Introduction
The combination of docetaxel, cisplatin and
5-fluorouracil (DCF regimen) is currently used to treat human
malignant diseases, including various progressive head and neck
squamous cell carcinomas and advanced gastric and esophageal
cancers (1,2). However, a high incidence of grade
III/IV adverse events (AEs) resulting from these compounds works
against the therapeutic efficacy of the DCF program (3). Therefore, optimizing the conventional
DCF program may lead to a comparable efficacy, but with lower
toxicity.
The oral anti-cancer precursor molecule,
capecitabine, is metabolized to 5-fluorouracil by thymidine
phosphorylase (TP) in tumor cells, and its efficacy has been
established in multiple clinical trials (4). It is more effective than (or at least
not inferior to) 5-fluorouracil against a variety of malignant
solid tumor types (5). Therefore,
capecitabine may be ideal for replacing intravenous 5-fluorouracil.
Oxaliplatin is a third-generation, platinum-containing anti-cancer
drug and several clinical studies have reported that the one-year
survival rate of patients who had received an anti-cancer regimen
containing oxaliplatin was significantly higher than that of
patients who had received cisplatin regimens, while less toxicity
was documented (6). In addition,
oxaliplatin and docetaxel can increase TP activity in tumor cells
(7) and may have a synergistic
anti-tumor effect when concurrently applied with capecitabine.
Therefore, replacement of cisplatin and 5-fluorouracil in the
traditional DCF regimen with oxaliplatin and capecitabine,
respectively (DOX regimen) may offer better efficacy and fewer
AEs.
In the present phase-I clinical trial, the safety
and tolerability of this modified DOX regimen for treating advanced
gastric cancer were estimated. Capecitabine was gradually increased
from 1,500 mg/m2 [day 1 (d1)-d7],
while the dose of the other drugs was kept constant (docetaxel, 75
mg/m2 and oxaliplatin, 100 mg/m2,
d1). The present study aimed to determine the
dose-limiting toxic dose and maximum tolerated dose (MTD) of the
DOX regimen.
Materials and methods
Study design
The present study reported on a single-center,
non-randomized, open-label, single-arm phase-I trial enrolling
patients (n=24) with advanced gastric cancer who were seen at the
Affiliated Cancer Hospital of Guangxi Medical University (Nanning,
China) between October 2009 and December 2012. All of the patients
provided written informed consent and the trial was approved by the
ethics committee of the Affiliated Cancer Hospital of Guangxi
Medical University (Nanning, China). This trial was registered in
the Chinese Clinical Trial Registry (ChiCTR-ONRC-13,004,023).
Inclusion criteria were as follows: i) Advanced
gastric cancer confirmed histologically or cytologically; ii) no
previous treatment with docetaxel, oxaliplatin or capecitabine, and
the last chemotherapy cycle completed at least 4 weeks prior to
enrollment; iii) an age of 18–70 years; iv) an Eastern Cooperative
Oncology Group Performance Status (ECOG-PS) ≤1; v) estimated
survival of at least 3 months; vi) blood parameters as follows:
White blood cells ≥4.0×109/l, absolute neutrophil count
≥2.0×109/l, hemoglobin ≥100 g/l, platelets
≥100.0×1012/l; vii) aspartate aminotransferase and
alanine aminotransferase ≤2.5 times the upper limit of normal
levels in liver function tests, and normal renal function; and
viii) written informed consent.
