Introduction
Pre and postoperative chemotherapy in addition to
surgery have significantly increased the survival rate for patients
with osteosarcoma (1,2). However, over the last 20 years,
attempts at more intense chemotherapeutic therapy using
conventional cancer agents have not improved the survival rate
significantly. Furthermore, in spite of an aggressive surgical and
chemotheraputic treatment strategy, patients with unresectable
primary osteosarcoma and those with distant metastases still have a
poor prognosis (3–5). The prognosis strongly correlates with
the tumor histological response to preoperative chemotherapy in
osteosarcoma (6,7). However, this valuable standard
criterion is available only following surgery, which means that
histological evaluation of tumor necrosis during the course of
chemotherapy requires repeated invasive biopsies. The quantitative
evaluation of preoperative radiological changes using
diffusion-weighted imaging (DWI), dynamic magnetic resonance
imaging, thallium-201 scintigraphy and positron emission tomography
with computed tomography (PET/CT) has been challenged (8–12). The
operative treatment and neoadjuvant chemotherapy of suspected poor
responders may then be intensified earlier, potentially increasing
their survival rates and decreasing the risk rates of iatrogenic
toxicity.
DWI is currently the only imaging method to
non-invasively measure the local diffusion characteristics of water
molecules in vivo. It is able to reflect the spatial
composition and the functional status of water exchange among
various tissues in pathophysiological states from the molecular
level. The apparent diffusion coefficient (ADC) is used to measure
water diffusion and has a decreasing tendency in highly cellular
tissue. DWI has been used to classify the subtype of
musculoskeletal tumors (13–16). As the signal of water diffusion is
directly associated with the tumor cellularity, necrotic areas in
the tumor increase a local diffusion signal. This phenomenon has
been demonstrated in clinical and experimental practice (17–20).
Although the ADC value on DWI may be a promising tool, due to the
scant data currently available, there is no routine practice for
DWI to predict the chemotherapeutic response of osteosarcoma.
The objective of the present study was to provide an
up-to-date summary of the role of DWI for the preoperative
assessment of the chemotherapy response of osteosarcoma. The mean
difference of post-neoadjuvant chemotherapy ADC between good and
poor histological responders of osteosarcoma was assessed using a
systematic literature search and meta-analysis.
Materials and methods
Literature search
A systematic literature search was performed
following the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) statement (21). The main research question, consisting
of the Target Population (including previous tests), Index Test,
Comparator Test, Outcome and Study design (PICOS) strategy, was
formulated into a search query. A combination of the terms
‘diffusion-weighted imaging’ and ‘osteosarcoma’ was searched
without a time limitation, on four electric literature databases:
MEDLINE, EMBASE, Web of Science and Cochrane Library.
Study selection
Two reviewers (TF and MJP) evaluated potentially
relevant articles for eligibility. The decision of article
inclusion or exclusion was hierarchical and firstly made on the
basis of the article title, then of the article abstract and
finally of the whole article. If either reviewer judged the article
title and subsequently the article abstract to be potentially
eligible, the two reviewers independently evaluated the whole
article for eligibility using predetermined inclusion or exclusion
criteria.
The inclusion criteria were i) articles published in
English; ii) diffusion-weighted imaging was used to predict
histological response following preoperative chemotherapy in
osteosarcoma; iii) All ADC values or the mean ADC values were
described; and iv) when parts of data were presented in more than
one article, the most recent article was used.
Data extraction
The same investigators independently reviewed the
included articles in consensus to extract study information for the
meta-analysis.
Quality evaluation
The quality of study designs was assessed using the
Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool
(22).
