Introduction
Granular cell tumor (GCT) is a benign rare tumor
that usually affects head and neck. GCT was first identified in
tongue in 1854 by Weber (1) and then
described in breast by Abrikossoff (2) by the name of granular cell myoblastoma.
GCTs were considered to arise from Schwann cells, histiocytes,
fibroblasts, myocytes or intestinal mesenchymal cells. Nowadays,
the accepted theory claims nerve sheath origin of the tumour
(3,4). These tumors may occur throughout the
body, usually in the head and neck, skin or subcutaneous tissues of
the trunk and upper extremities, breasts and female genital region.
5–8% of all cases of GCTs occur in the breast (5,6). They
are usually benign and solitary; however, approximately 2% occur as
malignant tumors, and 5–10% as multiple lesions (6). It is important to differentiate between
this tumor and breast carcinoma because they share similarities in
the diagnostic picture. Benign GCTs are treated with wide local
excision and are associated with a good prognosis. We report on our
findings in a patient with benign form of GTC in a rare location,
specifically in the axillary region.
Case report
A 43-year-old Asian woman felt a tumor-like lump in
the anterior axillary line outside of the right breast, and visited
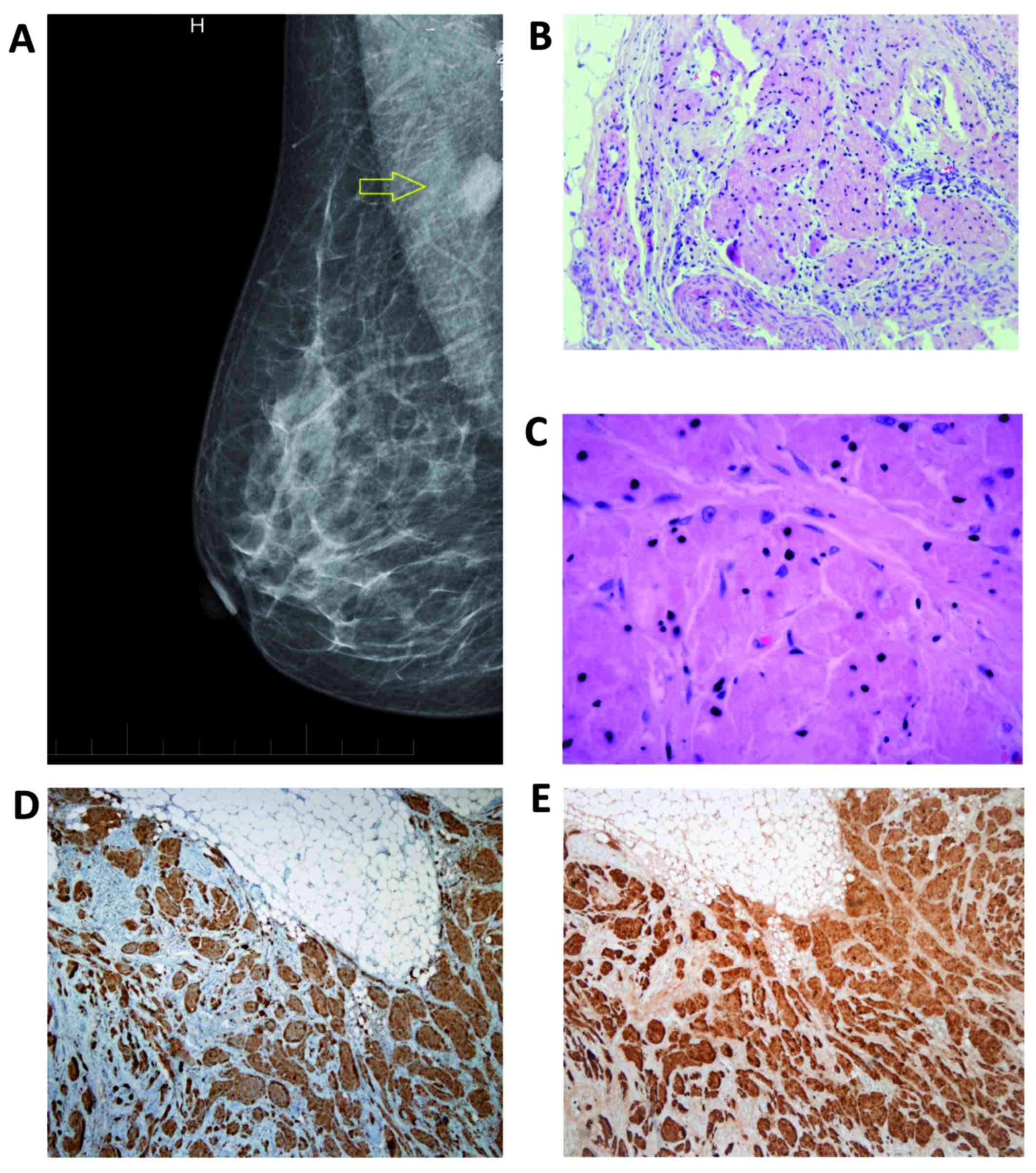
our Breast Unit in June, 2016. Mammography suggested a
circumscribed, round, radiopaque lesion with unsharp contours in
the right axillary region (Fig. 1A).
Breast ultrasonography revealed an oval-shaped, low-echoic tumor of
unclear aetiology. Infiltrating ductal carcinoma could not be
excluded. A ultrasound guided large core-needle biopsy of the tumor
was provided. Histologic examination of the core biopsy specimen
pointed to benign form of GCT. Lumpectomy was performed and benign
granular cell tumor was diagnosed by postoperative histopathologic
examination (Fig. 1B-E). The
