Contents
Introduction
IL-6/JAK/STAT3 pathway
Roles of IL-6/JAK/STAT3 pathway in CRC
Modulation of IL-6/JAK/STAT3 pathway in CRC
Further perspectives
Conclusions
Introduction
Janus kinase (JAK)/signal transducer and activator
of transcription 3 (STAT3) signaling pathway is involved in various
physiological processes, including immune function, cell growth,
differentiation and hematopoiesis (1). Accumulating evidence indicates that
abnormalities in the JAK/STAT3 pathway play a vital role in the
oncogenesis of several cancers. It was reported (2) that constitutive activation of JAK2
was found in childhood T cell acute lymphoblastic leukemia.
Constitutive activation of STAT3 is linked to cell proliferation in
breast carcinoma (3) and non-small
cell lung cancer (4), and also
inhibits apoptosis (5). Studies
have also revealed that oncogenesis can be altered by STAT3
activation (1). These published
reports all demonstrate the crucial importance of the JAK/STAT3
pathway in tumorigenesis and progression.
Colorectal cancer (CRC) is the third most common
cancer worldwide and it is reported that ∼530,000 patients die of
the disease each year (6).
Although much progress has been made in treatment, outcomes remain
poor as approximately half of patients receiving treatment still
die of the disease (7,8). Some studies have indicated that
elevated interleukin-6 (IL-6)/JAK/STAT3 signaling is one of the key
pathways involving in colorectum tumorigenesis, this signaling has
a critical role in various aspects including initiation,
development and formation in CRC. The role of IL-6 in tumorigenesis
has been well-established in CRC. Increased production of IL-6 has
been reported in tumor tissue itself and in the serum of patients
with CRC (9). Recent studies show
that cytokine-driven JAK/STAT3 pathways play an important role in
the processes of signal transduction, which are associated with the
hyperproliferative and invasive phenotype of CRC cells (10). Although our knowledge of
oncogenesis, protooncogene identification and tumor suppressor
genes involved in the tumorigenesis of CRC are growing, the
biologic and molecular mechanisms in CRC are still poorly
understood. Moreover, the molecular mechanisms that control CRC
progression are related to the alteration of different
proto-oncogenes, cytokines, tumor suppressor genes and their
receptors (11). Notably, these
abnormalities are involved in the JAK/STAT3 signal transduction
pathway.
In this review, we summarize the mechanisms and
roles of IL-6/JAK/STAT3 pathway in CRC and describe current
therapeutic strategies to treat CRC by targeting the IL-6/JAK/STAT3
pathway. Importantly, we discuss how to use current knowledge to
find potential therapeutic approaches.
IL-6/JAK/STAT3 pathway
Molecular cloning has identified two different forms
of cellular receptors of IL-6: an 80-kDa ligand-binding chain,
known as IL-6R (IL-6Ra, CD126) and a 130-kDa signal-transducing
chain, gp130 (IL-6Rb, CD130). In contrast to the ubiquitous
expression of gp130, IL-6R shows a highly limited expression
pattern and is mainly confined to hepatocytes, leukocyte subsets
and megakaryocytes (12). First,
IL-6 binds to the IL-6R on target cells, then the complex of IL-6
and IL-6R contacts the gp130, thereby boosting its dimerization and
the subsequent activation of intracellular signaling such as STAT3
phosphorylation by JAK. This so-called classical signaling pathway
is activated during early immune responses and in turn activates
the expression of various acute-phase proteins such as C-reactive
protein (13). We believe that
classical IL-6R signaling coordinates homeostatic properties of
IL-6, which may act as a cytokine with hormone-like
characteristics. In addition, a soluble type of the IL-6 receptor
(sIL-6R), which is produced by limited proteolysis by A disintegrin
and A metalloproteinase 10 (ADAM10) or ADAM17 of the membrane-bound
IL-6R and translation from an alternatively spliced mRNA, can still
bind to IL-6 and the complex of IL-6 and sIL-6R interplays with
gp130. This so-called IL-6 trans-signaling represents an
alternative to classical IL-6 signaling and allows IL-6 to regulate
a broad spectrum of target cells including neutrophils,
macrophages, epithelial cells and T cells (14). In our opinion, IL-6 trans-signaling
acts as a danger signal to enhance IL-6 responsiveness and drive
inflammatory events.
The signal transduction of IL-6 involves the
activation of JAK, then leads to the activation of transcription
factor STAT3 (15). Over 40
different cytokines or growth factors can activate STAT signaling
pathway. Normally STAT3 resides in the cytoplasm. STAT3 will be
phosphorylated and then forms dimers with other members of the STAT
family when activated by upstream signaling pathways such as JAK,
epidermal growth factor receptor and IL-6R activation. The
activated STAT3 complex will then translocate from the cytoplasm to
the nucleus initiating transcription of STAT3 target genes
including cyclin D1, Bcl-xL, c-myc, Mcl1 and vascular endothelial
growth factor (VEGF) by combining with consensus DNA elements
(16). STAT3 is known to play an
important role in promoting tumorigenesis of diverse human cancers
(17), since it is regarded as an
oncogenic transcriptional factor involving in cancer cell
proliferation, differentiation, invasion, inflammation and immune
function (Fig. 1).
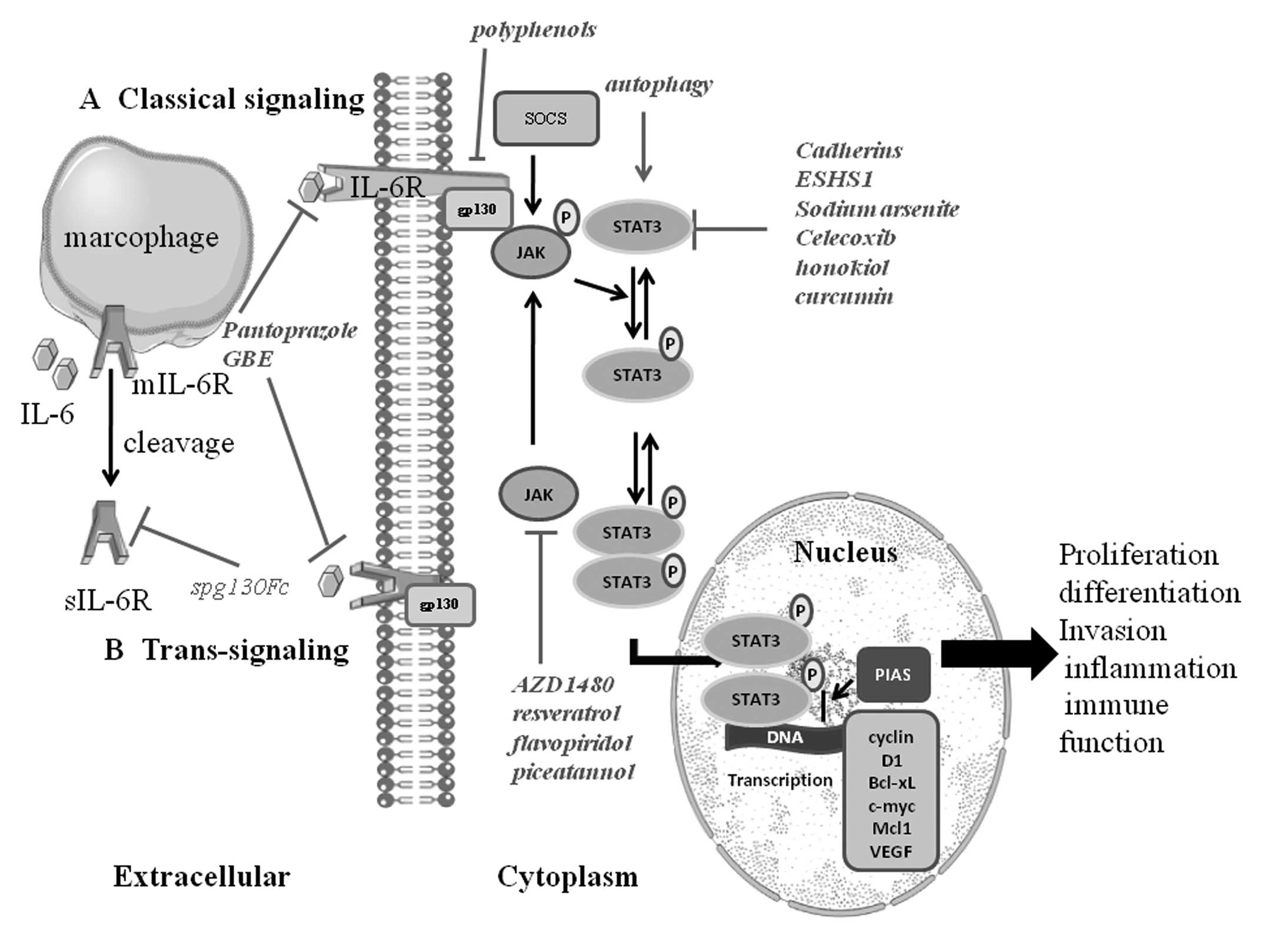 | Figure 1.IL-6/JAK/STAT3 signaling pathway.
