Introduction
Neuroendocrine tumors (NETs) have an average annual
incidence of 2 per 100,000 cases among all tumors of the
gastrointestinal tract. NETs primarily arise in the
bronchopulmonary or gastrointestinal tracts (e.g., pancreas, ileum
or appendix) and account for ~70% of all NETs found in the body
(1–3).
However, NETs are frequently reported as metastatic liver tumors,
although the liver itself is rarely described as the site of the
primary tumor. Since Edmondson first reported a case of primary
hepatic NET (PHNET) (4), <100
cases have been reported in the English-language literature
(3,5–9). The
clinicopathological characteristics of PHNETs reveal that (i) they
occur at a relatively young age (mean, 45 years), (ii) there is no
known gender predominance, (iii) the majority of the cases are
asymptomatic, (iv) the pathological diagnosis requires
immunohistochemistry and (v) various therapeutic approaches may be
attempted for PHNETs, such as hepatic lobectomy, systemic
chemotherapy, transhepatic arterial chemoembolization (tace),
radiofrequency ablation and liver transplantation (10–12). To
the best of our knowledge, there has been no report of PHNET with
multiple liver metastases in a patient aged >85 years to
date.
Case report
A 87-year-old man was referred to Kagawa University
Hospital (Kagawa, Japan) with multiple liver tumors identified on
abdominal ultrasound. The assessment performed on admission
included physical examination, computed tomography (CT) during
hepatic angiography and CT during arterial portography, and
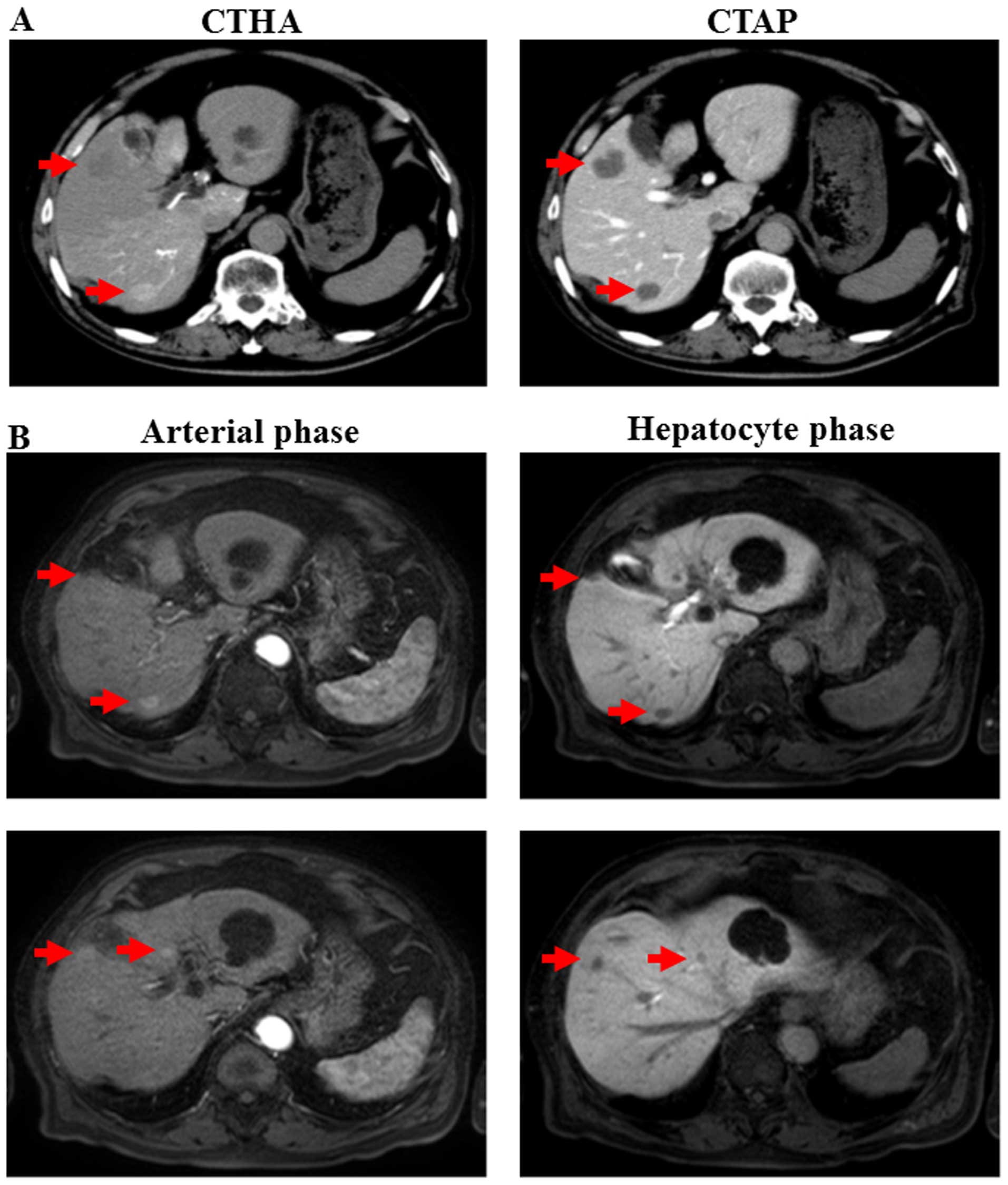
revealed multiple liver tumors (20 mm maximum diameter) (Fig. 1A). The liver tumors also exhibited
hyperechogenicity and hypoechogenicity with acoustic shadows on
ultrasonography (data not shown). On contrast-enhanced magnetic
resonance imaging (MRI) using gadolinium ethoxybenzyl
diethylenetriamine pentaacetic acid, certain tumors exhibited
contrast enhancement during the early phase and contrast washout
during the hepatocyte phase (Fig.
