Introduction
Liver cancer, also known as hepatic cancer,
originates in the liver. Higher rates of liver cancer occur where
hepatitis B and C are common, including East Asia and Sub-Saharan
Africa (1). Viral infection with
either hepatitis C virus (HCV) or Hepatitis B virus (HBV) is the
chief cause of liver cancer in the world, accounting for 80% of
hepatocellular carcinoma (HCC), with >600,000 deaths each year.
The viruses cause HCC with massive inflammation, fibrosis and
eventual cirrhosis within the liver. The current treatment
strategies of liver cancer include surgical resection, radiotherapy
and chemotherapy (2). However,
many liver cancer patients fail to respond to initial chemotherapy,
or they acquire drug resistance during the treatment (3). Normal cells divide as many times as
needed and stop (4). They attach
to other cells and stay in place in tissues. Cells become cancerous
when they lose their ability to stop dividing. Normal cells will
commit cell suicide (apoptosis) when they are no longer needed.
Until then, they are protected from cell suicide by several protein
clusters and pathways (5–7). However, liver cancer with high
mortality rate, still has no effective measures for treatment.
Therefore, the development of novel therapies for liver cancer is
urgently needed.
The Akt pathway is a signal transduction pathway
that promotes survival and growth in response to extracellular
signals (8). Activated Akt
mediates downstream responses, including cell survival, growth,
proliferation, cell migration and angiogenesis, by phosphorylating
a range of intracellular proteins. Studies have found that AKT
pathway plays an important or protective role in many tumors, such
as lung, breast, intestine cancer and pancreatic carcinoma
(9). Sometimes the genes along
these protective pathways are mutated, rendering the cell incapable
of committing suicide when it is no longer needed. This is one of
the steps that causes cancer in combination with other mutations.
Thus, the Akt pathway is stuck in the position and the cancer cell
does not commit suicide (10,11).
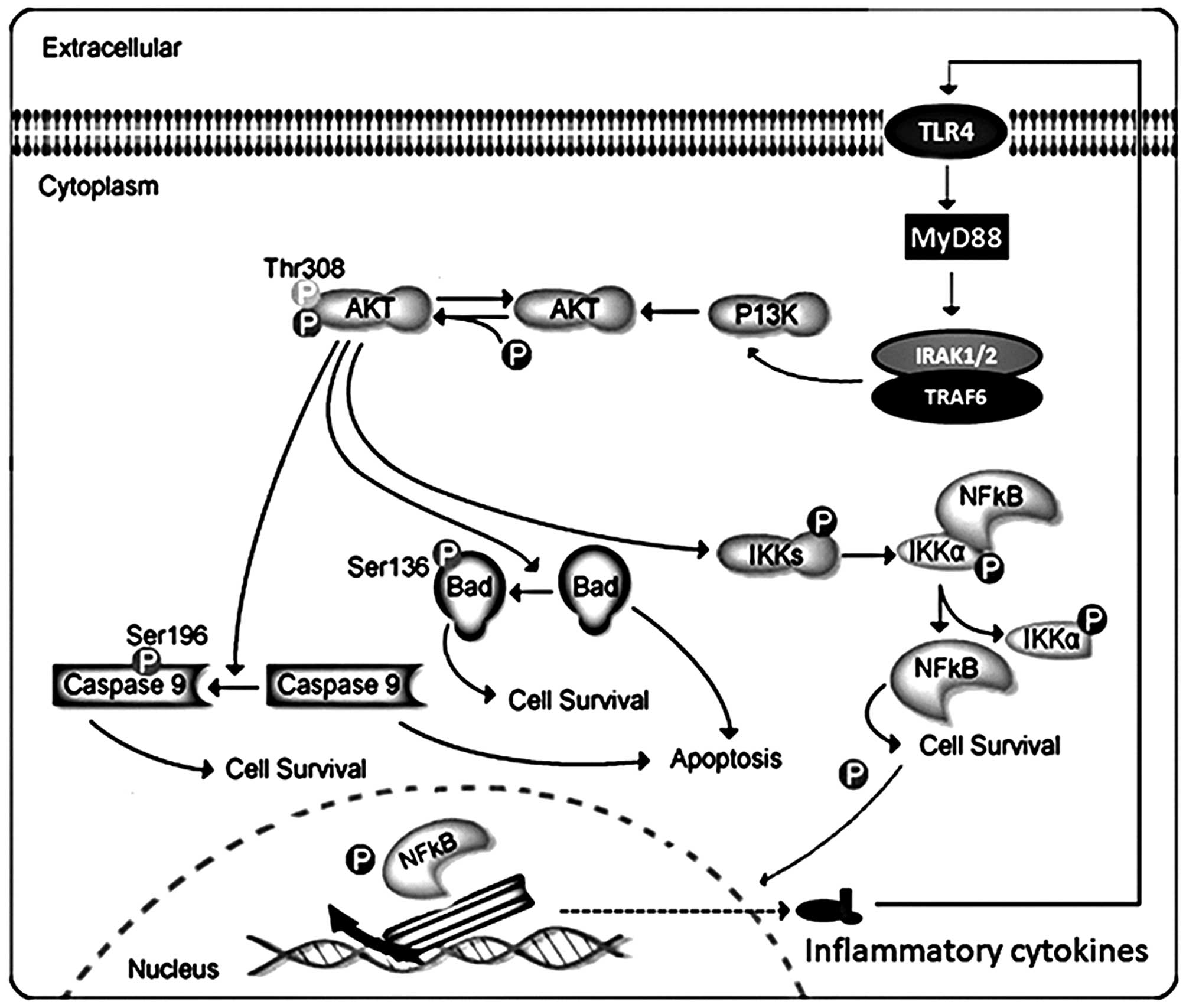
Akt can phosphorylate and activate the IκB kinase IKK-α, causing
degradation of IκB and nuclear translocation of NF-κB where it
promotes expression of cytokines, including interleukin (IL)-1β
(1β), interleukin-18 (IL-18), as well as tumor necrosis factor-α
(TNF-α), accelerating inflammation response. Akt, also, negatively
regulates pro-apoptotic proteins by direct phosphorylation
(12). For example,
phosphorylation of Bad, the Bcl-2 family member, causes
translocation from the mitochondrial membrane to the cytosol. Akt
phosphorylates caspase-9, preventing a caspase cascade leading to
cell death, modulating apoptosis. Thus, the association of Akt and
NF-κB and Bad signaling pathways play a critical role in the
inflammatory formation and apoptotic development (13). We investigated the progression of
liver cancer from Akt/NF-κB and Akt/Bad signaling pathway,
revealing Akt-regulated inflammation and apoptosis during the
development of hepatocellular carcinoma.
Hence, in the present study, in the first part we
investigated whether Akt-knockout could affect liver cancer
progression by interfering with NF-κB pathway activation, as well
as Bad signaling pathway relating to inflammation and apoptosis,
which might serve as a potential therapeutic target and prognostic
marker for the treatment of liver cancer.
Materials and methods
Animals
Five-week-old BALB/c Akt-knockout mice and nude
mice, were purchased from Vital River Laboratories (VRL; Beijing,
China), and were used for a liver cancer xenograft model of
MHCC97-H and Bel7402 cells. A total of 0.2 ml medium containing
5×106 MHCC97-H and Bel7402 cells was injected
subcutaneously into the left and right posterior flank regions of
each mouse. Mice were housed in a pathogen-free environment and
tumor growth was examined each week (for a total of 5 weeks).
