Introduction
Mucosal complications, such as stomatitis, including
aphtha and ulcerative lesions, are common with tyrosine kinase
inhibitors (TKIs) (1). In the
Lux-Lung-3 trial, 72.1% of the patients treated with afatinib
developed stomatitis, with 8.7% exhibiting grade >3 stomatitis
(2). Corticosteroids have been used
to treat this complication; however, a standard management for
TKI-induced stomatitis has not yet been established.
Azulene, a chamomile extract, possesses
anti-inflammatory properties (3–6). Sodium
azulene sulfonate (Azunol) is derived from azulene and is used to
treat chronic gastritis and ulcers (7,8). The
toxicological safety of Azunol preparations in the oral cavity has
been previously described (9,10), and
Azunol has been used for gargle and mouthwash preparations. To
evaluate the management of stomatitis, oral care with a mouthwash
and gargle liquid containing Azunol was administered to patients
treated with afatinib, a second-generation epidermal growth factor
receptor (EGFR)-TKI. With regard to Hangeshashinto, a traditional
herbal (Kampo) medicine, there have been previous reports of its
favorable effects on chemotherapy- and chemoradiotherapy-induced
stomatitis (11–14). Patients who developed stomatitis were
treated with a mouthwash containing Hangeshashinto, which has been
found to reduce CPT-11induced gastrointestinal tract complications
(15,16). The present study was conducted to
evaluate the efficacy of these medicines in controlling stomatitis
in a small cohort of lung cancer patients treated with
afatinib.
Patients and methods
Patients
This singlecenter, retrospective chart review study
was conducted at the Ryugasaki Saiseikai Hospital (Ryugasaki,
Japan). All patients who were treated with afatinib for EGFRmutated
nonsmallcell lung cancer (NSCLC) between January, 2015 and March,
2016 were included in this study. The study conformed to the
Ethical Guidelines for Clinical Studies issued by the Ministry of
Health, Labor and Welfare of Japan and ethical approval was
obtained from the Institutional Review Boards of Ryugasaki