Exclusion criteria were as follows: i) Allergies to
docetaxel, oxaliplatin or capecitabine; ii) inability to receive
oral capecitabine; iii) malabsorption syndromes; iv) serious heart
and liver dysfunction; v) brain metastases; and vii) any other
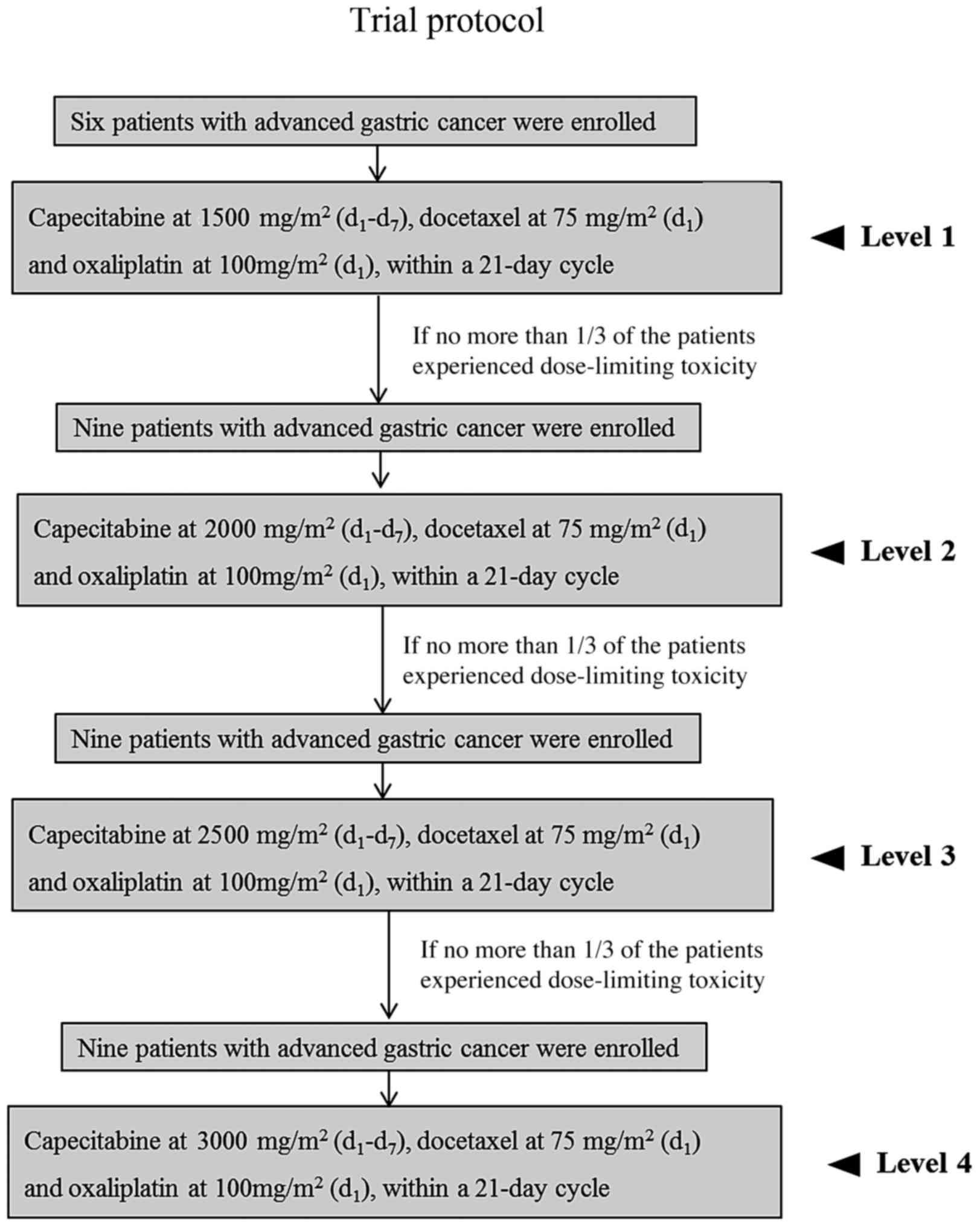
factor rendering the subject unsuitable for the trial. The trial
protocol is illustrated in Fig.
1.
Safety evaluation
The medical history of each patient was reviewed and
documented, and a physical examination, blood biochemistry,
electrocardiograms and other tests were performed seven days prior
to initiation of the DOX program. Routine blood examination was
performed on d1, d10 and d14 of
every cycle, and liver and renal function tests were performed
every week during treatment. AEs were evaluated according to the
Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE
4.0) (8).