1B). By contrast, no lesions were identified during positron
emission tomography (PET) imaging of the liver (data not shown). A
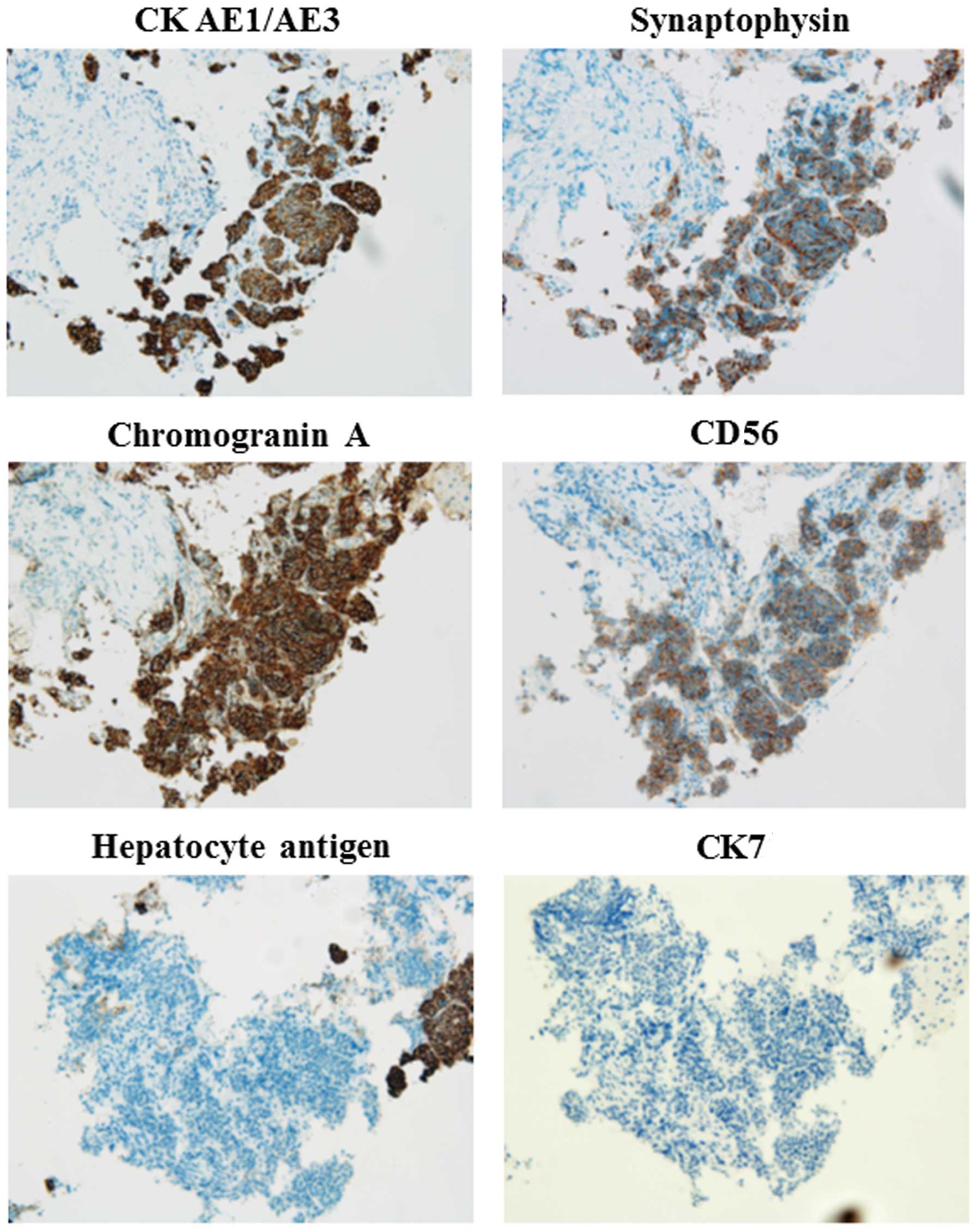
liver biopsy was performed and immunohistochemical staining
revealed enhanced expression of cytokeratin (CK) AE1/AE3,
synaptophysin, chromogranin A and CD56, and no expression of
hepatocyte antigen or CΚ7 (Fig. 2).
