Introduction
Alcohol consumption is the most common risk factor
of liver damage; and the main cause of liver disease. Alcoholic
liver disease (ALD) is an important global health issue as alcohol
consumption leads to hepatic steatosis, hepatitis, life-threatening
cirrhosis, and progressive fibrosis (1). Nowadays, many compounds such as
corticosteroids are used to treat ALD, but these drugs have adverse
effects, which include an elevated risk of infection, gastritis and
osteoporosis (2). Accordingly,
there is need for more effective drugs with fewer side effects to
treat ALD.
The liver is the main organ responsible for the
metabolism of alcohol, and many metabolites, such as, acetaldehyde;
and toxic lipid species, are formed during this process (3,4).
Several mechanisms, such as, steatosis, oxidative stress and
inflammatory factors, contribute to the pathogenesis of ALD.
Hepatic steatosis is the first manifestation of ALD, and is
characterized by lipid accumulation in hepatocytes (5). Much evidence supports the role of
oxidative stress in the pathogenesis of ALD (6). Cytochrome P450 2E1 (CYP2E1)
catalyzes the conversion of ethanol into acetaldehyde and is
markedly overexpressed in ALD (7). In the presence of ethanol exposure,
CYP2E1 causes the production of reaction oxygen species (ROS) and
depletes glutathione (GSH) (8).
In recent studies, NADPH oxidase (NOX) has been identified as a
major source of ROS production and an important cause of superoxide
dismutase (SOD) production. Furthermore, associations between NOX1
and p67phox, p47phox and p22phox have been associated with
alcoholic steatohepatitis, lipid accumulation and hepatic apoptosis
(9). NF-E2-related factor 2
(Nrf2) has been reported to play a major role in defense against
oxidative stress (10). In
addition, it has been shown that Nrf2 is regulated by the
phosphatidylinositol 3-kinase (PI3K)/Akt pathway (11), and that excessive ROS production
causes mitochondrial damage, cytochrome c (cyt c)
secretion, caspase activation and liver apoptosis (12).
Triticum aestivum (TA) is a major worldwide
crop and an excellent source of biologically active substances
(13,14). Previous studies on the
pharmacological properties of extracts of Triticum aestivum
have reported anti-inflammatory, antioxidative, anti-obesity and
anticancer effects (15–19). A previous study revealed that
TA-derived phenolic compounds possess antioxidant effects as they
produce high free-radical-scavenging activity and SOD-like activity
(20). In addition, a recent
study showed that TA-derived polysaccharides contain β-glucan which
reduces hyperglycemia and controls diabetes (21,22). Many studies have shown that
polysaccharides from different biological sources protect against
hepatic fibrosis and injury due to their anti-inflammatory and
antioxidant effects (23,24). However, no previous study has
examined the hepatoprotective effect of Triticum aestivum
sprout-derived polysaccharide (TASP) on ethanol-induced liver
injury or the mechanisms involved.
This study was performed to evaluate the effect of
TASP on ethanol-induced liver damage in C57BL/6 mice, and to
elucidate the molecular mechanisms responsible for its effects.
Materials and methods
Materials and reagents
Ethanol was purchased from Merck Millipore
(Darmstadt, Germany). Silymarin and Oil Red O (ORO) staining were
purchased from Sigma (St. Louis, MO, USA). TRIzol reagent and the
SuperScript III kit were obtained from Invitrogen (Carlsbad, CA,
USA). The protein assay kit (RIPA buffer) was from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The primary antibodies,
rabbit anti-[p-Akt (9271), Akt (9272) and Bcl-2 (2870)] were from
Cell Signaling Technology (Danvers, MA, USA). Rabbit
anti-[caspase-3 (sc-7148) and Bcl-2-associated X (Bax) (sc-6236)],
mouse anti-[β-actin (sc-47778) and CYP2E1 (sc-133491)], and the
protein assay kit (RIPA buffer) were obtained from Santa Cruz
Biotechnology, Inc. Primary antibodies at 1:1,000 and secondary
antibodies at 1:5,000 dilutions were used. For rabbit primary
antibodies: goat-anti-rabbit IgG HRP (#sc-2030) and for mouse
primary antibodies: goat-anti-mouse IgG HRP (#sc-2030) (both from
Santa Cruz Biotechnology) were used.
Extraction and purification of TASP
Triticum aestivum sprouts were supplied by
the National Institute of Crop Science (Jeonbuk, Korea) and were
freeze-dried. The extraction of water soluble polysaccharides from
Triticum aestivum sprouts (TASP) has been previously
described (16,17). Briefly, wheatgrass was crushed,
powdered, frozen and extracted with ethanol. The extract so
obtained was purified with a DEAE Sephadex A-25 column (using 0.15
M sodium chloride as the mobile phase) by high performance liquid
chromatography (HPLC; Waters, Milford, MA, USA) equipped with an
electrochemical detector (ELCD). The purified extract was
precipitated with ethanol for desalting, and the content of TASP
was measured using D-glucose as a standard using the
phenol-sulfuric acid method (22,25). The extract contained 98±1% total
carbohydrate and 93±1% non-starch polysaccharide. It contained
glucose, mannose, and galactose at a ratio of 1.4:1.0:2.1 as
determined by neutral sugar composition analysis (22).
Animals and treatment
Male C57BL/6 mice (6–8 weeks old) were purchased
from Samtako Bio Korea (Osan, Korea). The experimental procedures
were conducted using a protocol approved by the Institutional
Animal Care Committee of Chonbuk National University. Mice were
housed at 22±2°C, 50±5% RH and were provided a normal diet. After
an acclimatization period of one week, mice were divided into six
groups of 6 mice/group. All mice were treated for 10 days by oral
gavage: i) normal, mice fed with phosphate-buffered saline (PBS);
ii) EtOH, mice fed with 5% EtOH daily; iii) EtOH+silymarin, mice
fed with EtOH and treated with silymarin (100 mg/kg); iv) EtOH+TASP
(50), mice fed with EtOH plus TASP (50 mg/kg) treatment; v)
EtOH+TASP (100), mice fed with EtOH plus TASP (100 mg/kg)
treatment; vi) EtOH+TASP (200), mice fed with EtOH plus TASP (200
mg/kg) treatment. On the 11th day, all mice, except mice in the
normal control group, were administered an additional single dose
of 20% EtOH, and 12 h later mice were sacrificed under anesthesia.
Blood and liver tissue were collected for further experiments.
Recent studies used silymarin as a positive control to investigate
the hepatoprotective effect of natural compounds (26,27). Here, we selected silymarin with a
dose of 100 mg/kg as a positive control as it was reported that
silymarin, with this dose, significantly reduced the
alcohol-induced liver steatosis in mice (26).
Measurement of liver index (%)
Total body weights of mice were measured immediately
before sacrifice. Liver weight was measured and the liver index
percentage (%) was calculated by expressing liver weight as the
percentage of body weight.
Oil Red O (ORO) staining
Hepatic lipid accumulation was measured by ORO
staining. Briefly, portions of left lobes were immediately fixed in
10% neutral buffered formalin, embedded in frozen section and cut
serially into 10-µm sections, which were then stained with
Oil Red O staining. Pathological changes were observed under a
microscope. To measure total lipid, liver tissues were homogenized
in a chloroform/methanol mixture (2:1, v/v), and total
triglycerides (TG) and total cholesterol (TC) were measured using a
commercially available kit (Asian Pharmaceutical, Hwaseong-Si,
Korea).
Hematoxylin and eosin (H&E)
staining
Liver tissues were fixed with 10% neutral buffered
formalin, embedded in paraffin wax and cut serially into
10-µm-thick sections, which were then stained with H&E.
Histopathological alterations were observed under a microscope, and
photographed (magnification, ×100, Olympus CX21; Olympus America
Inc., Melville, NY, USA).
Liver enzyme analysis
Blood samples were centrifuged to obtain serum, and
serum samples were subjected to biochemical analysis. Serum enzyme
activities of alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) were measured using ALT/AST cassette test
kit (Alere Cholestech LDX® system; Alere Inc., San
Diego, CA, USA).
Measurement of hepatic GSH
Immediately after removal, liver tissues were washed
in cold isotonic saline. After removing excess saline by blotting,
tissues were weighed and homogenized in ice-cold 5% (w/v)
meta-phosphoric acid (20 ml/g tissue). Homogenates were centrifuged
at 14,000 × g for 15 min at 4°C and supernatants were stored at
−80°C for future use. Glutathione concentrations in liver tissue
were measured using the Glutathione (Total) kit (Enzo Life
Sciences, Inc., Farmingdale, NY, USA).