IL-6 activation has two modes of: (A) classical signaling pathway:
IL-6 binds to the IL-6R on target cells. Then the complex of IL-6
and IL-6R contacts the gp130; (B) trans-signaling pathway: a
soluble type of the IL-6 receptor (sIL-6R) binds to IL-6 and
interplays with gp130. Thereby both of them induce dimerization and
initiates signaling. The signal transduction of IL-6 involves the
activation of JAK, then leading to the activation of transcription
factors of the signal transducers and activators of STAT3. Normally
STAT3 resides in the cytoplasm. STAT3 is phosphorylated and then
forms dimers with other members of the STAT family when activated
by upstream signaling pathways such as JAK, EGFR, IL-6 receptor
activation. The activated STAT3 complex will then translocate from
the cytoplasm to the nucleus initiating transcription of STAT3
target genes (including cyclin D1, Bcl-xL, c-myc, Mcl1, survivin
and VEGF) by combining with consensus DNA elements. STAT3 is
regarded as an oncogenic transcriptional factor involved in cancer
cell proliferation, differentiation, invasion, inflammation and
immune function. Many factors can influence the range and duration
of STAT activation. The protein inhibitors of activated STATs
(PIAS) family of proteins are negative regulators of STAT-mediated
gene transcription. In addition, the suppressors of cytokine
signaling (SOCS) protein family affects the JAKs, and thus restrain
the phosphorylation of gp130, STATs and the JAKs themselves. |
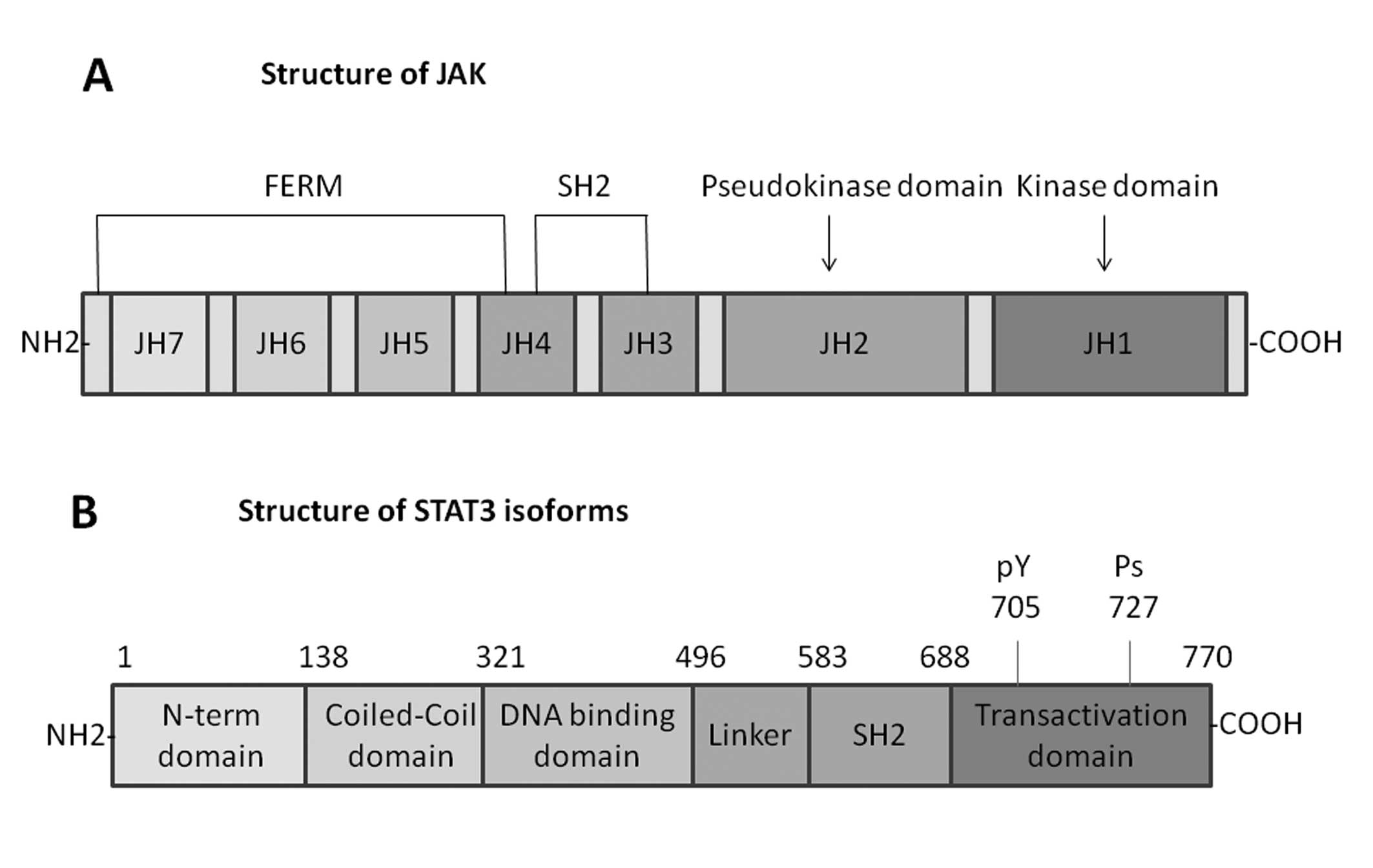
JAK family proteins
The JAK family proteins of cytoplasmic tyrosine
kinases include four mammalian members, which are related to the
cytoplasmic regions of signal transducing cytokine receptors. Three
of them, JAK1, JAK2 and TYK2, are expressed in various tissues, yet
JAK3 is expressed only in cells of the hematopoietic system
(18). The unique structure of the
JAKs easily differentiates them from other members of the protein
tyrosine kinase family. The most attractive feature of these
proteins is the presence of two JAK homology (JH) domains, JH1 and
JH2, which have extensive homology to tyrosine kinase domains. JH1
domain at the C-terminus appears to be a functional tyrosine kinase
domain, while the JH2 domain does not possess substantial tyrosine
kinase activity (19). The JH3-JH7
regions, which form the N-terminal half of the JAKs, are related to
binding to cytokine receptors. A portion of the N-terminal region
of JAKs has similar sequence with the so-called four-point-one,
ezrin, radixin and moesin (FERM) domains, and JAKs have been proved
to shield a divergent type of FERM domain. The SH2 domain also
contains JH3 and JH4 domains. The selectivity of STAT activation by
various ligands mainly depends on the highly specific interactions
between the SH2 domain and the phosphotyrosine residues on each
receptor (20) (Fig. 2).
