Introduction
The growth of solid tumour beyond certain size and
the systemic spread of cancer cells are dependent on the presence
and degree of angiogenesis in the tumour (1–3).
This realisation has led to discovery and development of
anti-angiogenesis agents, both naturally occurring (for example
endostatin, angiostatin, VEGI), synthetic and biological forms, for
example bevacizumab and thalidomide. The last decade has witnessed
the translation of anti-angiogenesis agents into clinical practice.
For example, some of the anti-angiogenesis drugs are now almost
routinely used on eligible patients (4–9).
Some of the anti-angiogenic and anti-cancer compounds are either
extracts from natural products or derivatives of natural products
(7–9).
Yangzheng Xiaoji is a traditional Chinese
medical formula that has been redeveloped in recent years and has
been shown to have anti-cancer actions in patients with certain
solid tumours. In a recent randomised doubled blinded study of
patients with primary liver cancer, patients who received
conventional chemotherapy combined with Yangzheng Xiaoji
(n=304) showed significantly increased rate of disease remission
(complete and partial remissions) compared with patients who
received chemotherapy alone (n=103) (23.3% vs 14%, respectively,
p<0.01) (10). In the study,
patients who received combinational therapy also had improved
quality of life, based on the Karnofsky method. The formula has
also been reported to be able to improve atypical dysplasia in the
stomach (11).
The mechanisms of the anti-cancer action of
Yangzheng Xiaoji are not clear. It has been shown that
patients who received the Yangzheng Xiaoji and chemotherapy
combination has less bone marrow suppression compared with those
who received chemotherapy (10).
It has been suggested therefore that one of mechanisms underlying
the clinical observations is that Yangzheng Xiaoji may
improve the immune function of the body. However, if the formula
has an direct effect on cancer cells is not clear.
In addition to the body’s defence, cancer
progression is also dependent on the biological characteristics of
cancer cells, including the rate of cell proliferation,
invasiveness, ability to degrade matrix and migration. Angiogenesis
is also a key to the distant spread of cancer cells. The latter
cell functions are also closely linked to the metastatic potential
of cancer cells. Naturally occurring compounds have been reported
to be able influence a number of these cell functions. For example,
Taxol, a plant alkaloid, was initially extracted from western yew
bark and is a widely used chemotherapeutic agent (12,13).
Fumagillin is also a natural product shown to be a strong
anti-angiogenic agent (8,14). Artenisinin, an compound extracted
from Qinhao, a Chinese medical herb used in the treatment of
malaria has also been indicated in cancer treatment (15,16).
In the present study, we report the direct effect of
Yangzheng Xiaoji extract on in vitro angiogenesis and
the adhesion and migration of vascular endothelial cells. With
little effect on the growth of endothelial cells, it has a marked
inhibitor effect on the microvessel-like tubules, cell-matrix
adhesion and cellular migration, in a concentration dependent
manner. Whilst the extract inhibited the phosphorylation of FAK, it
synergistically inhibited the angiogenesis with FAK inhibitor.
Materials and methods
Human endothelial HECV cells were purchased from
Interlab (Milan, Italy). The cells were maintained in Dubecco’s
modified Eagle’s medium (DMEM) (Sigma-Aldrich, Poole, Dorset, UK)
supplemented with penicillin, streptomycin and 10% foetal calf
serum (Sigma-Aldrich). The cells were incubated at 37°C, 5%
CO2 and 95% humidity. Matrigel (reconstituted basement
membrane) was purchased from Collaborative Research Products
(Bedford, MA, USA). A selective small inhibitor to FAK (FP573228)
was from Tocris (Bristol, UK). Antibody to Paxillin were from
Transduction Laboratories and phospho-specific antibodies
(anti-FAK, anti-pFAK and anti-pPaxillin) were from Santa-Cruz
Biotechnologies (Santa Cruz, CA, USA).
Preparation of extract DME25 from
Yangzheng Xiaoji for experimental use
Medicinal preparation of Yangzheng Xiaoji
(Yiling Pharmaceutical, Hebei) was subject to extraction using
DMSO, balanced salt solution and ethanol, on rotating wheel for 24
h at 4°C as we recently reported (17). Insolubles were removed after
centrifugation at 15,000 × g. DMSO preparation was found to be more
consistent, reproducible and with better yield compared with the
other two solvents. DMSO extract was hence used in the subsequent
experiments. The extract was standardised by quantifying the
optical density of the preparation using a spectrophotometer at a
wavelength of 405 nM. A master preparation of the extract which
gave 0.25 OD was stocked as the master stock and so named as DME25
for the experiments.
In vitro cell growth assay
This was based on a previous published method
(18). HECV cells were seeded into
96 well plates at a density of 3,000 cells/well. Triplicate plates
were set up for incubation periods of overnight, 3 days and 5 days.
Following sufficient incubation, the plates were removed from the
incubator, fixed in 4% formaldehyde (v/v) and stained with 0.5%
(w/v) crystal violet. The crystal violet stain was subsequently
extracted using a 10% acetic acid (v/v) allowing the detection of
cell density through spectrophotmeric analysis of the resulting
solutions absorbance using a Bio-Tek ELx800 multi-plate reader
(Bio-Tek Instruments Inc., VT, USA).
