Introduction
Neuroblastoma is a common childhood malignant tumor
of the sympathetic nervous system, accounting for up to 10% of
pediatric cancers and 15% of cancer-related mortality in children
(1–3). Neuroblastoma comprises a
heterogeneous group of tumors, in which the level of
differentiation is known to be of prognostic significance (4,5).
Histologically, neuroblastomas range from tumors containing
poorly-differentiated neuroblasts to those composed of
fully-differentiated sympathetic neurons (6,7).
Patients with poorly differentiated neuroblastomas have a
significantly poorer survival than those with neuroblastomas that
are shown to be well-differentiated on histological examination
(4,5,8).
Retinoic acid (RA) is an effective inducer of the
differentiation of neuroblastoma cells (9,10),
which has been used in clinical practice as a therapeutic agent in
high-risk neuroblastomas in order to improve the differentiation
state of the cells (11,12). In addition, GATA transcription
factors are involved in the regulation of hematopoiesis, and the
development of the cardiovascular, nervous, and urogenital systems
(13–17). The GATA family contains six
members, which are reported to be expressed in distinct
spatiotemporal patterns (18–20).
GATA2 and GATA3 are the only members of this family that are
present in the nervous system (21,22),
and the pattern of the expression of these two proteins is known to
overlap. GATA3 has been reported to be involved in the development
of serotonergic neurons during formation of the ear, and in the
development of the caudal raphe nuclei and the peripheral nervous
system (22–27). The present study investigated the
role of GATA3 in neuroblastoma proliferation and
differentiation.
Materials and methods
Cell culture
SHEP1, SK-N-DZ, SK-N-AS, and SK-N-SH human
neuroblastoma cells were grown in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
SK-N-BE (2), IMR32, BE (2)-C and SY5Y human neuroblastoma cell
lines were grown in a 1:1 mixture of DMEM and Ham’s nutrient
mixture F12 (F12/DMEM), supplemented with 10% FBS and nonessential
amino acids. LAN-6 and SMS-KCNR human neuroblastoma cell lines were
grown in RPMI-1640 supplemented with 10% FBS. The growth media and
FBS were obtained from Invitrogen Life Technologies (Carlsbad, CA,
USA). All cells were obtained from American Type Culture Collection
(Manassus, VA, USA) and cultured at 37°C in a 5% CO2
humidified incubator. The 293GPG retroviral packaging cell line was
cultured as described previously (28).
Retroviral production and infection
The retroviral constructs, pBabe-green fluorescent
protein (GFP) and pBabe-GATA3, were used in the overexpression
experiments. Retroviruses were produced using the 293GPG packaging
cell line as described previously (28). At 24 h following the final round of
retroviral infection, cells were cultured in the growth medium
containing 1.0 μg/ml puromycin for three days, and drug-resistant
cells were pooled. The percentage of retrovirus-infected cells
ranged between 80 and 90%, as estimated in parallel infections
using the retrovirus-expressing GFP. Over-expression of relevant
proteins was verified by an immunoblotting assay.
Immunoblot analysis
Following RA (Sigma-Aldrich, St. Louis, MO, USA)
treatment or retroviral infection, cells in the exponential growth
phase at 70–80% confluence were harvested at various time points
and washed once with ice-cold phosphate-buffered saline. Cell
pellets were suspended in SDS sample buffer and boiled for 10 min
prior to centrifugation at 211 × g for 10 min. Samples were
subjected to 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
and transferred to a polyvinylidene fluoride membrane (EMD
Millipore, Billerica, MA, USA). The membrane was probed with
antibodies and binding was visualized using enhanced
chemiluminescence (ECL; Beyotime Institute of Biotechnology,
Haimen, China). The following primary antibodies were used: Rabbit
polyclonal anti-GATA3 (1:100; H-48, sc-9009; Santa Cruz
Biotechnology Inc., Dallas, TX, USA), mouse monoclonal anti-Mash1
(1:100; clone 24B72D11.1; BD Pharmingen, San Diego, CA, USA),
rabbit polyclonal anti-peripherin (1:2,000; AB1530; Chemicon
International, Inc., Billerica, MA, USA) and mouse monoclonal
anti-α-tubulin (1:10,000; B-5-1-2; Sigma-Aldrich). Horseradish
peroxidase-conjugated goat anti-mouse and goat anti-rabbit IgG
(1:5,000, ICN, Bryan, OH, USA) were used as secondary
antibodies.