surgically removed specimen was fixed using 10% neutral buffered
formalin for 24 h. The fixed specimen was trimmed with a scalpel to
fit into a tissue cassette. It was processed in an automated tissue
processing machine (BenchMark XT; Ventana Medical Systems, Inc.,
Tucson, AZ, USA). The processing included dehydration, clearing,
and embedding, in which the specimens were infiltrated with
paraffin wax to create paraffin blocks. These blocks were cut with
a rotary microtome (Leica RM2235, Leica Biosystems GmbH, Nussloch,
Germany) to produce 5 µm sections. The sections were then stained
with hematoxylin and eosin (H&E). Histological evaluation of
the surgical specimens revealed tumor cells with a distinctive
granular eosinophilic cytoplasm associated with typical nuclei,
without increase in nuclear division or another signs of
malignancy. Benign breast parenchyma and mature fat tissue was
present between the tumor structures (Fig. 1B and C). Definitive diagnosis of GCT
was established using immunohistochemistry (IHC). The
immunostaining for S100 protein (mouse monoclonal antibody to S100;
clone 4C4.9; cat. no. ab4066; 1:100 dilution, incubation for 30 min
at 25°C; Abcam, Cambridge, UK) and CD68 (mouse monoclonal antibody,
clone C68/684; cat. no. ab201340; 1:100 dilution, incubation for 30
min at 25°C; Abcam, Cambridge, UK) revealed positive results
(Fig. 1D and E). IHC analysis of the
tumor with anti-cytokeratins antibody (mouse monoclonal antibody to
pan Cytokeratin; clone AE1/AE3 + 5D3; cat. no. ab86734; 1:200
dilution, incubation for 30 min at 37°C; Abcam, Cambridge, UK)
showed negative results. There was also diffuse steroid receptors
(estrogen and progesteron receptor) negativity. The post-operative
course was uneventful and the patient was discharged home on
post-operative day 7. The patient is now 4 months post-surgery and
remains disease-free. Written informed consent was obtained from
the patient.