No other primary lesion was detected and the patient was diagnosed
with PHNETs. The mindbomb E3 ubiquitin protein ligase-1 index was
~2% in most of the tumor. The patient underwent TACE with a
combination of miriplatin (total dose of 84 mg) mixed with gelatin
sponge particles and lipiodol. The patient was treated using 3
courses of TACE, and partial response was identified during the
follow-up examination, 21 months after the liver biopsy.
Discussion
NETs commonly develop in the gastrointestinal tract,
pancreas and bronchopulmonary tract. In the majority of those
cases, NETs detected in the liver have metastasized from different
organs, and primary NETs originating in the liver are quite rare
(13). PHNETs may be difficult to
diagnose, even with pathological evidence, and must be
differentiated from metastatic liver tumors. Therefore, clinical
characteristics and imaging methods, including CT and MRI, are also
crucial for definitively diagnosing PHNETs. In the present study,
we diagnosed multiple liver tumors as PHNETs by histology and by
applying various imaging modalities, such as CT, MRI and PET.
Non-specific symptoms, such as abdominal pain and
distension, may be associated with the early stages of this
disease. Additionally, several patients with gastrointestinal NETs
suffer from carcinoid syndrome (14).
This syndrome occurs in <10% of patients with gastrointestinal
NETs and is associated with liver metastasis. Of note, carcinoid
syndrome is rarely observed in PHNET patients (15).
PHNETs primarily occur in patients between aged
40–50 years and are usually located in the right lobe of the liver
(10). In the present study, the
onset age of PHNET was 87 years and the tumors were located in both
lobes of the liver. PHNETs may slowly metastasize to the other side
of the hepatic lobe. To date, this is the first report of a PHNET
patient aged >85 years.
Although the origin of PHNETs has not been
elucidated, it has been hypothesized that NET cells may spread to
the intrahepatic biliary tract and undergo malignant
transformation, or that malignant stem cells may be
transdifferentiated to NET cells (16). As PHNETs are rare, slow-growing and
asymptomatic, early-stage diagnosis may be difficult. In the
present study, a patient aged 87 years presented with multiple
intrahepatic tumors that were diagnosed as PHNETs. Therefore, it is
crucial to elucidate the etiology and mechanism underlying the
development of these tumors.
Immunohistochemical analysis is the most effective
method for the diagnosis of PHNETs. In our study, representative
immunohistochemical markers, such as CK AE1/AE3, synaptophysin,
chromogranin A and CD56, were positive (Fig. 2). Additionally, hepatocyte antigen and
CK7, which are not expressed in NETs, were negative (Fig. 2). Sundin et al (17) reported that synaptophysin and
chromogranin A were useful markers for a definitive diagnosis of
PHNETs. Moreover, the quantification of chromogranin A in the
plasma may be used for the follow-up evaluation of NETs (1). These reports suggest that
immunohistochemical markers are powerful tools for diagnosing
PHNETs.
Surgical treatment is the only curative method, with
5- and 10-year survival rates of 78 and 59%, respectively (18). The majority of the PHNET patients
underwent surgical resection if surgery was indicated. In our case,
multiple PHNETs were detected in both lobes of the liver, and
surgical resection was not considered to be an option. Therefore,
TACE was performed with cisplatin. Yao et al (19) reported that hepatic chemoembolization
for NETs effectively improves the clinical symptoms and achieves
tumor control. TACE may be the most effective therapy for PHNETs
with intrahepatic metastasis.
Radiofrequency ablation (RFA) may be used to treat
HCCs sized <5 cm (20,21) when <3 tumors are present (21). Our case had at least 4 tumors in both
hepatic lobes (Fig. 1); thus, RFA was
not indicated. However, RFA may be useful for treating unresectable
PHNETs.
In conclusion, PHNETs are rare, particularly in
elderly individuals. immunohistochemistry is key to accurately
diagnosing PHNETs. Surgery is the only curative option, but TACE
and/or RFA may be considered as alternative approaches in the case
of unresectable PHNETs.