Cell growth and differentiation
assays
For differentiation assays, RA was dissolved in
dimethyl sulfoxide (DMSO) and 10 mM stock solutions were prepared.
SK-N-SH cells were treated with 1 μM RA. DMSO (0.1%; Sigma-Aldrich)
was used as negative control. Cell growth was observed under a
microscope (Olympus IX71; Olympus, Tokyo, Japan) and determined by
MTT analysis (Sigma-Aldrich)
Patient data analysis
Patient data and gene expression datasets were
obtained from the Oncogenomics Section Data Center (http://pob.abcc.ncifcrf.gov/cgi-bin/JK).
Kaplan-Meier analysis and resulting survival curves were created
using GraphPad Prism (version 6.0; GraphPad Software, Inc, La
Jolla, CA, USA). All data and P-values (log-rank test) for these
experiments were downloaded online (http://pob.abcc.ncifcrf.gov/cgi-bin/JK) and all cutoff
values for separating the groups with high and low expression were
determined using the online database algorithm (29). A good prognosis of neuroblastoma
patients was considered to be associated with better survival.
Soft agar clonogenic assay and sphere
formation assay
Cells were mixed in 0.3% Noble agar (Sigma-Alrdich)
in DMEM supplemented with 10% FBS and plated at 4,000 cells/well
into 6-well plates, which contained a solidified bottom layer
composed of 0.6% Noble agar in the same growth medium. At 14 days,
colonies were stained with 5 mg/ml MTT and photographed (Olympus,
IX71; Olympus). For sphere formation assays, cells were plated at
4,000 cells/well in serum-free DMEM, and supplemented with 20 ng/ml
epidermal growth factor and basic fibroblast growth factor
(Invitrogen Life Technologies) in Matrigel ultra-low attachment
plates (Thermo Fisher Scientific, Pitsburgh, PA, USA). Spheres that
arose within 1–2 weeks were counted.
In vivo tumorigenic assay
For the tumorigenic assays, six female NOD/SCID mice
(4 weeks old) were used and were maintained under SPF conditions.
For the tumorigenic assays, the mice were randomly divided into two
groups, control group and GATA3-overexpressing group. Mice were
injected subcutaneously in both flanks with 1×107
SK-N-SH cells or SK-N-SH-GATA3 cells in 200 μl DMEM. At one week
following the injection of tumor cells, tumor growth was estimated
using calipers, and tumor volume was calculated using the formula
4/3πr3, where r is the radius of the tumor. Tumors were
removed and weighed following three weeks of tumor growth. The
present study was approved by the Institutional Animal Care and Use
Committee of Southwest University (Chonqing, China).
Statistical analysis
Data are presented as the mean ± standard deviation.
Two-tailed Student’s t-test was conducted for paired samples and
was performed using GraphPad Prism version 6.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). P≤0.05 was considered to
indicate a statistically significant difference.