STAT family proteins
STAT family proteins have identified seven mammalian
members: STAT1, STAT2, STAT3, STAT4, STAT5 (STAT5α and STAT5β), and
STAT6. Every STAT protein has several conserved domains including
the N-terminal coiled-coil domain, DNA binding domain, a linker,
Src homology 2 (SH2) domain, and a C-terminal transactivation
domain, which are closely related to its functions (21). Several studies have indicated that
the N-terminal domain promotes dimerized STAT molecules to
polymerize and bind multiple DNA sites that are related to
oncogenic growth signaling pathways (22). The DNA-binding domain is the
central region of each STAT protein (except for STAT2) and
regulates the DNA-binding specificity for each STAT protein
(23). A linker domain, region of
500–575, is α-helical before the classical SH2 domain. The
conserved SH2 domain exists in the region between 600 and 700 amino
acid residues and it is crucial to form dimers between two
activated STAT monomers via reciprocal phospho-tyrosine-SH2-domain
interactions (20). The C-terminal
transactivation domain is associated with transcriptional
complexes, and builds structures when binding with interacting
partners. The tyrosine residue of position 705 in this STAT domain
plays an important role in STAT activation. Additionally, at
position 727 (except STAT2 and STAT6), a conserved serine, is a
phosphorylation site and regulates STAT transcriptional activity
(24) (Fig. 2).
Roles of IL-6/JAK/STAT3 pathway in CRC
IL-6 in CRC
IL-6, a pleiotropic inflammatory cytokine, not only
has central roles in immune and inflammatory response, but also is
regarded as a key growth factor for malignancy (25). Cancer cells utilize the autocrine
production of growth and survival factors to upregulate growth and
survival pathways. The increased expression of IL-6 in cancer cells
manifests that IL-6 is an important autocrine growth factor
promoting tumorigenesis (26).
Tissue or infection will cause more secretion of IL-6. Thereby,
IL-6 participates in the regulation of the acute phase response,
the differentiation of monocytes to macrophages, the proliferation
and apoptotic resistance of T cells and Th2 cytokine production
(27). The IL-6 signaling pathway
is considered as one of the most important ways linking
inflammation to cancer (28).
The role of IL-6 in tumorigenesis has been
well-established in a wide range of human cancers including
lymphoma, glioma, melanoma, breast, ovarian, prostate, renal,
pancreatic cancer, as well as CRC. An increasing number of studies
have found that IL-6 levels elevate in tumor tissue itself and in
the serum of patients with CRC (9). Additionally, a review survey by
Knüpfer and Preiss shows that IL-6 expression is closely related to
tumor stage, size, metastasis and survival of patients with CRC
(29). IL-6/sIL-6R and
inflammation function in the pathogenesis of CRC (30). IL-6 plays an important role in
recruitment of immune cells that produce pro-inflammatory
cytokines, and also in modulating Th17 and Treg cells in CRC
(31). Moreover, IL-6 was reported
to be localized at the sites of macrophage infiltration, suggesting
an interaction between IL-6 and immune cells in the tumor
microenvironment (32). Two
recognized IL-6 polymorphisms have been reported to associate with
a significantly reduced risk of CRC. In a study of 46 patients with
CRC, multivariate analysis showed that the blood
granulocyte/lymphocyte ratio and the serum IL-6 level were
independent risk factors for poor prognosis, indicating that both
factors may be significantly predictive for CRC cancer progression
(33).
The risk of developing precancerous lesions and
invasive carcinoma will increase exponentially when under the
duration of inflammation in inflammatory bowel disease (IBD)
patients such as Crohn’s disease (CD) and ulcerative colitis (UC),
and those who have not controlled inflammation will have higher
risk of development of CAC (27).
There is a 17.8% risk for CRC in patients with UC within 30 years
(34). The cumulative risk for the
development of CRC in patients with large bowel involvement of CD
is ∼8.3% over a period of 30 years (35). Similar to CRC, IL-6 expression is
increased in patients with IBD (36). Many studies have manifested that
IL-6 plays central roles in the pathogenesis of IBD, mainly due to
its effect on immune cell function (27). IL-6 trans-signaling has been proved
to activate T cells in the lamina propria of patients with IBD and
leads to resistance of these cells against apoptosis via
upregulation of anti-apoptotic factors such as Bcl-2 and Bcl-xL
(37). According to the
association of IL-6 expression with CRC prognosis and the increased
expression of IL-6 in patients with IBD, IL-6 is known as a
connection between chronic inflammation and tumor development. New
data revealed a direct effect of a functional relevance for IL-6
acting on tumor cells in sporadic CRC, which is likely mediated by
trans-signaling, as intestinal epithelial cells usually do not
express mIL-6R (38).
JAK/STAT3 in CRC
Similarly to many malignant tumors, the
hyperproliferative and invasive phenotype of CRC cells has showed
to be related to abnormalities on the level of signal transduction.
Cytokine-driven JAK/STAT3 pathways play crucial roles in these
processes (10). Generally, the
molecular mechanisms that regulate development of CRC are
associated with the expression of different proto-oncogenes, tumor
suppressor genes, cytokines, and their receptors, including Ras,
Src, p27kip1, p16ink4a, interleukin and
epidermal growth factor receptor. These alterations markedly refer
to the JAK/STAT3 signal transduction pathway (39). Though few studies of abnormal
expression of JAK/STAT3 have been shown in CRC, STAT3 activity is
constitutively upregulated in diverse human tumors including CRC
(11).
So far several studies have indicated that elevated
malignancy and invasive behaviour of CRC cells are closely
associated with STAT3 activity (40). STAT3 is originally characterized as
a mediator of IL-6 receptor signaling (41) with extremely widespread functions
throughout the organism (reviewed in ref. 42) and it is the only embryonic lethal
knockout within the STAT family (43). Not only cytokine and growth factor
receptors, but several viral or cellular oncogenes such as src,
fps, polyoma virus middle T-antigen, and sis are also known to
activate STAT3 (reviewed in ref. 44). A constitutively active artificial
variant of STAT3 generated by forced dimerization was shown to
behave as an oncoprotein to induce tumorigenesis in nude mice
(45). STAT3 activity is thought
linked to elevated malignancy and invasive behaviour of CRC cells.