Electric cell-substrate impedance sensing
(ECIS) based cellular adhesion and migration assays
ECIS-Zθ instrument (Applied Biophysics Inc., NJ,
USA) were used for cell adhesion and motility (wounding assay)
assays in the study (19,20). Cell modelling was carried out using
the ECIS RbA modelling software, supplied by the manufacturer. The
96W1E ECIS arrays were used in the present study. ECIS measures the
interaction between cells and the substrate to which they were
attached via gold-film electrodes placed on the surface of culture
dishes. Following treating the array surface with a cysteine
solution, the arrays were incubated with complete medium for 1 h.
The same number of the respective cells was added to each well. In
the cell adhesion assay, the adhesion was tracked immediately after
adding the cells into the arrays. For cell migration assay, the
arrays with cells were allowed to reach confluence after 3 h. The
monolayer of the cells was electrically wounded at 2,000 mA for 20
sec. Impedance and resistance of the cell layer were immediately
recorded for a period of up to 20 h. For signalling transduction
inhibitor assays, the respective inhibitors was included in the
wells. Adhesion and migration were modelled using the ECIS RbA cell
modelling software as we recently reported (21,22).
In vitro tubule formation assay
In vitro microvessel tubule formation was
assessed using a Matrigel endothelial cell tubule formation assay.
This was modified from a previously reported method (23,24).
Briefly, 250 μg of Matrigel was seeded, in serum-free medium, into
a 96-well plate and placed in an incubator for a minimum of 40 min
to set. Following this, 35,000 HECV were seeded onto the Matrigel
layer and incubated for 4–5 h, in the presence or absence of DME25,
FAK inhibitor or their combination. Tubule formation that occurred
over the incubation period was visualised under low magnification
and images captured. Total tubule perimeter per field was
quantified using ImageJ software.
Cell-matrix adhesion
Cell culture plate (96-well) was first coated with 2
μg Matrigel and allowed to air dry. After rehydration for 1 h, the
wells were gently washed with DMEM medium, HECV cells (20,000 per
well) were added together with the respective treatments. Wells
were gently washed with BSS 5 times, before cells were fixed with
4% formalin and stained with crystal violet. Adherent cells were
counted and shown as number of adherent cell per high power field
of an upright microscope.
Immunofluorescent staining (IFC)
HECV cells were seeded at a density of 20,000 cells
per well in a 16-well chamber slide (LAB-TEK Fisher Scientific UK,
Longhborough, UK), together with the respective treatment for 2 h
(25,26). Medium was carefully aspirated from
the wells and the cells were fixed in 4% formalin for 20 min.
Following fixation, the cells were permeabilised for 5 min in a
0.1% Triton X-100 BSS solution. A blocking solution of (Tris buffer
25 mM Tris, pH 7.4) with 10% semi-skimmed milk was used to block
non-specific binding for 40 min. Cells were subsequently washed
twice with wash buffer before probing for specific antibodies to
FAK and phopho-FAK (SC-1688 and SC-11766, respectively, from
Santa-Cruz Biotechnologies, Inc., Santa Cruz, CA, USA). Primary
antibodies were made up in the Tris buffer with 3% milk at a 1:100
concentration for 1 h. The primary antibody was then completely
removed by washing the cells 5 times in the same buffer. FITC
conjugated anti-mouse and anti-rabbit secondary antibodies
(Sigma-Aldrich) was subsequently added to the cells and the slides
were incubated on a shaker platform in the dark for 1 h. The slides
were finally washed 3 times to remove unbound secondary antibody,
mounted with Fluor-save (Calbiochem-Novabiochem Ltd., Nottingham,
UK) and visualised under an Olympus BX51 fluorescent microscope at
×100 objective magnification.
SDS-PAGE and western blotting
Cells were grow to confluence in a 25 cm3
tissue culture flask, detached and lysed in HCMF buffer containing
1% Triton X-100, 2 mM CaCl2, 100 μg/ml
phenylmethylsulfonyl fluoride, 1 mg/ml leupeptin, 1 mg/ml aprotinin
and, 10 mM sodium orthovanadate on a rotor wheel for 1 h before
being spun at 13,000 x g to remove insolubles. The protein levels
in the samples were subsequently quantified using the Bio-Rad DC
Protein assay kit (Bio-Rad Laboratories, CA, USA).
Once sufficient separation had occurred the proteins
were blotted onto a Hybond-C Extra nitrocellulose membrane
(Amersham Biosciences UK Ltd., Bucks, UK), blocked in 10% milk and
probed for the expression of specific proteins. Anti-pFAK and
anti-pPaxillin were used to probe the phophorylated FAK and
paxillin, respectively (27). In
addition to this, GAPDH expression was also assessed using an
antibody specific to this molecule (Santa Cruz Biotechnology Inc.)
to assess total protein levels and uniformity throughout the test
samples. Protein bands were then visualised through the Supersignal
West Dura Extended Duration substrate chemiluminescent system
(Perbio Science UK Ltd., Cramlington, UK) and detected using a
UVIProChem camera system (UVItec Ltd., Cambridge, UK).
Results
DME25 inhibited formation of
microvessel-like tubules without affecting the growth of
endothelial cells
Using an in vitro tubule formation assay, it
was shown that DME25 (shown in Fig.
1 are 1:1000 dilution) significantly reduced tubule length
compared with control (p=0.046). This was seen at concentrations at
which no growth inhibition was achieved at the concentrations
without cytotoxicity on HECV cells (Fig. 2). DME25 over a wide concentration
did not have a significant influence on the growth of endothelial
cells.