Measurement of hepatic SOD
To measure SOD activities, liver tissues were kept
in cold PBS after removal. After repeated washing and blotting,
tissues were homogenized and cell pellets were collected. Cytosol
extracts were prepared and SOD activities were measured using a SOD
assay kit (Dojindo Molecular Technologies, Inc., Rockville, MD,
USA) by measuring absorbance at 450 nm using a microplate reader
(Zenyth 200rt; Anthos, Salzburg, Austria).
Real-time PCR
To determine the relative mRNA expression of genes
involved in oxidation, we performed real-time PCR using the
comparative CT method (28).
Total RNA was extracted from liver tissues using TRIzol®
RNA isolation reagent, according to the manufacturer's
instructions. cDNA was synthesized, using the SuperScript™ III
First Strand Synthesis system (Invitrogen). Real-time PCR was
performed using the ABI Real-Time PCR system (Applied Biosystem
Inc., Foster City, CA, USA) and SYBR-Green PCR Master Mix (Life
Technologies, Carlsbad, CA, USA). The temperature profile used for
PCR was 95°C for 10 min, followed by 40 cycles of denaturation at
95°C for 15 sec, and annealing for 60°C for 1 min (29). Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was the internal control. The primer
sequences used were: GAPDH, sense (5′-CAT GGC CTT CCG TGT TC-3′)
and antisense (5′-CCT GGT CCT CAG TGT AGC-3′); Nrf2, sense (5′-ACC
AAG GGG CAC CAT ATA AAA G-3′) and antisense (5′-CTT CGC CGA GTT GCA
CTC A-3′); hemeoxygenase-1 (HO-1), sense (5′-CAG AAC CAG CCT GAA
CTA GC-3′) and antisense (5′-TGG ATG TGT ACC TCC TTG GT-3′);
CYP2E1, sense (5′-CGT TGC CTT GCT TGT CTG GA-3′) and antisense
(5′-AAG AAA GGA ATT GGG AAA GGT CC-3′); p67phox, sense (5′-TCT CAT
GCA TGC CAA GAA AG-3′) and antisense (5′-CTT CAT GTT GGT TGC CAA
TG-3′); p47phox, sense (5′-GTC CCT GCA TCC TAT CTG GA-3′) and
antisense (5′-ATG ACC TCA ATG GCT TCA CC-3′) and p22phox, sense
(5′-GTG GAC TCC CAT TGA GCC TA-3′) and antisense (5′-GTG GAC TCC
CAT TGA GCC TA-3′).
Western blot analysis
Liver tissues were lysed in ice-cold RIPA buffer for
40 min and centrifuged (12,000 × g) for 20 min at 4°C (30,31). Briefly, 30 µg of lysates
were run on sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to polyvinylidene difluoride (PVDF)
membranes (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Membranes were then blocked with 5% skimmed milk in Tris-buffered
saline containing 0.1% Tween-20 (TBST) for 1 h at room temperature
(RT), probed with primary antibodies at 4°C overnight, washed with
TBST four times, and incubated with horseradish
peroxidase-conjugated secondary antibody for 45 min at RT. After
three washes with TBST, proteins were visualized using an enhanced
chemiluminescence detection kit (Millipore, Billerica, MA,
USA).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
Hepatic apoptosis was determined using a
commercially available TUNEL assay kit (Millipore). Briefly, liver
tissues were fixed in 10% buffered formalin and 5-µm thick
frozen sections were obtained, and mounted on slides. Slides were
washed in PBS for 30 min at 37°C, and incubated with proteinase K
for 30 min at 37°C. Slides were rinsed in PBS, treated with TUNEL
reaction mixture containing terminal deoxynucleotidyl transferase
in a humidified chamber at 37°C for 60 min in the dark, washed with
PBS, blocked with avidin-FITC in blocking buffer for 30 min at 37°C
in the dark, glass cover slipped, and analyzed under a fluorescent
