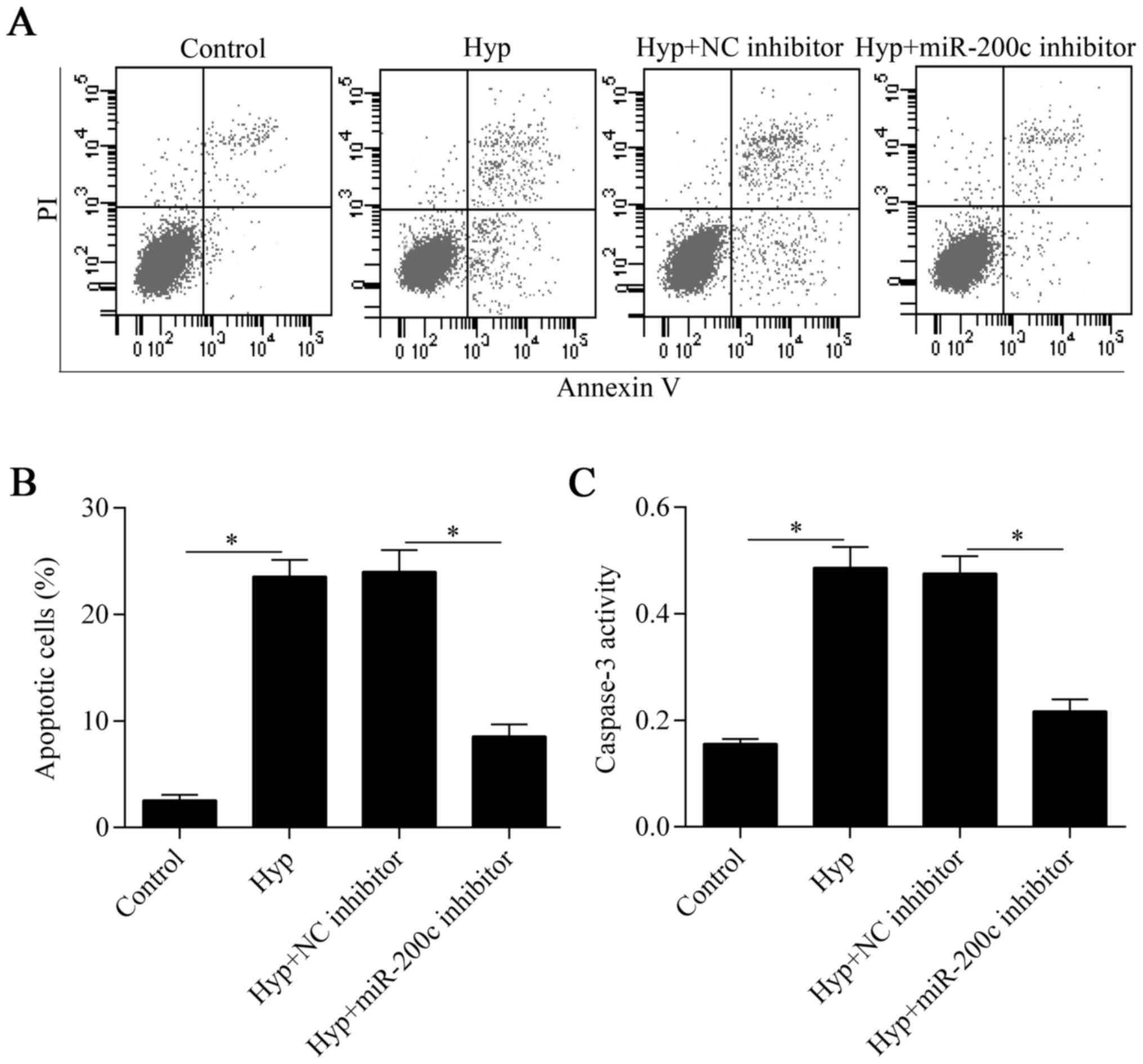
Downregulation of miR-200c inhibits
cardiomyocyte apoptosis induced by hypoxia
To verify the protective effect of miR-200c
inhibition on cardiomyocytes under hypoxia, we further investigated
the effect of miR-200c inhibition on hypoxia-induced apoptosis. The
Annexin V/PI apoptosis assay showed that hypoxia-induced apoptosis
was significantly suppressed by the downregulation of miR-200c
(Fig. 3A and B). Furthermore, the
increased activity of the pro-apoptotic protein, caspase-3, induced
by hypoxia was also markedly decreased by miR-200c inhibition
(Fig. 3C). Overall, these results
indicate that the downregulation of miR-200c suppresses
hypoxia-induced cardiomyocyte apoptosis.