Treatment
Dose escalation was performed in cohorts of six
patients beginning at an initial capecitabine dose of 1,500
mg/m2 (750 mg/m2, per os bidaily) for seven
days, docetaxel [75 mg/m2, intravenous (iv) infusion
over 1 h] and oxaliplatin (100 mg/m2 iv infusion over 2
h) on d1, within a 21-day cycle. Each cohort was
observed for 21 days at the set dose level. If no more than
one-third of the patients experienced a dose-limiting toxicity
(DLT), a subsequent cohort of nine additional patients was treated
at the next higher dose level. Dosing regimens were as follows:
Docetaxel, 75 mg/m2 (d1), oxaliplatin, 100
mg/m2 (d1) and capecitabine was dosed at
increasing levels (1,500 mg/m2 for level 1, 2,000
mg/m2 for level 2, 2,500 mg/m2 for level 3
and 3,000 mg/m2 for level 4, d1–7). DLT was
defined as the occurrence of any grade-IV hematological toxicity
during the first cycle or any non-hematological grade-III or -IV
toxicity, excluding nausea and alopecia, over the 2-week delay
prior to the next cycle. Skin toxicity, vomiting and diarrhea were
only regarded as DLTs if they remained at grade III or above
despite optimal treatment. After recovery from DLTs or other
toxicities attributed to the study medication, the patients
continued their chemotherapy treatment at a modified appropriate
dose. The MTD was defined as the highest dose at which no more than
one-third of the patients experienced DLTs. Chemotherapy was
discontinued if disease progression or intolerable AEs occurred, or
if the patients refused to continue the treatment.
Efficacy evaluation
Physical examination, X-ray and spiral computed
tomography were performed to evaluate measurable lesions within 14
days after initiation of therapy. Treatment responses were assessed
using Response Evaluation Criteria in Solid Tumors, version 1.1
(RECIST 1.1) every 6 weeks (9).
Results
Patient characteristics
Between October 2009 and December 2012, 24 patients
with advanced gastric cancer were recruited at the Department of
Medical Oncology of the Affiliated Cancer Hospital of Guangxi
Medical University (Nanning, China). The demographic data and
characteristics of the patients are shown in Table I. All patients completed at least two
cycles of chemotherapy with the DOX regimen, and 13 patients
completed at least six cycles. The median number of completed
chemotherapy cycles was 4.7.
 | Table I.Characteristics of the patients with
advanced gastric cancer. |
Table I.
Characteristics of the patients with
advanced gastric cancer.
| Patient
IDa | Age (years) | Gender | TNM
Stageb | ECOG
PSc | Completed cycles
(n) |
|---|
| 1–01 | 62 | F | IV (T4N1M1) | 1 | 6 |
| 1–02 | 61 | M | III (T4N2M0) | 1 | 6 |
| 1–03 | 48 | M | IV (T4N1M1) | 1 | 2 |
| 1–04 | 42 | M | IV (T3N1M1) | 1 | 6 |
| 1–05 | 64 | M | III (T3N2M0) | 0 | 2 |
| 1–06 | 59 | M | III (T3N2M0) | 1 | 5 |
| 2–07 | 59 | M | IV (T2N2M1) | 1 | 2 |
| 2–08 | 52 | M | III (T2N3M0) | 0 | 6 |
| 2–09 | 64 | M | IV (T3N2M1) | 1 | 6 |
| 2–10 | 54 | M | III (T4N1M0) | 1 | 4 |
| 2–11 | 69 | M | IV (T3N1M1) | 1 | 6 |
| 2–12 | 31 | M | III (T4N1M0) | 1 | 8 |
| 2–13 | 54 | M | IV (T4N1M1) | 1 | 2 |
| 2–14 | 62 | F | IV (T2N2M1) | 1 | 4 |
| 2–15 | 50 | M | IV (T4N2M1) | 1 | 4 |
| 3–16 | 45 | F | IV (T3N2M1) | 1 | 6 |