Results
High GATA3 expression predicts poor
survival in neuroblastoma patients
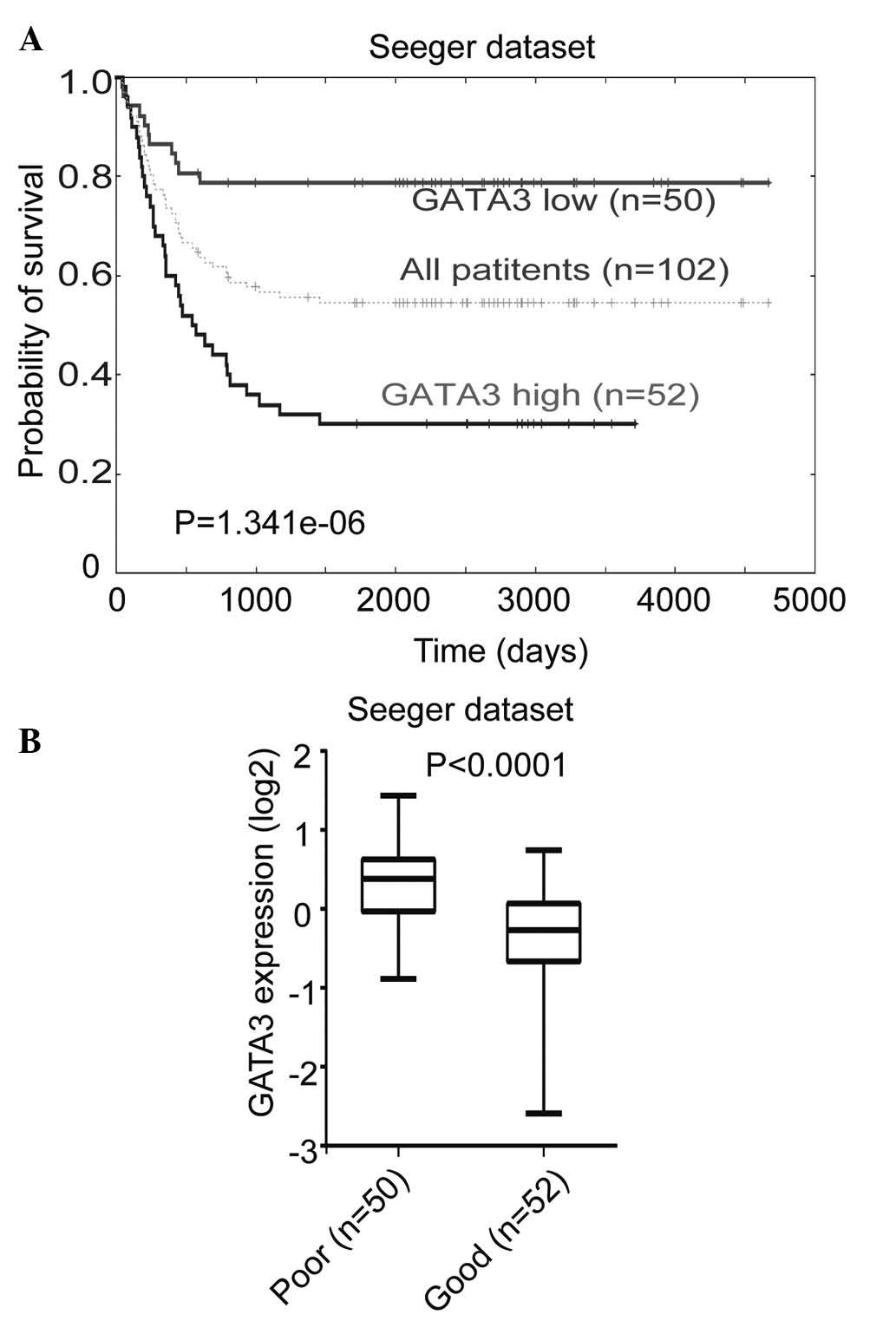
The correlation between GATA3 expression levels and
prognosis in primary neuroblastoma was investigated using the
Seeger microarray dataset, which is available from the online
Oncogenomics database. This dataset includes a cohort of 102
neuroblastoma patients with metastatic tumors lacking MYCN
amplification (30). Kaplan-Meier
analysis of progression-free survival for the Seeger dataset showed
that low GATA3 expression was associated with a good prognosis,
whereas high GATA3 expression was associated with a poor outcome
(Fig. 1A). Furthermore, a box plot
of GATA3 expression levels in tumors from patients with either a
good or a poor prognosis demonstrated the same result (Fig. 1B). This analysis indicated that
GATA3 is a prognostic marker in neuroblastoma, which is independent
of the status of MYCN amplification.
