Introduction
The role of the mitochondria in pathogenesis,
especially that related to oxidative phosphorylation (OXPHOS),
mainly concerns their role in the process of apoptosis, free
radical and ATP production (1).
The mitochondrial DNA (mtDNA) contains genes encoding for 2 types
of rRNA, 22 types of tRNA and 13 proteins taking part in the
process of OXPHOS.
Proteins involved in OXPHOS locate at the inner
mitochondrial membrane and constitute the respiratory chain. The
mitochondrial chain of electron transport is formed of four large
complexes of respiratory enzymes, the complexes I-IV. The proton
gradient generated as a result of the transport of reduction
equivalents starts the last link of the respiratory chain, which is
ATP synthase (F1F0 ATP-asa) also called complex V. The ATP synthase
is an enzyme that uses a stream of protons passing through the
inner membrane of the mitochondria to synthesize ATP. It consists
of a part located on the mitochondrial membrane (F0) and containing
a proton channel, and a catalytic component (F1) connected to F0
and locating on the side of the mitochondrial matrix (2). The F0 component contains 3–9 protein
subunits (9 in humans) including subunits 6 and 8, encoded by the
mtDNA genes ATP6 and ATP8, respectively.
The role of the mitochondria in the process of
carcinogenesis is highlighted by the results of recently published
studies. These studies revealed a mutation in the nuclear DNA
(nDNA) on the fumarate hydratase gene in myoma and kidney cancer,
as well as mutations in genes encoding 3/4 subunits of the succinic
dehydrogenase belonging to complex II of the respiratory chain, in
paraganglioma and pheochromocytoma (3,4).
Zhu et al (5) discovered at least one somatic
mutation in the mtDNA in 14/15 cases of breast cancer. To date, it
is unclear which functions are fulfilled by the mtDNA, and
especially the genes encoding proteins involved in OXPHOS, in the
context of cancer, although it seems that these genes play an
important part in the process. The purpose of this study was to
analyze mutations in the sequences encoding subunits 6 and 8 of the
ATP synthase and their effect on the biochemical properties,
structure and function of the enzyme.
Materials and methods
Samples and ethics
The tested material was DNA isolated from specimens
of ductal carcinoma (carcinoma ductale) Tp1-2Np0-1Mp0, blood and
non-cancerous tissue of mammary gland (control). The specimens were
collected from 50 patients who had been operated for breast cancer.
The patients had received no chemotherapy or hormone therapy and
were all perimenopausal. All participants provided informed consent
for the use of their biological material (blood taken for routine
laboratory testing and tissues removed during surgery) for research
purposes. The Institutional Review Board at the Medical University
of Lublin approved this study (approval no., KE-254/141/2009).
Nucleic acids isolation and polymerase
chain reaction (PCR)
DNA was extracted from tumor tissues and the
corresponding non-tumor tissues with the DNeasy Blood and Tissue
kit (Qiagen, Hilden, Germany). DNA was isolated on the automated
nucleic acid extraction system QIACube (Qiagen). DNA samples were
qualitatively and quantitatively assessed by electrophoretic
separation on an agarose gel and spectrophotometric measurements of
sample absorbance in a BioPhotometer spectrophotometer (Eppendorf,
Hamburg, Germany), respectively.
The isolated DNA was used to amplify fragments of
mitochondrial genes: mitochondrially encoded ATP synthase 6
(ATP6) and ATP8. The PCR primers for both: ATP8 and
ATP6 (868 bp fragment from 8,312 to 9,280 bp position) were:
forward (F), 5′-CCACTGTAAAGCTAACTTAGC-3′, position: 8,312–8,332 bp
and reverse (R), 5′-GTTAGG GGTCATGGGCTG, position: 9,263–9,280 bp.
They were designed based on sequences available from the complete
mitochondrial genome of Homo sapiens (GenBank accession no.,
AB055387) using the Primer3 program (http://frodo.wi.mit.edu/). The amplification products
were visualized in a 2% agarose gel. The amplicons were then
sequenced on both strands using the Applied Biosystems®
GeneAmp PCR system 9700 and the BigDye®Terminator Cycle
Sequencing kit (both from Applied Biosystems, Foster City, CA,
USA). The samples were subsequently purified on CentriSep™ columns
according to the manufacturer’s protocol or precipitated with
ethanol and sodium acetate according to the protocol of the BigDye
kit manufacturer (both from Thermo Fisher Scientific). Extension
products were separated on the Applied Biosystems® ABI
377 automated sequencer (Applied Biosystems).