Animals were euthanized and tumors were excised and subjected to
pathological examination. The animal studies were approved by the
First Affiliated Hospital Xiamen University, China.
Cell culture
Human liver cancer cell lines (MHCC97-H and Bel7402)
and normal liver cells (hhl-5) were grown and maintained in
RPMI-1640 medium containing 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin. Recombinant lentiviral particles encoding
Akt and short hairpin RNA targeting Akt were produced, concentrated
and titrated. Cells were seeded at 3×104 cells/ml and
were infected with recombinant lentivirus twice with an interval of
12 h and incubated for 24 h. After 24 h, the medium was refreshed
and the cells were cultured for another 24 h.
ELISA measurement
At the end of the experiments, blood was extracted
from the eyeball, the different serum concentration of the
inflammatory cytokines TNF-α, IFN-γ, IL-2, IL-1β, IL-10, IL-18 and
IL-6 were measured using ELISA kits according to the manufacturer's
instructions (R&D Systems, Inc., Minneapolis, MN, USA).
Inflammatory cell counts
The cell samples were centrifuged (4°C, 3,000 rpm
for 10 min) to pellet the cells. The cell pellets were resuspended
in PBS for the total cell counts using a hemacytometer, and
cytospins were prepared for differential cell counts by staining
with the Wright-Giemsa staining method.
Histopathological examination of cells
and tissues
Histopathological evaluation was performed on mice
and cells that were collected. Samples were fixed with 10% buffered
formalin, imbedded in paraffin and sliced. After hematoxylin and
eosin (H&E) staining, pathological changes of tissues were
observed under a light microscope. Some samples were also subjected
to immunohistochemical staining (Akt, NF-κB and Bad) according to
CST Technology Co. introductions, and performed by Shanghai Zhenda
Biotechnology, Co., Ltd. (Shanghai, China).
Immunofluorescence staining
After induction by conditioned culture medium, the
cells were fixed in 4% paraformaldehyde, permeabilized with 0.1%
Triton X-100 in PBS containing 0.5% BSA (PBS-BSA) for 30 min. The
cells were subsequently incubated with Akt, NF-κB and Bad for 30
min, followed by labeling with Alexa Fluor 488-conjugated rabbit
anti-mouse or goat anti-rabbit IgG antibody. The cells were viewed
under a fluorescent microscope.
Apoptosis analysis by terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL)
Apoptosis assay of samples was determined by TUNEL
used an In Situ Cell Death Detection Kit, Fluorescein (Roche
Applied Science, South San Francisco, CA, USA) according to the
manufacturer's protocol. The number of TUNEL-positive cells was
counted under a fluorescence microscope. The percentages of
apoptotic cells were calculated from the ratio of apoptotic cells
to total cells. Tissue sections were counterstained with
hematoxylin, then mounted and observed under light microscopy. The
experiment was performed independently three times for each cell
line.
Western blot analysis
Cell proteins were extracted using T-PER Tissue
Protein Extraction reagent kit (Thermo Fisher Scientific) according
to the manufacturer's instructions. Protein concentrations were
determined by BCA protein assay kit, and equal amounts of protein
were loaded per well on a 10% sodium dodecyl sulphatepolyacrylamide
gel. Subsequently, proteins were transferred onto polyvinylidene
difluoride membrane. The resulting membrane was blocked with
Tris-buffered saline containing 0.05% Tween-20 (TBS-T),
supplemented with 5% skim milk (Sigma) at room temperature for 2 h
on a rotary shaker followed by TBS-T washing. The specific primary
antibody, diluted in TBST, was incubated with the membrane at 4°C
overnight. Subsequently, the membrane was washed with TBS-T
followed by incubation with the peroxidase-conjugated secondary
antibody at room temperature for 1 h. The immunoactive proteins
were detected by using an enhanced chemiluminescence western
blotting detection kit. The bands were observed using GE Healthcare
ECL western blotting analysis system and exposed to X-ray film
(Kodak).
Real-time quantitative PCR analysis
Total RNA isolated from an individual mouse kidney
with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the
manufacturer's protocol, was evaluated for mRNA expression of Akt,
NF-κB, Bad, IKK-α, IκB-α, IL-1β, IL-18, Bcl-Xl, cytochrome
c, Apaf-1, caspase-9, caspase-3 and glyceraldehyde
3-phosphate dehydrogenase (GAPDH). For reverse transcription, the
SuperScript First-Strand Synthesis kit (RT-PCR; Invitrogen) was
used. The primers for real-time PCR are summarized in Table I. All primer sequences were checked
in GenBank to avoid inadvertent sequence homologies. They were
designed and synthesized by BioGenes GmbH (Berlin, Germany).
Reactions were performed using SYBR-Green PCR Master Mix (Applied
Biosystems) in a Roche LightCycler 480 detection system. As an
internal control, GAPDH levels were quantified in parallel with
target genes. Normalization and fold change for each of the genes
were calculated using the 2−ΔΔCT method. The sequences
of the genes are listed in Table
I.
 | Table IPrimer sequences of RT-PCR
analysis. |
Table I
Primer sequences of RT-PCR
analysis.
| Gene | Forward primers
(5′-3′) | Reverse primers
(5′-3′) |
|---|
| GAPDH |
CATTCAAGACCGGACAGAGG |
ACATACTCAGCACCAGCATCACC |
| Akt |
GTGTCCAGTGTAGAATGACTC |
ATCTGTCGGAGAACACACATG |
| NF-κB |
GCAAAGGGAACATTCCGATAT |
GCGACATCACATGGAAATCTA |
| IKK-α |
GAACCGGCACCTGACACC |
ACGACCTTCGTCAGTACCGA |
| IκB-α |
AGCACAAAGAGAGTGTCGC |
CGTCAGTCAGTGTGTATG |
| IL-1β |
GACAGCAAAGTGATAGGCC |
CGTCGGCAATGTATGTGTTGG |
| IL-18 |
GCAGCAGGTGAGTGGGCAGT |
CTGTACGCCTGGTTCGCTCTGT |
| Bcl-xL |
CATGCTGGGGCCGTACAG |
TTGTCCGACCTTTGGCAACT |
| Cytochrome
c |
CAGAAGGAAGTTAGGCC |
CGTCGCAGTGGATGATGTG |
| Apaf-1 |
CTTCTCACTGTCGACTACCGC |
GCGTCTCCTGTGCATTCG |
| Caspase-9 |
GACTCTTCCTGGTCTTACCATATT |
CTGCTATTGCAAGGACCCAATT |
| Caspase-3 |
GCAAGGACAAGATTCGATACT |
GCCAGACTACATGGAAATCTA |
Secondary colony-forming assay
Briefly, cancer cells were suspended in 0.9%
methylcellulose-based semisolid medium MethoCult H4100 (StemCell
Technologies, Inc., Beijing, China). After 14 days, individual
primary clones (450 cells) were trypsinized and re-plated in the
same conditions to examine the secondary colonyforming ability for
self-renewal.