microscope (magnification, ×400, Laser Scanning Microscope, LSM
510).
Statistical analysis
Results are expressed as means ± SEMs, and were
analyzed using GraphPad Prism software (version 5.0; GraphPad
Software, San Diego, CA, USA). For the evaluation of samples with
normal distribution, we used the two-tailed unpaired Student's
t-test; otherwise, the non-parametric data were measured by
Mann-Whitney test to obtain p-values. p-values <0.05 were
considered statistically significant.
Results
TASP improves the liver indices of
ethanol fed mice
Body weights, liver weights, and liver indices were
measured and compared (Table I).
The mean liver index in the ethanol group was 17.8% higher than
that in the normal controls. TASP co-treatment at 50, 100 and 200
mg/kg reduced this increase in mean lipid index to 5.7, 7.5 and
11.3%, respectively.
 | Table ITASP improves the liver index (%) in
ethanol fed mice. |
Table I
TASP improves the liver index (%) in
ethanol fed mice.
| Group | Body
weight (g) | Liver
weight (g) | Liver
index (%) |
|---|
| Control | 23.7±0.8 | 1.07±0.04 | 4.5±0.2 |
| EtOH | 22.4±0.6 | 1.20±0.05 | 5.3±0.3 |
| EtOH+silymarin | 22.0±0.7 | 1.07±0.05 | 4.9±0.2b |
| EtOH+TASP (50) | 23.0±1.2 | 1.16±0.04 | 5.0±0.3 |
| EtOH+TASP
(100) | 22.9±0.8 | 1.12±0.06 | 4.9±0.4a |
| EtOH+TASP
(200) | 23.0±0.6 | 1.09±0.05 | 4.7±0.4b |
TASP prevents ethanol-induced hepatic
steatosis and improves liver enzymes
To assess the role of TASP in hepatic steatosis
induced by ethanol intake, the qualitative measurement of hepatic
lipid accumulation was obtained by ORO staining and by measuring TG
and TC levels in liver tissues using a commercial kit (Asian
Pharmaceutical, Hwaseong-Si, Korea). The ethanol group was found to
show neutral lipid droplet accumulation in liver tissues as
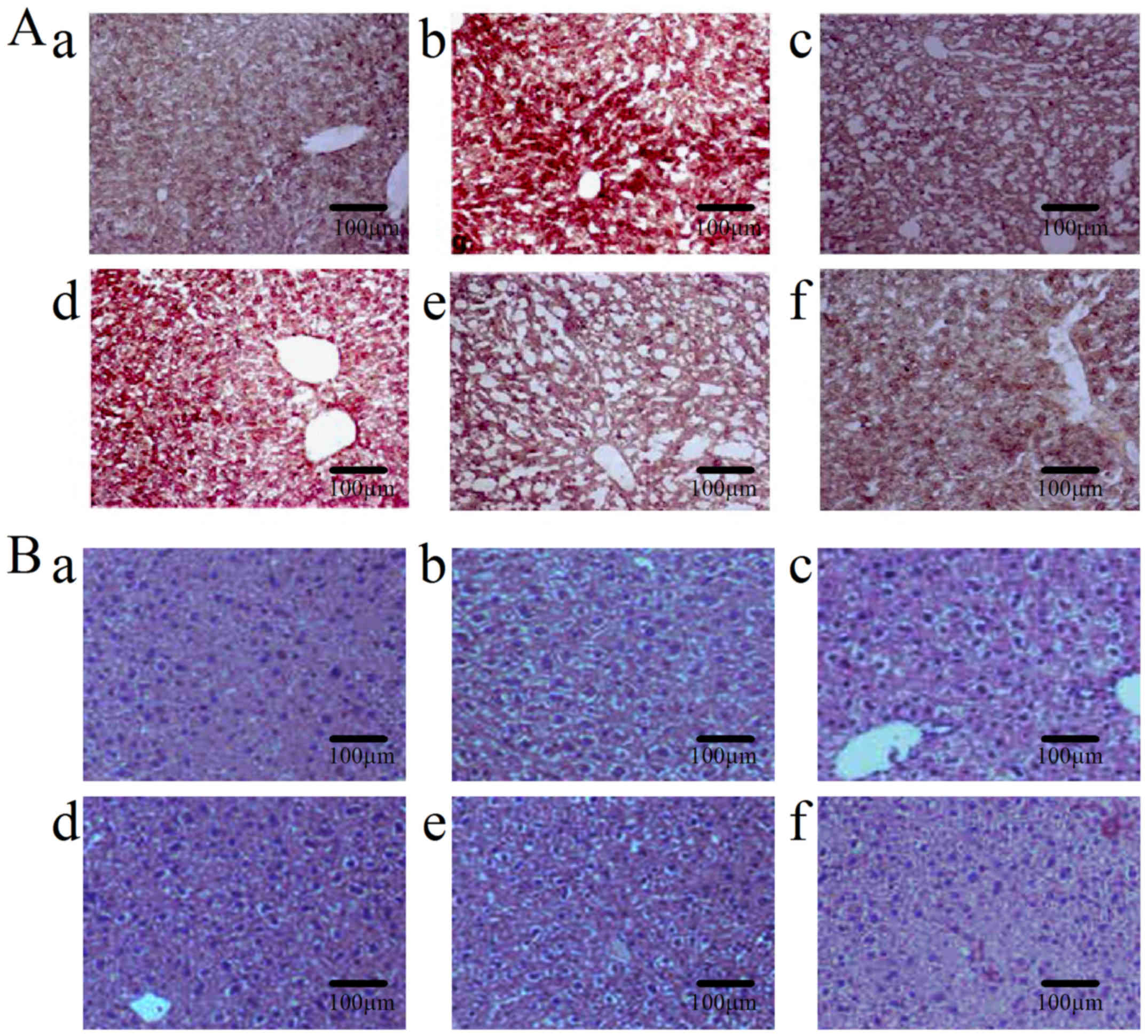
compared with the normal controls, whereas ORO staining showed that
this accumulation was markedly reduced in the three TASP groups
(Fig. 1A). Histological analysis
of excised livers was performed by using H&E staining. As shown
in Fig. 1B, livers in the control
group had a normal architecture, whereas livers in the ethanol
group showed excessive vacuolization and ballooning of liver
tissues with loss of hepatic architecture, and disappearance of
nuclei, but these histological changes were less pronounced in the
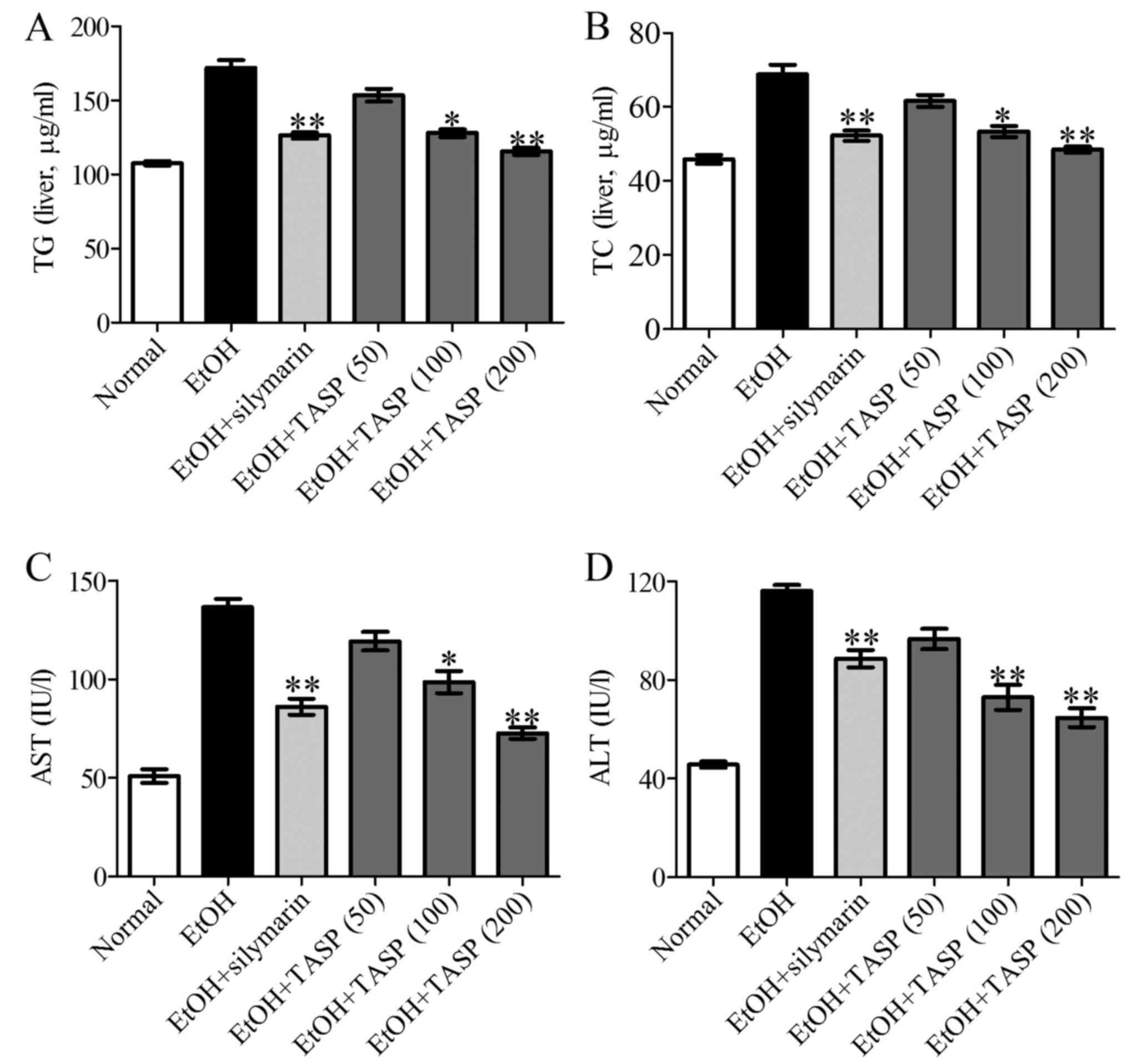
TASP-treated groups. Quantitative measurements of TG and TC levels
in the liver tissues confirmed these histological results and
showed a significant increase in lipid accumulation in the ethanol
fed group as compared with the normal controls. TASP
dose-dependently inhibited the TG and TC increases caused by
ethanol treatment and also TASP (200 mg/kg) more significantly
inhibited these increases than that of the silymarin group
(Fig. 2A and B). To determine the
effect of the TASP co-treatment on ethanol-induced hepatotoxicity,
we measured the serum activities of ALT and AST, which are the
clinical markers of liver damage. It was found that serum ALT and
AST activities were 54.4 and 104% higher, respectively, in the
ethanol group than these values in the normal control (Fig. 2C and D) and that co-treatment with
TASP at 200 mg/kg reduced these increases to 44.3 and 46.8%,
respectively, of the ethanol group. These data showed that TASP
improved liver steatosis and liver enzymes.