Prediction of changes at the protein
level
The impact of the identified mutations on the
physical and biochemical properties of the corresponding peptides
was assessed as follows: The probability of deleterious mutations,
i.e. a functional effect of non-synonymous (amino acid-changing)
protein-coding single nucleotide polymorphisms (SNPs), was
determined using the PANTHER classification system (6), which estimates the value of
substitution position-specific evolutionary conservation (subPSEC)
and the probability of a deleterious effect on protein function
(Pdeleterious, probability of functional impairment) on
the basis of alignment of evolutionarily related proteins.
SubPSEC=−3 was used as the cut-off. A SubPSEC=−0.3 corresponds to
50% probability that the SNP will have a negative impact on the
function of the protein (Pdeleterious=0.5).
The presence of transmembrane helices in the studied
proteins was predicted using the TMHMM 2.0 program (7). The spatial structure of the proteins
corresponded, and the location and orientation of the α-helices
were predicted using the Pfam database (8). Determination of helicity per residue
was performed using the Agadir program (9). The grand average of hydropathy
(GRAVY) value (10) and the
theoretical isoelectric point (pI) were calculated using the
software program tool ProtParam (11). Conservation data were downloaded
from the ConSurf-DB (12). The
ConSurf-DB is a database that contains precomputed conservation
scores for all structures in the Protein Data Bank (PDB). The
ConSurf server (13) was used to
identify functional regions in the proteins. The probability to
observe a given residue in the protein sequence was assessed using
a position-specific scoring matrix (PSSM) and the PSSM viewer
available from the National Center for Biotechnology Information
(http://www.ncbi.nlm.nih.gov/Class/Structure/pssm/pssm_viewer.cgi),
using conserved domain (CD) protein alignments. Positive PSSM
scores indicate that the given amino acid substitution occurs more
frequently in the protein alignment than expected by chance, while
negative scores indicate that the substitution occurs less
frequently than expected. Polymorphisms were considered to be
changes that occured in both blood and tumor cells in the same
patient. Mutations were a change characteristic for cancer cells
but did not occur in the patients’ blood.
Results
In the gene encoding the subunit 6 of the ATP
synthase (ATP6), we identified 8 nucleotide changes
(Table I) in 72% (36/50) of breast
cancer female patients. Five of these (G8557A, G8697A, T8793C,
G8854A and A8860G) are defined as polymorphisms in the Human
Mitochondrial Genome Database maintained at Uppsala University
(http://www.mtdb.igp.uu.se/; Uppsala,
Sweden). The remaining 3 have not been previously described in the
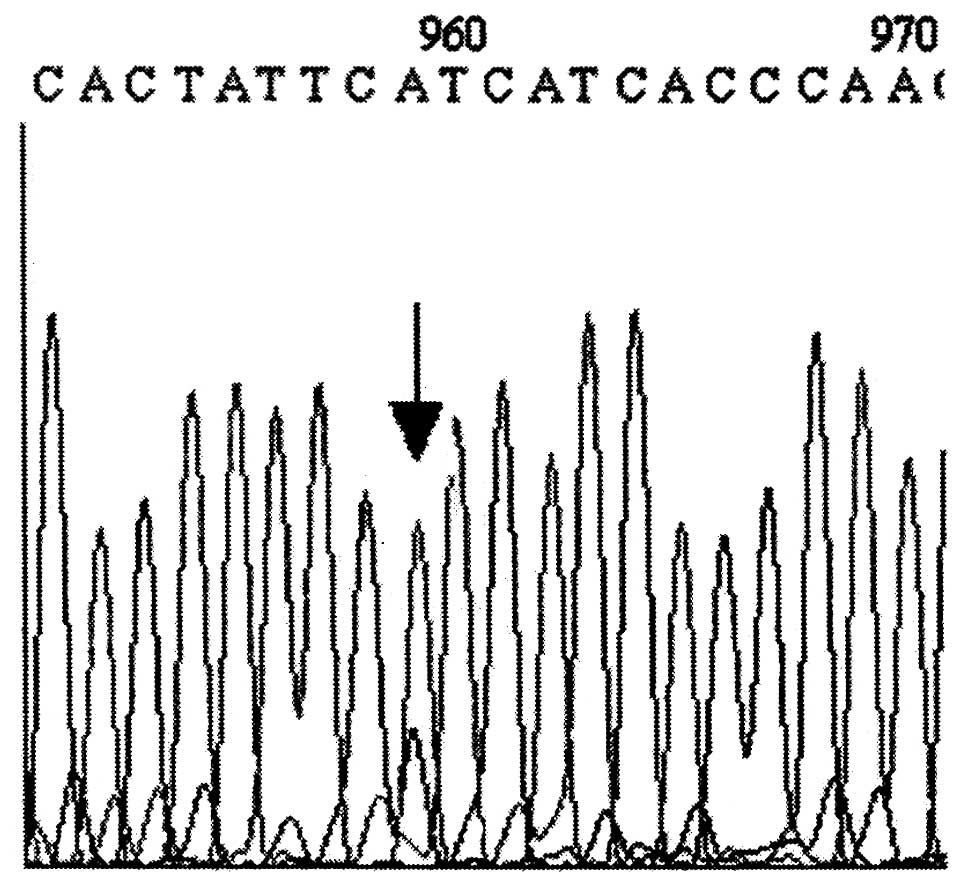
literature. Heteroplasmy occurred in 2 cases, at positions 8,429 of
the ATP8 gene (Fig. 1) and
at position 9,130 of ATP6 (Fig.