Flow cytometric analysis
For flow cytometric analysis, the hepatocytes were
obtained through shearing liver tissue, separating by collagenase
type II (Invitrogen) digestion and suspended in RPMI-1640 medium
(Gibco-BRL). Cell suspensions were centrifuged at 1,000 rpm for 5
min to remove cellular debris and impurities. Then, the hepatic
mononuclear cells (MNCs) were harvested and re-suspended in 70%
percoll (Sigma). MNCs were collected from the interphase, and
washed twice in Hank's buffer (Gibco-BRL). According to the
protocol of R&D kit systems (R&D Systems) for flow
cytometry, anti-CD4 FITC and anti-CD8 FITC antibodies were added to
the flow cytometry tube containing single-cell suspension, and the
cells were analyzed by Cytomics™ FC 500 MCL of Beckman-Coulter
(Beckman-Coulter, Inc., Brea, CA, USA).
Statistical analysis
Data are expressed as means ± SEM. Treated cells,
tissues and the corresponding controls were compared using GraphPad
Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA) by
a one-way ANOVA with Dunn's least significant difference tests.
Differences between groups were considered significant at
P<0.05.
Results
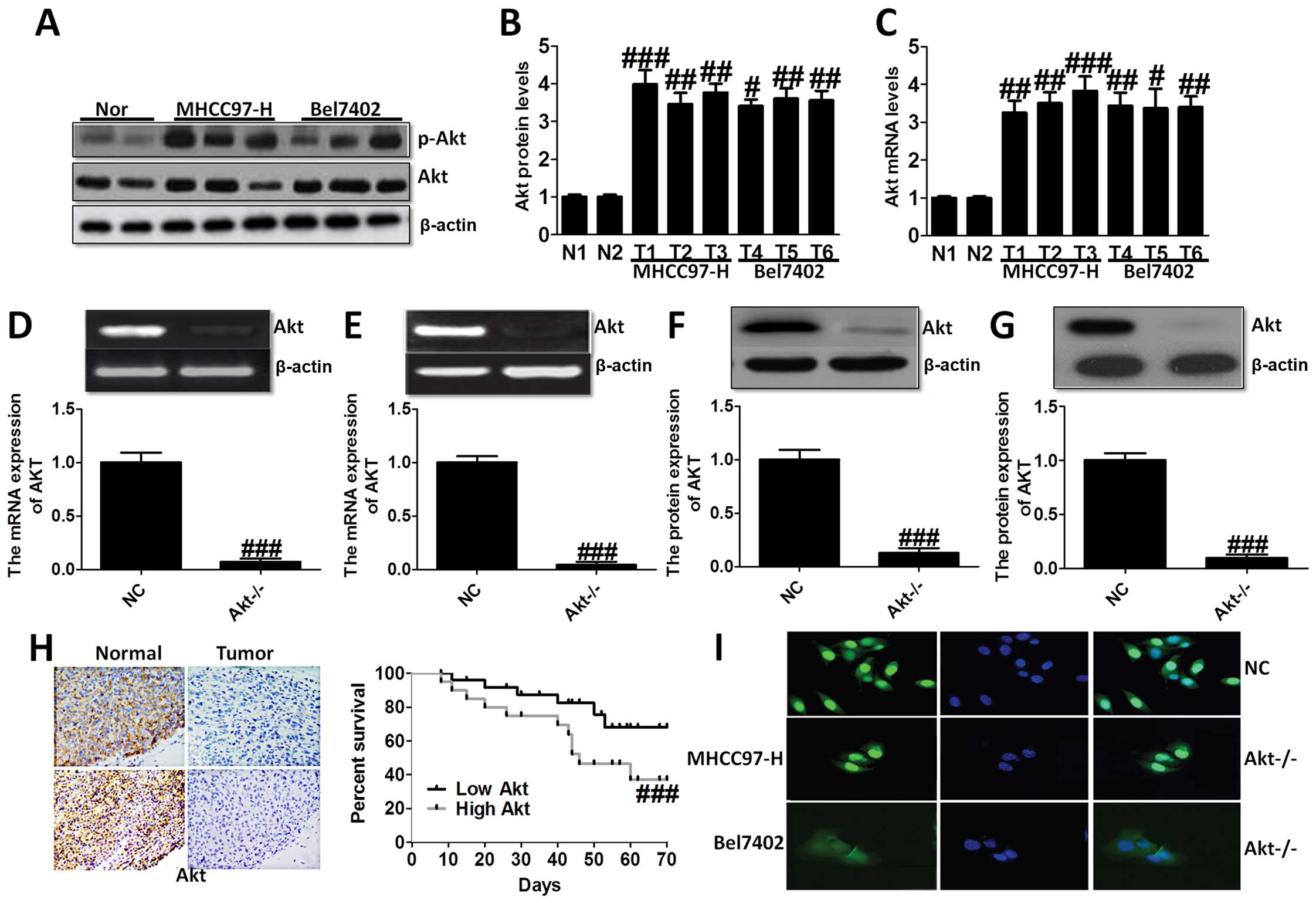
Akt is overexpressed in liver cancer cell
lines and tissues
In the present study, we evaluated the Akt
expression in liver cancer cell lines and normal liver cells. As
shown in Fig. 1A–C, we found Akt
overexpressed in liver cancer cell lines compared to the normal
cells via western blot analysis and RT-PCR analysis, demonstrating
that Akt is likely to play an essential role in the progression of
liver cancer. Thus, we aimed at Akt-knockout, and studied Akt
expression in MHCC97-H (Fig. 1D and
F) and Bel7402 cells (Fig. 1E and
G). In order to confirm the role of Akt on liver cancer,
immunohistochemical staining analysis was used to demonstrate the
difference between the normal group, and tumor group, which showed
higher survival percent of low Akt levels (Fig. 1H). Immunofluorescence staining was
performed to further indicate Akt expression in NC group and
Akt−/− group of MHCC97-H and Bel7402 cells (Fig. 1I). These results suggested that Akt
was expressed highly in liver cancer cell lines and after knockout
of Akt, the survival percent of cells was improved compared to the
normal cells, suggesting that Akt might be considered as a target
for the treatment of liver cancer.