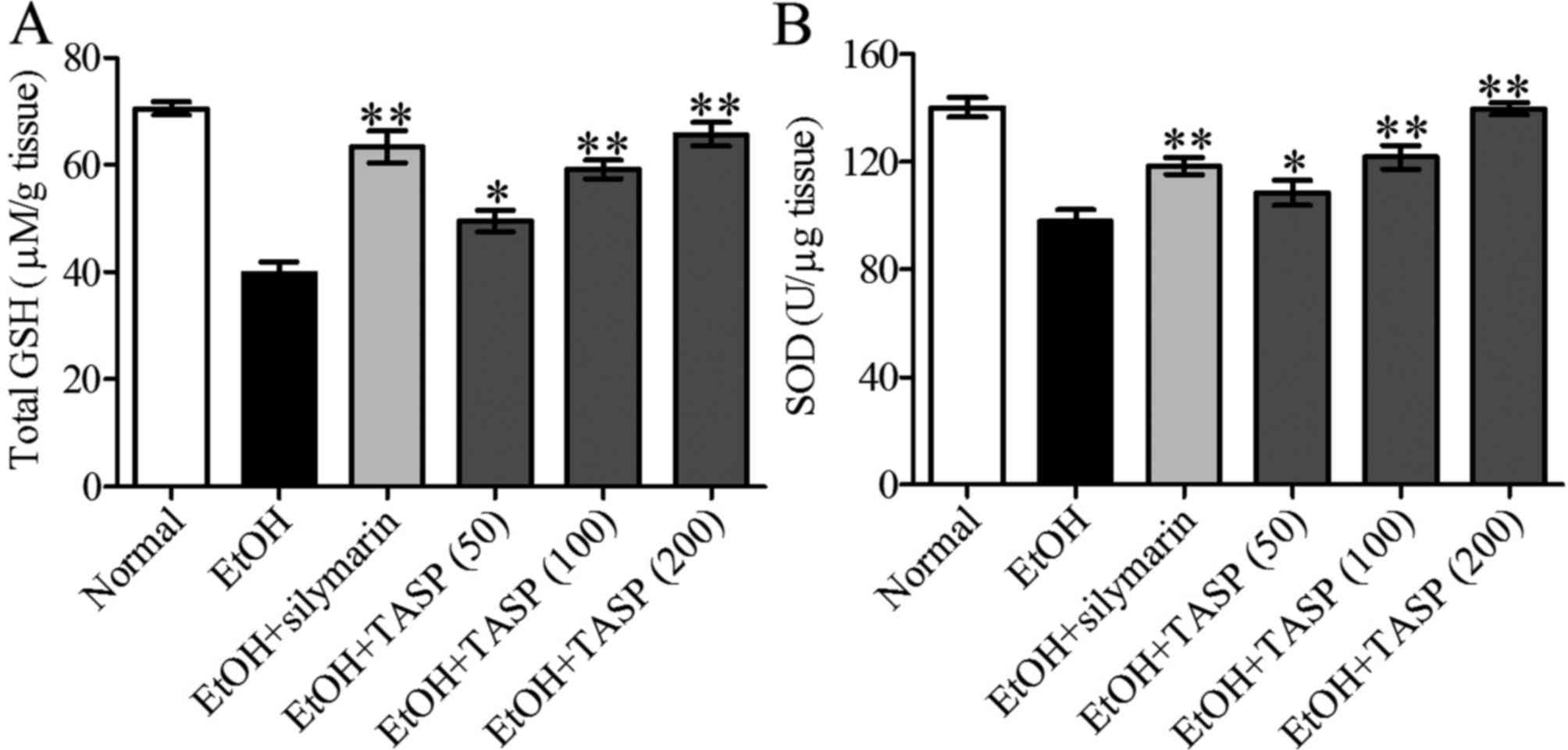
TASP improves the antioxidative status in
ethanol-treated mice
The activities of antioxidant enzymes in liver
tissues of mice were measured to investigate the protective effect
of TASP on ethanol-induced oxidative damage. As shown in Fig. 3, GSH and SOD activities were
significantly lower in the ethanol group than these values in the
normal controls. However, TASP co-treatment significantly and
dose-dependently increased these reductions, and 200 mg/kg of TASP
co-treatment had a greater effect than that noted in the silymarin
group.
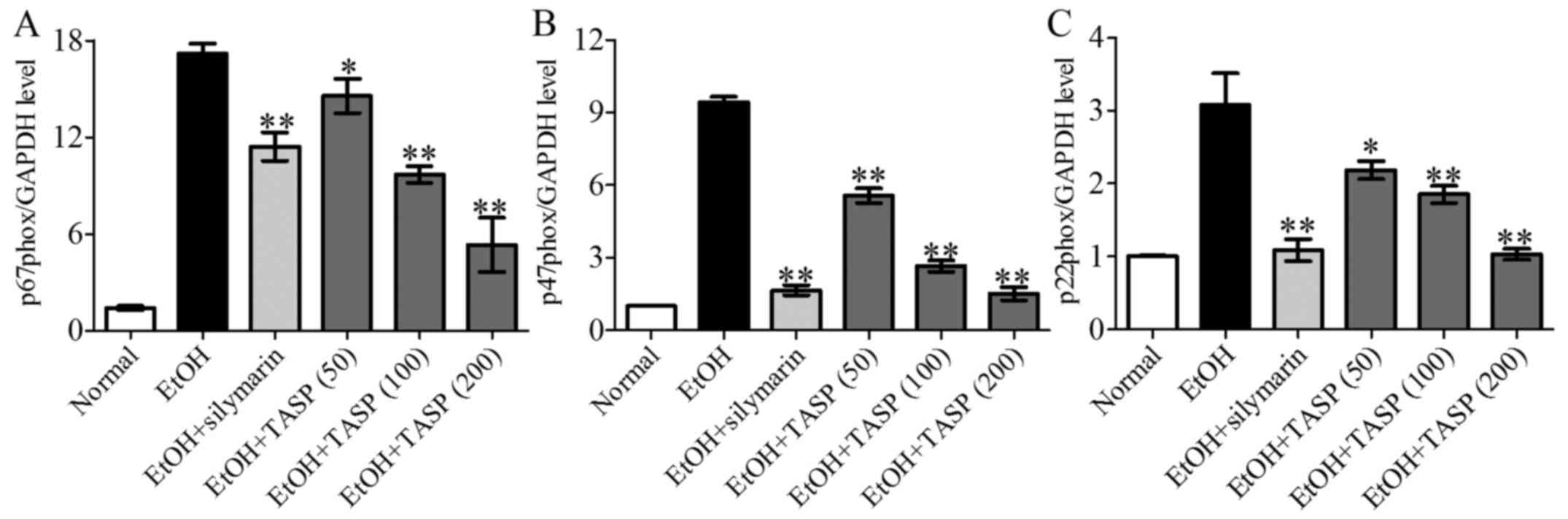
NOX is the major source of ROS which is involved in
hepatic steatatosis and apoptosis (9,32).
We determined the effect of TASP on the genes of NOX1, such as
p67phox, p47phox and p22phox. The results showed that ethanol fed
mice showed increased expression levels of p67phox, p47phox and
p22phox than these levels in the normal mice, whereas co-treatment
of TASP significantly decreased these increments in NOX1 genes
compared to the ethanol group (Fig.
4).
We also performed real-time PCR to assess the mRNA
expression of oxidative stress markers. TASP was found to
significantly and dose-dependently enhance the mRNA expression of
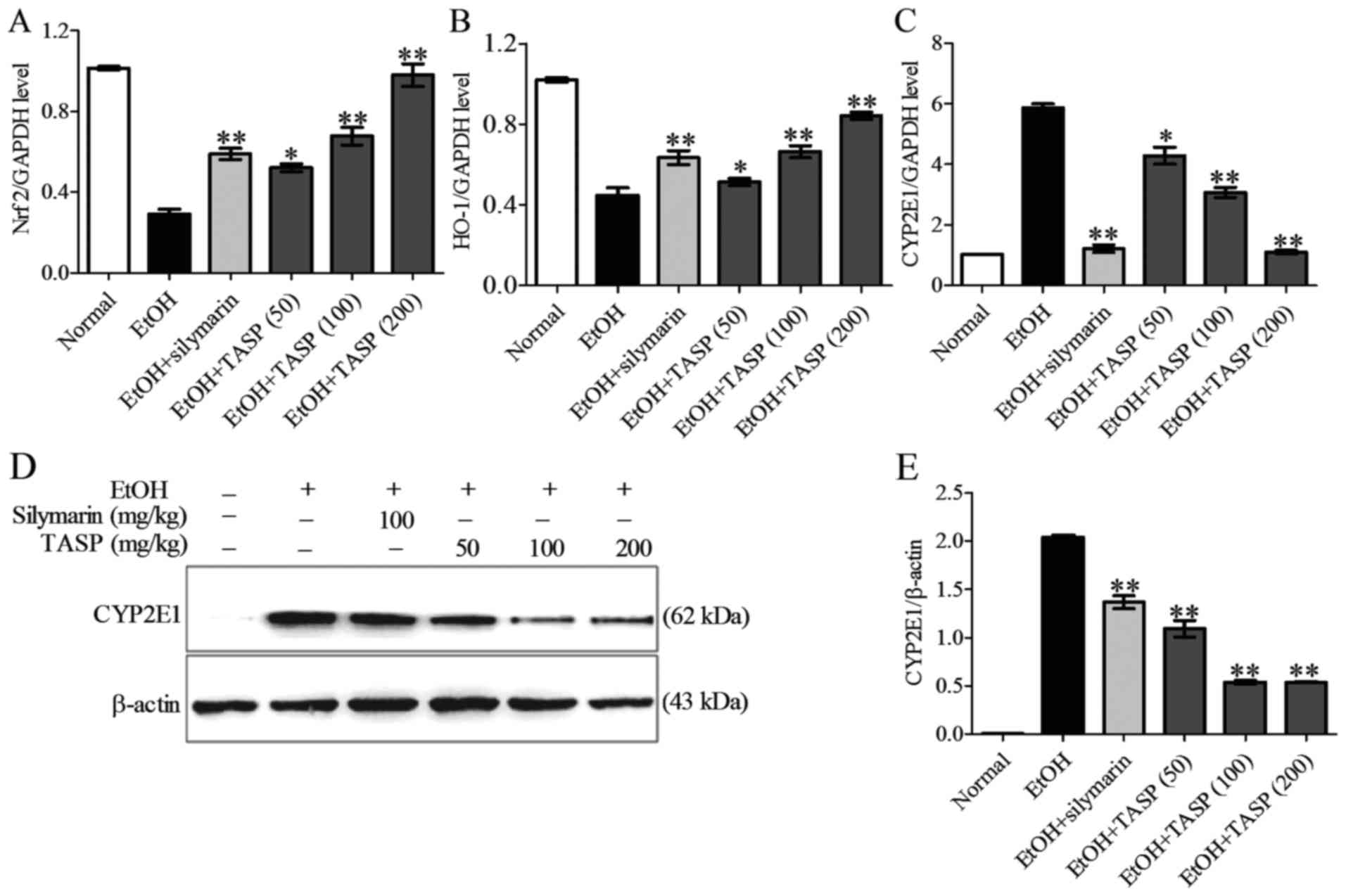
Nrf2 and HO-1 (Fig. 5A and B).