GATA3 is commonly expressed in
neuroblastoma cells
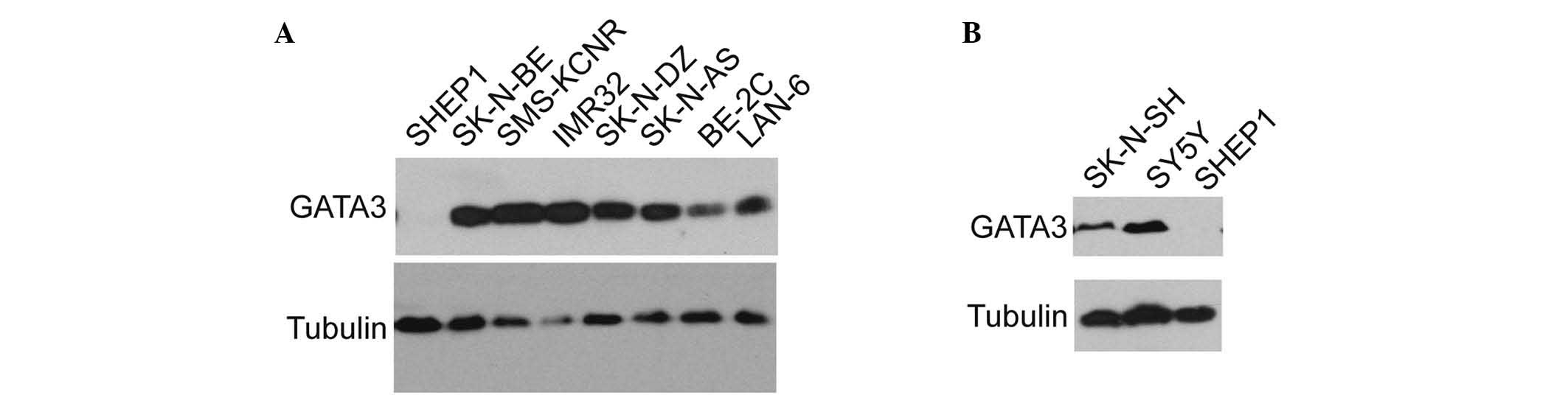
The expression of GATA3 in various neuroblastoma
cell lines was examined. GATA3 was found to be widely expressed in
the majority of neuroblastoma cell lines (Fig. 2A), including BE (2)-C, IMR32,
SK-N-DZ, SK-N-AS, and SK-N-BE, which are malignant cell lines. The
expression of GATA3 was also investigated in the SHEP1 cell line,
which is a benign neuroblastoma cell line with a highly
differentiated status (31,32).
The results indicated that GATA3 may be associated with the degree
of neuroblastoma differentiation and the consequent prognosis. The
SK-N-SH cells were a group of mixed cells which were isolated into
SY5Y and SHEP1 in different conditions (33), and SY5Y is a type of malignant cell
comparing with SHEP1 (32). As
hypothesized, GATA3 expression was relatively high in the SY5Y and
SK-N-SH cell lines, but there was no detectable expression in the
SHEP1 cell line (Fig. 2B). This
suggests that GATA3 may be used as a prognostic marker in
neuroblastoma.
GATA3 is associated with neuronal
differentiation in neuroblastoma cells
Since RA is commonly used to induce neuronal
differentiation in neuroblastoma (34), SK-N-SH cells were treated with RA
for 10 days. On examination the cells displayed morphological
features of neuronal differentiation, such as small and rounded
cell bodies, scant cytoplasm, and extensive neurite-like processes
(Fig. 3A). Furthermore, MTT,
sphere formation and soft agar analyses showed that RA treatment
resulted in the suppression of cell proliferation, tumorigenicity
and self-renewal of neuroblastoma cells (Fig. 3B–E). The GATA3 expression in this
process was measured, and the results showed that RA induction led
to marked downregulation of GATA3 expression with time (Fig. 3F). These findings suggest that
RA-induced neuronal differentiation is accompanied by GATA3
downregulation, which leads to weakened self-renewal of
neuroblastoma cells.