2). At position 9,130, heteroplasmy concerned only the control
tissue, and at position 8,429 it concerned only the neoplastic
cells.
 | Table IDifferences in the ATP6 and
ATP8 gene sequences between the Cambridge reference (ref.)
sequence and the sequences obtained from the mitochondrial DNA of
females with breast cancer. |
Table I
Differences in the ATP6 and
ATP8 gene sequences between the Cambridge reference (ref.)
sequence and the sequences obtained from the mitochondrial DNA of
females with breast cancer.
| No. of patients
(a) | Frequency
(mtDB)b | Mt. haplogroup | Cambridge ref. | BC cells | BC blood | Healthy breast
cells | Amino acid
change |
|---|
|
|---|
| A | G | C | T | del |
|---|
| ATP6
polymorphisms |
| 3 (23,82,84) | 21 | 2,681 | 2 | 0 | 0 | H | G8557 | G8557A | G8557A | G8557A | A11T |
| 6 | 2,698 | 0 | 0 | 0 | H2 | A8860 | A8860G | A8860G | A8860G | T112A |
| 2 (22,24) | 0 | 0 | 14 | 2,690 | 0 | M4 | T8793 | T8793C | T8793C | T8793C | Syn |
| 6 | 2,698 | 0 | 0 | 0 | H2 | A8860 | A8860G | A8860G | A8860G | T112A |
| 2 (3,26) | 3 | 2,701 | 0 | 0 | 0 | T2 | G8854 | G8854A | G8854A | G8854A | A110T |
| 6 | 2,698 | 0 | 0 | 0 | H2 | A8860 | A8860G | A8860G | A8860G | T112A |
| 22 (2,4–5,10–15,
17,19,21,27–31, 34,36–40,213,81) | 6 | 2,698 | 0 | 0 | 0 | H2 | A8860 | A8860G | A8860G | A8860G | T112A |
| ATP6
mutations |
| 2 (19,27) | 128 | 2,576 | 0 | 0 | 0 | JT | G8697 | G8697A | G8697 | G8697A | Syn |
| 2 (13,15) | 0 | 0 | 0 | 0 | 0 | n.a. | G8858 | G8858C | G8858 | G8858 | G111A |
| 1 (28) | 0 | 0 | 0 | 0 | 0 | n.a. | T9119 | T9119G | T9119 | T9119G | L198R |
| 1 | 0 | 2,703 | 0 | 0 | n.a. | C9130 | C9130G | C9130 | C9130G | L202V |
| ATP8
polymorphisms |
| 3 (23,82,84) | 21 | 2,681 | 2 | 0 | 0 | H | G8557 | G8557A | G8557A | G8557A | Syn |
| ATP8
mutations |
| 1 (202) | 1 | 0 | 2,702 | 0 | 0 | n.a. | C8429 | C8429A | C8429 | C8429 | L22I |
| 2 (1,30,213) | 0 | 0 | 0 | 0 | 0 | n.a. | A8439 | A8439C | A8439 | A8439 | Q25P |
| 2 (4,22) | 0 | 0 | 5 | 2,699 | 0 | H11 | T8448 | T8448C | T8448 | T8448C | M28T |
| 1 (81) | 1 | 2,703 | 0 | 0 | 0 | n.a. | G8519 | G8519A | G8519 | G8519 | E52K |
The polymorphisms G8557A, A8860G and G8854A caused a
change in the encoded amino acid (Tables I and II). The polymorphism A8860G is
associated with the H2 mitochondrial haplogroup. It was present in
35/50 breast cancer patients. The polymorphism A8860G changing the
polar threonine into a non-polar alanine at position 112 concerns
α-helix 3 and influences the GRAVY value. According to PANTHER and
PSSM analysis, the greatest effect on the function of the protein
is exerted by polymorphism A110T, for which subPSEC was estimated
at 2.90892 and Pdeleterious at 0.47725. As shown in
Tables III and IV, both amino acid changes L198R and
L202V can impact the function of the protein (subPSEC>-3 and
Pdel>0.5). The deleterious effect of these mutations
confirms their effect on the change of the biochemical properties
of the protein, including the percentage of α-helix 6 (Table II). As far as the effect of the
L198R mutation on the protein conformation is concerned, TMHMM
analysis showed that there is a shift in the amino acids
constituting the 4 last intramembrane α-helices. A change in the
sections of α-helices occurrence started from α-helice 3.