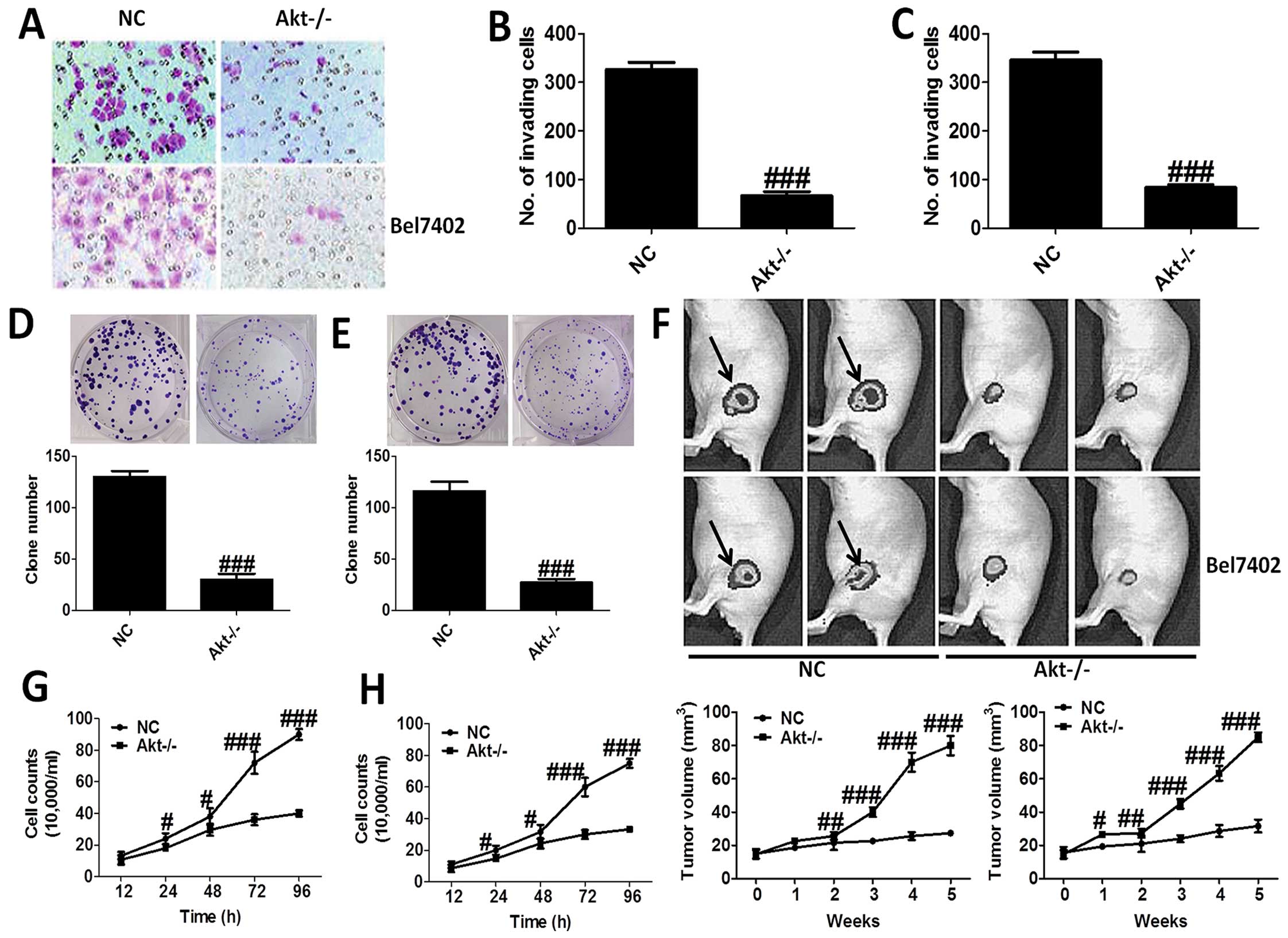
The effects of Akt on the progression of
liver cancer
H&E staining was applied to reveal the role of
Akt-knockout in MHCC97-H and Bel7402 cells (Fig. 2A–C), which showed that Akt-knockout
significantly decrease the number of invading cells. In addition,
loss of Akt activity attenuated liver cancer cell growth in
vitro, and the clone number of cells is shown in Fig. 2D and E. Moreover, nude mice were
used to test the role Akt on cancer development directly, which
indicated that compared to the NC group, mice treated with
Akt-knockout of MHCC97-H and Bel7402 cells showed markedly lower
tumor volume (Fig. 2F). After
treatment, we tested the cell counts. The results showed that the
number of cell counts in culture from 12 to 96 h was highly reduced
in Akt−/− cells, in both MHCC97-H (Fig. 2G) and Bel7402 cells (Fig. 2H). The results proved that Akt is
of great importance for the progression and development of liver
cancer.
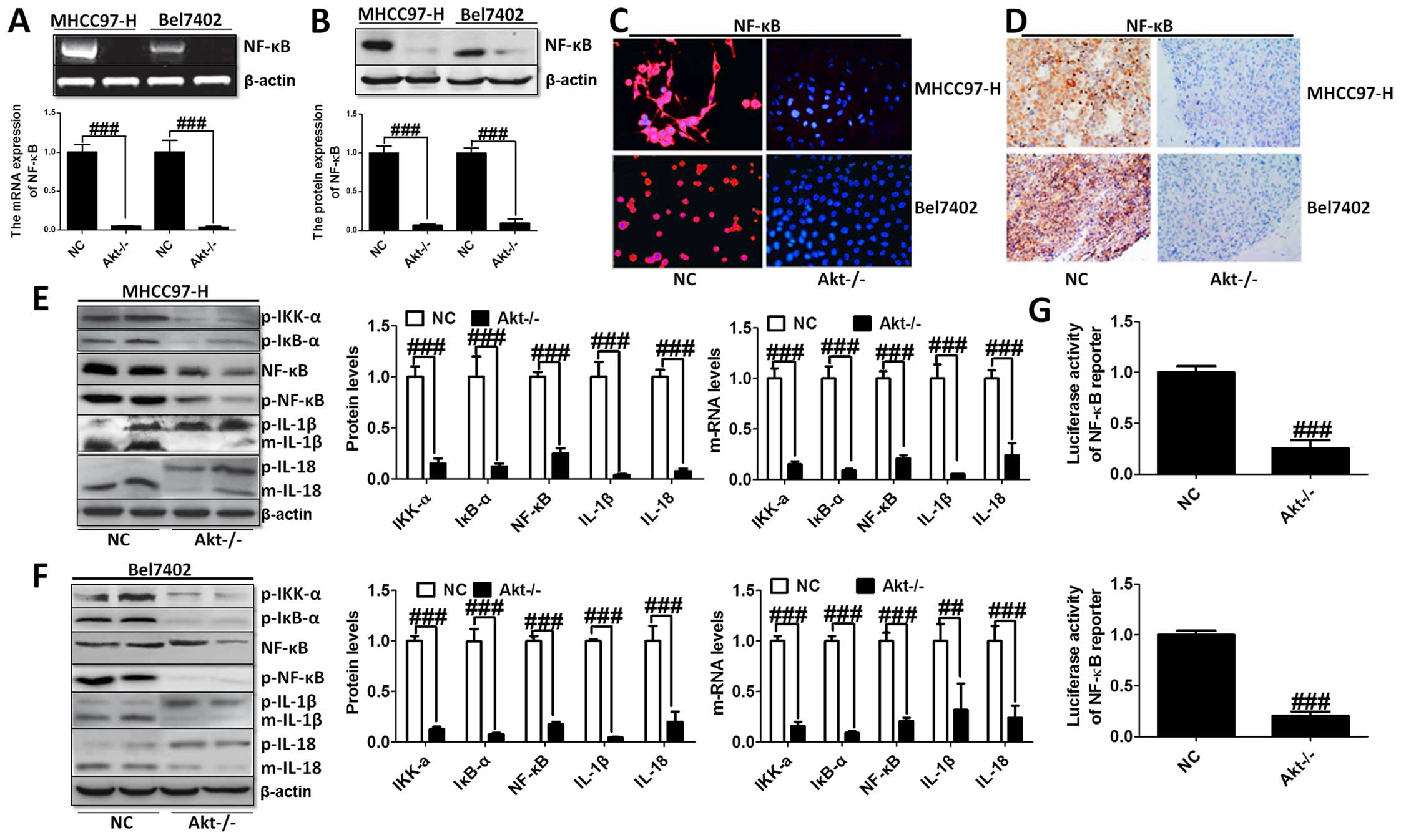
The effects of Akt on NF-κB-regulated
inflammation response
The knockout of Akt inhibited NF-κB expression was
assessed using western blot and RT-PCR analysis with significant
statistical difference compared to the control group (Fig. 3A and B). The immunofluorescence
(Fig. 3C) and immunohistochemical
staining (Fig. 3D) analysis showed
that NF-κB liver cancer cells were markedly decreased in both cell
lines. Moreover, the transcriptional activity of NF-κB was
determined by the luciferase assay when Akt was knocked out.