STAT3 activation can increase the expression of matrix
metalloproteinase (MMP), which also potentially promote CRC cells
invasion and metastasis via proteolytic degradation of the
extracellular matrix (46). The
first evidence has be provided that Aloin may inhibit tumor
angiogenesis and growth by inhibiting STAT3 activation (47). Also recently it has been
demonstrated that STAT3 signaling is important for the development
of CRC and promotes angiogenesis by regulating VEGF-A and MMP2
expression (48). However, some
research showed that STAT3 activity may also have negative effects
on the development of colon cancer. From ApcMin mouse
models, STAT3 was demonstrated to inhibit tumor cell invasiveness
and adenoma to carcinoma transition (49).
Modulation of IL-6/JAK/STAT3 pathway in
CRC
Although in recent years significant progress was
made in CRC treatment, CRC still remains the leading cause of
cancer related to death in the worldwide. Therefore, new
therapeutic methods, especially for patients with advanced disease,
are greatly required. Therapeutics targeting the IL-6/JAK/STAT3
pathway are hopeful strategies because more and more evidence shows
that IL-6/JAK/STAT3 pathway has a critical role in various aspects
including initiation, development and formation in CRC.
Regulation of IL-6
IL-6 is an important tumor promoting cytokine that
enforces proliferation and anti-apoptotic effects in tumor cells.
Clinical and experimental data strongly propose a contribution of
IL-6 signaling to the development of both sporadic and
colitis-associated CRC development. In this regard, several
components of the IL-6 signaling pathway such as IL-6R have been
proposed as promising targets for CRC therapy.
Several studies have reported that IL-6 and STAT3
play an important role in the survival of intestinal epithelial
cells and development of inflammation-associated cancer, anti-IL-6R
antibody (anti-IL-6RAb) has been reported as an inhibitor to
suppress CRC development (50).
Subsequently, IL-6 ligand-blocking antibody was produced to express
functions in antitumor and anti-inflammatory activities. CNTO-328,
a human-mouse chimeric antibody, was generated from a murine anti
IL-6 monoclonal antibody (McAb). CNTO-328 has a long half-life (∼2
weeks) without remarkable immunogenicity and it also has a strong
affinity with recombinant as well as native IL-6 (51). It is capable of blocking the signal
transduction pathway of IL-6/IL-6R/gp130 by inhibiting the binding
of IL-6 to the IL-6R, ultimately achieving antitumor and
anti-inflammatory effects (52).
In addition, the humanized anti-IL-6R McAb of IgG1 class, named
anti-IL-6RAb (tocilizumab), was produced by grafting the
complementarity-determining regions of a mouse antihuman IL-6R
antibody onto human IgG1. The mechanism of anti-IL-6RAb is to
control IL-6-mediated signal transduction by inhibiting the process
of IL-6 binding to membrane-bound IL-6R and sIL-6R (53). Tocilizumab was reported to treat
Castelman’s disease and rheumatoid arthritis and may be used in
cancers (54). Recently, it was
shown that when expression of IL-6 and IL-1β decreases, the
IL-6/STAT3 signaling pathway also weakens in tumors in oroxylin
A-treated mice. Through this mechanism it was demonstrated that
oroxylin A inhibits colitis-associated carcinogenesis in an
azoxymethane/dextran sodium sulfate mouse model and in the colon
cancer cell line HCT-116 (55)
(Table I).
 | Table I.Current therapies targeting the
IL-6/JAK/STAT3 pathway in CRC. |
Table I.
Current therapies targeting the
IL-6/JAK/STAT3 pathway in CRC.
| Strategy | Compound | Disease | Phase |
|---|
| IL-6
inhibition | CNTO328 | Solid cancer
including CRC | Phase I + II |
| Oroxylin A | Experimental model
of cancer | Preclinical |
| IL-6R inhibition
and SIL-6R inhibition | Anti-IL-6R antibody
(tocilizumab) | Experimental model
of cancer | Preclinical |
| Stat3
inhibition | Pien Tze
Huang, ethanol extract of Hedyotis diffusa Willd,
Spica Prunellae | CRC | Clinical |
| JAK/STAT signaling
inhibition | Trichostatin A,
bufalin | Experimental
CRC | Preclinical |
The above treatments work in both classical and
trans-signaling, and therefore also block physiological functions
of IL-6. In contrast, modulation of sgp130Fc is a specific
inhibition of trans-signaling. Sgp130Fc is a designer cytokine that
specifically binds IL-6/sIL-6R complexes, and therefore it only
blocks trans-signaling. It has been shown to be effective for the
treatment of experimental CAC with sgp130Fc in the study by Becker
et al (56). The substance
will soon enter clinical development and it will be interesting to
evaluate its effect on human cancer (57).
Regulation of JAK/STAT3
JAK/STAT3 has been shown in many aspects of CRC
development, including cell growth, survival, invasion and
migration (39,58). Therefore, inhibiting JAK/STAT3 is a
valuable regulative strategy for cancers.
The suppressor of cytokine signaling (SOCS) proteins
family comprise of eight members: SOCS 1-7, and cytokineinducible
SH2-containing (CIS)-1 (with similar structure to the other SOCS
proteins). So far research has mainly focused on CIS and SOCS1-3,
which have been found to serve as negative regulators of the
JAK/STAT signaling pathway. However, the various family members
seem to have different specific mechanism. CIS, first defined
member, was proved to interact with STAT5 and then regulate the
JAK2/STAT5 pathway. SOCS1 and SOCS3 are the most effective in
inhibiting IL-6-mediated signaling pathways. Evidence has shown
that both of above members affect the JAKs, thus restraining the
phosphorylation of gp130, STATs and the JAKs themselves (59). Some studies reported that SOCS3
expression was decreased in a variety of inflammation-associated
human cancers and cancer cell lines, which was correlated with
strong STAT3 activity in these cells (60). New data demonstrate that sodium
butyrate inhibits JAK2/STAT signaling through upregulation of SOCS1
and SOCS3, which are regulated by histone deacetylase 8 (61). Likewise, a study shows that
honokiol increases the activity and protein expression of
SH2-containing tyrosine phosphatase-1 further blocking the STAT3
pathway (62) (Fig. 2). It has been demonstrated that,
like in various other tumors with high STAT3 activity, DNA
methylation influences epigenetic regulation leading to the decline
in SOCS3 expression in CRC (32).
In addition, data exist suggesting that trichostatin A may increase
SOCS1 and SOCS3 expression by inducing histone modifications and
ultimately inhibit JAK2/STAT3 signaling in CRC cells (63) (Table
I).
The protein inhibitors of activated STATs (PIAS)
family of proteins are negative regulators of STAT-mediated gene
transcription. The family of PIAS consists of five mammalian
components: PIAS1, PIAS3, PIASxα, PIASxβ and PIASy. Since Gu/RNA
helicase II-binding protein (PIAS1) was recognized, it is widely
accepted that PIASs are transcriptional co-regulator proteins
important to the JAK/STAT pathway (64). Inhibitory molecules from PIAS can
interact with activated STATs, but they inhibit different STAT
proteins by distinct types. For instance, PIAS3 inhibits
STAT3-mediated gene expression (after IL-6 stimulation), whereas
PIAS1 blocks STAT1-dependent signaling and directly inhibits the
STAT-DNA complex activity with other transcriptional suppressive
co-operators (65). Recent studies
have shown that curcumin controls activation of PIAS-3 to restrain
JAK-STAT signaling, thus weakens STAT-3 phosphorylation and tumor
cell growth (66) (Fig. 1). Inhibitor of activated STAT3
protein-PIAS3 expression was also reduced in various cancers
including prostate, gastric, brain, and CRC (67).