 | Table IIComparison of protein properties
related to the non-synonymous protein-coding SNP in females with
breast cancer. |
Table II
Comparison of protein properties
related to the non-synonymous protein-coding SNP in females with
breast cancer.
| Amino acid
change | Theoretical
isoelectric point (pI) | Aliphatic
index | Instability
index | Grand average of
hydropathy (GRAVY) | Percentage α helix
(start and end of helix) | Region |
|---|
| ATP6 |
| A11T | 10.09 | 146.37 | 32.38 | 0.963 | 4.67 (5–27) | Transmembrane |
| T112A | | | | | 0.57 (98–119) | |
| A110T | 10.09 | 146.37 | 31.90 | 0.963 | 0.54 (98–119) | Transmembrane |
| T112A | | | | | | |
| G111A | 10.09 | 147.26 | 33.13 | 0.984 | 0.90 (98–119) | Transmembrane |
| T112A | | | | | | |
| T112A | 10.09 | 146.81 | 32.75 | 0.984 | 0.57 (98–119) | Transmembrane |
| L198R | 10.28 | 144.65 | 30.67 | 0.939 | 14.73
(191–222) | Transmembrane |
| L202V | | | | | | |
| T112A | | | | | | |
| L202A | 10.09 | 145.93 | 31.52 | 0.965 | 24.58
(191–222) | Transmembrane |
| WT | 10.09 | 146.37 | 32.75 | 0.963 | 4.61
(5–27)
0.42 (98–119)
41.36 (191–222) | Transmembrane |
| ATP8 |
| Q26P | 9.92 | 78.82 | 52.51 | −0.332 | 0.51 (1–68) | Transmembrane |
| M28T | 9.92 | 78.82 | 46.61 | −0.399 | 3.26 (1–68) | Transmembrane |
| L22I | 9.92 | 78.82 | 46.61 | −0.388 | 2.28 (1–68) | Transmembrane |
| E52K | 10.19 | 78.82 | 42.66 | −0.404 | 3.24 (1–68) | Mitochondrial
matrix |
| WT | 9.92 | 78.82 | 51.40 | −0.360 | 4.80 | Transmembrane |
 | Table IIIProbabilities of functional effects
for non-synonymous protein-coding SNPs. |
Table III
Probabilities of functional effects
for non-synonymous protein-coding SNPs.
| Protein | subPSEC |
Pdeleterious | Substitution | MSA position | Pwt |
Psubstitution | NIC |
|---|
| ATP6 | −2.18038 | 0.30584 | A11T | 61 | 0.09977 | 0.28345 | 3.734 |
| −2.90892 | 0.47725 | A110T | 165 | 0.18319 | 0.02946 | 3.843 |
| −2.28754 | 0.32905 | G111A | 166 | 0.36732 | 0.11781 | 3.843 |
| −1.43811 | 0.17338 | T112A | 167 | 0.18992 | 0.15938 | 3.843 |
| −3.79435 | 0.68876 | L198R | 258 | 0.24713 | 0.015 | 3.931 |
| −3.91748 | 0.71453 | L202V | 262 | 0.64613 | 0.03379 | 3.931 |
| ATP8 | −4.58997 | 0.83061 | Q25P | 25 | 0.71968 | 0.00887 | 2.049 |
| −2.74712 | 0.43711 | M28T | 28 | 0.23526 | 0.02328 | 2.049 |
| −0.89758 | 0.10886 | L22I | 22 | 0.22628 | 0.28675 | 2.049 |
| −1.91322 | 0.25223 | E52K | 52 | 0.28393 | 0.07158 | 2.049 |
 | Table IVResidue frequencies and PSSM scores
determined using the PSSM viewer. |
Table IV
Residue frequencies and PSSM scores
determined using the PSSM viewer.