Meta-analysis
The mean difference in ADC value following
neoadjuvant chemotherapy between good and poor histological
responders was assessed in the 5 studies. Additionally, the ADC
ratio was calculated by using the following formula to assess the
relative change in the pre- and post-neoadjuvant chemotherapy ADC
values of osteosarcomas: ADC ratio=(postchemotherapy
ADC-prechemotherapy ADC)/prechemotherapy ADC ×100. The mean
difference in ADC ratio was correlated between good and poor
histological responders in 3 of the 5 studies. Heterogeneity of the
mean difference of each study was evaluated using the inconsistency
index I-square (I2) test as well as the
χ2 test. An I2 >50% and/or
P<0.10 was considered to be statistically significant. The Der
Simonian and Laird random effect model was applied if significant
heterogeneity between studies was observed, while a fixed effect
model was used in the absence of significant between-study
heterogeneity. Publication bias was estimated using funnel plot
asymmetry tests. All meta-analysis was performed using Review
Manager software, version 5 (Cochrane Collaboration, Oxford, UK).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Literature search and study
selection
The PICOS main research question was P, patients
with osteosarcoma treated by the combination of chemotherapy and
surgery; I, preoperative DWI assessment for chemotherapy response;
C, histological assessment for chemotherapy response; O, mean
difference of ADC and ADC ratio; S, retrospective and prospective
cohort studies. Using the predefined electric literature databases,
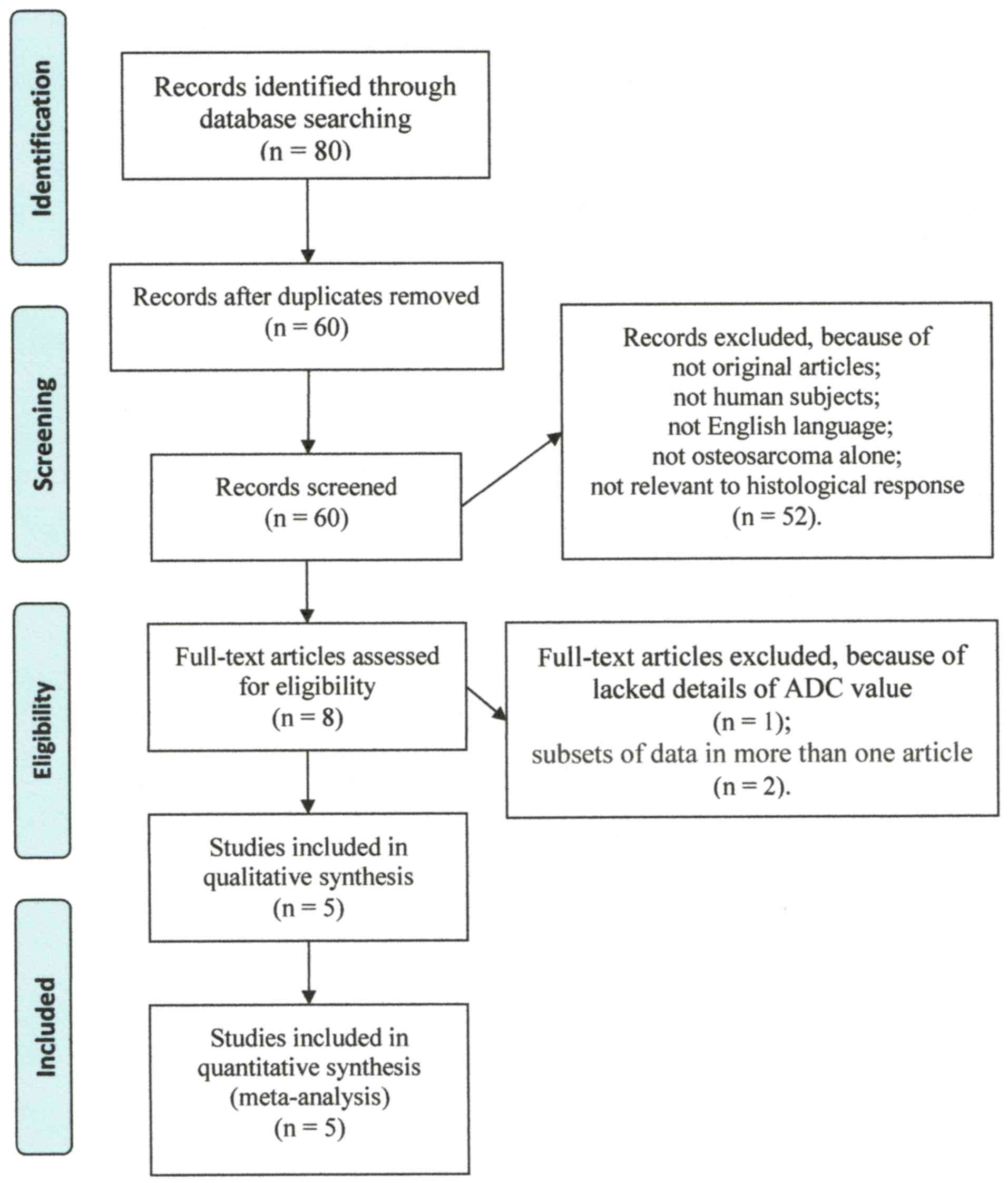
we identified 80 potentially eligible articles, of which 72 were
excluded due to duplication or after reviewing the article title
and abstract. Subsequently, 3 articles were excluded after
reviewing the whole article (23–25).