Novel agents directly inhibiting STAT3 were designed
mainly to target the SH2 domain, which block either STAT3
phosphorylation or dimerization. These contain designed small
molecules and peptidomimetics. Studies from many preclinical cancer
models, many of these agents with high specificity to disrupt STAT3
function have been found to inhibit cancer growth (68). However, these agents have not been
applied in clinic (69).
Continuous unregulated STAT3 could increase cell proliferation and
reduce cell apoptosis, then leading to development of various
cancers including CRC (39,70).
Therefore, affecting the STAT3 pathway and its target gene
expression could balance cell apoptosis with proliferation, which
seems a promising strategy for the development of novel anticancer
therapies. Recent studies have showed that treatment with some
well-known traditional Chinese formulas such as Pien Tze
Huang (71), ethanol extract of
Hedyotis diffusa Willd (72), Spica Prunellae (73) would lead to the inhibi tion of
cancer cell proliferation and the promotion of apoptosis in CRC
mouse tumor tissues through suppression of STAT3 phosphorylation
activation (Table I).
Since the IL-6/JAK/STAT3 pathway is important to
CRC, research has been conducted to reveal its potential role in
treating CRC. Abrogation of galectin-4 expression promotes cancer
cell proliferation and the downregulation of galectin-4 elicits
tumor promotion in vitro and in vivo through
activation of IL-6/NF-κB/STAT3 signaling, so regulation of
galectin-4 may be a direction to treat CRC (74). Organo-Mg inhibits
inflammation-related mouse colon carcinogenesis by modulating the
proliferative activities and chromosomal instability of CRC and
suppressing colonic inflammation suggests potential use of
organo-Mg for clinical chemoprevention trials of CRC in the
inflamed colon (75). Leptin
influences the growth and proliferation of cancer cells via
activation of various growth and survival signaling pathways
including JAK/STAT, PI3-kinase/AKT, and/or MAP kinases, showing
promise as a molecule to treat CRC (76). Ganetespib, a potent heat shock
protein 90 (HSP90) inhibitor disrupts angiogenesis in CRC through
inhibition of HIF-1α and STAT-3 CRC cell lines (HCT116 and HT29),
and these results collectively suggest that inhibition of HSP90 is
a promising anti-angiogenic strategy in CRC (77). Recently studies have showed that
bufalin not only inhibits the growth of CRC SW620 cells, but also
induces apoptosis of SW620 cells through inhibition of JAK/STAT3
signaling pathway (78), thus,
more clinical studies are required to confirm the efficacy of
bufalin to treat human CRC.
Further perspectives
To date, many studies have recorded potential
therapies in IL-6/JAK/STAT3 pathway to treat many cancers. However,
limited attention has been paid to CRC, even though IL-6/JAK/STAT3
pathway plays a vital role in CRC. The studies in other diseases
have indicated the influence on relieving the pathology in
IL-6/JAK/STAT3 pathway, and it indicates a promising future in CRC,
while more studies need to be performed to verify the
hypothesis.
Some studies have demonstrated that modulating the
expression of IL-6 can prevent the development of other diseases.
Pantoprazole decreased the secretion of IL-6 and caused cell death
specifically in gastric cancer. Therefore, it may be a potential
substance to treat CRC, which shows similar mechanism of tumor
progression to gastric cancer (79). Diet-derived polyphenols suppressed
angiogenesis by regulating the expression of IL-6 signal
transducing receptor (IL-6Rα) and SOCS3 protein, which is also the
signaling pathway inhibiting development of CRC (80). Ginkgo biloba extract (GBE)
inhibited high-glucose-induced endothelial inflammation by
restraining redox-dependent IL-6 pathways, so GBE may be a
potential target to relieve intestinal inflammation, which relates
to inflammation-associated CRC (81).
Another method is to specifically inhibit JAK
activation, which involves the activation of transcription factor
STAT3. Some preclinical trials have utilized a number of natural
products such as resveratrol, flavopiridol and piceatannol to
inhibit pathways involved in inflammation, whose mechanisms include
inhibition of STAT3 phosphorylation, reduction of the cytokine
production and direct inhibition of the JAK (69). The role of JAK inhibition in solid
tumors was also tested preclinically. The JAK1/2 inhibitor AZD1480
suppressed tumor development in models of IL-6-driven breast,
ovarian, and prostate cancers (82). Currently, there is little clinical
data on the use of JAK inhibition in CRC. However, AZD1480 and
these natural products may be potential substances to treat CRC in
the future.
Regulating the activation of STAT3 can prevent the
progress of diseases. Three classical cadherins, E-cadherin,
N-cadherin and cadherin 11, can control survival via the
gp130/STAT3 pathway, thus we may control cadherins to inhibit the
progress of CRC through the STAT3 pathway (83). Enoyl-CoA hydratase short chain 1
(ESHS1) specifically inhibited STAT3 activity and decreased
expression of several target genes of STAT3 (84). Additionally, sodium arsenite
inhibited self-renewal and induced apoptosis in mouse embryonic
stem cells, which was enhanced also by suppressing the STAT3
pathways simultaneously (85).
Therefore, functions of ECHS1 and sodium arsenite may also come
true in intestinal cells, which prevent the development of CRC via
the STAT3 pathway. Celecoxib induced cell apoptosis and cell cycle
arrest on nasopharyngeal carcinoma, which was partly mediated by
the STAT3 pathway. So it may be used as the promising target to
induce intestinal cell apoptosis and treat CRC, but more studies
should be performed (86).
Advanced glycation end product-specific receptor-mediated autophagy
contributed to pancreatic tumorigenesis and bioenergetics via the
IL6-pSTAT3 pathway, so inhibiting the receptor could be a potential
therapeutic manner to control tumorigenesis of CRC and other solid
tumors (87) (Fig. 1 and Table II).
 | Table II.Potential therapies targeting of the
IL-6/JAK/STAT3 pathway in CRC. |
Table II.
Potential therapies targeting of the
IL-6/JAK/STAT3 pathway in CRC.
| Strategy | Compound | Indication |
|---|
| IL-6
inhibition | Pantoprazole | Gastric cancer |
| GBE | Endothelial
inflammation |
| IL-6R inhibition
and SIL-6R inhibition | Polyphenols | Suppress
angiogenesis |
| REGN-88 | Rheumatoid
arthritis |
| JAK inhibition | AZD1480 | Solid tumors |
| | Hematologic
malignancies |
| Resveratrol
flavopiridol piceatannol | Reduce cytokine
production |
| CEP-701 | |
| XL019 | Myelofibrosis |
| INCB018424 | Myeloproliferative
disorders |
| | Hematologic
malignancies |
| STAT3
inhibition | Cadherins | Control
survival |
| ESHS1 | |
| Sodium
arsenite | Induce
apoptosis |
| Celecoxib | Induce cell
apoptosis on nasopharyngeal carcinoma |
| Honokiol | |
| Curcumin | |
| STAT3 decoy | Head and neck
cancer |
Some studies show that combining with inhibition of
the IL-6/STAT3 signaling can enhance the effect of
chemoradiotherapy. Treatment together with IL-6 inhibition enhanced
the radiation response of prostate cancer (88). Ganoderic acid A, inhibition of the
JAK-STAT3 signaling pathway, increased chemosensitivity of HepG2
cells to cisplatin (89).