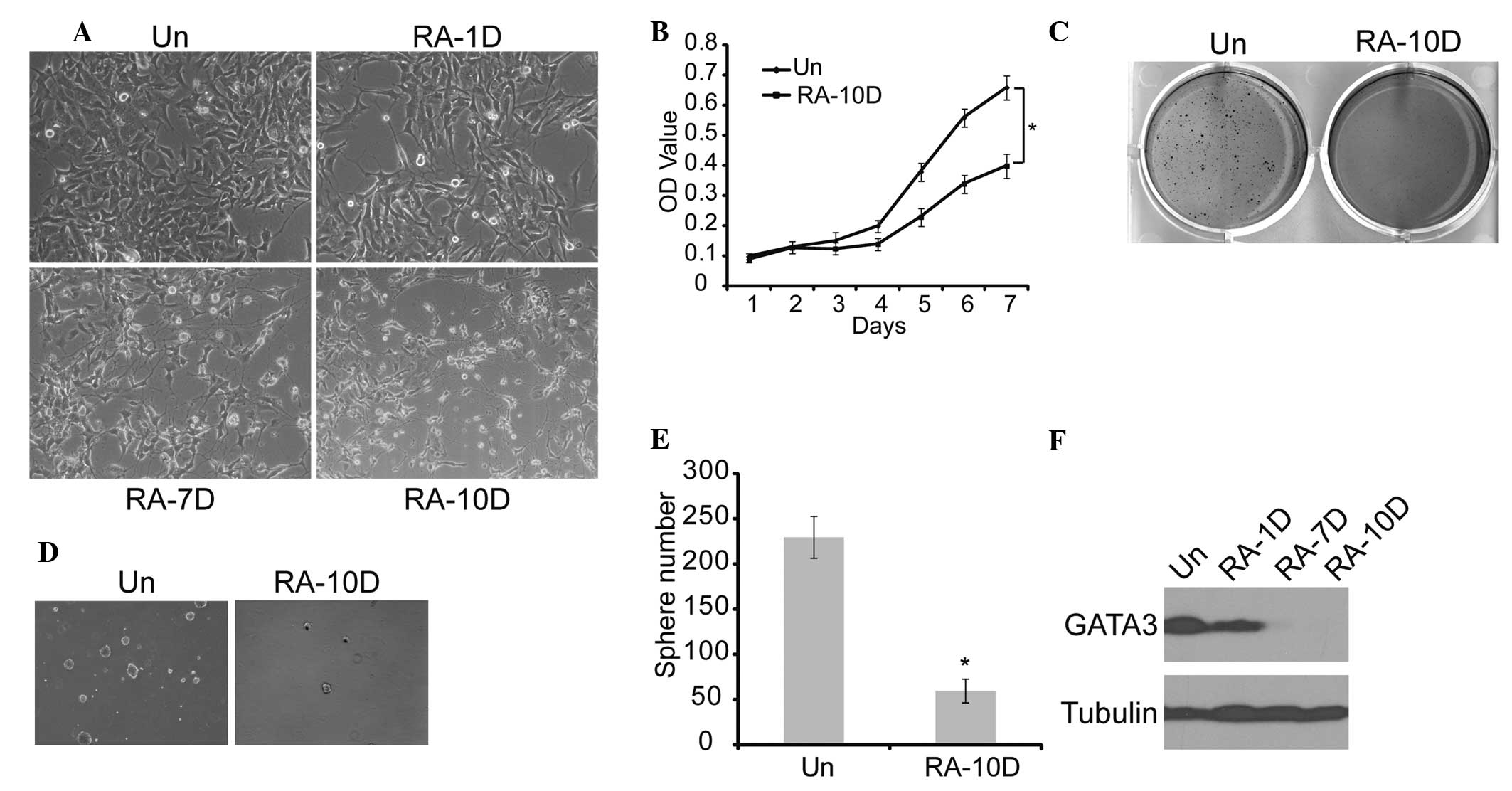 | Figure 3Association between GATA3 expression
and neuronal differentiation in neuroblastoma cells. (A)
Morphological examination of SK-N-SH cells treated with RA or DMSO
(magnification, ×20). (B) SK-N-SH cells were treated with RA or
DMSO, and cell proliferation was analyzed with an MTT assay. (C)
SK-N-SH cells were plated at 4×103 cells per well in
six-well culture plates. At days 14 to 21 soft agar colonies grew
from the cells treated with DMSO. Cells treated with RA were
observed to give rise to small and scanty colonies in soft agar.