Five articles with 106 patients who fulfilled all of the inclusion
criteria were selected for the meta-analysis (Table I) (26–30). The
detailed procedure of study selection and its exclusion reasons in
the meta-analysis is presented in Fig.
1.
 | Table I.Summary of the studies included in the
meta-analyses. |
Table I.
Summary of the studies included in the
meta-analyses.
| First author | Year | Journal | Country | N | Study design | Enrolment | (Refs.) |
|---|
| Baunin | 2012 | Skeletal radiol | France | 14 | Prospective | N/D | (26) |
| Byun | 2013 | J. Nucl med | Korea | 27 | Prospective | Consecutive | (27) |
| Oka | 2010 | Skeletal radiol | Japan | 22 | Retrospective | N/A | (28) |
| Uhl | 2006 | Pediatr radiol | Germany | 8 | Prospective | N/D | (29) |
| Wang | 2013 | PLoS One | China | 35 | Prospective | N/D | (30) |
Study description and quality
evaluation
Table II presents
the clinical characteristics of the 5 studies included in the
meta-analysis. All included studies fulfilled ≥5 ‘low’ answers in
the 7 domains of the QUADAS-2 tool for methodological quality
assessment. Common weaknesses concentrated on the domain of patient
selection (Table III).
 | Table II.Clinical characteristics of the
patients included in the meta-analysis. |
Table II.
Clinical characteristics of the
patients included in the meta-analysis.
| First author | Year | M/F | Age, years
(mean/range) | Field, strength
tesla | b-values,
s/mm2 | Assessors | Blindness | (Refs.) |
|---|
| Baunin | 2012 | N/D | N/D | N/D | 0, 900 | 2 | N/D | (26) |
| Byun | 2013 | 15/12 | 20.6/14–23 | 3.0 | 0, 800 | 2 | N/D | (27) |
| Oka | 2010 | 8/14 | 15.3/8–29 | 1.5 | 0, 1000 | 4 | Blind | (28) |
| Uhl | 2006 | N/D | 14.7/11–19 | 1.5 | 0, 700 | 2 | Blind | (29) |
| Wang | 2013 | 18/17 | 26.8/7–65 | 1.5 | 0, 700 | 2 | N/D | (30) |
 | Table III.Quality assessment of diagnostic
accuracy studies-2. |
Table III.