| 3–17 | 41 | F | IV (T4N1M1) | 0 | 2 |
| 3–18 | 49 | M | IV (T3N1M1) | 1 | 2 |
| 3–19 | 62 | M | III (T2N3M0) | 0 | 6 |
| 3–20 | 57 | M | III (T2N3M0) | 0 | 6 |
| 3–21 | 36 | M | IV (T4N1M1) | 1 | 6 |
| 3–22 | 48 | F | IV (T3N3M1) | 1 | 6 |
| 3–23 | 41 | F | IV (T3N1M1) | 1 | 4 |
| 3–24 | 46 | M | IV (T4N1M1) | 1 | 6 |
MTD
Table II lists the
toxicities observed at each dosing level. No DLTs occurred in the
first six-patient cohort (docetaxel, 75 mg/m2,
d1; oxaliplatin, 100 mg/m2, d1;
capecitabine, 1,500 mg/m2, d1–7). Of the nine
patients who were treated at level 2 (docetaxel, 75
mg/m2, d1; oxaliplatin, 100 mg/m2,
d1; capecitabine, 2,000 mg/m2,
d1–7), two patients experienced DLTs with grade-IV
leukopenia and/or neutropenia. At dose level 3 (docetaxel, 75
mg/m2, d1; oxaliplatin, 100 mg/m2,
d1; capecitabine, 2,500 mg/m2,
d1–7), three patients developed DLTs, which were
grade-III nausea and vomiting in one patient and grade-IV
leukopenia and neutropenia in the other two patients. Thus, the MTD
of capecitabine had been reached and could not be increased
further. Accordingly, the recommended dose for the DOX program was
docetaxel, 75 mg/m2, d1; oxaliplatin, 100
mg/m2, d1; and capecitabine, 2,000
mg/m2, d1–7.
 | Table II.Adverse events according to the
NCI-CTC. |
Table II.
Adverse events according to the
NCI-CTC.
| Adverse event | Level 1 (n=6) All
grades, n (%) | Level 2 (n=9) All
grades, n (%) | Level 3 (n=9) All
grades, n (%) | Total (n=24) n
(%) |
|---|
| Hematological |
|
|
|
|
|
Leukopenia | 4 (66.7) | 5 (55.6) | 6 (66.7) | 15 (62.5) |
|
Neutropenia | 3 (50.0) | 5 (55.6) | 5 (55.6) | 13 (54.2) |
|
Thrombocytopenia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Anemia | 1 (16.7) | 3 (33.3) | 1 (11.1) | 5 (20.8) |
|
Non-hematological |
|
|
|
|
|
Nausea | 3 (50.0) | 4 (44.4) | 7 (77.8) | 14 (58.3) |
|
Vomiting | 3 (50.0) | 4 (44.4) | 6 (66.7) | 13 (54.2) |
|
Anorexia | 1 (16.7) | 3 (33.3) | 0 (0.0) | 4 (16.7) |
|
Peripheral neuritis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Hand-foot syndrome | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Diarrhea | 0 (0.0) | 1 (11.1) | 0 (0.0) | 1 (4.2) |
|
Pyrexia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Fatigue | 0 (0.0) | 0 (0.0) | 5 (55.6) | 5 (20.8) |
|
Impaired hepatic function | 0 (0.0) | 0 (0.0) | 2 (22.2) | 2 (8.3) |
|
Mucositis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Toxicity
The most common toxicities observed in the present
trial were leukopenia (62.5%), and other hematological AEs included
neutropenia (13/24, 54.2%) and anemia (5/24, 20.8%). The AEs of the
digestive tract included nausea (14/24, 58.3%), vomiting (13/24,
54.2%), anorexia (4/24, 16.7%) and diarrhea (1/24, 4.2%). The
majority of the gastrointestinal side effects were grade I/II,
although the occurrence of grade-III nausea and vomiting required
intravenous nutrition therapy in one patient. Grade-IV leukopenia
and neutropenia were observed in two patients treated with
capecitabine at 2,500 mg/m2 (d1–7). After
treatment with granulocyte colony-stimulating factor, the symptoms
improved, and the patients completed the next chemotherapy cycle
with 20% less capecitabine, which prevented additional AEs. Other
non-hematological toxicities are listed in Table II.