Fig. 3G shows that NF-κB
transcriptional activity was reduced markedly compared to the NC
group. In addition, the upstream and downstream signals of NF-κB
were determined by western blot analysis, and RT-PCR demonstrating
that cytokines, involving IL-1β and IL-18, were downregulated
greatly, in accordance with the trend of NF-κB alteration (Fig. 3E and F).
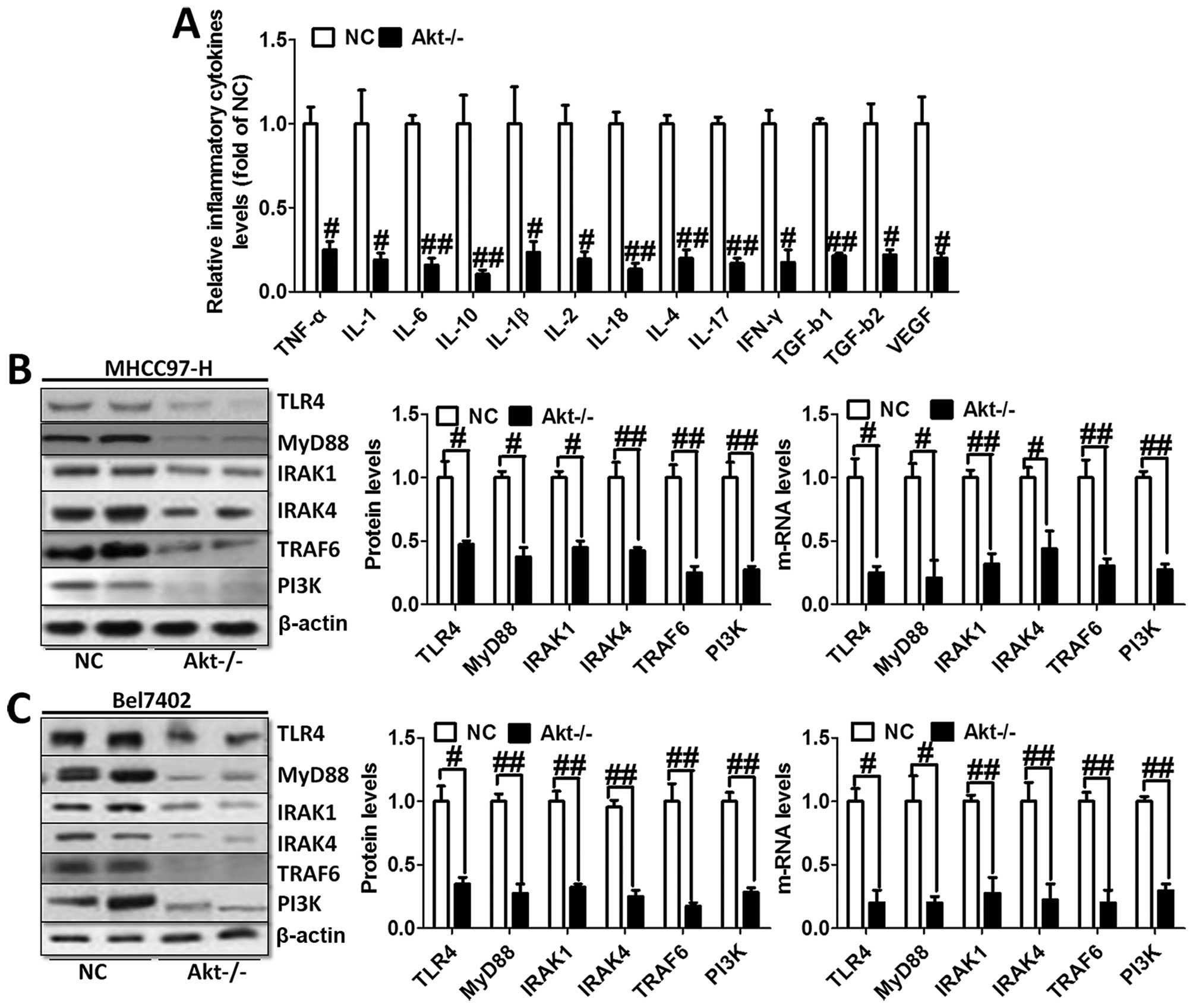
Furthermore, we evaluated the levels of inflammatory
cytokines in the serum of animal models. As shown in Fig. 4A, TNF-α, IL-1, IL-6, IL-10, IL-1β,
IL-2, IL-18, IL-4, IL-17, IFN-γ, TGF-b1, TGF-b2 and VEGF were
tested through ELISA kits. The results showed obvious cytokine
reduction in Akt-knockout mice compared to the normal liver cancer
mice. These results indicated that Akt regulated liver cancer
progression via NF-κB activation and its downstream signals
contributed to the inflammation response.
We investigated TLR4/MyD88 signaling pathway
contribution to the activation of NF-κB. The results showed
significant reduction of TLR4 and MyD88 via western blot analysis
and RT-PCR analysis in both MHCC97-H and Bel7402 cells (Fig. 4B). In addition, the downstream
signals of TLR4/MyD88 pathway were decreased, including IRAK1,
IRAK4 as well as TRAF6 (Fig. 4B and
C), which was consistent with the expression trend of NF-κB and
inflammation-related cytokines. In this regard, we finally tested
the expression level of PI3K in both cell types, displaying reduced
phenomenon (Fig. 4B and C). These
results possibly suggest to us that TLR4/MyD88 signaling pathway
was regulated, in turn, after the overexpression of
inflammation-associated factors. Activated PI3K phosphorylated Akt,
leading to aggravation of inflammation response and apoptosis,
which was likely to form a vicious cycle. Thus, knockout of Akt is
related to the progression of the inflammation response.