Quality assessment of diagnostic
accuracy studies-2.
|
| Risk of bias | Applicability
concerns |
|
|---|
|
|
|
|
|
|---|
| First author | Patient
selection | Index test | Reference
standard | Flow and
timing | Patient
selection | Index test | Reference
standard | (Refs.) |
|---|
| Baunin | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | (26) |
| Byun | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | (27) |
| Oka | High risk | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | (28) |
| Uhl | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | (29) |
| Wang | Unclear risk | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | (30) |
Meta-analysis
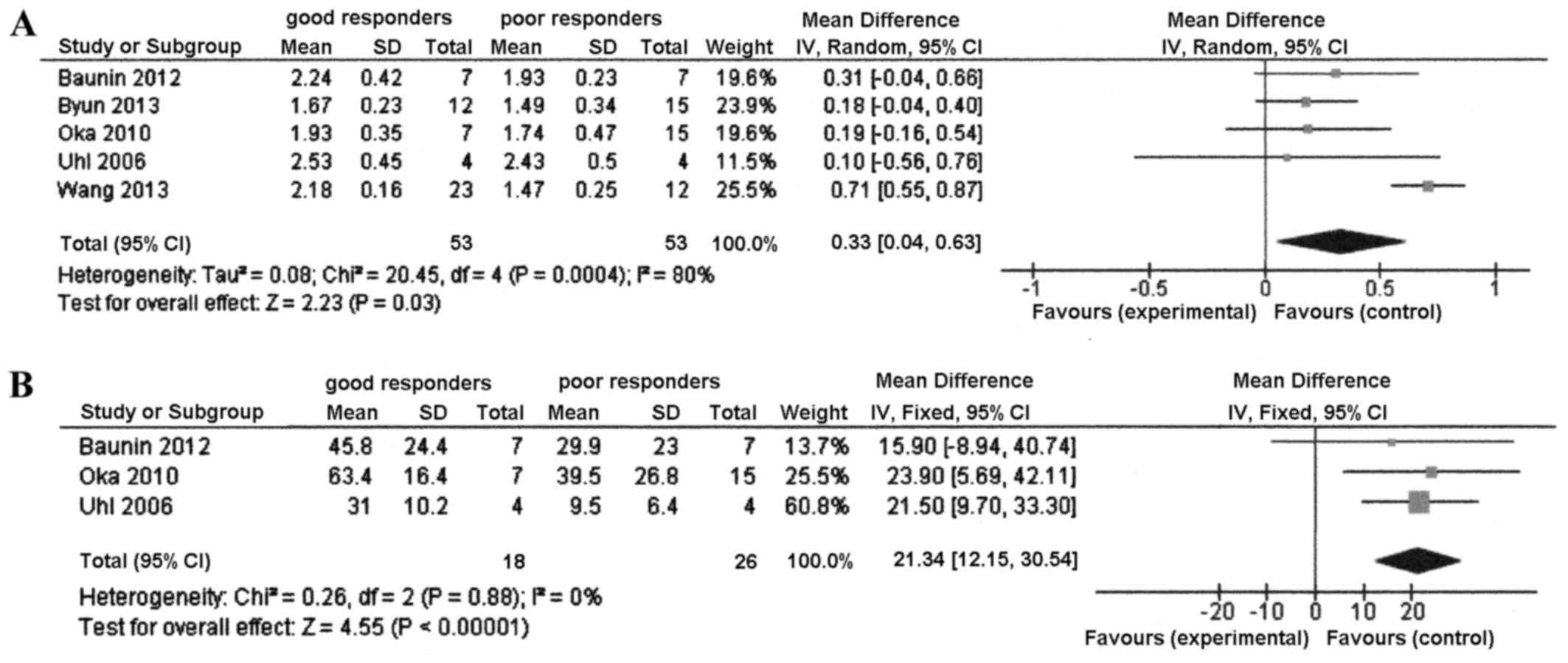
There was significant heterogeneity among the 5
studies in terms of the mean difference in ADC between good and
poor responders (P=0.0004 and I2=80%). Therefore, the
random effect model was used. Significant mean differences were
identified between good and poor responders in the ADC value (mean
difference, 0.33; 95% CI, 0.04–0.63; P=0.03). The good responders
had a higher ADC value than the poor responders (Fig. 2A).