Efficacy
All of the patients enrolled in the present trial
were available for efficacy evaluation after two cycles of
chemotherapy. While none of the patients had complete remission,
two patients had partial remission (PR), 16 had stable disease and
six had progressive disease. Accordingly, the effective rate of the
DOX program was 75.0% (18/24).
Discussion
The DCF program has been used as an effective
regimen for diverse solid tumor types, including advanced gastric,
esophageal as well as head and neck cancers. However, a high
incidence (82%) of grade-III–IV neutropenia has been documented
with this regimen (1). In addition,
cycle delays occurred in 64% of the patients treated with a
standard DCF program, and dose reductions were required for 41% of
the patients (1). As many as 29.3%
of the patients refused to continue the DCF program, and so only
12.2% of the subjects completed eight cycles (10). The prescribed implementation of the
DCF regimen was therefore obstructed by its toxicity, particularly
in elderly patients (age, ≥65 years) (1). Thus, an alternative chemotherapeutic
regimen with a broad spectrum of anti-tumor action, but lacking
overlapping mechanisms associated with AEs, is urgently required.
In the present study, the DCF protocol was modified by replacing
cisplatin and 5-fluorouracil with oxaliplatin and capecitabine,
respectively, and the tolerability, treatment-associated toxicity,
as well as efficacy of this regimen were then examined.
Oxaliplatin is a third-generation platinum compound,
which is as effective as cisplatin, but causes less
gastrointestinal reactions and nephrotoxicity (6). Gu et al (11) have reported a synergistic effect of
oxaliplatin with taxanes. Neurotoxicity is typically responsible
for the DLT attributable to oxaliplatin, which manifests as
peripheral sensory nerve abnormalities (12). It is well known that the incidence
and severity of neurotoxicity induced by oxaliplatin increases in a
dose-dependent manner (12). Due to
the high incidence rate of neurotoxicity previously observed at
doses of 130–200 mg/m2 (11) the dose of oxaliplatin used in the
present study was fixed at 100 mg/m2. Indeed, in the
present study, none of the patients experienced any obvious
neurotoxicity at the set oxaliplatin dose.
By contrast, neutropenia is the most frequent AE of
docetaxel at 70–75 mg/m2 as part of combination regimens
(13). A prospective randomized
trial confirmed that there were no significant differences with
regard to survival for patients who received docetaxel at 75 and
100 mg/m2 as second-line treatments of non-small cell
lung cancer (14). In another
randomized phase-II clinical trial, docetaxel was reduced from 85
to 75 mg/m2 due to AEs (10). Thus, in the present study, the dose
of docetaxel was fixed at 75 mg/m2. In the present
study, neutropenia was identified in 54.2% of the patients, and was
mild and temporary in most patients; however, one patient in the
second dose-level cohort, and two patients in the third dose-level
cohort, developed grade-IV neutropenia, which required treatment
with granulocyte colony-stimulating factor.