Therefore, the inhibition may be applied to treat CRC and other
solid tumors needing chemoradiotherapy.
Putoczki et al have revealed that the related
cytokine IL-11 might have a stronger correlation with elevated
STAT3 activation in human gastrointestinal cancers in genetic mouse
models (90). Therefore, targeting
the interference with IL-11 could be a potential therapeutic
strategy for the treatment of gastrointestinal cancers.
Conclusions
The continuous evidence of recent years makes us
convinced that IL-6/JAK/STAT3 pathway plays a crucial role in
colorectum tumorigenesis. The use of inhibitors of this signal
transduction pathway has provided critical information for a better
understanding of molecular mechanisms of pathology and developing
new therapeutic methods of CRC. Thus, strategies targeting the
IL-6/JAK/STAT3 pathway have emerged as attractive options to treat
CRC.
Many studies have showed the potential therapy of
IL-6/STAT3 signaling pathway to various diseases. However,
experimental research on the treatment of CRC in IL-6/JAK/STAT3
signaling pathway is limited, even if this signaling is one of the
key pathways involved in colorectum tumorigenesis. The studies in
other diseases have demonstrated the influence on relieving the
pathology in IL-6/JAK/STAT3 pathway, and it indicates a promising
future in CRC, while more research needs to be carried out to
confirm the hypothesis.
Abbreviations:
|
IL
|
interleukin;
|
|
IL-6R
|
IL-6 receptor;
|
|
JAK
|
Janus-activated kinase;
|
|
STAT3
|
signal transducer and activator of
transcription 3;
|
|
CRC
|
colorectal cancer;
|
|
ADAM
|
A dsintegrin and A
metalloproteinase;
|
|
VEGF
|
vascular endothelial growth
factor;
|
|
TGF-β
|
transforming growth factor-β;
|
|
Treg
|
regulatory T;
|
|
Th
|
T helper;
|
|
MDSCs
|
myeloid derived suppressor cells;
|
|
DCs
|
dendritic cells;
|
|
CAC
|
colitis-associated cancer;
|
|
IBD
|
inflammatory bowel disease;
|
|
CD
|
Crohn’s disease;
|
|
JH
|
JAK homology;
|
|
FERM
|
four-point-one, ezrin, radixin and
moesin;
|
|
SH2
|
Src homology 2;
|
|
MMP
|
matrix metalloproteinase;
|
|
McAb
|
monoclonal antibody;
|
|
SOCS
|
suppressor of cytokine signaling;
|
|
CIS
|
cytokine-inducible SH2-containing;
|
|
PIAS1
|
Gu/RNA helicase II-binding
protein;
|
|
GBE
|
Ginkgo biloba extract;
|
|
ESHS1
|
enoyl-CoA hydratase short chain 1
|
References
|
1.
|
Niwa Y, Kanda H, Shikauchi Y, et al:
Methylation silencing of SOCS-3 promotes cell growth and migration
by enhancing JAK/STAT and FAK signalings in human hepatocellular
carcinoma. Oncogene. 24:6406–6417. 2005.PubMed/NCBI
|
|
2.
|
Lacronique V, Boureux A, Valle VD, et al:
A TEL-JAK2 fusion protein with constitutive kinase activity in
human leukemia. Science. 278:1309–1312. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zhang F, Li C, Halfter H and Liu J:
Delineating an oncostatin M-activated STAT3 signaling pathway that
coordinates the expression of genes involved in cell cycle
regulation and extracellular matrix deposition of MCF-7 cells.
Oncogene. 22:894–905. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Alvarez JV, Greulich H, Sellers WR,
Meyerson M and Frank DA: Signal transducer and activator of
transcription 3 is required for the oncogenic effects of
non-small-cell lung cancer-associated mutations of the epidermal
growth factor receptor. Cancer Res. 66:3162–3168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zamo A, Chiarle R, Piva R, et al:
Anaplastic lymphoma kinase (ALK) activates Stat3 and protects
hematopoietic cells from cell death. Oncogene. 21:1038–1047. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Khong TL, Thairu N, Larsen H, Dawson PM,
Kiriakidis S and Paleolog EM: Identification of the angiogenic gene
signature induced by EGF and hypoxia in colorectal cancer. BMC
Cancer. 13:5182013.PubMed/NCBI
|
|
7.
|
Guthrie GJ, Roxburgh CS, Horgan PG and
McMillan DC: Does interleukin-6 link explain the link between
tumour necrosis, local and systemic inflammatory responses and
outcome in patients with colorectal cancer? Cancer Treat Rev.
39:89–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
9.
|
Chung YC and Chang YF: Serum interleukin-6
levels reflect the disease status of colorectal cancer. J Surg
Oncol. 83:222–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Gordziel C, Bratsch J, Moriggl R, Knosel T
and Friedrich K: Both STAT1 and STAT3 are favourable prognostic
determinants in colorectal carcinoma. Br J Cancer. 109:138–146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Dambacher J, Beigel F, Seiderer J, et al:
Interleukin 31 mediates MAP kinase and STAT1/3 activation in
intestinal epithelial cells and its expression is upregulated in
inflammatory bowel disease. Gut. 56:1257–1265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kishimoto T: IL-6: from its discovery to
clinical applications. Int Immunol. 22:347–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Rose-John S: Coordination of interleukin-6
biology by membrane bound and soluble receptors. Adv Exp Med Biol.
495:145–151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Culig Z: Cytokine disbalance in common
human cancers. Biochim Biophys Acta. 1813:308–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Muller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Aoki Y, Feldman GM and Tosato G:
Inhibition of STAT3 signaling induces apoptosis and decreases
survivin expression in primary effusion lymphoma. Blood.
101:1535–1542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Leeman RJ, Lui VW and Grandis JR: STAT3 as
a therapeutic target in head and neck cancer. Expert Opin Biol
Ther. 6:231–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Haan C, Kreis S, Margue C and Behrmann I:
Jaks and cytokine receptors - an intimate relationship. Biochem
Pharmacol. 72:1538–1546. 2006.PubMed/NCBI
|
|
19.
|
Rane SG and Reddy EP: JAKs, STATs and Src
kinases in hematopoiesis. Oncogene. 21:3334–3358. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Subramaniam A, Shanmugam MK, Perumal E, et
al: Potential role of signal transducer and activator of
transcription (STAT)3 signaling pathway in inflammation, survival,
proliferation and invasion of hepatocellular carcinoma. Biochim
Biophys Acta. 1835:46–60. 2013.PubMed/NCBI
|
|
21.
|
Fan Y, Mao R and Yang J: NF-kappaB and
STAT3 signaling pathways collaboratively link inflammation to
cancer. Protein Cell. 4:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Xu Q, Briggs J, Park S, et al: Targeting
Stat3 blocks both HIF-1 and VEGF expression induced by multiple
oncogenic growth signaling pathways. Oncogene. 24:5552–5560. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ma J and Cao X: Regulation of Stat3
nuclear import by importin alpha5 and importin alpha7 via two
different functional sequence elements. Cell Signal. 18:1117–1126.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shuai K: The STAT family of proteins in
cytokine signaling. Prog Biophys Mol Biol. 71:405–422. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33(Suppl 1): S79–S84. 2013.