The mean difference in ADC ratio between good and
poor responders was calculated with the fixed effects model, as
there was no heterogeneity among the studies (P=0.88;
I2=0%). There was a significant mean difference between
the good and poor responders in the ADC ratio of the 3 studies
(mean difference, 21.3; 95% CI, 12.2–30.5; P<0.00001) (Fig. 2B).
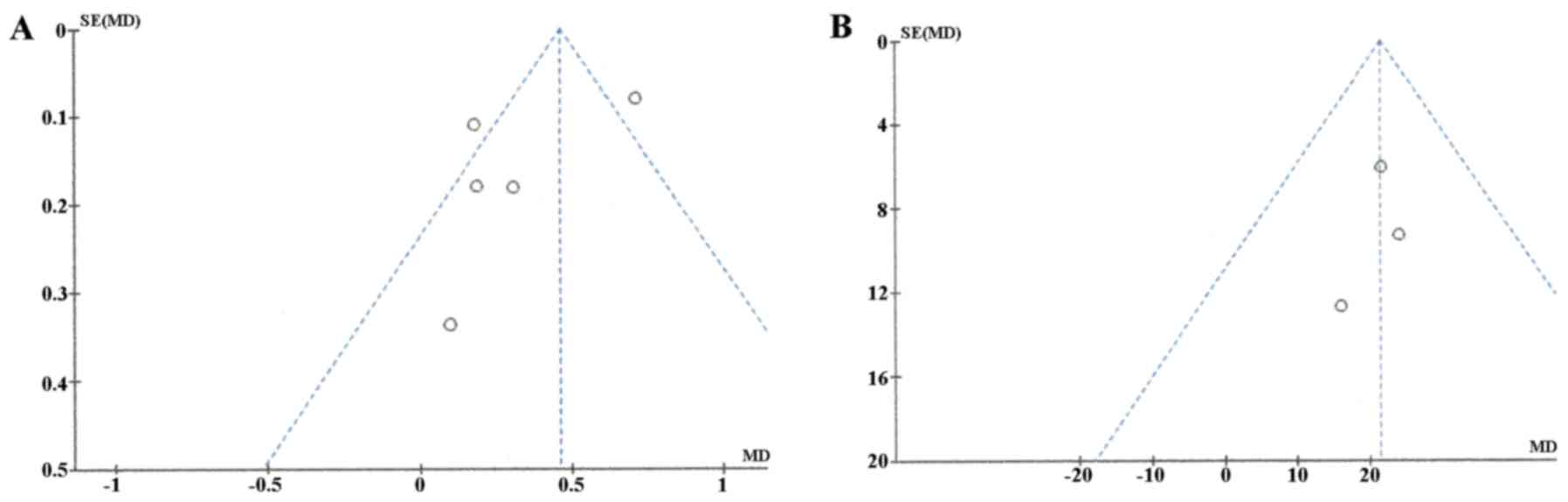
Publication bias was assessed using funnel plot
asymmetry tests (Fig. 3). The plot
of ADC meta-analysis among the 5 studies was asymmetric, indicating
that there was some possible publication bias. However, the plots
of ADC ratio meta-analysis was symmetric, suggesting a low risk of
publication bias.
Discussion
Certain studies revealed that an increased ADC
following neoadjuvant chemotherapy is associated with good
histological response (24,25,27,29,30).
However, there have been conflicting results. Other studies have
not identified a significant association between ADC value and
tumor necrosis (23,26,28).
Therefore, the predictive value of ADC remained undetermined. This
meta-analysis focused on not only the ADC but also the ADC ratio
for evaluating the chemotherapy response, which to the best of our
knowledge had not previously been studied. The present study found
that the good responders demonstrated a higher ADC and a higher ADC
ratio than the poor responders.