Discussion
GCTs account for an incidence of 0.5% among soft
tissue tumors (3–6). It may occur throughout the body,
usually in the head and neck, skin or subcutaneous tissues of the
trunk and upper extremities. Recently, Costa Almeida et al
(7) published a case report of GCT
on the upper limb and reviewed 21 patients with this GCT
localization from the literature. When occurring in the breast, as
it occurs in 5–8% of all cases of GCT, they present mostly as
painless rounded nodules (4,6). GCT of the breast may mimic breast
cancer both clinically and radiologically (8–10). These
lesions have been defined as ranging from a round
well-circumscribed mass to an indistinct or spiculated lesion on
mammography (8,9). Microcalcifications are not normally a
feature of GCTs. On ultrasound, GCTs can present as solid, poorly
marginated lesions with marked posterior shadowing or as more
benign-appearing well-circumscribed solid masses (8,9). MRI
findings in a patient with GCT were described by Scaranelo et
al (10). In contrast to
clinical findings, mammography, ultrasonography and MRI positron
emission tomography (PET) with 2-[fluorine-18]
fluoro-2-deoxy-d-glucose (FDG) can correctly differentiate GCT from
a malignant tumor (11). Fine-needle
aspiration cytology and frozen section methods are inadequate for
definitive diagnosis of GCT (12).
The large core-needle biopsy did accurately predict a GCT in our
patient.
The appearance of a GCT in axillary region is
extremely rare. Aoyama et al (3), reported in their study of six cases of
GCTs, a 54-year-old woman with a GCT in the left axillary cavity,
which is very similar to our patient. Another patient with similar
location of GCT in the upper outer quadrant of the right breast
which appeared to be attached to the underlying pectoralis major
muscle, was described by Patel et al (9). The mammographic appearence of the GCT
was very similar to the patient from our case report. A pediatric
GCT in a 15-year-old female in the right upper outer quadrant with
no associated lymphadenopathy was recently refered by Heinzerling
et al (13). Delaloye et
al (14) reported a rare case of
benign GCT of the breast associated with multiple similar lesions
of the scalp, the right shoulder, the right flank, the abdominal
wall and the vulva, treated with wide excision. Al-Ahmadie et
al (15) documented in their
report a GCT of the breast coexisting with an ipsilateral
infiltrating ductal carcinoma, infiltrated each other. Coates et
al (16) reported a case of a
patient with a large infra-mammary fold GCT, the management of
which required a multidisciplinary operative approach due to
extensive chest wall invasion. Malignant forms of GCTs are very
rare (1–3% of all GCT cases). Chen et al (6) and Akahane et al (12) described malignant GCTs in breast.
Criteria for malignancy are not consistent; adjacent tissue and/or
vascular invasion, high mitotic activity, and size >4–5 cm were
discussed, but only the presence of metastases was accepted as
explicit criterion (6,7). No data exist about the efficacy of
adjuvant therapy in GCT treatment.
GCTs are macroscopically, solid, firm tumours with a
yellowish-white cross sectional surface (10,11,13). The
histogenesis of GCT remains uncertain, however the hypothesis of a
neural or neuroectodermal origin is supported by the presence of
the S-100 protein, typically expressed by these neoplastic cells,
and by the similar ultrastructural features of the tumour cells and
Schwann cells (Fig. 1E). On
pathological examination they can be identified using both
microscopic and immunohistochemical features. The cells have a
distinctive granular eosinophilic cytoplasm associated with typical
nuclei (Fig. 1B and C).
Immunohistochemically they are positive for S100 protein, CD68
(Fig. 1D) and neuron specific
endolase (NSE). Fine and Li (17)
suggested interaction between expression of calretinin and the
alpha-subunit of inhibin in granular cell tumors.
In conclusion, GCT of the breast is a usually benign
disease of the breast which may mimic breast cancer both clinically
and radiologically. Its presentation in axillary region is very
rare. The definitive diagnosis is made by immunohistochemical
examination. Clinicians should be aware of this finding in the
differential diagnosis of breast and axillary masses to prevent
overtreatment.
References
|
1
|
Weber CO: Anatomische untersuchung einer
hypertrophischen zunge nebst bemerkungen über die neubildung
quergestreifter muskelfasern anatomical examination of a
hypertrophic tongue as well as remarks on the new formation of
transverse muscle fibers. Virchows Arch A Pathol Anat. 7:115–125.