View Article : Google Scholar
|
|
26.
|
Grivennikov S and Karin M: Autocrine IL-6
signaling: a key event in tumorigenesis? Cancer Cell. 13:7–9.
2008.PubMed/NCBI
|
|
27.
|
Neurath MF and Finotto S: IL-6 signaling
in autoimmunity, chronic inflammation and inflammation-associated
cancer. Cytokine Growth Factor Rev. 22:83–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Naugler WE and Karin M: The wolf in
sheep’s clothing: the role of interleukin-6 in immunity,
inflammation and cancer. Trends Mol Med. 14:109–119. 2008.
|
|
29.
|
Knupfer H and Preiss R: Serum
interleukin-6 levels in colorectal cancer patients - a summary of
published results. Int J Colorectal Dis. 25:135–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Atreya R and Neurath MF: Involvement of
IL-6 in the pathogenesis of inflammatory bowel disease and colon
cancer. Clin Rev Allergy Immunol. 28:187–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Jones SA, Richards PJ, Scheller J and
Rose-John S: IL-6 transsignaling: the in vivo consequences. J
Interferon Cytokine Res. 25:241–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Li Y, de Haar C, Chen M, et al:
Disease-related expression of the IL6/STAT3/SOCS3 signalling
pathway in ulcerative colitis and ulcerative colitis-related
carcinogenesis. Gut. 59:227–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Shimazaki J, Goto Y, Nishida K, Tabuchi T,
Motohashi G and Ubukata H: In patients with colorectal cancer,
preoperative serum interleukin-6 level and granulocyte/lymphocyte
ratio are clinically relevant biomarkers of long-term cancer
progression. Oncology. 84:356–361. 2013. View Article : Google Scholar
|
|
34.
|
Jess T, Rungoe C and Peyrin-Biroulet L:
Risk of colorectal cancer in patients with ulcerative colitis: a
meta-analysis of population-based cohort studies. Clin
Gastroenterol Hepatol. 10:639–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Canavan C, Abrams KR and Mayberry J:
Meta-analysis: colorectal and small bowel cancer risk in patients
with Crohn’s disease. Aliment Pharmacol Ther. 23:1097–1104.
2006.
|
|
36.
|
Atreya R and Neurath MF: New therapeutic
strategies for treatment of inflammatory bowel disease. Mucosal
Immunol. 1:175–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Atreya R, Mudter J, Finotto S, et al:
Blockade of interleukin 6 trans signaling suppresses T-cell
resistance against apoptosis in chronic intestinal inflammation:
evidence in crohn disease and experimental colitis in vivo. Nat
Med. 6:583–588. 2000. View
Article : Google Scholar
|
|
38.
|
Yamamoto K and Rose-John S: Therapeutic
blockade of interleukin-6 in chronic inflammatory disease. Clin
Pharmacol Ther. 91:574–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Xiong H, Zhang ZG, Tian XQ, et al:
Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle
arrest, and reduces tumor cell invasion in colorectal cancer cells.
Neoplasia. 10:287–297. 2008.PubMed/NCBI
|
|
40.
|
Xiong H, Hong J, Du W, et al: Roles of
STAT3 and ZEB1 proteins in E-cadherin down-regulation and human
colorectal cancer epithelial-mesenchymal transition. J Biol Chem.
287:5819–5832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Akira S, Nishio Y, Inoue M, et al:
Molecular cloning of APRF, a novel IFN-stimulated gene factor 3
p91-related transcription factor involved in the gp130-mediated
signaling pathway. Cell. 77:63–71. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Levy DE and Lee CK: What does Stat3 do? J
Clin Invest. 109:1143–1148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Takeda K, Noguchi K, Shi W, et al:
Targeted disruption of the mouse Stat3 gene leads to early
embryonic lethality. Proc Natl Acad Sci USA. 94:3801–3804. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar
|
|
45.
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
et al: Stat3 as an oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar
|
|
46.
|
Zugowski C, Lieder F, Muller A, et al:
STAT3 controls matrix metalloproteinase-1 expression in colon
carcinoma cells by both direct and AP-1-mediated interaction with
the MMP-1 promoter. Biol Chem. 392:449–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Pan Q, Pan H, Lou H, Xu Y and Tian L:
Inhibition of the angiogenesis and growth of Aloin in human
colorectal cancer in vitro and in vivo. Cancer Cell Int. 13:692013.
View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Qian WF, Guan WX, Gao Y, et al: Inhibition
of STAT3 by RNA interference suppresses angiogenesis in colorectal
carcinoma. Brazilian journal of medical and biological research =
Revista brasileira de pesquisas medicas e biologicas / Sociedade
Brasileira de Biofisica [et al]. 44:1222–1230.
2011.PubMed/NCBI
|
|
49.
|
Lee J, Kim JC, Lee SE, et al: Signal
transducer and activator of transcription 3 (STAT3) protein
suppresses adenoma-to-carcinoma transition in Apcmin/+ mice via
regulation of Snail-1 (SNAI) protein stability. J Biol Chem.
287:18182–18189. 2012.PubMed/NCBI
|
|
50.
|
Grivennikov S, Karin E, Terzic J, et al:
IL-6 and Stat3 are required for survival of intestinal epithelial
cells and development of colitis-associated cancer. Cancer Cell.
15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Guo Y, Xu F, Lu T, Duan Z and Zhang Z:
Interleukin-6 signaling pathway in targeted therapy for cancer.
Cancer Treat Rev. 38:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Puchalski T, Prabhakar U, Jiao Q, Berns B
and Davis HM: Pharmacokinetic and pharmacodynamic modeling of an
anti-interleukin-6 chimeric monoclonal antibody (siltuximab) in
patients with metastatic renal cell carcinoma. Clin Cancer Res.
16:1652–1661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Tanaka T, Narazaki M and Kishimoto T:
Therapeutic targeting of the interleukin-6 receptor. Annu Rev
Pharmacol Toxicol. 52:199–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Garnero P, Thompson E, Woodworth T and
Smolen JS: Rapid and sustained improvement in bone and cartilage
turnover markers with the anti-interleukin-6 receptor inhibitor
tocilizumab plus methotrexate in rheumatoid arthritis patients with
an inadequate response to methotrexate: results from a substudy of
the multi-center double-blind, placebo-controlled trial of
tocilizumab in inadequate responders to methotrexate alone.
Arthritis Rheum. 62:33–43. 2010.
|
|
55.
|
Yang X, Zhang F, Wang Y, et al: Oroxylin A
inhibits colitis-associated carcinogenesis through modulating the
IL-6/STAT3 signaling pathway. Inflamm Bowel Dis. 19:1990–2000.
2013.PubMed/NCBI
|
|
56.
|
Becker C, Fantini MC, Schramm C, et al:
TGF-beta suppresses tumor progression in colon cancer by inhibition
of IL-6 trans-signaling. Immunity. 21:491–501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Waetzig GH and Rose-John S: Hitting a
complex target: an update on interleukin-6 trans-signalling. Expert
Opin Ther Targets. 16:225–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Xiong H, Su WY, Liang QC, et al:
Inhibition of STAT5 induces G1 cell cycle arrest and reduces tumor
cell invasion in human colorectal cancer cells. Lab Invest.