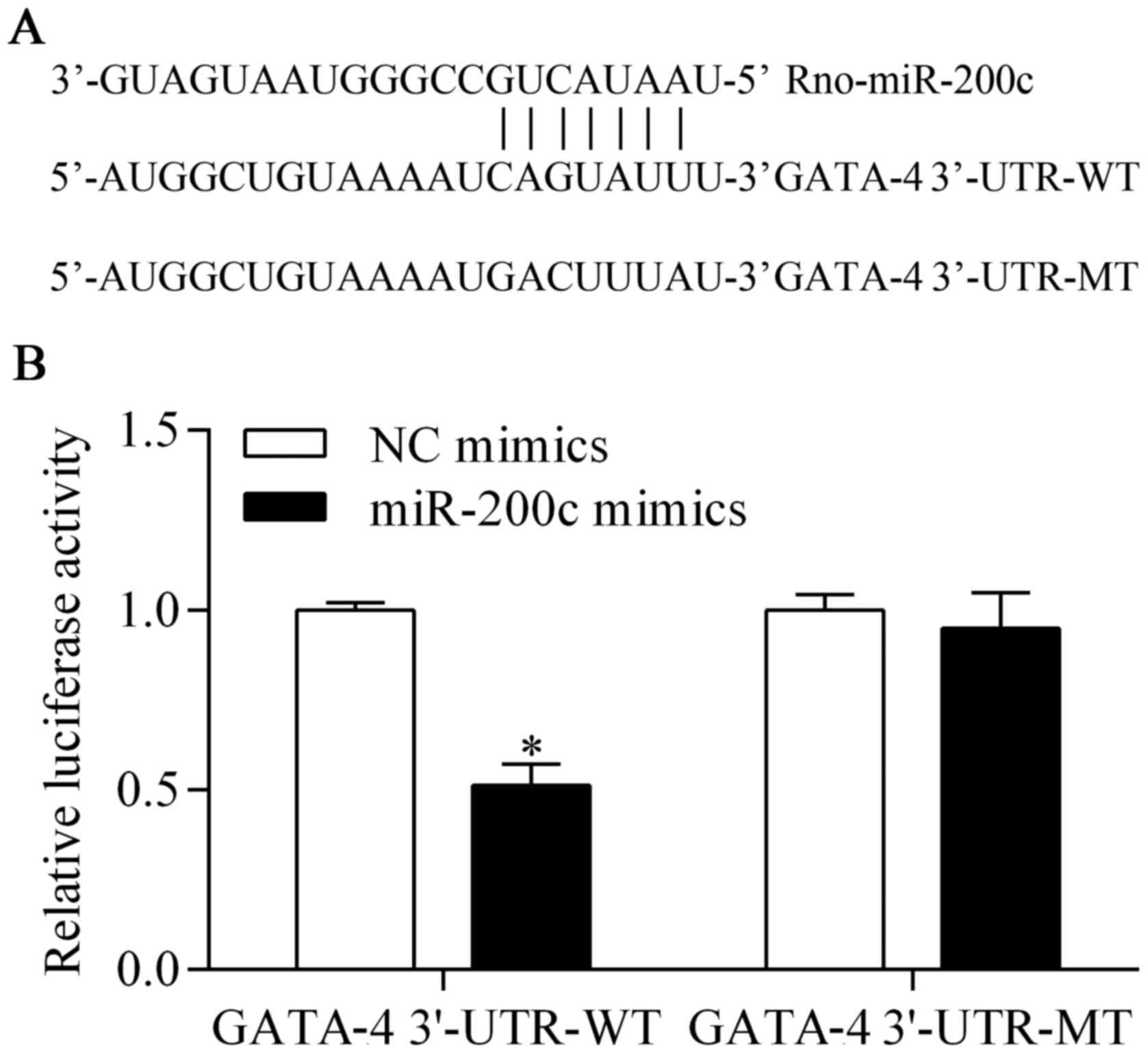
GATA-4 is a potential target of
miR-200c
To investigate the underlying mechanism by which the
suppression of miR-200c provides a protective effect against
hypoxia, we predicted the target genes of miR-200c with
bioinformatics analysis. We found that GATA-4, an important
transcription factor for cardiomyocyte survival (23,24), was a potential target gene of
miR-200c. The 3′-UTR of GATA-4, harboring the complementary
seed-matched binding sites of the miR-200c binding site (GATA-4
3′-UTR-WT) and a mutant (GATA-4 3′-UTR-MT), are shown in Fig. 4A. To confirm the presence of the
interaction between miR-200c and GATA-4 3′-UTR, GATA-4 3′-UTR-WT or
GATA-4 3′-UTR-MT was cloned into the pmirGLO vector that was used
for the luciferase reporter assay. The results showed that the
luciferase activity of pmirGLO-GATA-4 3′-UTR-WT was significantly
decreased by miR-200c overexpression (Fig. 4B). However, the luciferase
activity of pmirGLO-GATA-4 3′-UTR-MT was not obviously affected by
miR-200c overexpression (Fig.