| Protein
(position) | Residue | Raw frequency | Weighted
frequency | PSSM score |
|---|
| ATP6a |
| 11 | T | 0.70 | 0.62 | 6 |
| A | 0.11 | 0.10 | 1 |
| 110 | A | 0.83 | 0.73 | 5 |
| T | 0.03 | 0.08 | 1 |
| 111 | G | 0.90 | 0.83 | 6 |
| A | 0.09 | 0.15 | 2 |
| 112 | T | 0.68 | 0.76 | 6 |
| A | 0.32 | 0.24 | 2 |
| 198 | L | 0.97 | 0.95 | 6 |
| R | - | - | −4 |
| 202 | L | 1.00 | 0.99 | 6 |
| V | - | - | −1 |
| ATP8b |
| 22 | I | 0.60 | 0.52 | 6 |
| L | 0.13 | 0.15 | 2 |
| 25 | Q | 0.98 | 0.89 | 9 |
| P | - | - | −5 |
| 28 | M | 0.06 | 0.09 | 4 |
| T | 0.04 | 0.10 | 1 |
| 52 | E | 0.78 | 0.65 | 7 |
| K | 0.04 | 0.15 | 2 |
The L202V mutation does not affect the amino acids
that constitute the α-helices. The deleterious effect of this
mutation was confirmed by PSSM analysis. A leucine is the preferred
amino acid encoded at position 198 (L198R) with PSSM=6, in contrast
to arginine (PSSM=−4). At position 202 of the conservative domain
of ATP6, the preferred amino acid was leucine (PSSM=6), and it was
replaced by valine (PSSM=−1).
In the ATP8 gene, we identified 5 changes,
including the missense mutation A8439C that has not been described
before. This mutation concerned a change of the preferred polar
amino acid glutamine at position 25 (PSSM=9) into a non-polar one
containing a methyl group and an aliphatic side chain, proline
(PSSM =−5). The effect of this change on the protein was confirmed
by the high Pdeleterious value (0.83). In addition, the
biochemical properties of the protein were affected, as evidenced
by the change in the instability index, increasing from 51.40 to
52.51, the grand average of hydropathy, decreasing from −0.336 to
−0.332, and the percentage of α-helix, decreasing from 4.8 to 0.51.
Overall, these changes indicate that this mutation affects the
function of ATP8.
Discussion
The interest in the role of mitochondria in
carcinogenesis was initiated from findings on respiratory deficits
in dividing cells, especially in cells with intensive proliferation
rates. It is known that respiratory deficits can further impact
cell differentiation and can cause neoplastic transformation.
Mutations in the mtDNA can be favorable or adaptive, neutral or
harmful, i.e. pathogenic. Favorable mutations in the human mtDNA
are a result of the adaptation process to the constantly changing
external conditions and climate during human evolution. It has been
suggested that these adaptive mutations, which have occurred in the
mtDNA of ancient human populations during migrations to other
continents, are associated with predisposition to certain diseases
(4,14,15).
To date, the majority of mutations within the mtDNA
have been identified in prostate cancer samples (4,16),
and mostly concern the cytochrome C oxidase subunit 1
(COI). The correlation between mutations in COI and
prostate cancer showed an increased incidence of prostate cancer
among Afro-Americans where mutations occurred frequently compared
to Caucasian Americans (16,17).
In addition, an increased occurrence of homoplasmic mutations in
the COI subunit were found in a European population among
subjects with prostate cancer in comparison to the controls (11 vs.
7.8%), both in neoplastic cells and lymphocytes (16).