The effects of Akt on Bad-regulated
apoptosis in liver cancer
We supposed that apoptosis accelerates the
progression of liver cancer. Thus, Bad, which is essential to
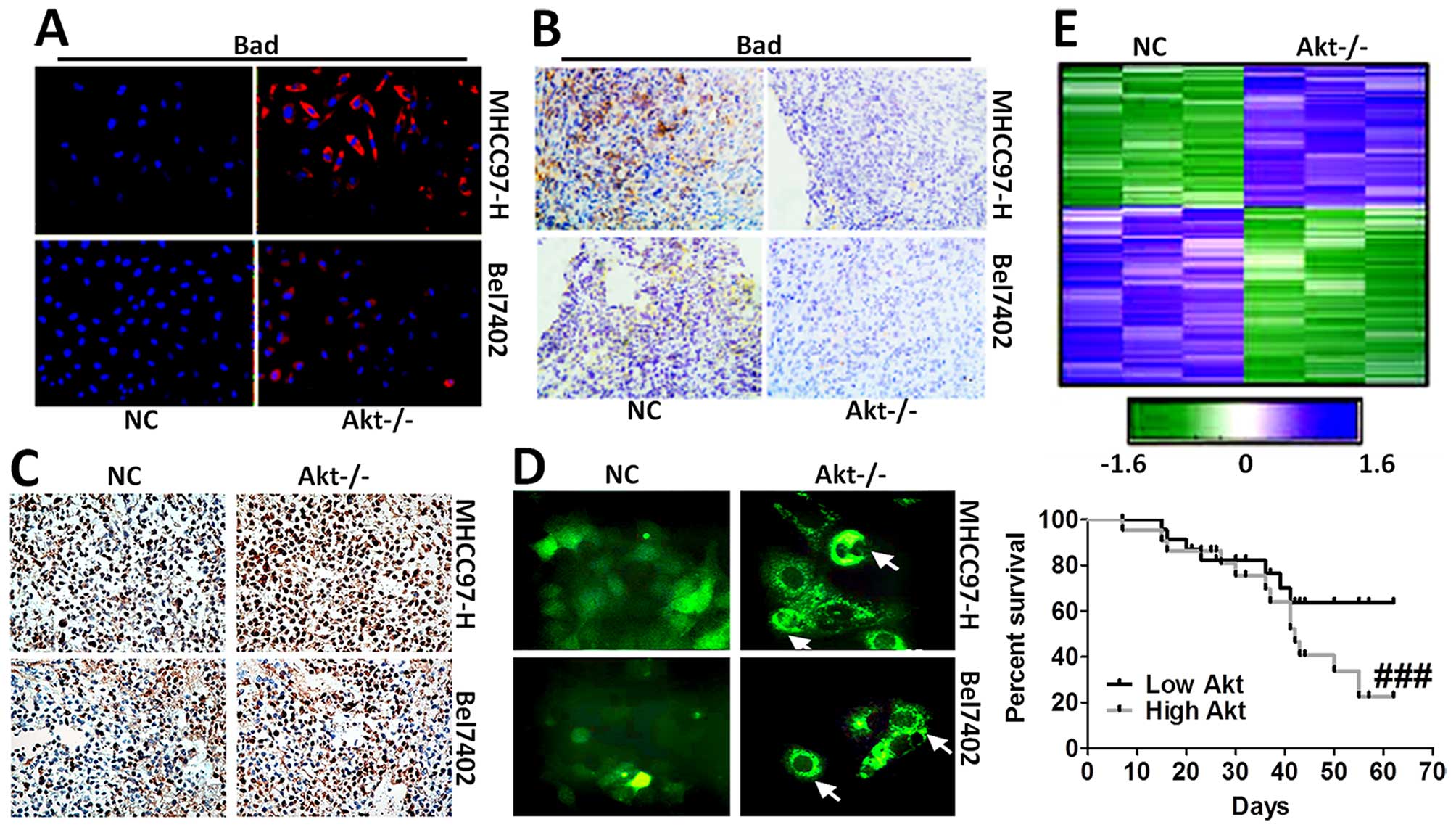
apoptosis, was tested via immunofluorescence staining (Fig. 5A) and immunohistochemical staining
analysis (Fig. 5B). The results
showed that cells expressing Bad was higher in Akt-knockout cells.
In addition, mice without Akt expression displayed serious cell
death in liver cancer cells, as shown in Fig. 5C and D. Hierarchical clustering of
microarray data from MHCC97-H and Bel7402 cells treated with
Akt-knockout was analyzed. Additionally, Kaplan-Meier survival plot
of liver cancer animals comparing a positive correlation with the
Akt-knockout gene signature or negative correlation with the
Akt-knockout gene signature was prepared, and is shown in Fig. 5E, demonstrating that the gene
signature produced by Akt-knockout conferred a marked improvement
in survival.
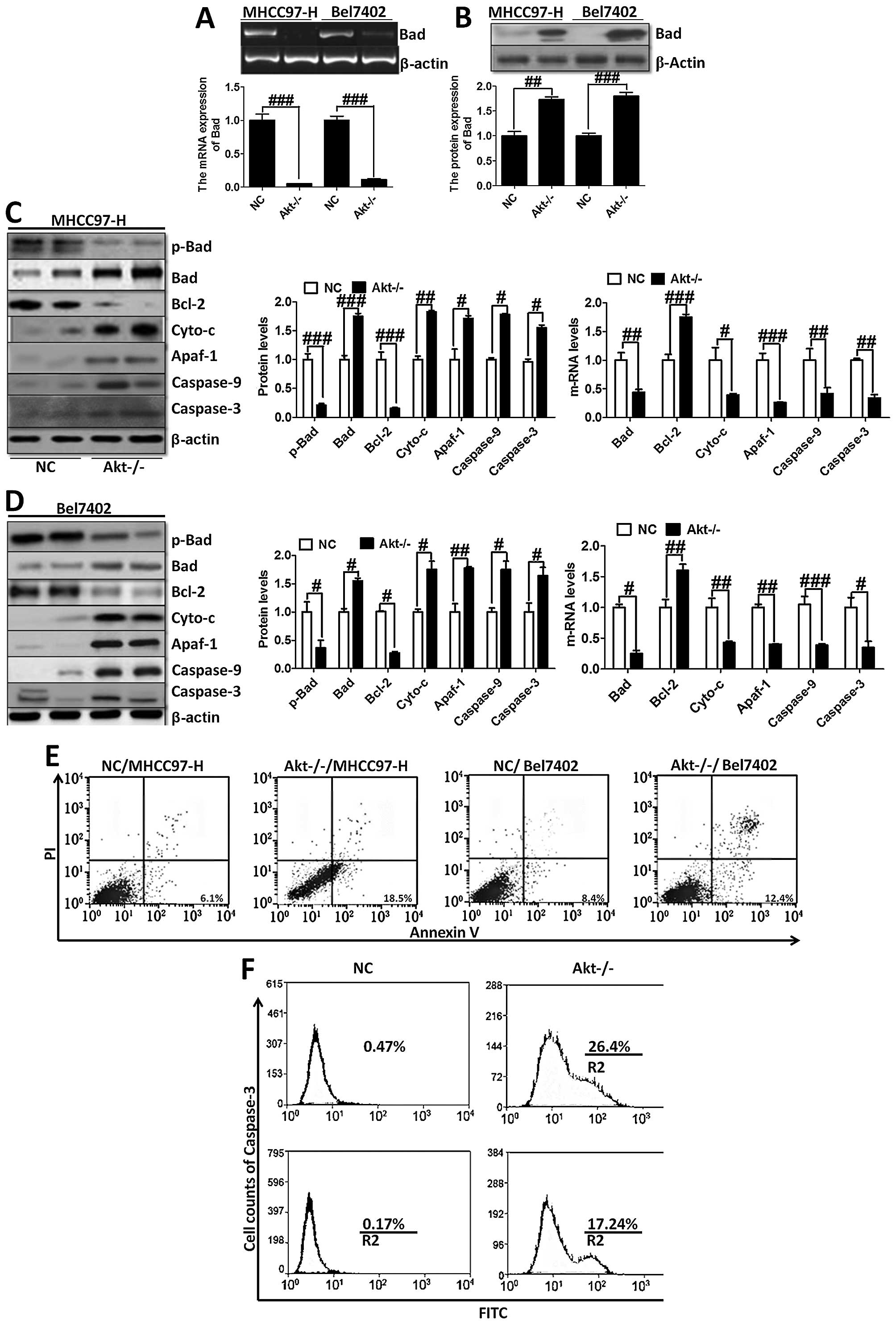
Furthermore, RT-PCR and western blot analysis were
used to analyze Bad expression (Fig.