4B). These results suggest that miR-200c directly targets the
3′-UTR of GATA-4. We then examined the direct effect of miR-200c on
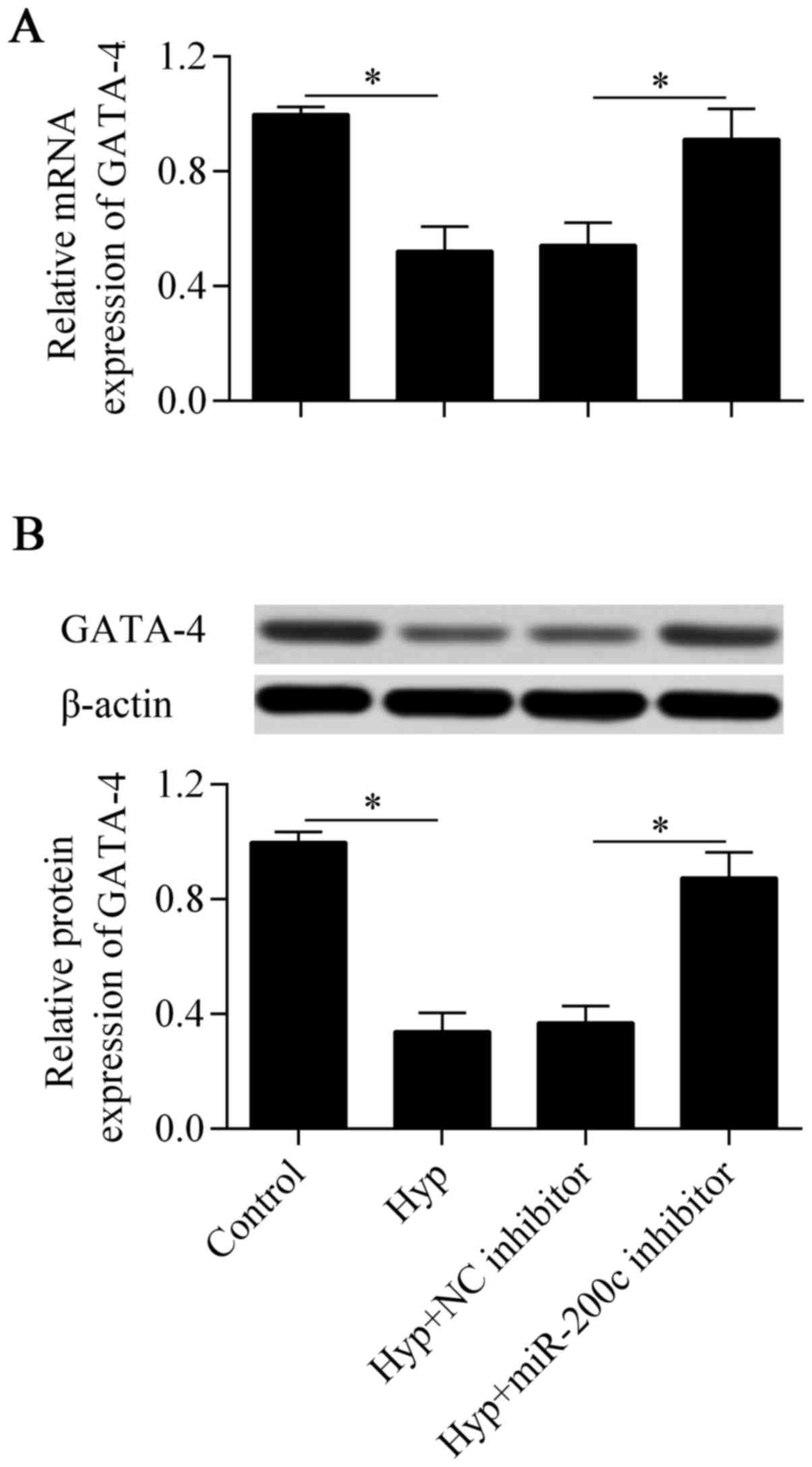
GATA-4 expression by RT-qPCR and western blot analysis. The results
showed that the downregulation of miR-200c significantly increased
the mRNA (Fig. 5A) and protein
(Fig. 5B) expression of GATA-4,
which were decreased by hypoxia in the cardiomyocytes. Taken
together, these results suggest that GATA-4 is a direct target gene
of miR-200c in cardiomyocytes.
Downregulation of miR-200c promotes the
expression of Bcl-2
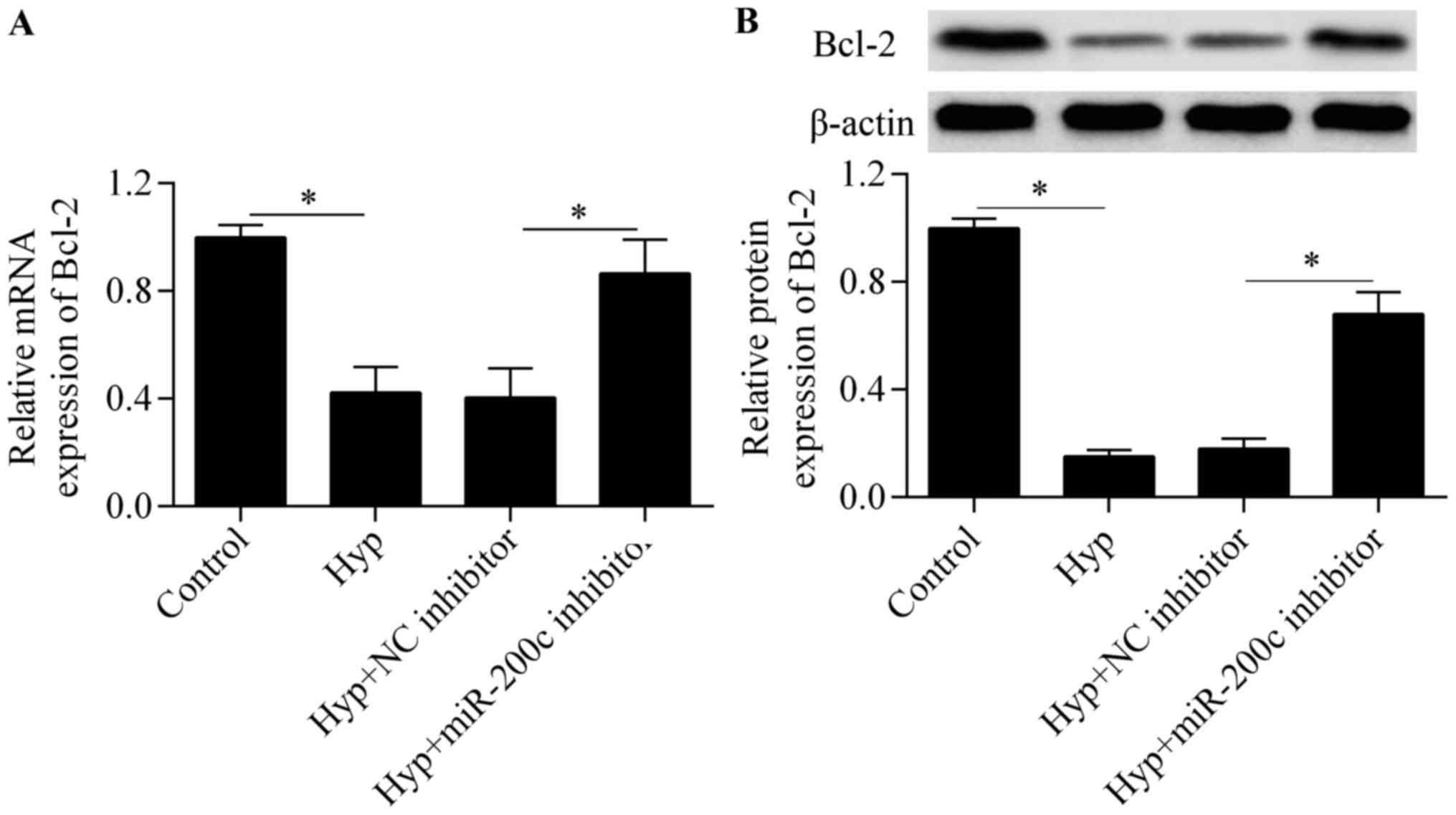
To further investigate the molecular basis of
miR-200c in regulating cardiomyocyte survival, we detected the
expression of the anti-apoptotic gene Bcl-2, a downstream gene of
GATA-4 (23). The results showed
that the downregulation of miR-200c significantly upregulated the
mRNA (Fig. 6A) and protein
(Fig. 6B) expression of Bcl-2 in
response to hypoxia, implying that miR-200c regulates Bcl-2
expression.
Knockdown of GATA-4 abolishes the
protective effect of miR-200c inhibition
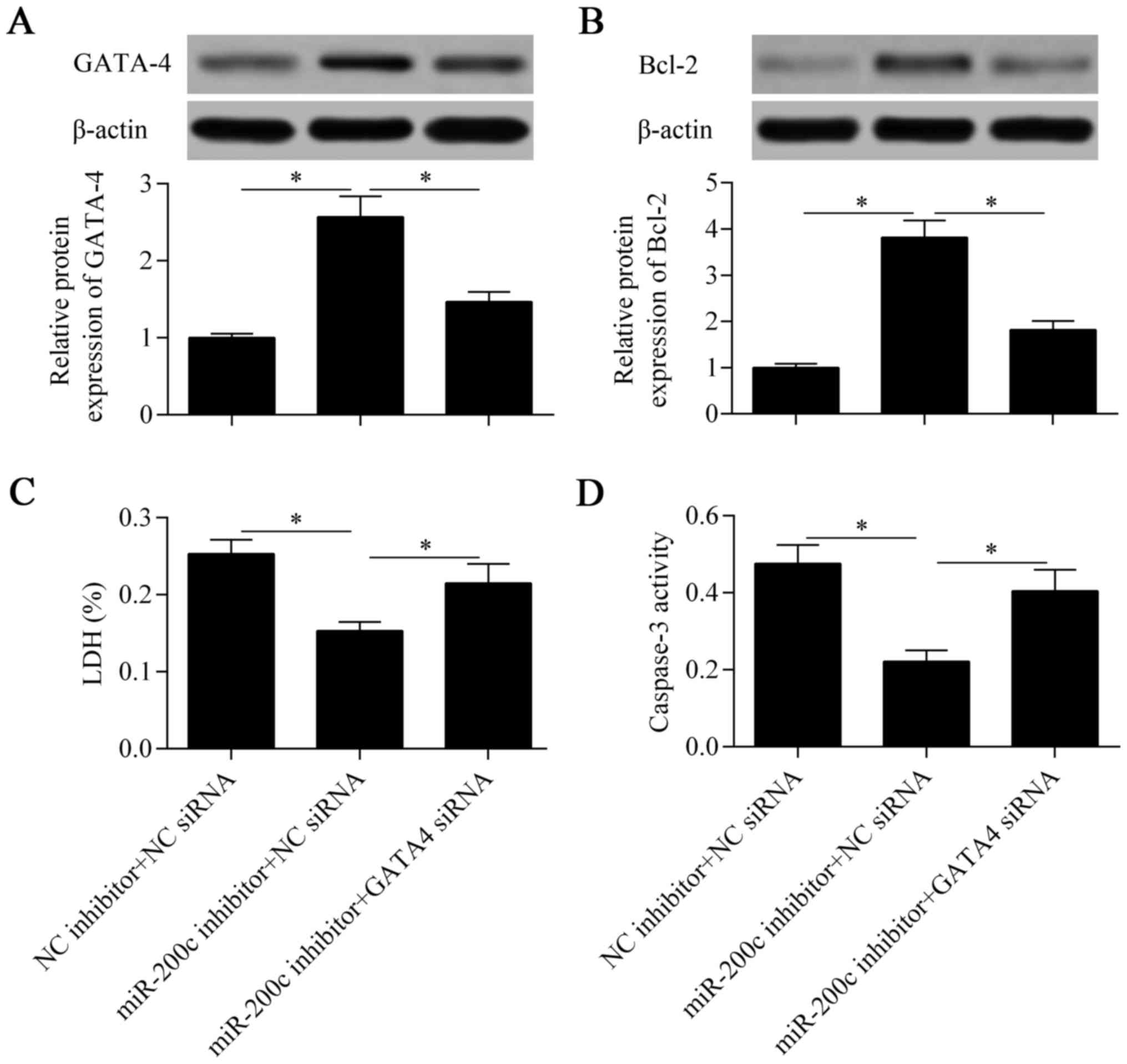
To verify that GATA-4 contributes to the miR-200c
inhibition-mediated protective effect against hypoxia, we silenced
the expression of GATA-4 at the same time as suppressing miR-200c.
The results showed that the promotive effect of miR-200c
suppression on GATA-4 expression was significantly abolished by the
knockdown of GATA-4 (Fig. 7A).
Similarly, the increased expression of Bcl-2 induced by miR-200c