The role of mtDNA somatic mutations in neoplastic
progression is still being examined for numerous neoplasms. It is
highly probable that mutations in the conservative regions,
replication loci, transcription promoters or binding sites for
transcription factors may negatively affect the amount of the
mitochondrial transcript. On the other hand, mutations in OXPHOS
mtDNA genes do not necessarily cause changes in the encoded
protein. In pancreatic cancer, among 49 changes in protein-coding
regions, 26 did not affect the encoded amino acid. The remaining 23
caused an amino acid replacement in the genes coding for rRNA, NADH
dehydrogenase (subunits ND1 to ND5), cytochrome B, complex
IV of the oxidoreductase cytochrome C, and ATP synthase
(subunits 6 and 8, and D-loop) (18). In a Chinese population, the
ATP6 gene of osteosarcoma cells harbored mutations in 24/39
patients (19). Furthermore, mtDNA
mutations were detected in urinary cancer, and these were more
frequent in the respiratory complex-coding regions (20).
In numerous experiments, the identified
polymorphisms and mutations within the mtDNA concerned a transition
of the types T-to-C and G-to-A, which may imply that they are a
result of oxidative stress. The nucleotide guanine, especially in
the mtDNA, is preferentially damaged as a result of oxidative
stress and the harmful activity of OXPHOS in the nDNA. In our data,
6/13 changes were of type T-to-C and G-to-A, and occurred in 24% of
the patients (12/50). In colon cancer, 70% of the identified
mutations were a result of reactive oxygen species activity and
concerned replacements of type T-to-C and G-to-A (21). The majority of these mutations were
somatic and homoplasmic, similarly to another study on ovarian
cancer (22). In our study, we
found a G-to-A replacement in the ATP6 gene at positions
8,557, 8,697 and 8,854; these changes are typical changes occurring
upon exposure to free radicals.
Somatic cells contain hundreds to several thousand
mitochondria, each containing 1–10 gene copies of mtDNA, which, due
to its structure (lack of protective activity of histones and the
globular, coiled structure of mtDNA), often undergoes spontaneous
mutations. It is known that neoplastic cells are of monoclonal
origin. It is difficult to explain how, among the many haplotypes
of mtDNA, one type is eventually fixed in neoplastic cells, a
phenomenon commonly detectable in the form of homoplasmy in the
affected tissues (16) and during
aging (3). The question is whether
the homoplasmic mutations appearing in the mtDNA arise de
novo or have been inherited. These homoplasmic mutations become
apparent with the emergence of clinical symptoms a long period
after the mutations’ appearance (when the mutated DNA comes to
prevail); these symptoms commonly show slow progression rates. In
the examined material in our study, most of the detected changes
were of homoplasmic type. In 4 cases (Table I), the transitions were related to
both control and tumor tissues and were not detected in blood
samples of the patients. It is possible that homoplasmy is
associated with mosaicism in these individuals, resulting from
paternal mtDNA inheritance, and thus, these mutations may not be
associated with the tumor. It can however not be excluded that
certain changes occurring in the cells of the tissue from which the
tumor originates promote the process of carcinogenesis.
Heteroplasmy occurred in 2 cases in our data: at
position 9,130 of the ATP6 gene, heteroplasmy concerned only
the control tissue, while at position 8,429 it concerned only
neoplastic cells (Figs. 1 and
2). Heteroplasmy in the control
tissue can be a result of changes taking place in the cells of the
tissue from which the neoplasm has originated. The mutation at
position 9,130 was predicted to affect the function of the protein
by both PANTHER and PSSM analysis. It may be that this mutation is
beneficial to the function of the mitochondria, which consequently
may lead to the elimination of wild-type mtDNA. On the other hand,
mutation at position 8,429 has been described in the literature as
a polymorphism, and based on PANTHER and PSSM analysis, it is not
expected to affect the function of the protein. It is also notable
that the replacement L221 was predicted to affect the α-helix
(change of helix percentage from 4.80 to 2.28) and the GRAVY value.
It was suggested that the mutated mtDNA is replicated at higher
rates compared to the wild-type one (1,3).
Carcinogenesis involves thousands of successive generations of
cells. The process lasts long enough to cause the replacement of
‘wild-type’ mtDNA by mutant mtDNA. It is believed that
mitochondrial replication is controlled, and that signaling
originating from the functionally changed mitochondria triggers
their excessive replication as a result of an improvement in their
function (4). This suggests that
every mutation in the mtDNA affects their function. However, it is
difficult to determine the effects on mitochondrial function
exerted by silent mutations, which do not cause a change in the
protein. In the examined data herein, two such changes were
identified, one in the ATP8 and one in the ATP6
gene.