DME25 exerted an inhibitory effect on
cell-matrix adhesion
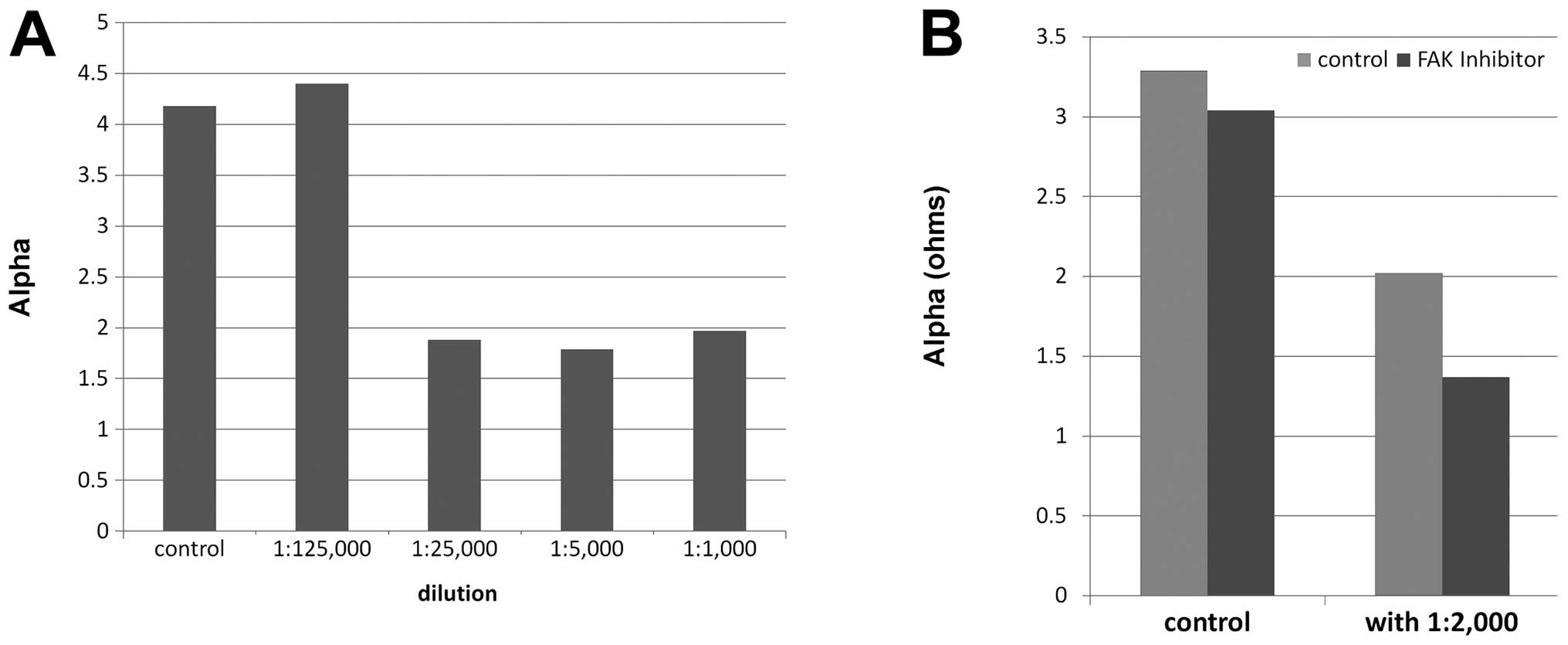
DME25 demonstrated a concentration-dependent
inhibitory effect on the adhesion of HECV cells, with marked
inhibitory effects seen at dilutions of 1:5,000 or lower (Figs. 3A and 4). Using 3D modelling, it was seen that
the inhibitory effects by DME25 were seen across the frequencies
tested (Figs. 3B and 5). Using conventional cell-matrix
adhesion method, DME25 had a significant inhibitory effect on the
adhesion (Fig. 3C and D).