suppression was eliminated by the knockdown of GATA-4 (Fig. 7B). As expected, the protective
effect of miR-200c suppression against hypoxia was also markedly
reversed by the knockdown of GATA-4 (Fig. 7C and D). Taken together, these
results suggest that downregulation of miR-200c protects
cardiomyocytes against hypoxia through the promotion of GATA-4
expression.
Discussion
Overcoming hypoxia-induced cardiomyocyte apoptosis
is a main obstacle for the treatment of ischemic heart disease. In
this study, we report that miR-200c is a novel regulator of
hypoxia-induced cardiomyocyte apoptosis. We found that miR-200c was
highly upregulated in cardiomyocytes subjected to hypoxia
treatment. Our data demonstrated that the downregulation of
miR-200c provided protective effects against hypoxia in
cardiomyocytes by upregulating GATA-4 and Bcl-2 expression,
indicating a potential therapeutic target for ischemic heart
disease.
Numerous studies have demonstrated that miR-200c
induces the apoptosis of various types of cancer cells (27,32,33). miR-200c sensitizes tumor cells to
apoptosis by targeting Fas-associated phosphatase-1 (34). Similarly, miR-200c was
significantly increased after spinal cord injury and reactive
oxygen species were found to enhance miR-200c expression, which
induced apoptosis in microglial cells (34). In endothelial cells, miR-200c
overexpression induced by reactive oxygen species promoted cell
growth arrest, senescence and apoptosis by targeting zinc-finger