There are two hypothesis concerning the effects of
silent mutations: according to the first, silent mutations carry
with them ‘unidentified’, difficult to detect mutations that lead
to a selective prevalence of the mutated genome and eventually, to
the replacement of the wild-type genome with the mutated one. It is
also possible that, while the mitochondrial genome does not undergo
recombination, mutations are fixed, which results in genetic
hitchhiking and causes heteroplasmy and, in the following
generations, homoplasmy (4,21,23,24).
The second hypothesis suggests that mitochondria control their own
replication. In the case of a change in their function, there is
increased replication of the ‘mutated’ mitochondrion and
eventually, this leads to its prevalence in the cell (4,25).
It appears that besides mutations in the mtDNA, the
additional occurrence of polymorphisms is important, which may
cause a slight, almost undetectable increase in the production of
free radicals. In the examined material, we detected 5 types of
polymorphisms. Polymorphism at position 8,860 appeared as many as
33 times in 50 cases. It is connected with the mitochondrial
haplogroup H2. In the study of Aikhionbare et al (26) concerning ovarian cancer, this
polymorphism appeared in 96/102 of the examined tumors. In the
remaining patients (17/50) an adenine occurred at this position.
Two other polymorphisms seem to be connected with an increased risk
of breast and endometrial cancer: the polymorphism at position
10,398 (G-to-A) of the ND3 gene, associated with the
mitochondrial haplogroup N, changes the codon A114T, and is
reported to associate with an increased incidence of breast cancer
(14,27). Setiawan et al (28) did not support this association in
their study. On the other hand, the polymorphism at position 16,189
(T-to-C) was associated with endometrial cancer (29). In our previous study (15), we showed the occurrence of
polymorphisms in mt tRNA genes in women with breast cancer,
including polymorphism 12,308G, which is associated with chronic
progressive external ophthalmoplegia (1). It cannot be excluded that
polymorphisms may favour the occurrence of selective advantage of
the mutated mtDNA.
In the present study, we identified a total of 13
changes in the mtDNA of breast cancer patients, including 4 that
have not been described in the literature before. The majority of
the changes were homoplasmic, of the missense type. Polymorphisms,
especially those of the missense type, can affect the function of
the mitochondria, especially if they lie on conservative domains of
the mitochondrial proteins. These changes can promote the selective
prevalence of mutated mtDNA over the wild-type mtDNA, which may be
involved in the process of carcinogenesis. Changes occurring in the
mtDNA during carcinogenesis may result from cell adaptation
processes to new conditions.
References
|
1
|
Wallace DC: Bioenergetics in human
evolution and disease: implications for the origins of biological
complexity and the missing genetic variation of common diseases.
Philos Trans R Soc Lond B Biol Sci. 368:2012.02672013.
|
|
2
|
Féthière J, Venzke D, Diepholz M, et al:
Building the stator of the yeast vacuolar-ATPase: specific
interaction between subunits E and G. J Biol Chem. 279:40670–40676.
2004.PubMed/NCBI
|
|
3
|
Czarnecka A and Bartnik E: The role of the
mitochondrial genome in ageing and carcinogenesis. J Aging Res.
2011:1364352011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grzybowska-Szatkowska L and Slaska B:
Mitochondrial DNA and carcinogenesis (review). Mol Med Rep.
6:923–930. 2012.
|
|
5
|
Zhu W, Qin W, Bradley P, Wessel A, Puckett
CL and Sauter ER: Mitochondrial DNA mutations in breast cancer
tissue and in matched nipple aspirate fluid. Carcinogenesis.
26:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomas PD, Campbell MJ, Kejariwal A, Mi H,
et al: PANTHER: A library of protein families and subfamilies
indexed by function. Genome Res. 13:2129–2141. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sonnhammer ELL, von Heijne G and Krogh A:
A hidden Markov model for predicting transmembrane helices in
protein sequences. Proc Int Conf Intell Syst Mol Biol; 6:175–182.
1998.
|
|
8
|
Finn RD, Mistry J, Tate J, Coggill P, et
al: The Pfam protein families database. Nucleic Acids Res.
38:D211–D222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muñoz V and Serrano L: Elucidating the
folding problem of helical peptides using empirical parameters. Nat
Struct Mol Biol. 1:399–409. 1994.
|
|
10
|
Kyte J and Doolittle RF: A simple method
for displaying the hydropathic character of a protein. J Mol Biol.