Ethanol increased both the mRNA and protein expression of CYP2E1
and these increases were dose-dependently reduced by co-treatment
of TASP (Fig. 5C–E). TASP also
dose-dependently reduced the ethanol-induced mRNA expression of
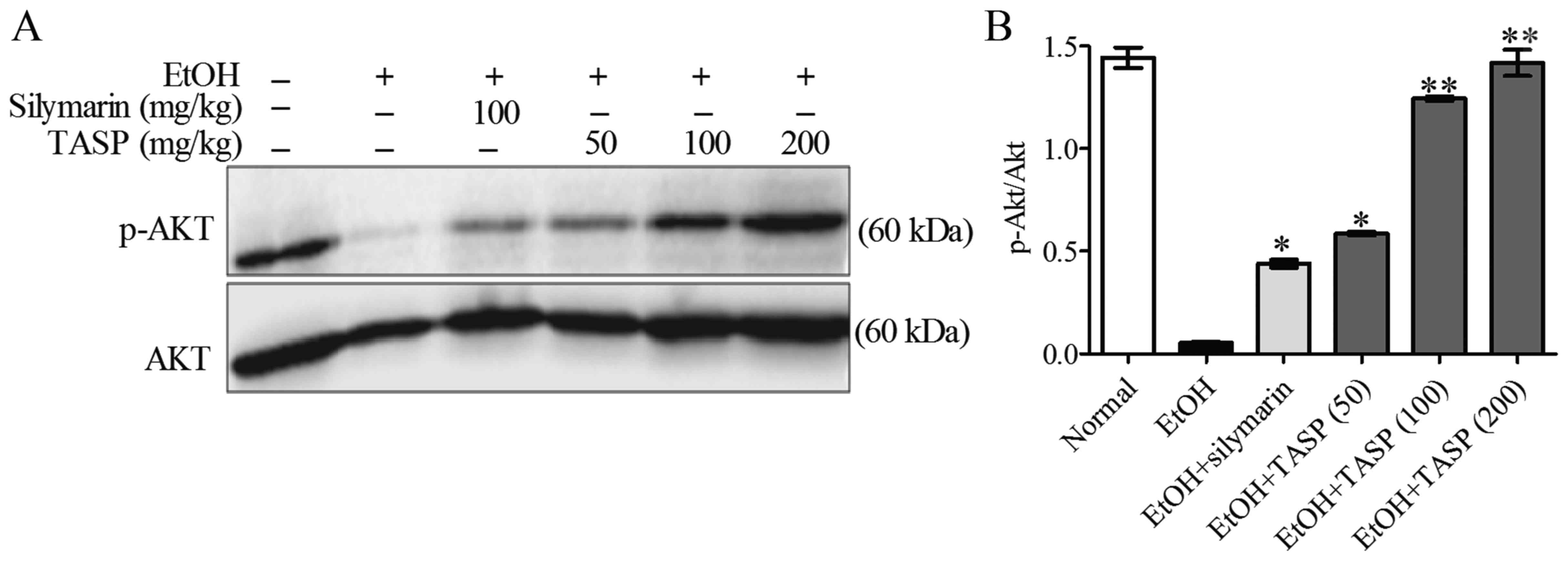
NADPH oxidase more significantly than silymarin. In a previous
study, the PI3K/Akt pathway was shown to be upstream of Nrf2 and to
play an important role in the synthesis of GSH (33). Therefore, to elucidate the
mechanism responsible for the antioxidative effect of TASP, we
investigated the phosphorylation of Akt. Western blot analysis was
performed to determine the phosphorylation of Akt by using total
Akt protein expression as a control (32). It was found that ethanol decreased
Akt phosphorylation compared to that noted in the normal controls,
and TASP significantly and dose-dependently reduced this increase
and did so to a significantly greater extent than silymarin
(Fig. 6).
TASP reduces ethanol-induced apoptosis in
liver tissues
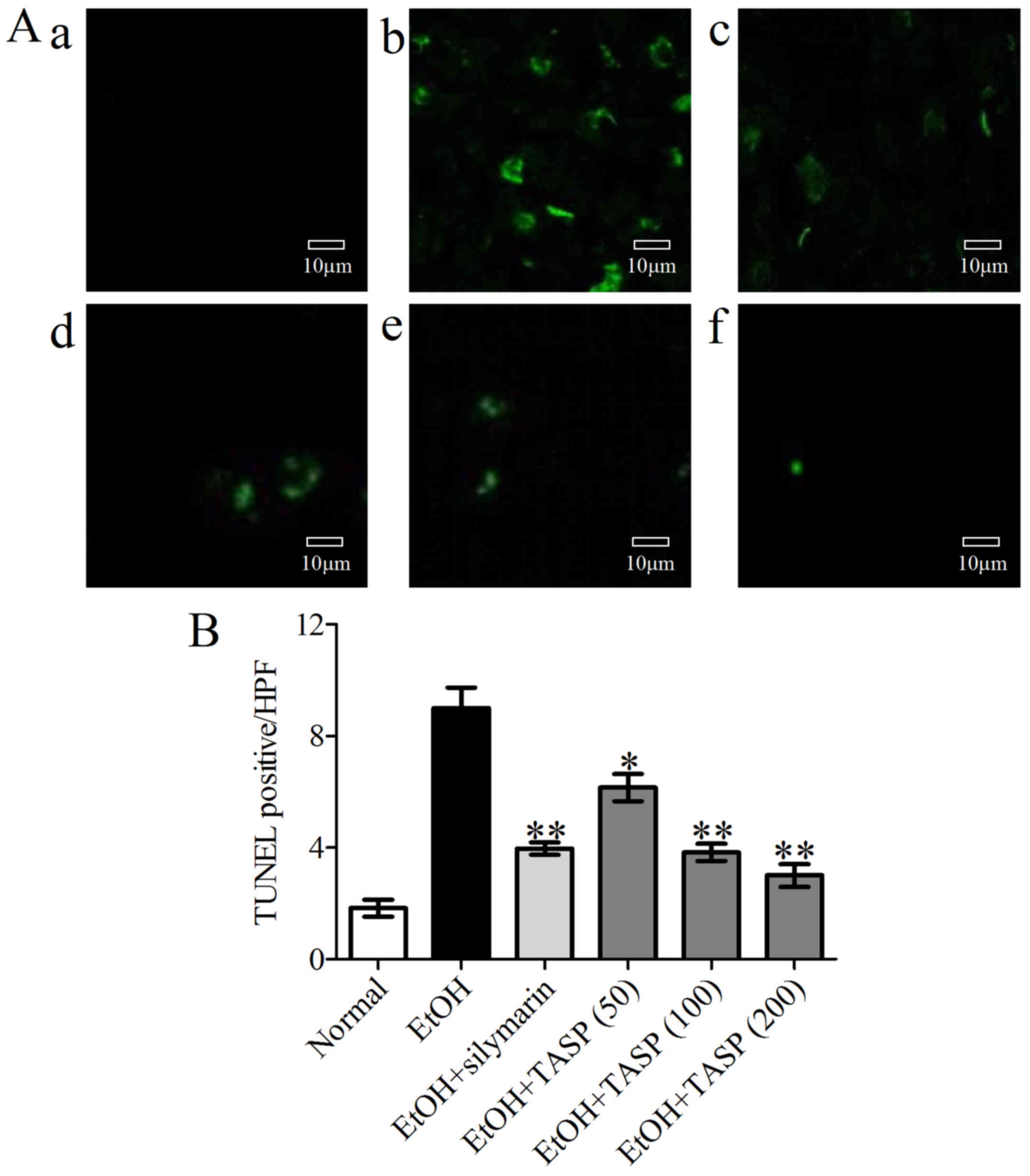
TUNEL staining was used to examine apoptotic bodies
in the liver tissues. A large number of TUNEL-positive hepatocytes
were observed in the ethanol group, but these numbers were
significantly and dose-dependently reduced by TASP (Fig. 7).