E-box binding homeobox 1 (35).
Downregulation of miR-200c preserved endothelial function in
diabetic mice by targeting zinc-finger E-box binding homeobox 1 and
inhibiting COX-2 (36). miR-200c
was found to increase in the gracilis muscle following ischemic
injury (29). The inhibition of
miR-200c attenuated hepatic ischemia or reperfusion injury
(37). Moreover, miR-200c was
found to be upregulated in the ischemic cortex after ischemic
preconditioning or focal cerebral ischemia (30). Downregulation of miR-200c
attenuated infarct volume and neurologic deficit in mice following
cerebral ischemia by targeting Reelin (31), and inhibited cardiomyocyte
hypertrophy in high glucose-treated cardiomyocytes (38). However, no data have indicated the
role of miR-200c in regulating cardiomyocyte apoptosis. In this
study, we found that miR-200c was highly upregulated by hypoxia in
cardiomyocytes. The downregulation of miR-200c suppressed
hypoxia-induced cardiomyocyte apoptosis, indicating an important
role of miR-200c in regulating cardiomyocyte survival in response
to stress injury.
To investigate the potential mechanism underlying
the regulation of cardiomyocyte apoptosis by miR-200c, we aimed to
identify the potential target gene of miR-200c in cardiomyocytes.
We identified GATA-4, an important transcriptional factor for
cardiomyocyte survival (23,24), to be a target gene of miR-200c.
GATA-4 was found to be suppressed by anthracyclines in
cardiomyocytes and the restoration of GATA-4 attenuated
cardiomyocyte apoptosis (39).