1954. View Article : Google Scholar
|
|
2
|
Abrikossoff AI: Weitere untersuchungen
über myoblastenmyome. Virchows Arch Pathol Anat Physiol Klin Med.
280:723–740. 1931. View Article : Google Scholar
|
|
3
|
Aoyama K, Kamio T, Hirano A, Seshimo A and
Kameoka S: Granular cell tumors: A report of six cases. World J
Surg Oncol. 10:2042012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pergel A, Yucel AF, Karaca AS, Aydin I,
Sahin DA and Demirbag N: A therapeutic and diagnostic dilemma:
Granular cell tumor of the breast. Case Rep Med. 2011:9721682011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gogas J, Markopoulos C, Kouskos E, Gogas
H, Mantas D, Antonopoulou Z and Kontzoglou K: Granular cell tumor
of the breast: A rare lesion resembling breast cancer. Eur J
Gynaecol Oncol. 23:333–334. 2002.PubMed/NCBI
|
|
6
|
Chen J, Wang L, Xu J, Pan T, Shen J, Hu W
and Yuan X: Malignant granular cell tumor with breast metastasis: A
case report and review of the literature. Oncol Lett. 4:63–66.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costa Almeida CE, Caroço T, Silva M and
Albano MN: Abrikossoff's tumour on the upper limb: A rare
presentation. BMJ Case Rep. 2017:pii: bcr-2017-222006. 2017.
|
|
8
|
Yang WT, Edeiken-Monroe B, Sneige N and
Fornage BD: Sonographic and mammographic appearances of granular
cell tumors of the breast with pathological correlation. J Clin
Ultrasound. 34:153–160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel A, Lefemine V, Yousuf SM and
Abou-Samra W: Granular cell tumour of the pectoral muscle mimicking
breast cancer. Cases J. 1:1422008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scaranelo AM, Bukhanov K, Crystal P,
Mulligan AM and O'Malley FP: Granular cell tumour of the breast:
MRI findings and review of the literature. Br J Radiol. 80:970–974.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoess C, Freitag K, Kolben M, Allgayer B,
Laemmer-Skarke I, Nathrath WB, Avril N, Roemer W, Schwaiger M and
Graeff H: FDG PET evaluation of granular cell tumor of the breast.
J Nucl Med. 39:1398–1401. 1998.PubMed/NCBI
|
|
12
|
Akahane K, Kato K, Ogiso S, Sakaguchi K,
Hashimoto M, Ishikawa A, Kato T, Fuwa Y, Takahashi A and Kobayashi
K: Malignant granular cell tumor of the breast: Case report and
literature review. Breast Cancer. 22:317–323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heinzerling NP, Koehler SM, Szabo S and
Wagner AJ: Pediatric granular cell tumor of the breast: A case
report and review of the literature. Case Rep Surg.
2015:5689402015.PubMed/NCBI
|
|
14
|
Delaloye JF, Seraj F, Guillou L, Genton
CY, Anciaux-Le Teno D, Schnyder P and De Grandi P: Granular cell
tumor of the breast: A diagnostic pitfall. Breast. 11:316–319.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Ahmadie H, Hasselgren PO, Yassin R and
Mutema G: Colocalized granular cell tumor and infiltrating ductal
carcinoma of the breast. Arch Pathol Lab Med. 126:731–733.
2002.PubMed/NCBI
|
|
16
|
Coates SJ, Mitchell K, Olorunnipa OB,
DeSimone RA, Otterburn DM and Simmons RM: An unusual breast lesion:
Granular cell tumor of the breast with extensive chest wall
invasion. J Surg Oncol. 110:345–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fine SW and Li M: Expression of calretinin
and the alpha-subunit of inhibin in granular cell tumors. Am J Clin
Pathol. 119:259–264. 2003. View Article : Google Scholar : PubMed/NCBI
|