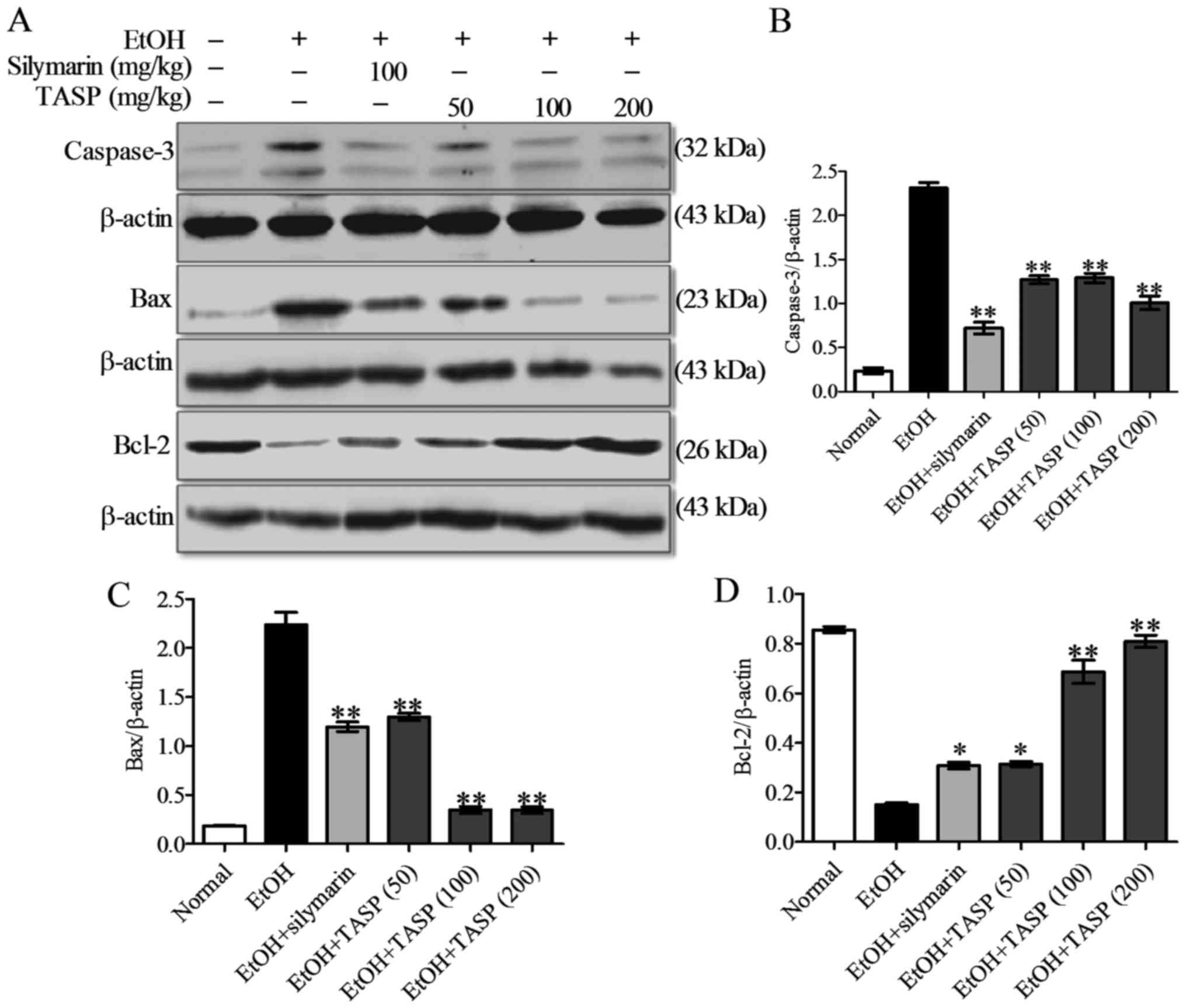
We also examined the effect of TASP on the protein
level of caspase-3 (a predominant downstream effector of apoptosis
activated by caspases-8 and -9, which trigger apoptosis via
extrinsic and intrinsic pathways, respectively) (12). As shown in Fig. 8, caspase-3 activation was detected
using western blot analysis showing that the top band corresponds
to full-length caspase-3 and the bottom band to cleaved caspase-3.
Caspase-3 protein levels were significantly higher in the
ethanol-fed group than that noted in the normal control or ethanol
non-fed group, but TASP co-treatment significantly downregulated
caspase-3 levels as compared with the ethanol group (Fig. 8A and B). We also found that
ethanol significantly increased Bax protein levels in the liver,
and that TASP co-treatment significantly suppressed this increase
(Fig. 8A and C). In contrast,
Bcl-2 protein levels were reduced by ethanol administration, and
TASP dose-dependently prevented this reduction (Fig. 8A and C). Our results showed that
TASP caused a significant decrease in caspase-3 activation and Bax
protein level. In addition, TASP significantly enhanced the Bcl-2
protein level in the liver tissues of the ethanol-fed mice when
compared with that of silymarin.
Discussion
It has been well-established that acute or chronic
alcohol consumption causes hepatic injury. Although medications are
available to treat ethanol-induced hepatic damage, the associated
side effects, for example, in the case of steroids, can cause
gastrointestinal symptoms. In contrast, drugs isolated from plants
and herbs have few side effects. In a recent study, TASP was found
to reduce hyperlipidemia and improve insulin resistance (21,22).
A study showed that polysaccharides derived from
plants protect against hepatic fibrosis and inhibit inflammation
(23). A similar hepatoprotective
effect was observed in rats treated with β-glucan-enriched
Euglena gracilis Z. (34).
In addition, a recent study showed that a fermentable marine
β-glucan protected against hepatotoxicity induced by LPS through
modulation of the immune response (35). However, the hepatoprotective
effect of TASP has not been previously studied, and this is the
first study to report the hepatoprotective effects of TASP on
ethanol-induced liver injury.
In the present study, we did not use polysaccharides
from rice or other plants as a negative control but selected the
nontoxic concentration in a dose-dependent manner as illustrated by
previous studies for determining the hepatoprotective effect of
polysaccharide from plants (23,24). Ethanol non-fed mice or normal mice
served as the negative control in this study. The absence of
polysaccharides from rice or other plants used as a negative
control is a limitation of our study. Ethanol-induced hepatic
injury was compared with the ethanol non-fed mice and the effect of
TASP was compared with ethanol-fed mice. Our result showed that
TASP significantly reduced ethanol-induced hepatic steatosis as
evidenced by reduced ORO staining and total lipid and total
cholesterol levels in liver tissues. These findings are similar to
those reported for the effect of luteolin (a phytoflavone) on
ethanol-induced liver injury (5).
Elevated serum activities of aminotransferase ALT
and AST are prominent signs of hepatic damage. In the present
study, TASP reduced ethanol-induced ALT and AST levels more
significantly than silymarin, a drug used to treat liver injury.
Moreover, H&E staining showed that TASP improved hepatic
architecture by preventing excessive vacuolization, loss of hepatic
architecture, and disappearance of nuclei. In line with our
results, it was previously reported that inhibition of ALT and AST
improved architecture in a mouse model of alcohol-induced liver
steatosis and injury (26).
GSH and SOD are antioxidant enzymes and scavengers
of free radicals and ROS in liver, and are the first line of
defense against oxidative injury. GSH plays an important role in
protection from oxidative stress by removing lipids and organic
peroxides (36), whereas SOD
defends against oxidative stress by removing superoxide radicals
(37). Alcohol increases
oxidative stress by increasing the intracellular levels of reactive
oxygen species and by reducing the activities of GSH and SOD
(37,38). Our results showed that oral
co-treatment with TASP enhanced GSH and SOD activities in liver
tissues more significantly than silymarin in the ethanol fed mice.