Activation of GATA-4 promoted cell survival and reduced cell death
induced by daunorubicin (40) and
doxorubicin (41). GATA-4 can
activate the anti-apoptotic signaling in cardiomyocytes and protect
cardiomyocytes against hypoxic injury and myocardial ischemia or
reperfusion injury (23,24). GATA-4 has becoming a promising
therapeutic target for the treatment of ischemic heart disease
(25,26). The co-culture of GATA-4
gene-engineered mesenchymal stem cells and cardiomyo-cytes
inhibited hypoxia-induced apoptosis (42,43). Moreover, exosomes derived from the
overexpression of GATA-4 in mesenchymal stem cells also showed
cardioprotection (44). It has
been reported that GATA-4 inhibits cardiomyocyte apoptosis by
activating Bcl-2 (23). Kobayashi
et al found that GATA-4 directly binds to the GATA site in
the promoter of Bcl-2 and positively regulated Bcl-2 expression in
cardiomyocytes in vivo and in vitro (23). A study also showed that GATA-4
regulated Bcl-2 expression in ovarian granulosa cell tumors
(45). Cardiac ankyrin repeat
protein has been reported to repress cardiomyocyte apoptosis
induced by hypoxia or reoxygenation by binding the Bcl-2 promoter
by interacting with GATA-4 (46).
These findings suggest that GATA-4 can serve as a promising
therapeutic target for preventing cardiomyocyte apoptosis. In this
study, we found that GATA-4 is a target gene of miR-200c.
Downregulation of miR-200c promoted the expression of GATA-4, thus
protecting cardiomyocytes from hypoxia-induced apoptosis.
Furthermore, silencing of GATA-4 abolished the miR-200c
inhibition-induced protective effects.
The activity of GATA-4 is controlled by various
post-translational modifications including ubiquitination,
phosphorylation and acetylation (20,47). Hypoxia induces the ubiquitination
of GATA-4 and the attenuation of GATA-4 ubiquitination by
erythropoietin increases cell viability under hypoxia (48). Recent studies also showed that
GATA-4 undergoes epigenetic regulation by miRNAs (49,50). Over expression of miR-26b was
found to suppress GATA-4 expression by targeting the 3′-UTR,
leading to increased cardiomyocyte apoptosis (51). miR-26a attenuated cardiac
hypertrophy via the targeting of GATA-4 in cultured cardiomyocytes
(52). Downregulation of miR-208a
suppressed doxorubicin-induced cardiomyocyte apoptosis by promoting
GATA-4 (53). In this study, we
found that miR-200c also targeted and regulated GATA-4 in
cardiomyocytes. The inhibition of miR-200c protected cardiomyocytes
against hypoxia-induced apoptosis by targeting GATA-4. In line with
our findings, miR-200c was found to regulate embryonic stem cell
renewal and differentiation by targeting GATA-4 (54). Our study indicates that miR-200c
may serve as a promising target for the development of miRNA-based
therapy for ischemic heart disease by targeting GATA-4.
In conclusion, our study demonstrated that miR-200c
is a hypoxia-response gene in cardiomyocytes and is induced by
hypoxia. Downregulation of miR-200c provides considerable
protective effects against hypoxia in cardiomyocytes by promoting
GATA-4 and Bcl-2 expression. Our study suggests a potential
therapeutic molecular target for the treatment of ischemic heart
disease. However, the precise role of miR-200c and GATA-4 in
regulating cardiomyocyte apoptosis remains to be fully elucidated
in vivo using animal models.
Glossary
Abbreviations
Abbreviations:
|
miRNAs
|
microRNAs or miRs
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
UTR
|
untranslated region
|
|
LDH
|
lactate dehydrogenase
|
|
PI
|
propidium iodide
|
References
|
1
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glinka YY and Youdim MB: Inhibition of
mitochondrial complexes I and IV by 6-hydroxydopamine. Eur J
Pharmacol. 292:329–332. 1995.PubMed/NCBI
|
|
3
|
Kitsis RN, Peng CF and Cuervo AM: Eat your
heart out. Nat Med. 13:539–541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang PM, Haunstetter A, Aoki H, Usheva A
and Izumo S: Morphological and molecular characterization of adult
cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res.
87:118–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Pan X, Fan Y, Hu X, Liu X, Xiang M
and Wang J: Dysregulated expression of microRNAs and mRNAs in
myocardial infarction. Am J Transl Res. 7:2291–2304. 2015.
|
|
9
|
Boon RA and Dimmeler S: MicroRNAs in
myocardial infarction. Nat Rev Cardiol. 12:135–142. 2015.