Saiseikai Hospital and Mito Medical Center, University of Tsukuba
(no. 16–16). Toxicity was graded according to the National Cancer
Institute Common Toxicity Criteria (CTC), version 3.0.
Oral care protocol
The oral care protocol included assessment of the
oral cavity by a doctor prior to TKI therapy, self-management or
nurse assistance if necessary [brushing the teeth, gums and tongue
using a soft toothbrush, and oral care plus 5 ml of Azunol gargle
liquid 4% mouthwash (Nippon Shinyaku Co., Ltd., Kyoto, Japan) taken
four times daily]. The appearance of the gingiva and teeth was
daily assessed by nurses. Treatment with Hangeshashinto was
initiated when mild mucositis occurred (grade >1 according to
the CTC criteria). The protocol was started immediately prior to
TKI therapy and was included in the medical records. The evaluation
of safety and efficacy was based on adverse events (AEs), physical
examination and laboratory examinations.
Results
Patient characteristics
During the study period, 14 NSCLC patients were
treated with afatinib. The patient characteristics are summarized
in Table I. Two patients with
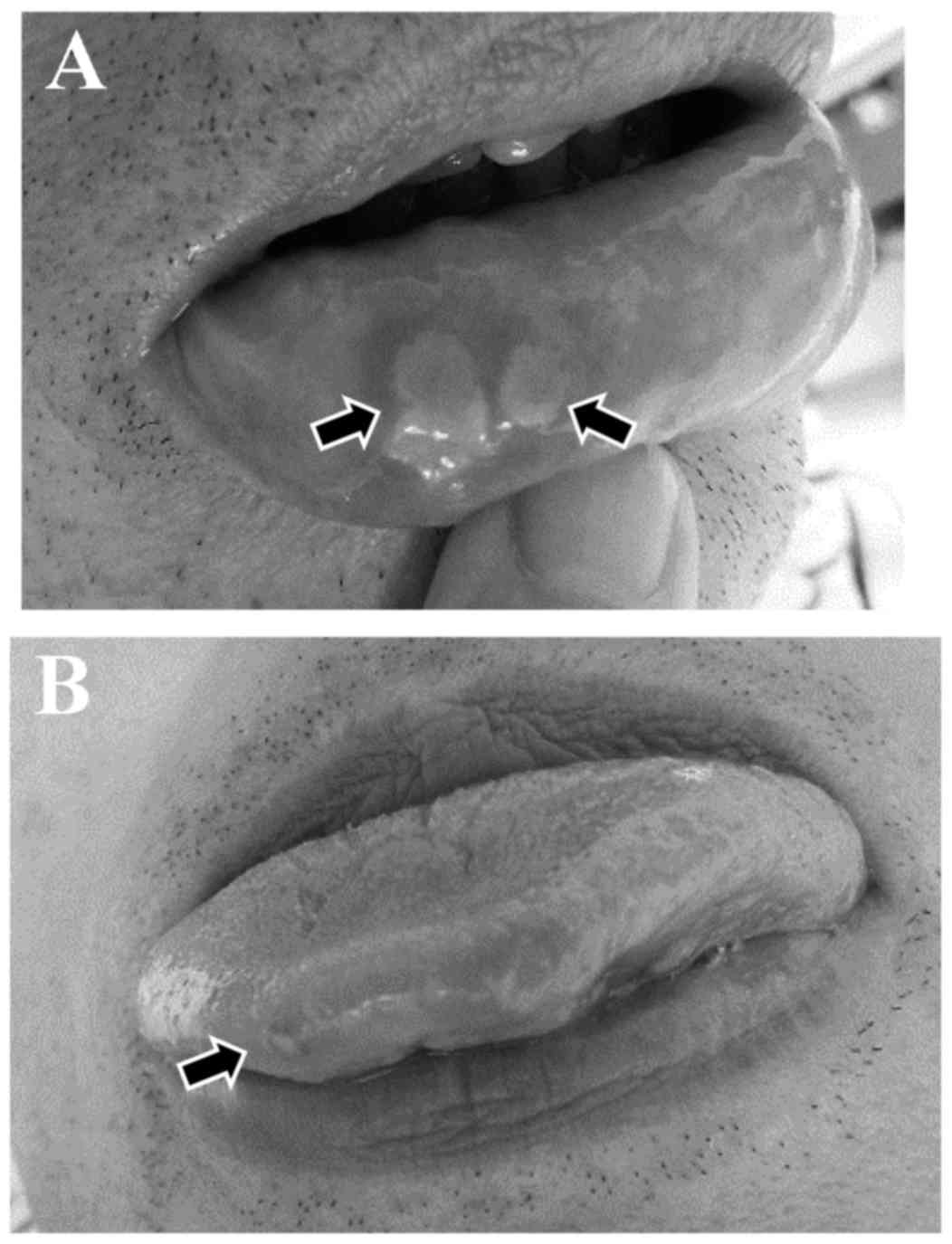
stomatitis prior to the initiation of Aznol mouthwash and
Hangeshashinto therapy are shown in Fig.
1. Of the 14 patients, 2 already had stomatitis (grade 1 and 2,
respectively) at the time of initiation of afatinib therapy. These
2 patients were treated with Aznol mouthwash and Hangeshashinto. Of
the remaining 12 NSCLC patients, 2 (1 man and 1 woman; 16.7%)
developed grade 2 stomatitis and grade 2 dysgeusia (‘sweet taste
failure’). All stomatitis patients developed the complication
within 2 weeks from the initiation of afatinib therapy. Afatinib
was discontinued for 1 week and the patients received Aznol
mouthwash and a prescription of Hangeshashinto. All the lesions
were completely alleviated 2 weeks after the initiation of Aznol
and Hangeshashinto therapy. Afatinib therapy was re-initiated, but
none of the patients developed stomatitis thereafter.
 | Table I.Characteristics of 14 patients treated
with afatinib. |
Table I.