(D) and (E) SK-N-SH cells were plated at 4×103 cells per
well in Matrigel ultra-low attachment plates. At days 14 to 21,
spheres grew from cells treated with DMSO, and were recorded. (F)
Western blot analysis of GATA3 expression in SK-N-SH cells treated
with RA or DMSO (magnification, ×10). α-tubulin levels are shown as
a loading control. Data in (B) and (E) are presented as the average
obtained from three independent experiments. Error bars, represent
standard deviation. *P≤0.05. RA, retinoic acid; Un,
untreated; DMSO, dimethyl sulfoxide; RA-(1D, 7D and 10D), one day,
seven days and ten days following RA treatment, respectively; OD,
optical density. |
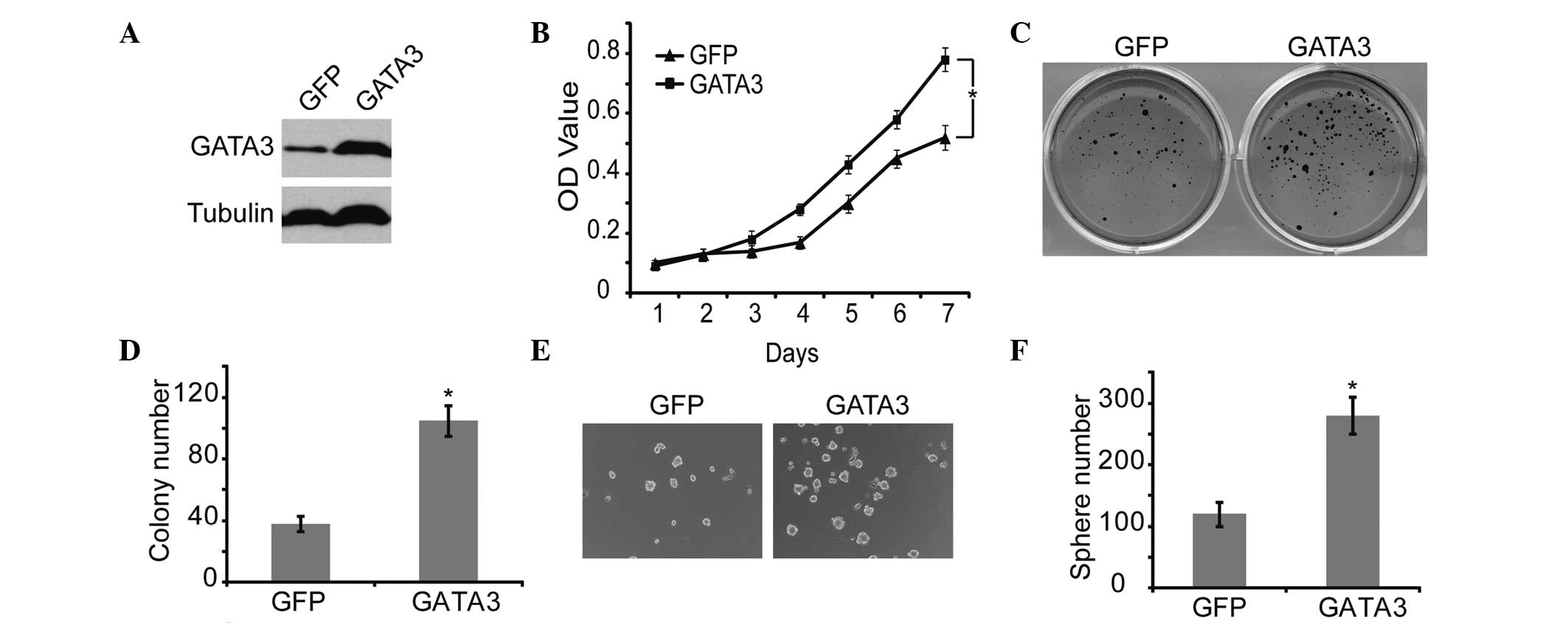
GATA3 promotes proliferation and
tumorigenicity of neuroblastoma cells
To confirm the correlation between GATA3 and
self-renewal of neuroblastoma cells, GATA3 was overexpressed in
neuroblastoma cells, using GFP as a control (Fig. 4A). GATA3 significantly increased
cell proliferation, which was verified by MTT analysis (Fig. 4B). In addition, GATA3 markedly
upregulated the self-renewal ability, including the colony forming
and sphere forming capability, of neuroblastoma cells (Fig. 4C and 4F). These results
demonstrated that high expression of GATA3 is associated with
increased self-renewal and cell proliferation in neuroblastoma
cells.