View Article : Google Scholar
|
|
10
|
Hang P, Guo J, Sun C and Du Z: MicroRNAs
as candidate drug targets for cardiovascular diseases. Curr Drug
Targets. Feb 29–2016.Epub ahead of print. PubMed/NCBI
|
|
11
|
Samanta S, Balasubramanian S, Rajasingh S,
Patel U, Dhanasekaran A, Dawn B and Rajasingh J: MicroRNA: a new
therapeutic strategy for cardiovascular diseases. Trends Cardiovasc
Med. 26:407–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zou Y, Liu W, Zhang J and Xiang D: miR-153
regulates apoptosis and autophagy of cardiomyocytes by targeting
Mcl-1. Mol Med Rep. 14:1033–1039. 2016.PubMed/NCBI
|
|
13
|
Xu H, Jin L, Chen Y and Li J:
Downregulation of microRNA-429 protects cardiomyocytes against
hypoxia-induced apoptosis by increasing Notch1 expression. Int J
Mol Med. 37:1677–1685. 2016.PubMed/NCBI
|
|
14
|
Xie J, Hu X, Yi C, Hu G, Zhou X and Jiang
H: MicroRNA 451 protects against cardiomyocyte anoxia/reoxygenation
injury by inhibiting high mobility group box 1 expression. Mol Med
Rep. 13:5335–5341. 2016.PubMed/NCBI
|
|
15
|
Ke ZP, Xu P, Shi Y and Gao AM: MicroRNA-93
inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by
targeting PTEN. Oncotarget. 7:28796–28805. 2016.PubMed/NCBI
|
|
16
|
Kelley C, Blumberg H, Zon LI and Evans T:
GATA-4 is a novel transcription factor expressed in endocardium of
the developing heart. Development. 118:817–827. 1993.PubMed/NCBI
|
|
17
|
Arceci RJ, King AA, Simon MC, Orkin SH and
Wilson DB: Mouse GATA-4: a retinoic acid-inducible GATA-binding
transcription factor expressed in endodermally derived tissues and
heart. Mol Cell Biol. 13:2235–2246. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang Q, De Windt LJ, Witt SA, Kimball TR,
Markham BE and Molkentin JD: The transcription factors GATA4 and
GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J
Biol Chem. 276:30245–30253. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Charron F, Tsimiklis G, Arcand M,
Robitaille L, Liang Q, Molkentin JD, Meloche S and Nemer M:
Tissue-specific GATA factors are transcriptional effectors of the
small GTPase RhoA. Genes Dev. 15:2702–2719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki YJ: Cell signaling pathways for the
regulation of GATA4 transcription factor: implications for cell
growth and apoptosis. Cell Signal. 23:1094–1099. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morimoto T, Hasegawa K, Kaburagi S, Kakita
T, Wada H, Yanazume T and Sasayama S: Phosphorylation of GATA-4 is
involved in alpha 1-adrenergic agonist-responsive transcription of
the endothelin-1 gene in cardiac myocytes. J Biol Chem.
275:13721–13726. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hautala N, Tokola H, Luodonpää M, Puhakka
J, Romppanen H, Vuolteenaho O and Ruskoaho H: Pressure overload
increases GATA4 binding activity via endothelin-1. Circulation.
103:730–735. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobayashi S, Lackey T, Huang Y, Bisping E,
Pu WT, Boxer LM and Liang Q: Transcription factor gata4 regulates
cardiac BCL2 gene expression in vitro and in vivo. FASEB J.
20:800–802. 2006.PubMed/NCBI
|
|
24
|
Kim Y, Ma AG, Kitta K, Fitch SN, Ikeda T,
Ihara Y, Simon AR, Evans T and Suzuki YJ: Anthracycline-induced
suppression of GATA-4 transcription factor: implication in the
regulation of cardiac myocyte apoptosis. Mol Pharmacol. 63:368–377.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rysä J, Tenhunen O, Serpi R, Soini Y,
Nemer M, Leskinen H and Ruskoaho H: GATA-4 is an angiogenic
survival factor of the infarcted heart. Circ Heart Fail. 3:440–450.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bian J, Popovic ZB, Benejam C, Kiedrowski
M, Rodriguez LL and Penn MS: Effect of cell-based intercellular
delivery of transcription factor GATA4 on ischemic cardiomyopathy.
Circ Res. 100:1626–1633. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shan W, Zhang X, Li M, Deng F and Zhang J:
Over expression of miR-200c suppresses invasion and restores
methotrexate sensitivity in lung cancer A549 cells. Gene.
593:265–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boominathan L: The tumor suppressors p53,
p63, and p73 are regulators of microRNA processing complex. PLoS
One. 5:e106152010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh CH, Jeng JC, Jeng SF, Wu CJ, Lu TH,
Liliang PC, Rau CS, Chen YC and Lin CJ: MicroRNA profiling in
ischemic injury of the gracilis muscle in rats. BMC Musculoskelet
Disord. 11:1232010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D,
Kang KM, Park KH, Bae EK, Kim M, Lee SK, et al: MicroRNAs induced
during ischemic preconditioning. Stroke. 41:1646–1651. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stary CM, Xu L, Sun X, Ouyang YB, White
RE, Leong J, Li J, Xiong X and Giffard RG: MicroRNA-200c
contributes to injury from transient focal cerebral ischemia by
targeting Reelin. Stroke. 46:551–556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Venkatadri R, Muni T, Iyer AK, Yakisich JS
and Azad N: Role of apoptosis-related miRNAs in resveratrol-induced
breast cancer cell death. Cell Death Dis. 7:e21042016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui J, Cheng Y, Zhang P, Sun M, Gao F, Liu
C and Cai J: Downregulation of miR200c promotes radiation-induced
thymic lymphoma by targeting BMI1. J Cell Biochem. 115:1033–1042.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu DS, Lv G, Mei XF, Cao Y, Wang YF, Wang
YS and Bi YL: MiR-200c regulates ROS-induced apoptosis in murine
BV-2 cells by targeting FAP-1. Spinal Cord. Dec 2–2014.Epub ahead
of print. PubMed/NCBI
|
|
35
|
Magenta A, Cencioni C, Fasanaro P,
Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F and
Capogrossi MC: miR-200c is upregulated by oxidative stress and
induces endothelial cell apoptosis and senescence via ZEB1
inhibition. Cell Death Differ. 18:1628–1639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang H, Liu J, Qu D, Wang L, Luo JY, Lau
CW, Liu P, Gao Z, Tipoe GL, Lee HK, et al: Inhibition of miR-200c
restores endothelial function in diabetic mice through suppression
of COX-2. Diabetes. 65:1196–1207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Y, Gu C and Huang X: Sevoflurane
protects against hepatic ischemia/reperfusion injury by modulating
microRNA-200c regulation in mice. Biomed Pharmacother.