6A and B). The trend of Bad expression was consistent with
immunofluorescence staining (Fig.
5A) and immunohistochemical staining (Fig. 5B), further indicating that
Akt-knockout played a critical role in regulating Bad. On the
contrary, we investigated the downstream signals of Bad, including
Bcl-2, cytochrome c, Apaf-1, caspase-9 and caspase-3
(Fig. 6C and D). Akt-knockout
induced significant reduction of the genes, except the upregulation
of Bcl-2, which plays an important role in anti-apoptosis in
MHCC97-H and Bel7402 cells. Determined by Annexin V/PI double
staining analysis, we found that knock out of Akt could suppress
overexpression of Akt-induced apoptosis in liver cancer cells,
showing higher apoptosis ratio in the indicated cancer cells
(Fig. 6E). Moreover, caspase-3
activation assay with the CaspGLOW fluorescein active caspase-3
staining kit with FACS analysis was applied to determine the effect
of Akt on apoptosis development, showing that Akt-knockout cells
had higher expression of caspase-3, which resulted in apoptosis in
liver cancer cells (Fig. 6F).
These data demonstrated that Akt-knockout serves as a survival
signal and induction of apoptosis in cancer cells.
Discussion
Liver cancer is the third leading cause of cancer
death after lung and stomach cancer world-wide. Liver cancer has
been emphasized as a serious threat to human health (14,15).
Survival rates in the developed world are high, however, in
developing countries survival rates are still poor, and
unsatisfactory. Moreover, the formation and development of liver
cancer is associated with high morbidity and mortality (16,17).
In previous research, some natural products have been used in
cells, in animal experiments, and partly tested in clinical studies
for preventing liver cancer providing useful information (18). However, the detailed underlying
molecular mechanisms controlling liver cancer are still not fully
explained. In addition, we are looking for the most effective
treatment for liver cancer-related diseases.
Akt is known to be implicated in several types of
cancer, including glioblastoma, ovarian, pancreatic and breast
cancer. Studies have also found that Akt is upregulated in terms of
mRNA production in the liver (19). The human liver cancer cell lines
MHCC97-H and Bel7402, and the normal liver cells hhl-5 were used to
investigate the expression of Akt in different cell lines (20). We found that the expression of Akt
in liver cancer cell lines was higher compared to in the normal
liver cells (Fig. 1A–C).
Akt-knockout liver cancer cells displayed lower expression of Akt
(Fig. 1D–I), which was used to
further indicate the effect or importance of Akt in the treatment
of liver cancer. Moreover, we found that the invading cells, clone
number of liver cancer cells and the cell counts cultured for
different times were downregulated after the knockout (Fig. 2A–H). Furthermore, xenografts in
nude mice suggested that the volume of the tumor in Akt-knockout
mice was ameliorated (Fig. 2F).
The above results showed that Akt is of great importance in the
development or progression for liver cancer. The activation of Akt
further enhanced the NF-κB pathway phosphorylation levels. As
previously reported (21) the increase of NF-κB
pathway phosphorylation leads to inflammatory responses and
cytokines production. Inflammatory abnormalities are a large group
of disorders that underly a vast variety of human diseases,
especially regulated by interleukins. Although the processes
involved are identical to tissue inflammation, systemic
inflammation is not confined to a particular tissue but involves
the endothelium and other organ systems. The RT-PCR, western blot
analysis, immunofluorescence, immunochemistry and luciferase
activity analysis also provided evidence (Fig. 3). The results showed that the
expression of NF-κB in Akt−/− liver cancer cells were
lower than in the liver cancer cells, demonstrating that inhibition
of overexpression of Akt could suppress the abnormal NF-κB
expression, subsequently improving cytokine expression, including
IL-1β and IL-18 (Fig. 3E and F).
Furthermore, as shown in Fig. 4A,
many cytokines were tested to further clarify the effect of Akt on
the levels of inflammatory factors, which play an essential role in
the progression of liver cancer. Then, overexpression of cytokines,
in turn, further activated the TLR4/MyD88 signaling pathway,
contributing to the downstream signals expression. Subsequently,
activation of PI3K phosphorylated Akt, which led to the
phosphorylation of NF-κB, and finally intensified the inflammation
response, which is a feature of cancer progression. A previous
study (21) has also shown that expression of NF-κB
results in cell survival. However, in the present study we supposed
that knockout of Akt reduced NF-κB expression and lessened p-NF-κB
expression, accordingly damaging cancer cell survival and
decreasing the feature of inflammation response in liver cancer
cells, which was similar to the former research. Thus, the
development and progression of liver cancer could be inhibited
through knockout of Akt.
The Bcl-2-associated death promoter (BAD) protein is
a pro-apoptotic member of the Bcl-2 gene family which is involved
in initiating apoptosis. BAD is a member of the BH3-only family, a
subfamily of the Bcl-2 family (22). It does not contain a C-terminal
transmembrane domain for outer mitochondrial membrane and nuclear
envelope targeting, unlike most other members of the Bcl-2 family
(23). When Bad is phosphorylated
by Akt, it forms the Bad-(14-3-3) protein heterodimer. Bax are
believed to initiate apoptosis by forming a pore in the
mitochondrial outer membrane that allows cytochrome c to
escape into the cytoplasm and activate the pro-apoptotic caspase
cascade (24). The anti-apoptotic
Bcl-2 and Bcl-xL proteins inhibit cytochrome c release
through the mitochondrial pore and also inhibit activation of the
cytoplasmic caspase cascade by cytochrome c (25,26).
These studies have suggested that activated Akt could phosphorylate
Bad, contributing to cell survival, and that caspase-9 and
caspase-3, play essential roles in apoptosis. The results in the
present study, similarly found that Akt-knockout liver cancer cells
showed lower p-Bad expression and higher Bad expression, cutting
down Bcl-xL expression and promoting the down stream signals, such
as cytochrome c, Apaf-1, caspase-9 and caspase-3 expression,
subsequently contributing to apoptosis in cancer cells (Figs. 5 and 7). Western blot and RT-PCR analysis
further confirmed the results that Akt−/− enhanced Bad
expression. In addition, Annexin V and caspase-3 staining kit with
FACS analysis (Fig. 6E and F)
demonstrated that the Akt-knockout might affect caspase-3 in an
indirect manner. As we mentioned above, the AKT signaling pathway
might be associated with the progression of liver cancer. These
data indicated that Akt is important upstream of NF-κB and Bad
might impact inflammatory responses and apoptosis (Fig. 7). Thus, inhibition of Akt
activation might be an essential strategy to suppress liver cancer
progression via inflammation response and apoptosis.
Taken together, liver cancer has been emphasized as
a threat to human health and life, particularly in developing
countries. Considerable number of risk factors contribute to the
occurrence and progression of liver cancer (1–3,27).