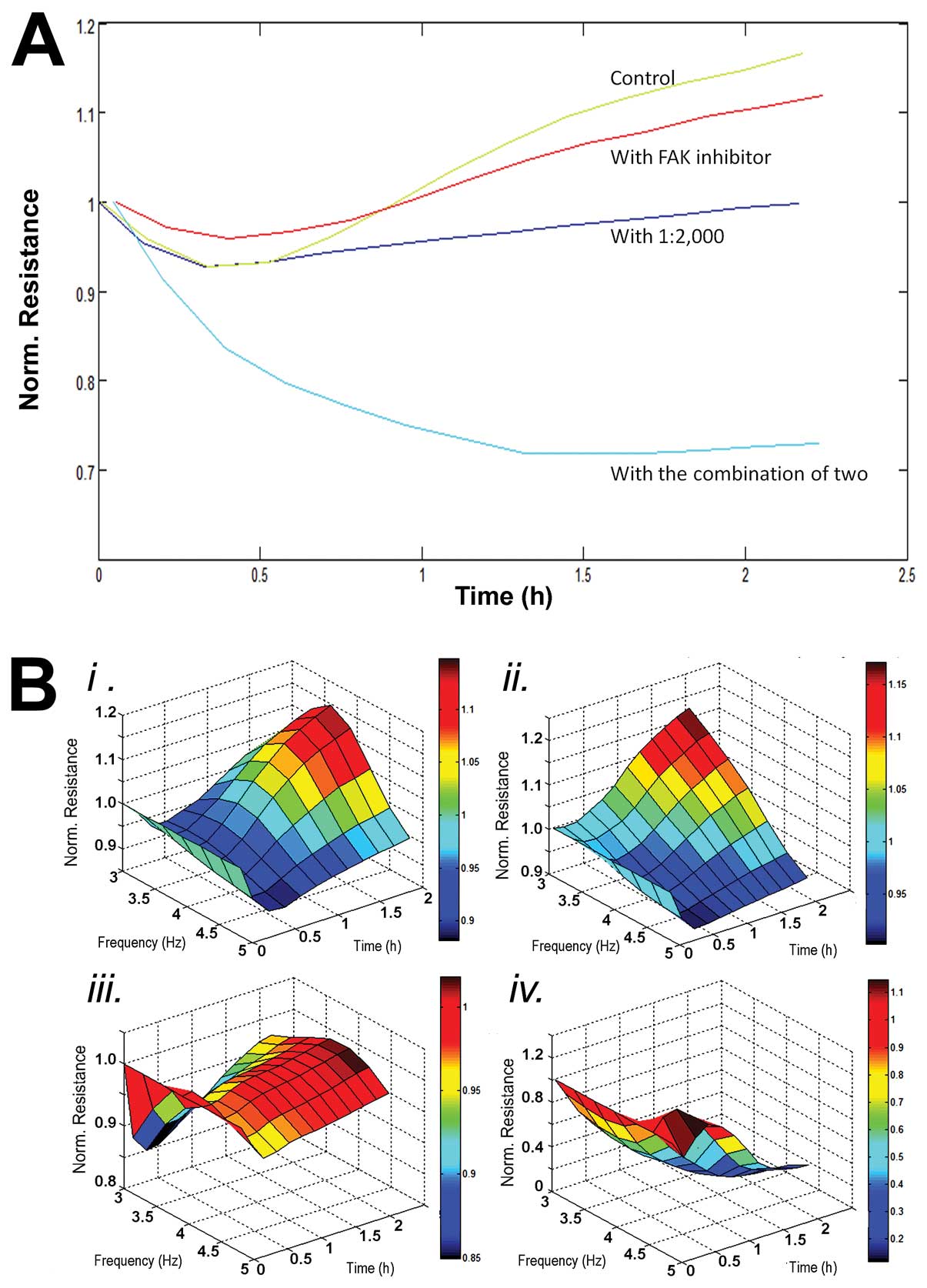
Endothelial cell migration was reduced by
DME25
In a similar fashion to cell-matrix adhesion,
cellular migration was similarly inhibited by the presence of DME25
and was further inhibited when FAK inhibitor was used together with
DME25 (Fig. 6).
DME25 and FAK inhibitor had a synergistic
effect on the adhesion, migration and tubule formation of
endothelial cells
FAK inhibitor has a marked effect on the adhesion of
HECV cells (Figs. 3C and D,
4B, 5). When administered together with DME25,
the inhibitory effect appears to be synergistically strengthened as
seen in these figures.
FAK inhibitor appears to have an inhibitory effect
on tubule formation although this is not statistically significant
(p=0.14) (Fig. 1E). However, the
combination between DME25 and FAK inhibitor had a marked inhibition
on tubule formation, compared with control, with FAK inhibitor
along and DME25 along (p=0.006, p=0.041, p=0.011,
respectively).
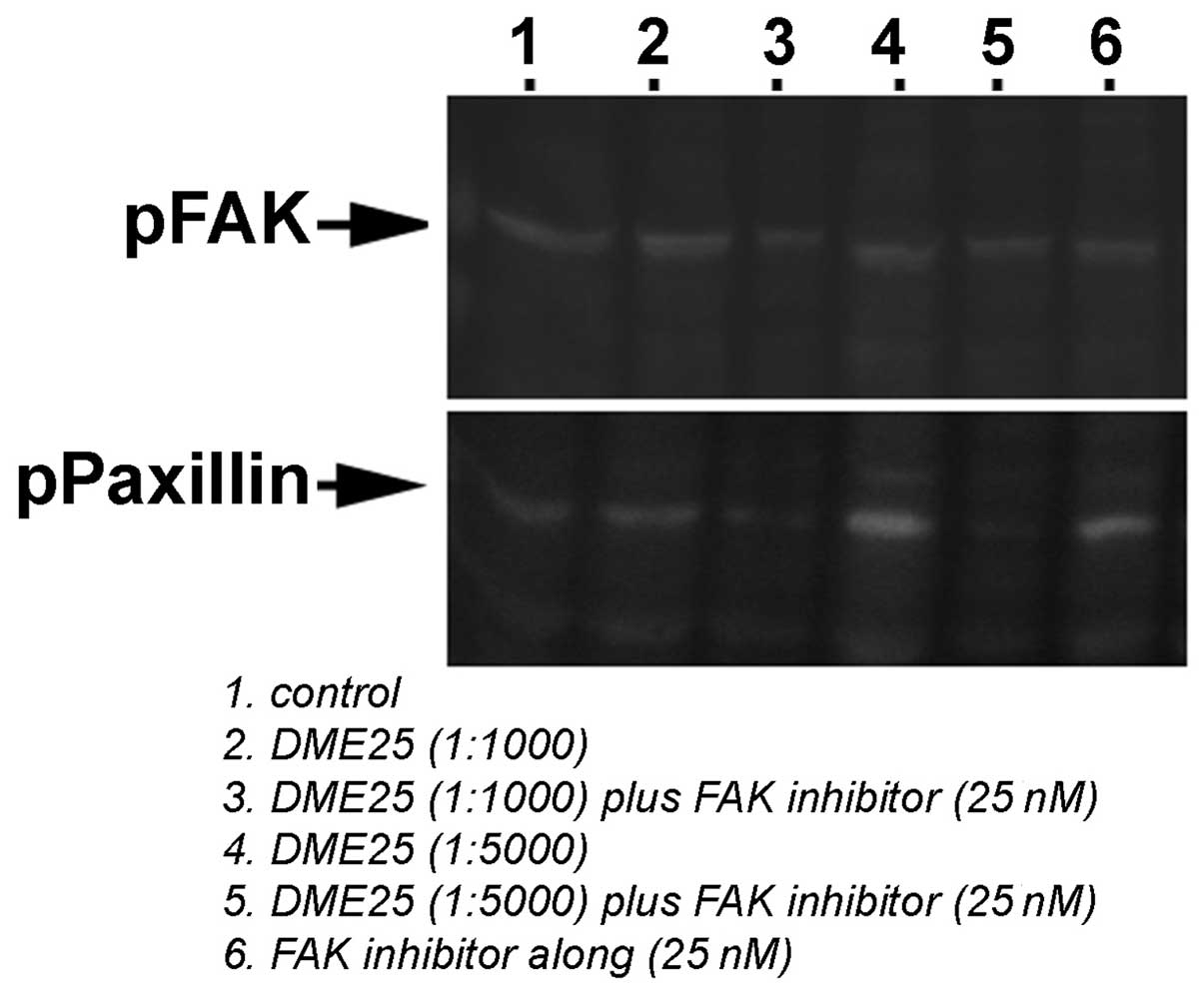
DME25 inhibited phosphorylation of FAK in
endothelial cells
We further evaluated the effect of DME25 on the
activation of FAK and paxillin in HECV cells, namely tyrosine
phophorylation in these proteins, using phospho-tyrosine specific
antibodies. As shown in Fig. 7,
DME25 suppressed phosphorylation of FAK and produced more profound
inhibition together with FAK inhibitor. Neither DME25 nor FAK
inhibitor or their combinations had marked effect on the
phosphorylation of paxillin.
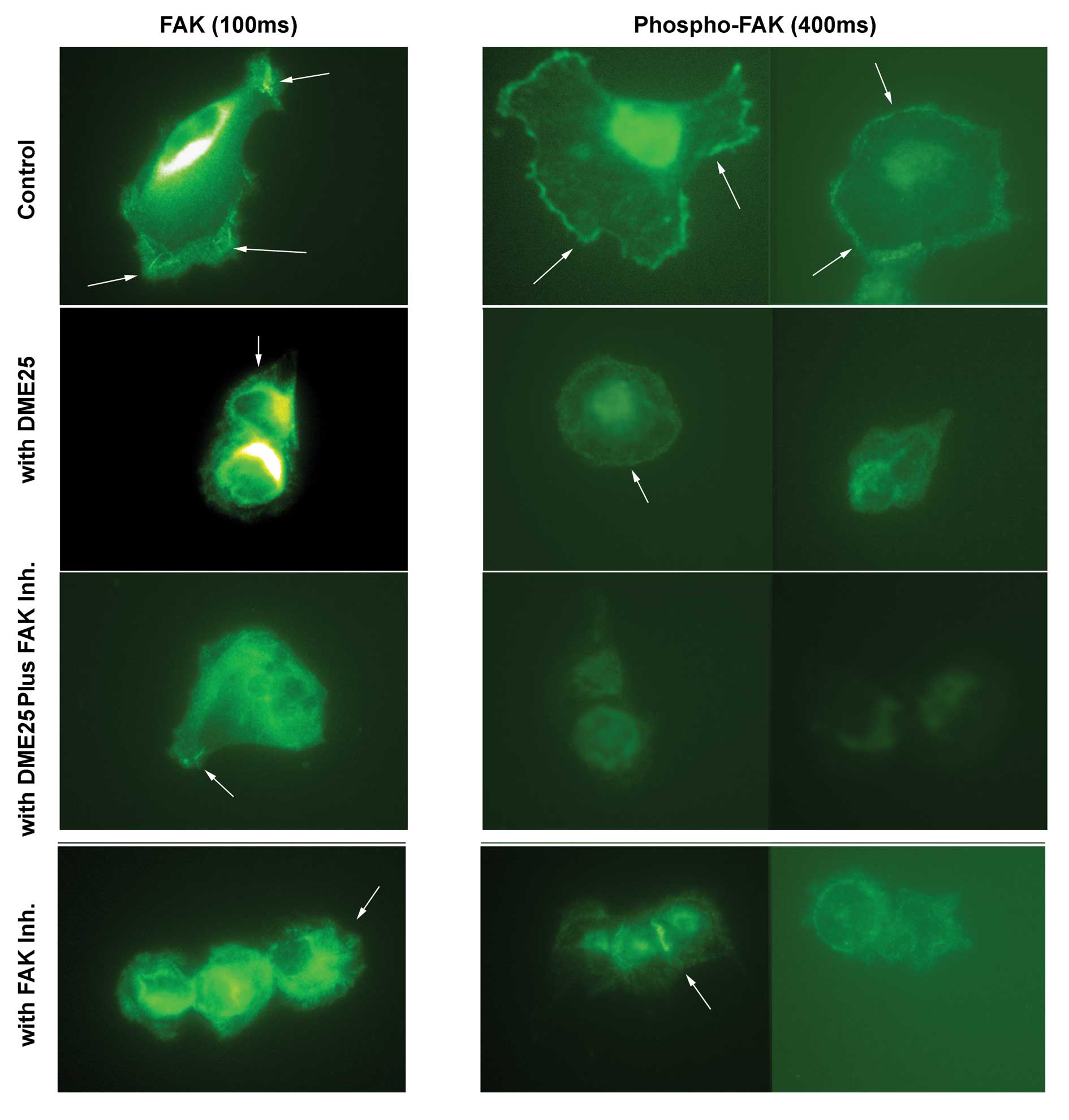
Using immunofluorescence method, FAK was seen to be
stained strongly in control cells at the focal adhesion sites
(Fig. 8, left panel, indicated by
arrows). Addition of DME25, FAK inhibitor and the combination of
DMA25 and FAK inhibitor render the cells with less focal adhesion
complex although the degree of staining was unchanged compared with
control (Fig. 8, left panel). It
is very interesting to observe the marked changes of phosphorylated
FAK which was stained with phosphorylation specific anti-pFAK
antibody. As shown in Fig. 8
(right panel, with extended exposure time), control cells had
visible stainings of pFAK at the focal adhesion sites in control