The current study has several limitations. Firstly,
only 3 articles with 44 patients were selected for the ADC ratio
study. Further studies based on these promising results are
warranted. Secondly, it was not possible to completely exclude
potential bias, despite the following efforts. To minimize bias in
the study selection and the data extraction, this study was
performed blindly and independently. To be sure that all the
selected articles were high-quality, only articles with a ‘low’
answer for the 7 domains in the QUADAS-2 quality assessment tool of
≥5 were selected. Publication bias was also assessed using a funnel
plot, and there was some possible publication bias in the ADC
meta-analysis among the 5 studies. Further prospective assessment
of DWI to evaluate the chemotherapeutic response of osteosarcoma is
required in order to exclude potential bias completely. Thirdly,
significantly high heterogeneity of the diagnostic performance of
DWI was found in the ADC meta-analysis among the 5 studies. This
heterogeneity might be attributable to the methodological
differences between the articles, such as the DWI acquisition
technique, interpretation scheme or reference standard. For
example, there was variation in the combinations of b-value and in
the choice of region of interest among the 5 studies. Additional
investigations with larger cohorts and the methodology of MR
techniques that are both standardized and optimized are necessary
to better characterize the benefit of this new technology for
patients with osteosarcoma.
Recently, PET or PET/CT has become one of the most
intensively-investigated imaging modalities for the monitoring of
preoperative chemotherapy effects (12). Byun et al (27) reported the equivalent potential of
PET/CT and DWI to predict the histologic response to neoadjuvant
chemotherapy in 28 patients with extremity osteosarcoma, using
sequential imaging capture. The combination of PET/CT and DWI may
yield varied biological information, such as changes in glucose
metabolism and cellularity, which means that it has the potential
to overcome the functional limitations of individual PET/CT and
DWI. Several studies have suggested 40–60% of the standardized
uptake value (SUV) ratio as the PET/CT cutoff point for a good
response to neoadjuvant chemotherapy of osteosarcoma (12,27,31), but
the optimal ADC cutoff point for good responders to neoadjuvant
chemotherapy of osteosarcoma has yet to be reported. Furthermore,
there is no standard means to measure ADC values. Further studies
are required in order to obtain the standardized and optimized ADC
values of DWI.
In conclusion, the present meta-analysis has
demonstrated that the ADC and ADC ratio are useful for predicting
the histologic response of patients to preoperative chemotherapy in
osteosarcoma. This method may have promising potential as a
preoperative non-invasive modality. For poor responders to
preoperative chemotherapy, a more radical tumor resection should be
performed and the postoperative chemotherapeutic regimen should be
altered. For good responders, a minimally invasive surgical
procedure can be selected with a low risk of local recurrence.
These results have the potential to change the present therapeutic
strategy for osteosarcoma based on the role of DWI prior to and
following adjuvant chemotherapy.
Acknowledgements
The present study is supported by the Practical
Research for Innovative Cancer Control from Japan Agency For
Medical Research and development (AMED) and Japan Society for the
Promotion of Science (JSPS) KAKENHI (grant no. 26462267).
References
|
1
|
Bacci G, Briccoli A, Ferrari S, Longhi A,
Mercuri M, Capanna R, Donati D, Lari S, Forni C and DePaolis M:
Neoadjuvant chemotherapy for osteosarcoma of the extremity:
Long-term results of the Rizzoli's 4th protocol. Eur J Cancer.
37:2030–2039. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lewis IJ, Nooij MA, Whelan J, Sydes MR,
Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van
Glabbeke M, et al: Improvement in histologic response but not
survival in osteosarcoma patients treated with intensified
chemotherapy: A randomized phase III trial of the European
Osteosarcoma Intergroup. J Natl Cancer Inst. 99:112–128. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eselgrim M, Grunert H, Kühne T, Zoubek A,
Kevric M, Bürger H, Jürgens H, Mayer-Steinacker R, Gosheger G and
Bielack SS: Dose intensity of chemotherapy for osteosarcoma and
outcome in the Cooperative Osteosarcoma Study Group (COSS) trials.