Similarly, it was previously reported that GSH and SOD augmentation
reduced acute alcohol-induced oxidative damage in mice (39). In addition, we observed that TASP
reduced ethanol-induced NOX (a primary source of SOD production)
more significantly than silymarin.
CYP2E1 is a member of the cytochrome P450 enzyme
family, and when active produces ROS. Furthermore, CYP2E is highly
expressed by the acute and chronic intake of alcohol (38,39), and plays an important role in
alcohol-induced liver injury, by increasing oxidative stress and
lipid accumulation in the liver (40,41). On the other hand, Nrf2 is an
important transcription factor which induces cytoprotective signals
in the presence of oxidative stimuli and also regulates metabolism
of hepatic lipid (42,43). In line with other studies, our
results showed that TASP significantly decreased ethanol-induced
CYP2E1 expression and enhanced the mRNA expression of the
antioxidant genes, Nrf2 and HO-1.
Akt is the primary mediator of the PI3K-initiated
signaling pathway, and when phosphorylated acts as an antiapoptotic
signaling molecule (33,44). Several studies have reported that
PI3K/Akt signaling is important for Nrf2 translocation, and thus,
for the regulation of GSH (11).
According to a previous report, the ratio of p-Akt/Akt showed that
the hepatic p-Akt level reduced in alcohol-fed mice was due to the
change in the phosphorylation level of p-Akt rather than a change
in total protein of Akt (32).
Hence we determined the p-Akt/Akt ratio rather than p-Akt/β-actin
in this study. TASP showed a hepatoprotective effect in
ethanol-induced liver injury by upregulation of Akt phosphorylation
which controls antioxidative markers.
Apoptosis is a feature of alcohol-induced liver
injury (8,45). Excessive ROS production causes
damage to mitochondria and causes leakage of cyt c, caspase
activation, and apoptosis (12).
Many studies have assessed the cell death in liver tissues by TUNEL
assay counterstained with 4′,6-diamidino-2-phenylindole (DAPI), a
nuclear staining (46,47). In this study, cell death was
assessed by TUNEL assay that detects DNA breaks but counterstaining
with DAPI was not performed. In the late stage of apoptosis, all
nuclei break and DAPI may not be able to stain them anymore
(48). Thus, the use of the TUNEL
assay is a reliable marker for apoptosis (33). Apoptosis may be more clearly
assessed using DAPI as a counterstain in the TUNEL assay but we
assessed proteins using only TUNEL assay as it can detect apoptosis
easily. Our results showed a large number of TUNEL-positive
hepatocytes in the ethanol-treated group. Furthermore, TASP (200
mg//kg) was found to be more effective in this respect than
silymarin. Also, we performed western blot analysis to measure key
proteins, such as Bax and Bcl-2 associated with apoptosis (33), to further illustrate the effect of
TASP in ethanol-induced apoptosis.
Caspase-3 is the downstream executioner of
programmed cell death activated by both the intrinsic and extrinsic
pathways (18,33). In the present study, caspase-3
protein level in liver tissues was enhanced by ethanol
administration, but this increase was diminished by TASP
co-treatment. In addition, TASP decreased caspase-3 and Bax protein
(a pro-apoptotic protein) levels and increased Bcl-2 (an
antiapoptotic protein) levels. Similarly, in a previous study,
peptides were observed to inhibit hepatocyte apoptosis in an
ethanol-induced liver injury model (33).
Taken together, our results suggest that TASP is a
natural hepatoprotective agent that protects against
alcohol-induced liver damage. The underlying mode of action of TASP
appears to involve antioxidant and antiapoptotic effects and
reduced hepatic steatosis. Further studies are required to
elucidate the molecular mechanisms underlying the protective
effects of TASP.
Acknowledgments
The study was supported by a 2017 grant from
Wonkwang University (Jeonbuk, Korea).
References
|
1
|
Park SH, Kim CH, Kim DJ, Park JH, Kim TO,
Yang SY, Moon YS, Kim TN, Kim HK, Park HY, et al: Prevalence of
alcoholic liver disease among Korean adults: Results from the
fourth Korea National Health and Nutrition Examination Survey,
2009. Subst Use Misuse. 46:1755–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arteel G, Marsano L, Mendez C, Bentley F
and McClain CJ: Advances in alcoholic liver disease. Best Pract Res
Clin Gastroenterol. 17:625–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawaratani H, Tsujimoto T, Douhara A,
Takaya H, Moriya K, Namisaki T, Noguchi R, Yoshiji H, Fujimoto M
and Fukui H: The effect of inflammatory cytokines in alcoholic
liver disease. Mediators Inflamm. 2013:4951562013. View Article : Google Scholar
|
|
4
|
Zamora Nava LE, Aguirre Valadez J,
Chávez-Tapia NC and Torre A: Acute-on-chronic liver failure: A
review. Ther Clin Risk Manag. 10:295–303. 2014.PubMed/NCBI
|
|
5
|
Liu G, Zhang Y, Liu C, Xu D, Zhang R,
Cheng Y, Pan Y, Huang C and Chen Y: Luteolin alleviates alcoholic
liver disease induced by chronic and binge ethanol feeding in mice.
J Nutr. 144:1009–1015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knockaert L, Descatoire V, Vadrot N,
Fromenty B and Robin MA: Mitochondrial CYP2E1 is sufficient to
mediate oxidative stress and cytotoxicity induced by ethanol and
acetaminophen. Toxicol In Vitro. 25:475–484. 2011. View Article : Google Scholar
|
|
7
|
Yamashita H, Goto M, Matsui-Yuasa I and
Kojima-Yuasa A: Ecklonia cava polyphenol has a protective effect
against ethanol-induced liver injury in a cyclic AMP-dependent
manner. Mar Drugs. 13:3877–3891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu T, Zheng L, Xu L, Yin L, Qi Y, Xu Y,
Han X and Peng J: Protective effects of dioscin against
alcohol-induced liver injury. Arch Toxicol. 88:739–753. 2014.
|
|
9
|
Jiang JX and Török NJ: NADPH oxidases in
chronic liver diseases. Adv Hepatol. 2014:7429312014.
|
|
10
|
Xu W, Hellerbrand C, Köhler UA, Bugnon P,
Kan YW, Werner S and Beyer TA: The Nrf2 transcription factor
protects from toxin-induced liver injury and fibrosis. Lab Invest.
88:1068–1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma X, Zhao YL, Zhu Y, Chen Z, Wang JB, Li
RY, Chen C, Wei SZ, Li JY, Liu B, et al: Paeonia lactiflora Pall.
protects against ANIT-induced cholestasis by activating Nrf2 via
PI3K/Akt signaling pathway. Drug Des Devel Ther. 9:5061–5074.
2015.PubMed/NCBI
|
|
12
|
Neuman MG: Apoptosis in liver disease. Rom
J Gastroenterol. 11:3–7. 2002.PubMed/NCBI
|
|
13
|
Lee SH, Xin MJ, Luyen BTT, Cha JY, Im JY,
Kwon SU, Lim SW, Suh JW, Kim YH, Kim DK, et al: Inhibitory effect
of Triticum aestivum ethanol extract on lipid accumulation in
3T3-L1 preadipocytes. Yakhak Hoeji. 55:478–484. 2011.
|
|
14
|
Wojakowska A, Perkowski J, Góral T and
Stobiecki M: Structural characterization of flavonoid glycosides
from leaves of wheat (Triticum aestivum L.) using LC/MS/MS
profiling of the target compounds. J Mass Spectrom. 48:329–339.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benedetti S, Primiterra M, Tagliamonte MC,
Carnevali A, Gianotti A, Bordoni A and Canestrari F: Counteraction
of oxidative damage in the rat liver by an ancient grain (Kamut
brand khorasan wheat). Nutrition. 28:436–441. 2012. View Article : Google Scholar
|
|
16
|
Luyen BT, Tai BH, Thao NP, Cha JY, Lee YM
and Kim YH: A new phenolic component from Triticum aestivum sprouts
and its effects on LPS-stimulated production of nitric oxide and
TNF-α in RAW 264.7 cells. Phytother Res. 28:1064–1070. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luyen BT, Thao NP, Tai BH, Lim JY, Ki HH,
Kim DK, Lee YM and Kim YH: Chemical constituents of Triticum
aestivum and their effects on adipogenic differentiation of 3T3-L1
preadipocytes. Arch Pharm Res. 38:1011–1018. 2015. View Article : Google Scholar
|
|
18
|
Poudel B, Ki HH, Luyen BT, Lee YM, Kim YH
and Kim DK: Triticumoside induces apoptosis via caspase-dependent
mitochondrial pathway and inhibits migration through
down-regulation of MMP2/9 in human lung cancer cells. Acta Biochim
Biophys Sin (Shanghai). 48:153–160. 2016. View Article : Google Scholar
|
|
19
|
Poudel B, Nepali S, Xin M, Ki HH, Kim YH,
Kim DK and Lee YM: Flavonoids from Triticum aestivum inhibit
adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol
Med Rep. 12:3139–3145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang JY, Jeong EY, Kim DK and Lee HS:
Antioxidant and α-glucosidase inhibitory effects in Triticum
aestivum sprouts treated to chilling temperature. J Korean Soc Appl
Biol Chem. 54:644–648. 2011.