Capecitabine is activated through its conversion
into 5-fluorouracil by TP in malignant tissues. In tumor cells, TP
is present at higher concentrations compared with normal cells,
which enables capecitabine to exert a high anti-tumor efficacy,
while exhibiting low toxicity to normal cells. A meta-analysis
based on the REAL-2 and ML 17,032 trials compared the efficacy of
capecitabine- and 5-fluorouracil-containing regimens in treating
advanced stomach and esophageal cancer, revealing a significant
superiority of capecitabine in terms of increasing overall survival
(OS) and the objective response rate (ORR), whereas no significant
difference was identified regarding progression-free survival
(15). Capecitabine is an oral drug,
and its dose can therefore be adjusted to avert toxicity, rendering
it a safer and more convenient therapy. For example, the two
patients in the third dose-level cohort who had suffered grade-IV
neutropenia were able to complete the next chemotherapy cycle with
a reduction of capecitabine by 20%, and this adjustment prevented
the reoccurrence of similar AEs. The observations of the present
study also suggested that the incidence and severity of neutropenia
were associated with the dosage of capecitabine. Of note, docetaxel
and oxaliplatin are able to lead to an upregulation of TP
expression to enhance the anti-cancer activity of capecitabine,
therefore exerting synergic effects (16,17).
The main AEs induced by capecitabine have been
identified as gastrointestinal reactions and hand-foot syndrome
(7), a finding that was confirmed by
the observations of the present study. The standard dosing regimen
for capecitabine comprised two doses (1,000 mg/m2)
administered on a daily basis for 14 days, followed by a seven-day
interval; this was repeated every 21 days. However, as the majority
of the patients treated with capecitabine were not able to complete
14 consecutive days of therapy due to AEs (7), this dosing regimen remains
controversial. Previous pre-clinical studies have indicated that
continuous administration of capecitabine for approximately seven
days offered maximal anti-cancer efficacy, whereas administration
beyond this duration only produced more toxicity while not
increasing efficacy (18).
Amarantidis et al (19) used
a combination of docetaxel, oxaliplatin and capecitabine as a
first-line treatment for advanced gastric cancer. Capecitabine
(2,750 mg/m2) was administered orally, divided into two
daily doses given on d1–7. Cycles were repeated every
two weeks and this regimen was efficacious and safe. However, a
retrospective analysis by Hennessy et al (20) suggested that this dose was too high
for patients to tolerate. Therefore, in the present phase-I
clinical trial, the initial dose of capecitabine was set at 1,500
mg/m2, divided into two daily doses given at
d1–7.
In the present study, none of the patients at dose
level 1 (capecitabine, 1,500 mg/m2) and two of the nine
patients at dose level 2 (capecitabine, 2,000 mg/m2)
experienced DLTs. Furthermore, of the nine patients who were
treated with capecitabine at 2,500 mg/m2 (dose level 3),
one patient experienced severe nausea and vomiting (grade III) and
two developed severe leukopenia and neutropenia (grade IV).
Accordingly, the MTD of capecitabine was established at 2,000
mg/m2. Therefore, the following regimen is recommended
for a phase-II trial: Docetaxel (75 mg/m2,
d1), oxaliplatin (100 mg/m2, d1)
and capecitabine (2,000 mg/m2, d1–7).
The combination of docetaxel (25 mg/m2,
d1 and d8), oxaliplatin (50 mg/m2,
d1 and d8) and capecitabine (1,250
mg/m2, d1–14) has been demonstrated to be a
tolerable and potent day-care regimen for advanced gastroesophageal
cancer (21). In addition, a recent
study has reported that combination chemotherapy comprising
docetaxel (60 mg/m2, d1), oxaliplatin (100
mg/m2, d1) and capecitabine (1,000
mg/m2, d1–21) was effective as a first-line
treatment for metastatic gastric cancer (22). The present study has revealed that a
reduced duration of capecitabine treatment (from 14 to 7 days) plus
docetaxel and oxaliplatin offered comparable efficacy against
advanced gastric cancer with tolerable side-effects. In the present
study, the effective rate of the DOX regimen was 75%, an outcome
similar to that of earlier clinical trials using these drug
combinations (23–26), in which the median time to
progression and median OS were not inferior to those achieved by
the DCF regimen. However, overall clinical outcome for patients
treated with the DOX regimen determined by the present study should
be evaluated in a phase-II trial to compare it with that for
patients treated with other DOX regimens or the conventional DCF
regimen.