cells. Both DME25 and FAK inhibitor resulted in reduction of
staining of pFAK. However, cells treated with the combination of
DME25 and FAK inhibitor almost completely lost staining of pFAK
(Fig. 8 right panel).
Discussion
Anti-angiogenesis therapies, for example Avastin,
have now been used as new line of therapies in solid tumours and
have been shown to have their clinical worthiness in some tumour
types. A few of the traditional anti-cancer compounds have also
been found to have their role in anti-angiogenesis. Yangzheng
Xiaoji is a new formula developed from traditional Chinese
medicine and has been shown to have clinical benefit in patients
with cancers, namely liver cancer and gastric cancer, two of the
leading cancer types in China (10,11).
The precise mechanism(s) of the formula is not clear, although
there has been indication that it may have some immune protective
effects when administered during chemotherapy. However, in a recent
preliminary study (17),
Yangzheng Xiaoji has been shown to have an inhibitory effect
on the adhesion and migration of cancer cells. These two cell
functions are also critical during the angiogenic process of
endothelial cells.
The present study attempted to examine the potential
effect of Yangzheng Xiaoji on angiogenesis. Our initial
screening met with a surprising finding that extract from
Yangzheng Xiaoji, DME25 markedly inhibited in vitro
tubule formation from vascular endothelial cells. We further
demonstrated that the extract has a concentration-dependent
inhibitory effect on cell-matrix adhesion and cellular migration.
These results are interesting and appear to be connected.
Cell-matrix adhesion is an important part during cellular migration
and both the adhesion and migration are essential during
angiogenesis and in particular during tubule formation in the model
of the present study, namely sandwich based tubule formation assay.
Orchestrated adhesion to matrix and migration over matrix are
necessary for the endothelial cells to join and form vessel-like
tubules. Thus, it is plausible to suggest that the effect on
adhesion and migration is likely to be the key contributing factor