89:717–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Trengove MC and Ward AC: SOCS proteins in
development and disease. Am J Clin Exp Immunol. 2:1–29. 2013.
|
|
60.
|
Isomoto H: Epigenetic alterations in
cholangiocarcinoma-sustained IL-6/STAT3 signaling in
cholangio-carcinoma due to SOCS3 epigenetic silencing. Digestion.
79(Suppl 1): 2–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Gao SM, Chen CQ, Wang LY, et al: Histone
deacetylases inhibitor sodium butyrate inhibits JAK2/STAT signaling
through upregulation of SOCS1 and SOCS3 mediated by HDAC8
inhibition in myeloproliferative neoplasms. Exp Hematol.
41:261–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Liu SH, Wang KB, Lan KH, et al:
Calpain/SHP-1 interaction by honokiol dampening peritoneal
dissemination of gastric cancer in nu/nu mice. PloS One.
7:e437112012. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Xiong H, Du W, Zhang YJ, et al:
Trichostatin A, a histone deacetylase inhibitor, suppresses
JAK2/STAT3 signaling via inducing the promoter-associated histone
acetylation of SOCS1 and SOCS3 in human colorectal cancer cells.
Mol Carcinog. 51:174–184. 2012. View Article : Google Scholar
|
|
64.
|
Shuai K and Liu B: Regulation of
gene-activation pathways by PIAS proteins in the immune system. Nat
Rev Immunol. 5:593–605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Chung CD, Liao J, Liu B, et al: Specific
inhibition of Stat3 signal transduction by PIAS3. Science.
278:1803–1805. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Saydmohammed M, Joseph D and Syed V:
Curcumin suppresses constitutive activation of STAT-3 by
up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in
ovarian and endometrial cancer cells. J Cell Biochem. 110:447–456.
2010.PubMed/NCBI
|
|
67.
|
Brantley EC, Nabors LB, Gillespie GY, et
al: Loss of protein inhibitors of activated STAT-3 expression in
glioblastoma multiforme tumors: implications for STAT-3 activation
and gene expression. Clin Cancer Res. 14:4694–4704. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB
and Tweardy DJ: Stat3 signaling in acute myeloid leukemia:
ligand-dependent and-independent activation and induction of
apoptosis by a novel small-molecule Stat3 inhibitor. Blood.
117:5701–5709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Fletcher S, Drewry JA, Shahani VM, Page BD
and Gunning PT: Molecular disruption of oncogenic signal transducer
and activator of transcription 3 (STAT3) protein. Biochem Cell
Biol. 87:825–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Bromberg J and Wang TC: Inflammation and
cancer: IL-6 and STAT3 complete the link. Cancer Cell. 15:79–80.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Zhuang Q, Hong F, Shen A, et al: Pien
Tze Huang inhibits tumor cell proliferation and promotes
apoptosis via suppressing the STAT3 pathway in a colorectal cancer
mouse model. Int J Oncol. 40:1569–1574. 2012.
|
|
72.
|
Cai Q, Lin J, Wei L, et al: Hedyotis
diffusa Willd inhibits colorectal cancer growth in vivo via
inhibition of STAT3 signaling pathway. Int J Mol Sci. 13:6117–6128.
2012. View Article : Google Scholar
|
|
73.
|
Lin W, Zheng L, Zhuang Q, et al: Spica
prunellae promotes cancer cell apoptosis, inhibits cell
proliferation and tumor angiogenesis in a mouse model of colorectal
cancer via suppression of stat3 pathway. BMC Complement Altern Med.
13:1442013. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Kim SW, Park KC, Jeon SM, et al:
Abrogation of galectin-4 expression promotes tumorigenesis in
colorectal cancer. Cell Oncol. 36:169–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Kuno T, Hatano Y, Tomita H, et al:
Organo-magnesium suppresses inflammation-associated colon
carcinogenesis in male Crj: CD-1 mice. Carcinogenesis. 34:361–369.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Uddin S, Hussain AR, Khan OS and Al-Kuraya
KS: Role of dysregulated expression of leptin and leptin receptors
in colorectal carcinogenesis. Tumour Biol. Sep 7–2013.Epub ahead of
print.
|
|
77.
|
Ganji PN, Park W, Wen J, et al:
Antiangiogenic effects of ganetespib in colorectal cancer mediated
through inhibition of HIF-1alpha and STAT-3. Angiogenesis.
16:903–917. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Zhu Z, Li E, Liu Y, et al: Inhibition of
Jak-STAT3 pathway enhances bufalin-induced apoptosis in colon
cancer SW620 cells. World J Surg Oncol. 10:2282012. View Article : Google Scholar : PubMed/NCBI
|
|
79.
|
Huang S, Chen M, Ding X and Zou X: Proton
pump inhibitor selectively suppresses proliferation and restores
the chemosensitivity of gastric cancer cells by inhibiting STAT3
signaling pathway. Int Immunopharmacol. 17:585–592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Lamy S, Akla N, Ouanouki A, Lord-Dufour S
and Beliveau R: Diet-derived polyphenols inhibit angiogenesis by
modulating the interleukin-6/STAT3 pathway. Exp Cell Res.
318:1586–1596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Chen JS, Chen YH, Huang PH, et al: Ginkgo
biloba extract reduces high-glucose-induced endothelial adhesion by
inhibiting the redox-dependent interleukin-6 pathways. Cardiovas
Diabetol. 11:492012. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Hedvat M, Huszar D, Herrmann A, et al: The
JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and
oncogenesis in solid tumors. Cancer Cell. 16:487–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Geletu M, Arulanandam R, Chevalier S, et
al: Classical cadherins control survival through the gp130/Stat3
axis. Biochim Biophys Acta. 1833:1947–1959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Chang Y, Wang SX, Wang YB, et al: ECHS1
interacts with STAT3 and negatively regulates STAT3 signaling. FEBS
Lett. 587:607–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Ivanov VN, Wen G and Hei TK: Sodium
arsenite exposure inhibits AKT and Stat3 activation, suppresses
self-renewal and induces apoptotic death of embryonic stem cells.
Apoptosis. 18:188–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86.
|
Liu DB, Hu GY, Long GX, Qiu H, Mei Q and
Hu GQ: Celecoxib induces apoptosis and cell-cycle arrest in
nasopharyngeal carcinoma cell lines via inhibition of STAT3
phosphorylation. Acta Pharmacol Sin. 33:682–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87.
|
Kang R, Tang D, Lotze MT and Zeh HJ III:
AGER/RAGE-mediated autophagy promotes pancreatic tumorigenesis and
bioenergetics through the IL6-pSTAT3 pathway. Autophagy. 8:989–991.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
Wu CT, Chen MF, Chen WC and Hsieh CC: The
role of IL-6 in the radiation response of prostate cancer. Radiat
Oncol. 8:1592013. View Article : Google Scholar : PubMed/NCBI
|
|
89.
|
Yao X, Li G, Xu H and Lu C: Inhibition of
the JAK-STAT3 signaling pathway by ganoderic acid A enhances
chemosensitivity of HepG2 cells to cisplatin. Planta Med.
78:1740–1748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90.
|
Putoczki TL, Thiem S, Loving A, et al:
Interleukin-11 is the dominant IL-6 family cytokine during
gastrointestinal tumorigenesis and can be targeted therapeutically.
Cancer Cell. 24:257–271. 2013. View Article : Google Scholar : PubMed/NCBI
|