157:105–132. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gasteiger E, Hoogland C, Gattiker A,
Duvaud S, Wilkins MR, Appel RD and Bairoch A: Protein
identification and analysis tools on the ExPASy server. The
Proteomics Protocols Handbook. Walker JM: Humana Press; New York,
NY: pp. 571–607. 2005, View Article : Google Scholar
|
|
12
|
Goldenberg O, Erez E, Nimrod G and Ben-Tal
N: The ConSurf-DB: pre-calculated evolutionary conservation
profiles of protein structures. Nucleic Acids Res. 37:D323–D327.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Glaser F, Pupko T, Paz I, Bell RE,
Bechor-Shental D, Martz E and Ben-Tal N: ConSurf: identification of
functional regions in proteins by surface-mapping of phylogenetic
information. Bioinformatics. 19:163–164. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Canter JA, Kallianpur AR, Parl FF and
Millikan RC: Mitochondrial DNA G10398A polymorphism and invasive
breast cancer in African-American women. Cancer Res. 65:8028–8033.
2005.
|
|
15
|
Grzybowska-Szatkowska L and Slaska B:
Polymorphisms in genes encoding mt-tRNA in female breast cancer in
Poland. Mitochondrial DNA. 23:106–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petros JA, Baumann AK, Ruiz-Pesini E, et
al: MtDNA mutations increase tumorigenicity in prostate cancer.
Proc Natl Acad Sci USA. 102:719–724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brandon M, Baldi P and Wallace DC:
Mitochondrial mutations in cancer. Oncogene. 25:4647–4662. 2006.
View Article : Google Scholar
|
|
18
|
Jones JB, Song JJ, Hempen PM, Parmigiani
G, Hruban RH and Kern SE: Detection of mitochondrial DNA mutations
in pancreatic cancer offers a ‘mass’-ive advantage over detection
of nuclear DNA mutations. Cancer Res. 61:1299–1304. 2001.PubMed/NCBI
|
|
19
|
Guo XG, Liu CT, Dai H and Guo QN:
Mutations in the mitochondrial ATPase6 gene are frequent in human
osteosarcoma. Exp Mol Pathol. 94:285–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dasgupta S, Shao CB, Keane TE, et al:
Detection of mitochondrial deoxyribonucleic acid alterations in
urine from urothelial cells carcinoma patients. Int J Cancer.
131:158–164. 2012. View Article : Google Scholar
|
|
21
|
Habano W, Nakamura S and Sugai T:
Microsatellite instability in the mitochondrial DNA of colorectal
carcinomas: evidence for mismatch repair systems in mitochondrial
genome. Oncogene. 17:1931–1937. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu VW, Shi HH, Cheung AN, et al: High
incidence of somatic mitochondrial DNA mutations in human ovarian
carcinomas. Cancer Res. 61:5998–6001. 2001.PubMed/NCBI
|
|
23
|
Slaska B, Grzybowska-Szatkowska L, Surdyka
M, Nisztuk S, Rozanska D, Rozanski P, Smiech A and Orzelski M:
Mitochondrial D-loop mutations and polymorphisms are connected with
canine malignant cancers. Mitochondrial DNA. 25:238–243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slaska B, Grzybowska-Szatkowska L, Nisztuk
S, Surdyka M and Rozanska D: Mitochondrial DNA polymorphism in
genes encoding ND1, COI and CYTB in canine malignant cancers.
Mitochondrial DNA. Oct 9–2013. View Article : Google Scholar
|
|
25
|
Hofhaus G and Gattermann N: Mitochondria
harbouring mutant mtDNA - a cuckoo in the nest? Biol Chem.
380:871–877. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aikhionbare FO, Mehrabi S, Kumaresan K,
Zavareh M, Olatinwo M, Odunsi K and Partridge E: Mitochondrial DNA
sequence variants in epithelial ovarian tumor subtypes and stages.
J Carcinog. 6:12007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Czarnecka AM, Krawczyk T, Zdrozny M, et
al: Mitochondrial NADH-dehydrogenase subunit 3 (ND3) polymorphism
(A10398G) and sporadic breast cancer in Poland. Breast Cancer Res
Treat. 121:511–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Setiawan VW, Chu LH, John EM, et al:
Mitochondrial DNA G10398A variant is not associated with breast
cancer in African-American women. Cancer Genet Cytogenet.
181:16–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu VW, Wang Y, Yang HJ, et al:
Mitochondrial DNA variant 16189T>C is associated with
susceptibility to endometrial cancer. Hum Mutat. 22:173–174.
2003.
|