to the inhibition on tubule formation.
The other interesting finding of the present study
is that blocking FAK using a small FAK inhibitor markedly
strengthened the effect of Yangzheng Xiaoji extract and that
the extract itself has an inhibitory effect on the activation of
FAK, namely tyrosine phosphorylation of FAK, which was seen by both
western blotting and immunofluorescence methods. FAK pathway is
essential during cell-matrix adhesion and cellular adhesion over
extracellular matrix (27–30).
Upon interacting with matrix, cells utilise the membrane integrins
to bind to the matrix and trigger the activation of series
intracellular events, one of the key pathway is the activation of
focal adhesion kinase, which in turn leads to activating the
integrin interaction with the cytoskeletal system (31). This forms an essential component
during matrix adhesion and subsequent cell migration. FAK has been
shown to be amongst key signalling pathways during angiogenesis
(31–35). FAK inhibitors, such as the one used
in the present study, has been shown in early clinical trials to
have anti-cancer effects in patients with lung cancer and breast
cancer (36–40). Extract from herbs has been
previously shown to affect the activities of FAK in endothelial
cells (41,42). Together, it can be argued that one
of the key pathways that Yangzheng Xiaoji targets is FAK
pathway during the angiogenic process. However, these results
should be interpreted with caution for the following reasons.
First, Yangzheng Xiaoji is a mixture of herbal medicine. The
active ingredient(s) in the formula is yet to be found. The effect
seen in the present study may well be a mixed effect of the
extract. Second, angiogenesis requires a great deal more
coordination of endothelial cells, than cell adhesion and cellular
migration. Effects on other cellular events should also be
examined.
In conclusion, the anti-cancer traditional formula,
Yangzheng Xiaoji, has a profound effect on angiogenesis,
in vitro. This is seen together with the reduction of
cell-matrix adhesion and cellular migration and is likely to be
mediated by the focal adhesion kinase (FAK) pathway.