Characteristics of 14 patients treated
with afatinib.
| Characteristics | n (%) |
|---|
| Age (years) |
|
|
Median | 71 |
|
Range | 55 84 |
| Gender |
|
| Male | 7 (50.0) |
|
Female | 7 (50.0) |
| Performance
status |
|
| 0–1 | 10 (71.4) |
| 2–4 | 4 (28.6) |
| Histology |
|
|
Adenocarcinoma | 14 (100.0) |
| EGFR mutations |
|
| Ex21
L858R | 4 (28.6) |
| Ex19
del | 9 (63.3) |
| Ex18
G719X | 1 (7.1) |
Discussion
Stomatitis is one of the most common AEs encountered
in TKI therapy for NSCLC. Stomatitis may limit the patients'
ability to tolerate chemotherapy and their nutritional status is
compromised; it may also drastically affect cancer treatment and
the patients' quality of life. The exact pathophysiology underlying
the development of stomatitis has not been fully elucidated;
however, it may be classified as direct and indirect stomatitis
(17). TKIs may interfere with the
normal turnover of the epithelial cells, leading to mucosal injury;
it may also occur due to indirect invasion by microorganisms.
Afatinib is a second-line TKI, which covalently
binds to a tyrosine kinase, preventing tumor growth by continuously
inhibiting its phosphorylation; as a result, it exerts a strong
antitumor effect in EGFRmutated NSCLC patients (2,18).
However, afatinib is associated with certain AEs, such as skin
rash, paronychia and stomatitis (2,18). The
incidence of these AEs is not low, and these complications may
occasionally require treatment discontinuation. In the Lux-Lung 3
trial, a 70% incidence of mucositis was reportedin patients treated
with afatinib (2).
Oral care during treatment is crucial for preventing
the development of stomatitis. Several treatment options are
available for preventing and treating this condition, but none have
yet been established as standard treatment. Efforts are currently
focused on better understanding this condition, so that the optimal
therapeutic regimens may be selected. Although stomatitis is rarely
life-threatening, it interferes with cancer treatment to a great
extent. Progress in the prevention and management of stomatitis
will significantly improve the quality of life of the patients.
The mainstay of management of chemotherapy- or
radiation-induced stomatitis is thorough oral hygiene and rinsing
with certain remedies (19–21), although none have been established as
standard treatment at present. There have been some reports of the
favorable effects of Hangeshashinto on chemotherapy and
chemoradiotherapy-induced stomatitis (11–14). As
regards EGFR-TKIinduced stomatitis, to the best of our knowledge,
there have no reports evaluating oral care in the management of
stomatitis to date. Although the number of patients included in
this study was limited, the results indicating that the incidence
of stomatitis was decreased by Aznol mouthwash and Hangeshashinto
therapy are considered to be meaningful. Compared with skin rash
and paronychia, stomatitis appears to be overlooked, as some
patients have no such complaints. However, these lesions may be
identified upon careful oral assessment by certified nurses. We
herein demonstrated that Azunol mouthwash and Hangeshashinto
effectively controlled EGFR-TKI-induced stomatitis, without any
AEs. This preventive care and treatment may reduce the incidence of
oral complications associated with EGFR-TKI therapy.
References
|
1
|
Califano R, Tariq N, Compton S, Fitzgerald
DA, Harwood CA, Lal R, Lester J, McPhelim J, Mulatero C,
Subramanian S, et al: Expert Consensus on the Management of Adverse
Events from EGFR Tyrosine Kinase Inhibitors in the UK. Drugs.
75:1335–1348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sequist LV, Yang JC, Yamamoto N, O'Byrne
K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al:
Phase III study of afatinib or cisplatin plus pemetrexed in
patients with metastatic lung adenocarcinoma with EGFR mutations. J
Clin Oncol. 31:3327–3334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ge ZD, Auchampach JA, Piper GM and Gross
GJ: Comparison of cardioprotective efficacy of two thromboxane A2
receptor antagonists. J Cardiovasc Pharmacol. 41:481–488. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rekka E, Chrysselis M, Siskou I and
Kourounakis A: Synthesis of new azulene derivatives and study of
their effect on lipid peroxidation and lipoxygenase activity. Chem
Pharm Bull. 50:904–907. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akagi M, Matsui N, Mochizuki S and Tasaka
K: Inhibitory effect of egualen sodium: A new stable derivative of
azulene on histamine release from mast cell-like cells in the
stomach. Pharmacology. 63:203–209. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kourounakis AP, Rekka EA and Kourounakis
PN: Antioxidant activity of guaiazulene and protection against
paracetamol hepatotoxicity in rats. J Pharm Pharmacol. 49:938–942.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yano S, Horie S, Wakabayashi S, Mochizuki
S, Tomiyama A and Watanabe K: Increasing effect of sodium
3-ethyl-7-isopropyl-1-azulenesulfonate 1/3 hydrate (KT1-32), a
novel antiulcer agent, on gastric mucosal blood flow in
anesthetized. Res Commun Chem Pathol Pharmacol. 70:253–256.