In conclusion, the recommended doses for the DOX
regimen were docetaxel at 75 mg/m2 (d1),
oxaliplatin at 100 mg/m2 (d1) and
capecitabine at 2,000 mg/m2 (d1–7). These
doses were generally well tolerated, and the efficacy of this
regimen should be evaluated in a phase-II study.
Acknowledgements
This work was supported by the Guangxi Nature
Science Fund (no. 2010GXNSFA013243) and the Guangxi Health Care Key
Scientific Research Project (no. 200,967).
References
|
1
|
van Cutsem E, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, et al: Phase III study of docetaxel and cisplatin plus
fluorouracil compared with cisplatin and fluorouracil as first-line
therapy for advanced gastric cancer: A report of the V325 study
group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Herpen CM, Mauer ME, Mesia R, Degardin
M, Jelic S, Coens C, Betka J, Bernier J, Remenar E, Stewart JS, et
al: Short-term health-related quality of life and symptom control
with docetaxel, cisplatin, 5-fluorouracil and cisplatin (TPF),
5-fluorouracil (PF) for induction in unresectable locoregionally
advanced head and neck cancer patients (EORTC 24971/TAX 323). Br J
Cancer. 103:1173–1181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Awada A, Gil T, Whenham N, Van Hamme J,
Besse-Hammer T, Brendel E, Delesen H, Joosten MC, Lathia CD, Loembé
BA, et al: Safety and pharmacokinetics of sorafenib combined with
capecitabine in patients with advanced solid tumors: Results of a
phase 1 trial. J Clin Pharmacol. 51:1674–1684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haller DG, Tabernero J, Maroun J, de Braud
F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K and
Schmoll HJ: Capecitabine plus oxaliplatin compared with
fluorouracil and folinic acid as adjuvant therapy for stage III
colon cancer. J Clin Oncol. 29:1465–1471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montagnani F, Turrisi G, Marinozzi C,
Aliberti C and Fiorentini G: Effectiveness and safety of
oxaliplatin compared to cisplatin for advanced, unresectable
gastric cancer: A systematic review and meta-analysis. Gastric
Cancer. 14:50–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hameed H and Cassidy J: Use of
capecitabine in management of early colon cancer. Cancer Manag Res.
3:295–299. 2011.PubMed/NCBI
|
|
8
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The Common Terminology Criteria for
Adverse Events Version 4.0. J Am Acad Dermatol. 67:1025–1039. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roth AD, Fazio N, Stupp R, et al:
Docetaxel, Cisplatin, and Fluorouracil; docetaxel and cisplatin;
and epirubicin, cisplatin, and fluorouracil as systemic treatment
for advanced gastric carcinoma: A randomized phase II trial of the
Swiss group for clinical cancer research. J Clin Oncol.
25:3217–3223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu J, Yamamoto H, Lu X, Ngan CY, Tsujino
T, Konishi K, Takemasa I, Ikeda M, Nagata H, Hashimoto S, et al:
Low-dose oxaliplatin enhances the antitumor efficacy of paclitaxel
in human gastric cancer cell lines. Digestion. 74:19–27. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gamelin E, Gamelin L, Bossi L and
Quasthoff S: Clinical aspects and molecular basis of oxaliplatin
neurotoxicity: Current management and development of preventive
measures. Semin Oncol. 29 5 Suppl 15:S21–S33. 2002. View Article : Google Scholar
|
|
13
|
Piccart MJ and Di Leo A: Future
perspectives of docetaxel (Taxotere) in front-line therapy. Semin
Oncol. 24 4 Suppl 10:S10-S27–S10-S33. 1997.
|
|
14
|
Shepherd FA, Dancey J, Ramlau R, Mattson
K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R,
et al: Prospective randomized trial of docetaxel versus best
supportive care in patients with non-small-cell lung cancer
previously treated with platinum-based chemotherapy. J Clin Oncol.