References
|
1
|
Modlin IM, Kidd M, Latich I, Zikusoka MN
and Shapiro MD: Current status of gastrointestinal carcinoids.
Gastroenterology. 128:1717–1751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Modlin IM, Sandor A, Tang LH, Kidd M and
Zelterman D: A 40-year analysis of 265 gastric carcinoids. Am J
Gastroenterol. 92:633–638. 1997.PubMed/NCBI
|
|
3
|
Modlin IM, Shapiro MD and Kidd M: An
analysis of rare carcinoid tumors: Clarifying these clinical
conundrums. World J Surg. 29:92–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edmondson H: Tumor of the liver and
intrahepatic bile duct. Atlas of Tumor Pathology. section 7,
fascicle 25. Armed Forces Institute of Pathology. (Washington).
105–109. 1958.
|
|
5
|
Kehagias D, Moulopoulos L, Smirniotis V,
Pafiti A, Ispanopoulos S and Vlahos L: Imaging findings in primary
carcinoid tumour of the liver with gastrin production. Br J Radiol.
72:207–209. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin CW, Lai CH, Hsu CC, Hsu CT, Hsieh PM,
Hung KC and Chen YS: Primary hepatic carcinoid tumor: A case report
and review of the literature. Cases J. 2:902009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwartz G, Colanta A, Gaetz H, Olichney J
and Attiyeh F: Primary carcinoid tumors of the liver. World J Surg
Oncol. 6:912008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu H, Sun K, Ward SC, Schwartz M, Thung
SN and Qin L: Primary hepatic signet ring cell neuroendocrine
tumor: A case report with literature review. Semin Liver Dis.
30:422–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mima K, Beppu T, Murata A, Otao R, Miyake
K, Okabe H, Masuda T, Okabe K, Sugiyama S, Chikamoto A, et al:
Primary neuroendocrine tumor in the liver treated by hepatectomy:
Report of a case. Surg Today. 41:1655–1660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gurung A, Yoshida EM, Scudamore CH, Hashim
A, Erb SR and Webber DL: Primary hepatic neuroendocrine tumour
requiring live donor liver transplantation: Case report and concise
review. Ann Hepatol. 11:715–720. 2012.PubMed/NCBI
|
|
11
|
Yang K, Cheng YS, Yang JJ, Jiang X and Guo
JX: Primary hepatic neuroendocrine tumor with multiple liver
metastases: A case report with review of the literature. World J
Gastroenterol. 21:3132–3138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamberts SW, Hofland LJ and Nobels FR:
Neuroendocrine tumor markers. Front Neuroendocrinol. 22:309–339.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yalav O, Ülkü A, Akçam TA, Demiryürek H
and Doran F: Primary hepatic neuroendocrine tumor: Five cases with
different preoperative diagnoses. Turk J Gastroenterol. 23:272–278.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shetty PK, Baliga SV, Balaiah K and Gnana
PS: Primary hepatic neuroendocrine tumor: An unusual cystic
presentation. Indian J Pathol Microbiol. 53:760–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mehta DC, Warner RR, Parnes I and Weiss M:
An 18-year follow-up of primary hepatic carcinoid with carcinoid
syndrome. J Clin Gastroenterol. 23:60–62. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaya G, Pasche C, Osterheld MC, Chaubert P
and Fontolliet C: Primary neuroendocrine carcinoma of the liver: An
autopsy case. Pathol Int. 51:874–878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sundin A, Eriksson B, Bergström M,
Långström B, Oberg K and Orlefors H: PET in the diagnosis of
neuroendocrine tumors. An NY Acad Sci. 1014:246–257. 2004.
View Article : Google Scholar
|
|
18
|
Knox CD, Anderson CD, Lamps LW, Adkins RB
and Pinson CW: Long-term survival after resection for primary
hepatic carcinoid tumor. Ann Surg Oncol. 10:1171–1175. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao KA, Talamonti MS, Nemcek A, Angelos P,
Chrisman H, Skarda J, Benson AB, Rao S and Joehl RJ: Indications
and results of liver resection and hepatic chemoembolization for
metastatic gastrointestinal neuroendocrine tumors. Surgery.
130:677–682; discussion 682–685. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boonsirikamchai P, Loyer EM, Choi H and
Charnsangavej C: Planning and follow-up after ablation of hepatic
tumors: Imaging evaluation. Surg Oncol Clin N Am. 20:301–315, viii.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gamblin TC, Christians K and Pappas SG:
Radiofrequency ablation of neuroendocrine hepatic metastasis. Surg
Oncol Clin N Am. 20:273–279, vii-viii. 2011. View Article : Google Scholar : PubMed/NCBI
|