84:1126–1136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh GB, Raut SK, Khanna S, Kumar A,
Sharma S, Prasad R and Khullar M: MicroRNA-200c modulates DUSP-1
expression in diabetes-induced cardiac hypertrophy. Mol Cell
Biochem. 424:1–11. 2017. View Article : Google Scholar
|
|
39
|
Suzuki YJ and Evans T: Regulation of
cardiac myocyte apoptosis by the GATA-4 transcription factor. Life
Sci. 74:1829–1838. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suzuki YJ: Stress-induced activation of
GATA-4 in cardiac muscle cells. Free Radic Biol Med. 34:1589–1598.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kobayashi S, Volden P, Timm D, Mao K, Xu X
and Liang Q: Transcription factor GATA4 inhibits
doxorubicin-induced autophagy and cardiomyocyte death. J Biol Chem.
285:793–804. 2010. View Article : Google Scholar :
|
|
42
|
Li HX, Zhou YF, Zhao X, Jiang B and Yang
XJ: GATA-4 protects against hypoxia-induced cardiomyocyte injury:
effects on mitochondrial membrane potential. Can J Physiol
Pharmacol. 92:669–678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu B, Gong M, Wang Y, Millard RW, Pasha Z,
Yang Y, Ashraf M and Xu M: Cardiomyocyte protection by GATA-4 gene
engineered mesenchymal stem cells is partially mediated by
translocation of miR-221 in microvesicles. PLoS One. 8:e733042013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu B, Kim HW, Gong M, Wang J, Millard RW,
Wang Y, Ashraf M and Xu M: Exosomes secreted from GATA-4
overexpressing mesenchymal stem cells serve as a reservoir of
anti-apoptotic microRNAs for cardioprotection. Int J Cardiol.
182:349–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kyrönlahti A, Rämö M, Tamminen M,
Unkila-Kallio L, Butzow R, Leminen A, Nemer M, Rahman N, Huhtaniemi
I, Heikinheimo M, et al: GATA-4 regulates Bcl-2 expression in
ovarian granulosa cell tumors. Endocrinology. 149:5635–5642. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang N, Ye F, Zhu W, Hu D, Xiao C, Nan J,
Su S, Wang Y, Liu M, Gao K, et al: Cardiac ankyrin repeat protein
attenuates cardiomyocyte apoptosis by upregulation of Bcl-2
expression. Biochim Biophys Acta. 1863:3040–3049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pikkarainen S, Tokola H, Kerkelä R and
Ruskoaho H: GATA transcription factors in the developing and adult
heart. Cardiovasc Res. 63:196–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jun JH, Shin EJ, Kim JH, Kim SO, Shim JK
and Kwak YL: Erythropoietin prevents hypoxia-induced GATA-4
ubiquitination via phosphorylation of serine 105 of GATA-4. Biol
Pharm Bull. 36:1126–1133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yao CX, Wei QX, Zhang YY, Wang WP, Xue LX,
Yang F, Zhang SF, Xiong CJ, Li WY, Wei ZR, et al: miR-200b targets
GATA-4 during cell growth and differentiation. RNA Biol.
10:465–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sowa N, Horie T, Kuwabara Y, Baba O,
Watanabe S, Nishi H, Kinoshita M, Takanabe-Mori R, Wada H, Shimatsu
A, et al: MicroRNA 26b encoded by the intron of small CTD
phosphatase (SCP) 1 has an antagonistic effect on its host gene. J
Cell Biochem. 113:3455–3465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han M, Yang Z, Sayed D, He M, Gao S, Lin
L, Yoon S and Abdellatif M: GATA4 expression is primarily regulated
via a miR-26b-dependent post-transcriptional mechanism during
cardiac hypertrophy. Cardiovasc Res. 93:645–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Y, Wang Z and Xiao W: MicroRNA-26a
protects against cardiac hypertrophy via inhibiting GATA4 in rat
model and cultured cardiomyocytes. Mol Med Rep. 14:2860–2866.
2016.PubMed/NCBI
|
|
53
|
Tony H, Yu K and Qiutang Z: MicroRNA-208a
silencing attenuates doxorubicin induced myocyte apoptosis and
cardiac dysfunction. Oxid Med Cell Longev. 2015:5970322015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang HN, Chen SY, Hwang SM, Yu CC, Su MW,
Mai W, Wang HW, Cheng WC, Schuyler SC, Ma N, et al: miR-200c and
GATA binding protein 4 regulate human embryonic stem cell renewal
and differentiation. Stem Cell Res (Amst). 12:338–353. 2014.
View Article : Google Scholar
|