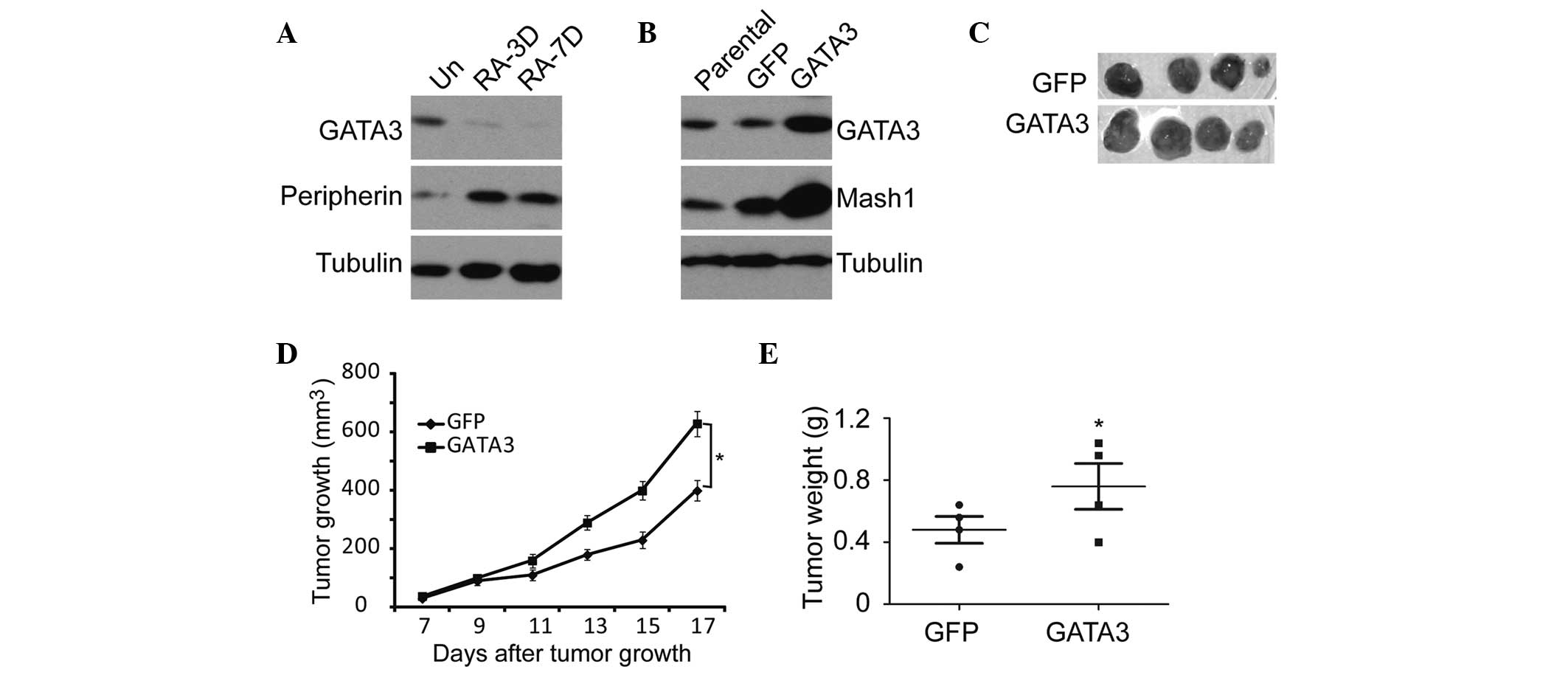
Furthermore, the RA-induced neuroblastoma
differentiation was accompanied by GATA3 downregulation and the
upregulation of peripherin, a neuronal differentiation marker
(35) (Fig. 5A). GATA3 upregulation led to a
significant increase in the expression of Mash1 (Fig. 5B), which is a potential stem cell
or progenitor cell marker (36,37).