18:2095–2103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okines AF, Norman AR, McCloud P, Kang YK
and Cunningham D: Meta-analysis of the REAL-2 and ML17032 trials:
Evaluating capecitabine-based combination chemotherapy and infused
5-fluorouracil-based combination chemotherapy for the treatment of
advanced oesophago-gastric cancer. Ann Oncol. 20:1529–1534. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sawada N, Ishikawa T, Fukase Y, Nishida M,
Yoshikubo T and Ishitsuka H: Induction of thymidine phosphorylase
activity and enhancement of capecitabine efficacy by taxol/taxotere
in human cancer xenografts. Clin Cancer Res. 4:1013–1019.
1998.PubMed/NCBI
|
|
17
|
Cassidy J, Tabernero J, Twelves C, Brunet
R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, et
al: XELOX (capecitabine plus oxaliplatin): Active first-line
therapy for patients with metastatic colorectal cancer. J Clin
Oncol. 22:2084–2091. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Traina TA, Dugan U, Higgins B, Kolinsky K,
Theodoulou M, Hudis CA and Norton L: Optimizing chemotherapy dose
and schedule by Norton-Simon mathematical modeling. Breast Dis.
31:7–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amarantidis K, Xenidis N, Chelis L,
Chamalidou E, Dimopoulos P, Michailidis P, Tentes A, Deftereos S,
Karanikas M, Karayiannakis A and Kakolyris S: Docetaxel plus
oxaliplatin in combination with capecitabine as first-line
treatment for advanced gastric cancer. Oncology. 80:359–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hennessy BT, Gauthier AM, Michaud LB,
Hortobagyi G and Valero V: Lower dose capecitabine has a more
favorable therapeutic index in metastatic breast cancer:
Retrospective analysis of patients treated at M. D. Anderson cancer
center and a review of capecitabine toxicity in the literature. Ann
Oncol. 16:1289–1296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goel G, Jauhri M, Negi A and Aggarwal S:
Feasibility study of docetaxel, oxaliplatin and capecitabine
combination regimen in advanced gastric or gastroesophageal
adenocarcinoma. Hematol Oncol Stem Cell Ther. 3:55–59. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Lauro L, Vici P, Belli F, Tomao S,
Fattoruso SI, Arena MG, Pizzuti L, Giannarelli D, Paoletti G, Barba
M, et al: Docetaxel, oxaliplatin and capecitabine combination
chemotherapy for metastatic gastric cancer. Gastric Cancer.
17:718–724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Cutsem E, Boni C, Tabernero J, Massuti
B, Middleton G, Dane F, Reichardt P, Pimentel FL, Cohn A, Follana
P, et al: Docetaxel plus oxaliplatin with or without fluorouracil
or capecitabine in metastatic or locally recurrent gastric cancer:
A randomized phase II study. Ann Oncol. 26:149–156. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stein A, Arnold D, Thuss-Patience PC,
Moehler M, Grothe W, Seufferlein T, Reinacher-Schick A, Geissler M,
Hofheinz RD and Schmoll HJ: Docetaxel, oxaliplatin and capecitabine
(TEX regimen) in patients with metastatic gastric or
gastro-esophageal cancer: Results of a multicenter phase I/II
study. Acta Oncol. 53:392–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sym SJ, Ryu MH, Kang HJ, Lee SS, Chang HM,
Lee JL, Kim TW, Yook JH, Oh ST, Kim BS and Kang YK: Phase I study
of 3-weekly docetaxel, capecitabine and oxaliplatin combination
chemotherapy in patients with previously untreated advanced gastric
cancer. Cancer Chemother Pharmacol. 66:373–380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rivera F, Massutí B, Salcedo M, Sastre J,
Martínez Galán J, Valladares-Ayerbes M, Serrano R, García de
Paredes ML, Manzano JL, Galán M, et al: Phase II trial of miniDOX
(reduced dose docetaxel-oxaliplatin-capecitabine) in ‘suboptimal’
patients with advanced gastric cancer (AGC). TTD 08–02. Cancer
Chemother Pharmacol. 75:319–324. 2015. View Article : Google Scholar : PubMed/NCBI
|