However, the underlying mechanisms of liver cancer are not yet
clarified, and treatments for liver cancer are still lacking, thus,
new strategies and studies are required for patients with the
disease. In the present study, we investigated in a liver cancer
animal model, and cells the Akt-regulated NF-κB and Bad signaling
pathway and its downstream signals. Also, the Akt activation could
be significantly upregulated in liver cancer cell lines, and
knockout of Akt was able to reduce inflammation-related cytokine
expression and apoptosis-associated signals, which might be
potential indicators for clinical treatment.
Acknowledgements
We are grateful for the support from the National
Natural Science Foundation of China (81301923 and 81371902), and
the National Natural Science Foundation of Fujian (2011D012 and
2015J01561). Medical Innovation Subject of Fujan Province of China
(2012-CX-30).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim YK, Lee GS, Jung EM, Hyun SH, Hwang WS
and Jeung EB: Generation of fibroblasts overexpressing
liver-specific PEPCK in a miniature pig model of human type 2
diabetes mellitus. Mol Med Rep. 6:45–50. 2012.PubMed/NCBI
|
|
3
|
Rampone B, Schiavone B, Martino A, Viviano
C and Confuorto G: Current management strategy of hepatocellular
carcinoma. World J Gastroenterol. 15:3210–3216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu G, Qi FZ, Zhang JH, Cheng GF, Cai Y and
Miao Y: Meta-analysis of surgical resection and radiofrequency
ablation for early hepatocellular carcinoma. World J Surg Oncol.
10:16320122012. View Article : Google Scholar
|
|
5
|
Salhab M and Canelo R: An overview of
evidence-based management of hepatocellular carcinoma: a
meta-analysis. J Cancer Res Ther. 7:463–475. 2011. View Article : Google Scholar
|
|
6
|
Zhou XD: Recurrence and metastasis of
hepatocellular carcinoma: progress and prospects. Hepatobiliary
Pancreat Dis Int. 1:35–41. 2002.
|
|
7
|
Guan M, Zhou X, Soulitzis N, Spandidos DA
and Popescu NC: Aberrant methylation and deacetylation of deleted
in liver cancer-1 gene in prostate cancer: potential clinical
applications. Clin Cancer Res. 12:1412–1419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lizarraga IM, Sugg SL, Weigel RJ and
Scott-Conner CE: Review of risk factors for the development of
contralateral breast cancer. Am J Surg. 206:704–708. 2013.
View Article : Google Scholar
|
|
9
|
Chapuis N, Tamburini J, Cornillet-Lefebvre
P, Gillot L, Bardet V, Willems L, Park S, Green AS, Ifrah N,
Dreyfus F, et al: Autocrine IGF-1/IGF-1R signaling is responsible
for constitutive PI3K/Akt activation in acute myeloid leukemia:
therapeutic value of neutralizing anti-IGF-1R antibody.
Haematologica. 95:415–423. 2010. View Article : Google Scholar :
|
|
10
|
Muders MH, Zhang H, Wang E, Tindall DJ and
Datta K: Vascular endothelial growth factor-C protects prostate
cancer cells from oxidative stress by the activation of mammalian
target of rapamycin complex-2 and AKT-1. Cancer Res. 69:6042–6048.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Opel D, Poremba C, Simon T, Debatin KM and
Fulda S: Activation of Akt predicts poor outcome in neuroblastoma.
Cancer Res. 67:735–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chagpar RB, Links PH, Pastor MC, Furber
LA, Hawrysh AD, Chamberlain MD and Anderson DH: Direct positive
regulation of PTEN by the p85 subunit of phosphatidylinositol
3-kinase. Proc Natl Acad Sci USA. 107:5471–5476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lopez-Carballo G, Moreno L, Masia S, Perez
P and Barettino D: Activation of the phosphatidylinositol
3-kinase/Akt signaling pathway by retinoic acid is required for
neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol
Chem. 277:25297–25304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang XW, Wang XL, Cao LQ, Jiang XF, Peng
HP, Lin SM, Xue P and Chen D: Green tea polyphenol
epigallocatechin-3-gallate enhances 5-fluorouracil-induced cell
growth inhibition of hepatocellular carcinoma cells. Hepatol Res.
42:494–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugiyama M, Sakahara H, Torizuka T, Kanno
T, Nakamura F, Futatsubashi M and Nakamura S: 18F-FDG
PET in the detection of extrahepatic metastases from hepatocellular
carcinoma. J Gastroenterol. 39:961–968. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Park JY, Kim do Y, Ahn SH, Han KH,
Seo HJ, Lee JD and Choi HJ: Prognostic value of 18F-FDG
PET for hepatocellular carcinoma patients treated with sorafenib.
Liver Int. 31:1144–1149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paudyal B, Oriuchi N, Paudyal P, Tsushima
Y, Higuchi T, Miyakubo M, Ishikita T, Nakajima T and Endo K:
Clinicopathological presentation of varying 18F-FDG
uptake and expression of glucose transporter 1 and hexokinase II in
cases of hepatocellular carcinoma and cholangiocellular carcinoma.
Ann Nucl Med. 22:83–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ng KK, Poon RT, Lo CM, Yuen J, Tso WK and
Fan ST: Analysis of recurrence pattern and its influence on
survival outcome after radiofrequency ablation of hepatocellular
carcinoma. J Gastrointest Surg. 12:183–191. 2008. View Article : Google Scholar
|
|
19
|
Berg M and Soreide K: EGFR and downstream
genetic alterations in KRAS/BRAF and PI3K/AKT pathways in
colorectal cancer: implications for targeted therapy. Discov Med.
14:207–214. 2012.PubMed/NCBI
|
|
20
|
Radisavljevic Z: AKT as locus of cancer
angiogenic robustness and fragility. J Cell Physiol. 228:21–24.
2013. View Article : Google Scholar
|
|
21
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balogova L, Maslanakova M, Dzurova L,
Miskovsky P and Stroffekova K: Bcl-2 proapoptotic proteins
distribution in U-87 MG glioma cells before and after hypericin
photodynamic action. Gen Physiol Biophys. 32:179–187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danial NN: BAD: undertaker by night,
candyman by day. Oncogene. 27(Suppl 1): S53–S70. 2008. View Article : Google Scholar
|
|
24
|
Levine B, Sinha S and Kroemer G: Bcl-2
family members: dual regulators of apoptosis and autophagy.
Autophagy. 4:600–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stauffer SR: Small molecule inhibition of
the Bcl-X(L)-BH3 protein-protein interaction: proof-of-concept of
an in vivo chemopotentiator ABT-737. Curr Top Med Chem. 7:961–965.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hojabrpour P, Waissbluth I, Ghaffari M,
Cox ME and Duronio V: CaMKII-gamma mediates phosphorylation of BAD
at Ser170 to regulate cytokine-dependent survival and
proliferation. Biochem J. 442:139–149. 2012. View Article : Google Scholar
|
|
27
|
Yin DL, Jiang HC, Liang YJ, Meng XZ, Wang
JB, Zheng TS and Liu LX: Precise hepatectomy guided by minimally
invasive surgery: a novel strategy for liver resection.
Hepatogastroenterology. 59:1951–1959. 2012.PubMed/NCBI
|