Acknowledgements
We wish to thank the Albert Hung
Foundation and Cancer Research Wales for supporting the study.
References
|
1.
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Fidler IJ: Critical determinants of cancer
metastasis: rationale for therapy. Cancer Chemother Pharmacol.
(Suppl 43): S3–S10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.
|
|
4.
|
Folkman J: Fighting cancer by attacking
its blood supply. Sci Am. 275:150–151. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bicknell R and Harris AL: Mechanisms and
therapeutic implications of angiogenesis. Curr Opin Oncol. 8:60–65.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
O’Reilly MS, Homgren L, Shing Y, Chen C,
Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH and Folkman J:
Angiostatin: a novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994.PubMed/NCBI
|
|
7.
|
Harris AL: Clinical trials of
anti-vascular agent group B streptococcus toxin (CM101).
Angiogenesis. 1:36–37. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yoshida T, Kaneko Y, Tsukamoto A, Han K,
Ichinose M and Kimura S: Suppression of hepatoma growth and
angiogenesis by a fumagillin derivative TNP470: possible
involvement of nitric oxide synthase. Cancer Res. 58:3751–3756.
1998.PubMed/NCBI
|
|
9.
|
Gregory RE and DeLisa AF: Paclitaxel: a
new antineoplastic agent for refractory ovarian cancer. Clin Pharm.
12:401–415. 1993.PubMed/NCBI
|
|
10.
|
Zhang SY, Gu CH, Gao XD and Wu YL: A
random, double-blinded and multicentre study of chemotherapy
assisted Yangzhengxiaoji capsule on treating primary hepatic
carcinoma. Chin J Diffic Compl Case. 8:461–464. 2009.
|
|
11.
|
Wang QL, Xuo CM, Wu XP, Li YX and Bi XJ:
Treatment of atypical gastric dysplasia using Yangzheng Xiaoji.
Chin J Diffic Compl Case. 7:38–39. 2009.
|
|
12.
|
Tran HT, Blumenschein GR Jr, Lu C, Meyers
CA, Papadimitrakopoulou V, Fossella FV, Zinner R, Madden T, Smythe
LG, Puduvalli VK, Munden R, Truong M and Herbst RS: Clinical and
pharmacokinetic study of TNP-470, an angiogenesis inhibitor, in
combination with paclitaxel and carboplatin in patients with solid
tumors. Cancer Chemother Pharmacol. 54:308–314. 2004.PubMed/NCBI
|
|
13.
|
Naganuma Y, Choijamts B, Shirota K,
Nakajima K, Ogata S, Miyamoto S, Kawarabayashi T and Emoto M:
Metronomic doxifluridine chemotherapy combined with the
anti-angiogenic agent TNP-470 inhibits the growth of human uterine
carcinosarcoma xenografts. Cancer Sci. 102:1545–1552. 2011.
View Article : Google Scholar
|
|
14.
|
Van Wijngaarden J, Snoeks TJ, van Beek E,
Bloys H, Kaijzel EL, van Hinsbergh VW and Löwik CW: An in vitro
model that can distinguish between effects on angiogenesis and on
established vasculature: actions of TNP-470, marimastat and the
tubulin-binding agent Ang-510. Biochem Biophys Res Commun.
391:1161–1165. 2010.PubMed/NCBI
|
|
15.
|
Woerdenbag HJ, Moskal TA, Pras N, Malingré
TM, el-Feraly FS, Kampinga HH and Konings AW: Cytotoxicity of
artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J
Nat Prod. 56:849–856. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Liu WM, Gravett AM and Dalgleish AG: The
antimalarial agent artesunate possesses anticancer properties that
can be enhanced by combination strategies. Int J Cancer.
128:1471–1480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ye Li, Ji K, Ji JF and Jiang WG:
Application of electric cell-substrate impedance sensing in
evaluation of traditional medicine on the cellular functions of
gastric and colorecctal cancer cells. Cancer Metastasis Biol Treat.
17:2012. View Article : Google Scholar
|
|
18.
|
Jiang WG, Hiscox S, Hallett MB, Horrobin
DF, Scott C and Puntis MCA: Inhibition of invasion and motility of
human colon cancer cells by gamma linolenic acid. Br J Cancer.
71:744–752. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Giaever I and Keese CR: Micromotion of
mammalian cells measured electrically. Proc Natl Acad Sci USA.
88:7896–7900. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Keese CR, Wegener J, Walker SR and Giaever
I: Electrical wound-healing assay for cells in vitro. Proc Natl
Acad Sci USA. 101:1554–1559. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Jiang WG, Ablin RJ, Kynaston HG and Mason
MD: The prostate transglutaminase (TGase-4, TGaseP) regulates the
interaction of prostate cancer and vascular endothelial cells, a
potential role for the ROCK pathway. Microvasc Res. 77:150–157.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Jiang WG, Martin TA, Lewis-Russell JM,
Douglas-Jones A, Ye L and Mansel RE: Eplin-alpha expression in
human breast cancer, the impact on cellular migration and clinical
outcome. Mol Cancer. 7:712008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Jiang WG, Hiscox SE, Parr C, Martin TA,
Matsumoto K, Nakamura T and Mansel RE: Antagonistic effect of NK4,
a novel hepatocyte growth factor variant, on in vitro angiogenesis
of human vascular endothelial cells. Clin Cancer Res. 5:3695–3703.