|
|
21
|
Lee SH, Lim SW, Lee YM, Lee HS and Kim DK:
Polysaccharide isolated from Triticum aestivum stimulates insulin
release from pancreatic cells via the ATP-sensitive K+
channel. Int J Mol Med. 29:913–919. 2012.PubMed/NCBI
|
|
22
|
Lee SH, LY, Lee HS and Kim DK:
Anti-oxidative and anti-hyperglycemia effects of Triticum aestivum
wheat sprout water extracts on the streptozotocin-induced diabetic
mice. Korean J Pharmacogn. 40:408–414. 2009.
|
|
23
|
Zhang K, Gao Y, Zhong M, Xu Y, Li J, Chen
Y, Duan X and Zhu H: Hepatoprotective effects of Dicliptera
chinensis polysaccharides on dimethylnitrosamine-induced hepatic
fibrosis rats and its underlying mechanism. J Ethnopharmacol.
179:38–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Q, Zhu M, Geng X, Wang H and Ng TB:
Characterization of polysaccharides with antioxidant and
hepatoprotective activities from the edible mushroom Oudemanisella
radicata. Molecules. 22:E2342017. View Article : Google Scholar
|
|
25
|
Lai DM, Høj PB and Fincher GB:
Purification and characterization of (1→3, 1→4)-beta-glucan
endohydrolases from germinated wheat (Triticum aestivum). Plant Mol
Biol. 22:847–859. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lian LH, Wu YL, Song SZ, Wan Y, Xie WX, Li
X, Bai T, Ouyang BQ and Nan JX: Gentiana manshurica Kitagawa
reverses acute alcohol-induced liver steatosis through blocking
sterol regulatory element-binding protein-1 maturation. J Agric
Food Chem. 58:13013–13019. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi MK, Han JM, Kim HG, Lee JS, Lee JS,
Wang JH, Son SW, Park HJ and Son CG: Aqueous extract of Artemisia
capillaris exerts hepatoprotective action in alcohol-pyrazole-fed
rat model. J Ethnopharmacol. 147:662–670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nepali S, Son JS, Poudel B, Lee JH, Lee YM
and Kim DK: Luteolin is a bioflavonoid that attenuates
adipocyte-derived inflammatory responses via suppression of nuclear
factor-κB/mitogen-activated protein kinases pathway. Pharmacogn
Mag. 11:627–635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poudel B, Ki HH, Lee YM and Kim DK:
Collagen I-induced dendritic cells activation is regulated by
TNF-alpha production through down-regulation of IRF4. J Biosci.
40:71–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Poudel B, Lim SW, Ki HH, Nepali S, Lee YM
and Kim DK: Dioscin inhibits adipogenesis through the AMPK/MAPK
pathway in 3T3-L1 cells and modulates fat accumulation in obese
mice. Int J Mol Med. 34:1401–1408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang ZH, Liu XQ, Zhang C, He W, Wang H,
Chen YH, Liu XJ, Chen X and Xu DX: Tlr4-mutant mice are resistant
to acute alcohol-induced sterol-regulatory element binding protein
activation and hepatic lipid accumulation. Sci Rep. 6:335132016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma Z, Hou T, Shi W, Liu W and He H:
Inhibition of hepatocyte apoptosis: An important mechanism of corn
peptides attenuating liverinjury induced by ethanol. Int J Mol Sci.
16:22062–22080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sugiyama A, Suzuki K, Mitra S, Arashida R,
Yoshida E, Nakano R, Yabuta Y and Takeuchi T: Hepatoprotective
effects of paramylon, a beta-1, 3-D-glucan isolated from Euglena
gracilis Z, on acute liver injury induced by carbon tetrachloride
in rats. J Vet Med Sci. 71:885–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Neyrinck AM, Mouson A and Delzenne NM:
Dietary supplementation with laminarin, a fermentable marine beta
(1-3) glucan, protects against hepatotoxicity induced by LPS in rat
by modulating immune response in the hepatic tissue. Int
Immunopharmacol. 7:1497–1506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fernández-Checa JC, Kaplowitz N,
García-Ruiz C, Colell A, Miranda M, Marí M, Ardite E and Morales A:
GSH transport in mitochondria: Defense against TNF-induced
oxidative stress and alcohol-induced defect. Am J Physiol.
273:G7–G17. 1997.PubMed/NCBI
|
|
37
|
Xiong ZE, Dong WG, Wang BY, Tong QY and Li
ZY: Curcumin attenuates chronic ethanol-induced liver injury by
inhibition of oxidative stress via mitogen-activated protein
kinase/nuclear factor E2-related factor 2 pathway in mice.
Pharmacogn Mag. 11:707–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han Y, Xu Q, Hu JN, Han XY, Li W and Zhao
LC: Maltol, a food flavoring agent, attenuates acute
alcohol-induced oxidative damage in mice. Nutrients. 7:682–696.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leung TM and Nieto N: CYP2E1 and oxidant
stress in alcoholic and non-alcoholic fatty liver disease. J
Hepatol. 58:395–398. 2013. View Article : Google Scholar
|
|
40
|
Jimenez-Lopez JM and Cederbaum AI:
CYP2E1-dependent oxidative stress and toxicity: Role in
ethanol-induced liver injury. Expert Opin Drug Metab Toxicol.
1:671–685. 2005. View Article : Google Scholar
|
|
41
|
Lu Y, Wu D, Wang X, Ward SC and Cederbaum
AI: Chronic alcohol-induced liver injury and oxidant stress are
decreased in cytochrome P4502E1 knockout mice and restored in
humanized cytochrome P4502E1 knock-in mice. Free Radic Biol Med.
49:1406–1416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang J, Tabbi-Anneni I, Gunda V and Wang
L: Transcription factor Nrf2 regulates SHP and lipogenic gene
expression in hepatic lipid metabolism. Am J Physiol Gastrointest
Liver Physiol. 299:G1211–G1221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu J, Wang X, Liu R, Liu Y, Zhang T, Fu H
and Hai C: Oleanolic acid co-administration alleviates
ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing
modulating in rats. Chem Biol Interact. 221:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abdelmegeed MA, Banerjee A, Jang S, Yoo
SH, Yun JW, Gonzalez FJ, Keshavarzian A and Song BJ: CYP2E1
potentiates binge alcohol-induced gut leakiness, steatohepatitis,
and apoptosis. Free Radic Biol Med. 65:1238–1245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cao YW, Jiang Y, Zhang DY, Wang M, Chen
WS, Su H, Wang YT and Wan JB: Protective effects of Penthorum
chinense Pursh against chronic ethanol-induced liver injury in
mice. J Ethnopharmacol. 161:92–98. 2015. View Article : Google Scholar
|
|
46
|
Gao X, Fan L, Li H, Li J, Liu X, Sun R and
Yu Z: Hepatic injury is associated with cell cycle arrest and
apoptosis with alteration of cyclin A and D1 in ammonium
chloride-induced hyperammonemic rats. Exp Ther Med. 11:427–434.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ducan MB, Yang C, Tanjore H, Boyle PM,
Keskin D, Sugimoto H, Zeisberg M, Olsen BR and Kalluri R: Type XVII
collagen is essential for survival during acute injury in mice. Dis
Model Mech. 4:942–951. 2013. View Article : Google Scholar
|
|
48
|
Collins JA, Schandi CA, Young KK, Vesely J
and Willingham MC: Major DNA fragmentation is a late event in
apoptosis. J Histochem Cytochem. 45:923–934. 1997. View Article : Google Scholar : PubMed/NCBI
|