1990.PubMed/NCBI
|
|
8
|
Okabe S, Takeuchi K, Mori Y, Furukawa O
and Yamada Y: [Effects of KT1-32 on acute gastric lesions and
duodenal ulcers induced in rats]. Nippon Yakurigaku Zasshi.
88:467–476. 1986.(in Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seki J, Mukai H and Sugiyama M: Studies on
the absorption of sodium guaiazulene-3-sulfonate. II. Absorption
mechanism from nasal and intestinal membrane. J Pharmacobiodyn.
8:337–343. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanzlik RP and Bhatia P: Metabolism of
azulene in rats. Xenobiotica. 11:779–783. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuda C, Munemoto Y, Mishima H, Nagata
N, Oshiro M, Kataoka M, Sakamoto J, Aoyama T, Morita S and Kono T:
Double-blind, placebo-controlled, randomized phase II study of
TJ-14 (Hangeshashinto) for infusional fluorinated-pyrimidine-based
colorectal cancer chemotherapy-induced oral mucositis. Cancer
Chemother Pharmacol. 76:97–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamashita T, Araki K, Tomifuji M, Kamide
D, Tanaka Y and Shiotani A: A traditional Japanese
medicine-Hangeshashinto (TJ-14)-alleviates chemoradiation-induced
mucositis and improves rates of treatment completion. Support Care
Cancer. 23:29–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aoyama T, Nishikawa K, Takiguchi N, Tanabe
K, Imano M, Fukushima R, Sakamoto J, Oba MS, Morita S, Kono T, et
al: Double-blind, placebo-controlled, randomized phase II study of
TJ-14 (hangeshashinto) for gastric cancer chemotherapy-induced oral
mucositis. Cancer Chemother Pharmacol. 73:1047–1054. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kono T, Kaneko A, Matsumoto C, Miyagi C,
Ohbuchi K, Mizuhara Y, Miyano K and Uezono Y: Multitargeted effects
of hangeshashinto for treatment of chemotherapy-induced oral
mucositis on inducible prostaglandin E2 production in human oral
keratinocytes. Integr Cancer Ther. 13:435–445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okumi H and Koyama A: Kampo medicine for
palliative care in Japan. Biopsychosoc Med Jan. 8:62014. View Article : Google Scholar
|
|
16
|
Ohnishi S and Takeda H: Herbal medicines
for the treatment of cancer chemotherapy-induced side effects.
Front Pharmacol. Feb;10(6): 142015.https://doi.org/10.3389/fphar.2015.00014
|
|
17
|
Naidu MU, Ramana GV, Rani PU, Mohan IK,
Suman A and Roy P: Chemotherapy-induced and/or radiation
therapy-induced oral mucositis-complicating the treatment of
cancer. Neoplasia. 6:423–431. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ke EE and Wu YL: Afatinib in the
first-line treatment of epidermal-growth-factor-receptor
mutation-positive non-small cell lung cancer: A review of the
clinical evidence. Ther Adv Respir Dis. 10:256–264. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bardy J, Molassiotis A, Ryder WD, Mais K,
Sykes A, Yap B, Lee L, Kaczmarski E and Slevin N: A double-blind,
placebo-controlled, randomised trial of active manuka honey and
standard oral care for radiation-induced oral mucositis. Br J Oral
Maxillofac Surg. 50:221–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogata J, Minami K, Horishita T, Shiraishi
M, Okamoto T, Terada T and Sata T: Gargling with sodium azulene
sulfonate reduces the postoperative sore throat after intubation of
the trachea. Anesth Analg. 101:290–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yen SH, Wang LW, Lin YH, Jen YM and Chung
YL: Phenylbutyrate mouthwash mitigates oral mucositis during
radiotherapy or chemoradiotherapy in patients with head-and-neck
cancer. Int J Radiat Oncol Biol Phys. 82:1463–1470. 2012.
View Article : Google Scholar : PubMed/NCBI
|