Pediatr Blood Cancer. 47:42–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyers PA, Gorlick R, Heller G, Casper E,
Lane J, Huvos AG and Healey JH: Intensification of preoperative
chemotherapy for osteogenic sarcoma: Results of the Memorial
Sloan-Kettering (T12) protocol. J Clin Oncol. 16:2452–2458. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huvos AG, Rosen G and Marcove RC: Primary
osteogenic sarcoma: Pathologic aspects in 20 patients after
treatment with chemotherapy en bloc resection, and prosthetic bone
replacement. Arch Pathol Lab Med. 101:14–18. 1977.PubMed/NCBI
|
|
7
|
Picci P, Bacci G, Campanacci M, Gasparini
M, Pilotti S, Cerasoli S, Bertoni F, Guerra A, Capanna R, Albisinni
U, et al: Histologic evaluation of necrosis in osteosarcoma induced
by chemotherapy. Regional mapping of viable and nonviable tumor.
Cancer. 56:1515–1521. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo J, Reddick WE, Glass JO, Ji Q, Billups
CA, Wu J, Hoffer FA, Kaste SC, Jenkins JJ, Flores XC Ortega, et al:
Dynamic contrast-enhanced magnetic resonance imaging as a
prognostic factor in predicting event-free and overall survival in
pediatric patients with osteosarcoma. Cancer. 118:3776–3785. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kubo T, Furuta T, Johan MP, Adachi N and
Ochi M: Percent slope analysis of dynamic magnetic resonance
imaging for assessment of chemotherapy response of osteosarcoma or
Ewing sarcoma: Systematic review and meta-analysis. Skeletal
Radiol. 45:1235–1242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inaki A, Taki J, Wakabayashi H, Sumiya H,
Zen Y, Tsuchiya H and Kinuya S: Thallium-201 scintigraphy for the
assessment of long-term prognosis in patients with osteosarcoma.
Ann Nucl Med. 26:545–550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kubo T, Shimose S, Fujimori J, Furuta T
and Ochi M: Quantitative (201)thallium scintigraphy for prediction
of histological response to neoadjuvant chemotherapy in
osteosarcoma; systematic review and meta-analysis. Surg Oncol.
24:194–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hongtao L, Hui Z, Bingshun W, Xiaojin W,
Zhiyu W, Shuier Z, Aina H, Yuanjue S, Daliu M, Zan S and Yang Y:
18F-FDG positron emission tomography for the assessment of
histological response to neoadjuvant chemotherapy in osteosarcomas:
A meta-analysis. Surg Oncol. 21:e165–e170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Rijswijk CS, Kunz P, Hogendoorn PC,
Taminiau AH, Doornbos J and Bloem JL: Diffusion-weighted MRI in the
characterization of soft-tissue tumors. J Magn Reson Imaging.
15:302–307. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baur A, Huber A, Arbogast S, Dürr HR, Zysk
S, Wendtner C, Deimling M and Reiser M: Diffusion-weighted imaging
of tumor recurrencies and posttherapeutical soft-tissue changes in
humans. Eur Radiol. 11:828–833. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herneth AM, Friedrich K, Weidekamm C,
Schibany N, Krestan C, Czerny C and Kainberger F: Diffusion
weighted imaging of bone marrow pathologies. Eur J Radiol.
55:74–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
MacKenzie JD, Gonzalez L, Hernandez A,
Ruppert K and Jaramillo D: Diffusion-weighted and diffusion tensor
imaging for pediatric musculoskeletal disorders. Pediatr Radiol.
37:781–788. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nonomura Y, Yasumoto M, Yoshimura R,
Haraguchi K, Ito S, Akashi T and Ohashi I: Relationship between
bone marrow cellularity and apparent diffusion coefficient. J Magn
Reson Imaging. 13:757–760. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Humphries PD, Sebire NJ, Siegel MJ and
Olsen ØE: Tumors in pediatric patients at diffusion-weighted MR
imaging: Apparent diffusion coefficient and tumor cellularity.