1999.PubMed/NCBI
|
|
24.
|
Sanders AJ, Ye L, Mason MD and Jiang WG:
The impact of EPLINα (epithelial protein lost in neoplasm) on
endothelial cells, angiogenesis and tumorigenesis. Angiogenesis.
13:317–326. 2010.
|
|
25.
|
Ye L, Martin TA, Parr C, Harrison GM,
Mansel RE and Jiang WG: Biphasic effects of 17-beta-estradiol on
expression of occludin and transendothelial resistance and
paracellular permeability in human vascular endothelial cells. J
Cell Physiol. 196:362–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Sanders AJ, Parr C, Martin TA, Lane J,
Mason MD and Jiang WG: Genetic upregulation of matriptase-2 reduces
the aggressiveness of prostate cancer cells in vitro and in vivo
and affects FAK and paxillin localisation. J Cell Physiol.
216:780–789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Matsuda S, Fujita T, Kajiya M, Takeda K,
Shiba H, Kawaguchi H and Kurihara H: Brain-derived neurotrophic
factor induces migration of endothelial cells through a
TrkB-ERK-integrin αVβ3-FAK cascade. J Cell Physiol. 227:2123–2129.
2012.PubMed/NCBI
|
|
28.
|
Gilmore AP and Romer LH: Inhibition of
focal adhesion kinase (FAK) signaling in focal adhesions decreases
cell motility and proliferation. Mol Biol Cell. 7:1209–1224. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Cai J, Parr C, Watkins G, Jiang WG and
Boulton M: Decreased pigment epithelium-derived factor expression
in human breast cancer progression. Clin Cancer Res. 12:3510–3517.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Braren R, Hu H, Kim YH, Beggs HE,
Reichardt LF and Wang R: Endothelial FAK is essential for vascular
network stability, cell survival, and lamellipodial formation. J
Cell Biol. 172:151–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Tavora B, Batista S, Reynolds LE, Jadeja
S, Robinson S, Kostourou V, Hart I, Fruttiger M, Parsons M and
Hodivala-Dilke KM: Endothelial FAK is required for tumour
angiogenesis. EMBO Mol Med. 2:516–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Peng X, Ueda H, Zhou H, Stokol T, Shen TL,
Alcaraz A, Nagy T, Vassalli JD and Guan JL: Overexpression of focal
adhesion kinase in vascular endothelial cells promotes angiogenesis
in transgenic mice. Cardiovasc Res. 64:421–430. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Li S, Butler P, Wang Y, Hu Y, Han DC,
Usami S, Guan JL and Chien S: The role of the dynamics of focal
adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl
Acad Sci USA. 99:3546–3551. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Lechertier T and Hodivala-Dilke K: Focal
adhesion kinase and tumour angiogenesis. J Pathol. 226:404–412.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Halder J, Lin YG, Merritt WM, Spannuth WA,
Nick AM, Honda T, Kamat AA, Han LY, Kim TJ, Lu C, Tari AM, Bornmann
W, Fernandez A, Lopez-Berestein G and Sood AK: Therapeutic efficacy
of a novel focal adhesion kinase inhibitor TAE226 in ovarian
carcinoma. Cancer Res. 67:10976–10983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Infante JR, Camidge DR, Mileshkin LR, Chen
EX, Hicks RJ, Rischin D, Fingert H, Pierce KJ, Xu H, Roberts WG,
Shreeve SM, Burris HA and Siu LL: Safety, pharmacokinetic, and
pharmacodynamic phase I dose-escalation trial of PF-00562271, an
inhibitor of focal adhesion kinase, in advanced solid tumors. J
Clin Oncol. 30:1527–1533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Stokes JB, Adair SJ, Slack-Davis JK,
Walters DM, Tilghman RW, Hershey ED, Lowrey B, Thomas KS, Bouton
AH, Hwang RF, Stelow EB, Parsons JT and Bauer TW: Inhibition of
focal adhesion kinase by PF-562,271 inhibits the growth and
metastasis of pancreatic cancer concomitant with altering the tumor
microenvironment. Mol Cancer Ther. 10:2135–2145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Cabrita MA, Jones LM, Quizi JL, Sabourin
LA, McKay BC and Addison C: Focal adhesion kinase inhibitors are
potent anti-angiogenic agents. Mol Oncol. 5:517–526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Chen JY, Tang YA, Huang SM, Juan HF, Wu
LW, Sun YC, Wang SC, Wu KW, Balraj G, Chang TT, Li WS, Cheng HC and
Wang YC: A novel sialyltransferase inhibitor suppresses
FAK/paxillin signaling and cancer angiogenesis and metastasis
pathways. Cancer Res. 71:473–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Jeon J, Lee J, Kim C, An Y and Choi C:
Aqueous extract of the medicinal plant Patrinia villosa Juss.
induces angiogenesis via activation of focal adhesion kinase.
Microvasc Res. 80:303–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Chung BH, Cho YL, Kim JD, Jo HS, Won MH,
Lee H, Ha KS, Kwon YG and Kim YM: Promotion of direct angiogenesis
in vitro and in vivo by Puerariae flos extract via activation of
MEK/ERK-, PI3K/Akt/eNOS-, and Src/FAK-dependent pathways. Phytother
Res. 24:934–940. 2010.PubMed/NCBI
|