Radiology. 245:848–854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lang P, Wendland MF, Saeed M, Gindele A,
Rosenau W, Mathur A, Gooding CA and Genant HK: Osteogenic
osteosarcoma: Noninvasive in vivo assessment of tumor necrosis with
diffusion-weighted MR imaging. Radiology. 206:227–235. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thoeny HC, De Keyzer F, Chen F, Ni Y,
Landuyt W, Verbeken EK, Bosmans H, Marchal G and Hermans R:
Diffusion-weighted MR imaging in monitoring the effect of a
vascular targeting agent on rhabdomyosarcoma in rats. Radiology.
234:756–764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and elaboration. PLoS Med.
6:e10001002009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Whiting PF, Rutjes AW, Westwood ME,
Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA and Bossuyt
PM: QUADAS-2: A revised tool for the quality assessment of
diagnostic accuracy studies. Ann Intern Med. 155:529–536. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bajpai J, Gamnagatti S, Kumar R, Sreenivas
V, Sharma MC, Khan SA, Rastogi S, Malhotra A, Safaya R and Bakhshi
S: Role of MRI in osteosarcoma for evaluation and prediction of
chemotherapy response: Correlation with histological necrosis.
Pediatr Radiol. 41:441–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayashida Y, Yakushiji T, Awai K, Katahira
K, Nakayama Y, Shimomura O, Kitajima M, Hirai T, Yamashita Y and
Mizuta H: Monitoring therapeutic responses of primary bone tumors
by diffusion-weighted image: Initial results. Eur Radiol.
16:2637–2643. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uhl M, Saueressig U, van Buiren M, Kontny
U, Niemeyer C, Köhler G, Ilyasov K and Langer M: Osteosarcoma:
Preliminary results of in vivo assessment of tumor necrosis after
chemotherapy with diffusion- and perfusion-weighted magnetic
resonance imaging. Invest Radiol. 41:618–623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baunin C, Schmidt G, Baumstarck K, Bouvier
C, Gentet JC, Aschero A, Ruocco A, Bourlière B, Gorincour G,
Desvignes C, et al: Value of diffusion-weighted images in
differentiating mid-course responders to chemotherapy for
osteosarcoma compared to the histological response: Preliminary
results. Skeletal Radiol. 41:1141–1149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Byun BH, Kong CB, Lim I, Choi CW, Song WS,
Cho WH, Jeon DG, Koh JS, Lee SY and Lim SM: Combination of 18F-FDG
PET/CT and diffusion-weighted MR imaging as a predictor of
histologic response to neoadjuvant chemotherapy: Preliminary
results in osteosarcoma. J Nucl Med. 54:1053–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oka K, Yakushiji T, Sato H, Hirai T,
Yamashita Y and Mizuta H: The value of diffusion-weighted imaging
for monitoring the chemotherapeutic response of osteosarcoma: A
comparison between average apparent diffusion coefficient and
minimum apparent diffusion coefficient. Skeletal Radiol.
39:141–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uhl M, Saueressig U, Koehler G, Kontny U,
Niemeyer C, Reichardt W, Ilyasof K, Bley T and Langer M: Evaluation
of tumour necrosis during chemotherapy with diffusion-weighted MR
imaging: Preliminary results in osteosarcomas. Pediatr Radiol.
36:1306–1311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang CS, Du LJ, Si MJ, Yin QH, Chen L, Shu
M, Yuan F, Fei XC and Ding XY: Noninvasive assessment of response
to neoadjuvant chemotherapy in osteosarcoma of long bones with
diffusion-weighted imaging: An initial in vivo study. PLoS One.
8:e726792013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Im HJ, Kim TS, Park SY, Min HS, Kim JH,
Kang HG, Park SE, Kwon MM, Yoon JH, Park HJ, et al: Prediction of
tumour necrosis fractions using metabolic and volumetric 18F-FDG
PET/CT indices, after one course and at the completion of
neoadjuvant chemotherapy, in children and young adults with
osteosarcoma. Eur J Nucl Med Mol Imaging. 39:39–49. 2012.
View Article : Google Scholar : PubMed/NCBI
|