Similar results were obtained from the in vivo
tumorigenicity analysis using the SK-N-SH neuroblastoma cells.
Overexpression of GATA3 in SK-N-SH cells significantly enhanced
tumor growth and development in NOD/SCID mice (Fig. 5C–E). These data indicate that GATA3
is not only a prognostic marker, but also an important mediator of
cell proliferation and differentiation.
Discussion
The current study provided a number of lines of
evidence to support the hypothesis that GATA3 acts as an important
mediator of neuroblastoma differentiation. GATA3 was shown to be
expressed at significantly lower levels following RA-induced
differentiation. Overexpression of GATA3 expression significantly
increased cell growth and self-renewal in neuroblastoma cells.
Furthermore, RA-induced neuronal differentiation resulted in the
upregulation of peripherin, a neuronal differentiation marker, and
the downregulation of GATA3. In turn, GATA3 overexpression
increased the expression of a marker of a self-renewal marker,
Mash1. These results suggest a possible molecular mechanism linking
neuronal differentiation and self-renewal. Using a gene expression
dataset of 102 metastatic neuroblastoma tumors, it was shown that
high GATA3 expression is a prognostic marker of poor outcome, which
supported the results of the other experiments.
MYCN is an important oncogene in the pathogenesis of
neuroblastoma (38), and is known
to regulate various cellular processes, including cell growth, cell
proliferation, cell differentiation and apoptosis (39,40).
The oncogene MYCN was originally identified in neuroblastoma cells
(41,42), and it has been reported as a
prognostic marker in patients with neuroblastoma (43). Amplification of MYCN occurs in 22%
of cases of neuroblastoma and is associated with advanced stages of
this disease and a poor prognosis (7). N-myc is currently the only marker
commonly used in the diagnosis of neuroblastoma. It is therefore
necessary to identify further genetic markers for neuroblastoma.
The current study showed that high GATA3 expression was correlated
with poor survival in patients with neuroblastoma. Low expression
of GATA3 was associated with a high degree of differentiation,
indicating that GATA3 may be of use as a prognostic marker in
neuroblastoma.
Neuroblastoma originates from precursor neuroblasts
of the sympathetic nervous system, and is characterized by a unique
capacity for complete spontaneous regression, at least partly
through the process of neuronal differentiation (8). In clinical practice, patients with
advanced neuroblastoma may be successfully treated by the
administration of RA, which induces tumor cells to differentiate
and leads to growth inhibition (9,11,44).
In the current study, neuronal differentiation, induced by RA, was
accompanied by GATA3 downregulation in neuroblastoma cells, whereas
upregulation of GATA3 was associated with increased self-renewal
and proliferation of neuroblastoma cells. In conclusion, the
present results confirmed that GATA3 has an important function in
neuroblastoma differentiation and proliferation. Therefore, GATA3
may be useful as a prognostic marker in patients with
neuroblastoma, and may also serve as a potential therapeutic target
for neuroblastoma.
Acknowledgements
This study was supported by the Research Fund for
the Doctoral Program of Higher Education of China (grant no.
20130182110003), the Natural Science Foundation of Chongqing (grant
no. cstc2013jcyjys0007) and the Fundamental Research Funds for the
Central Universities (grant no. SWU111014).
References
|
1
|
Zhu S, Yan X, Xiang Z, Ding HF and Cui H:
Leflunomide reduces proliferation and induces apoptosis in
neuroblastoma cells in vitro and in vivo. PLoS One. 8:e715552013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li T, Wang L, Ke XX, et al: DNA-damaging
drug-induced apoptosis sensitized by N-myc in neuroblastoma cells.
Cell Biol Int. 36:331–337. 2012. View Article : Google Scholar
|
|
3
|
Li T, Cui ZB, Ke XX, et al: Essential role
for p53 and caspase-9 in DNA damaging drug-induced apoptosis in
neuroblastoma IMR32 cells. DNA Cell Biol. 30:1045–1050. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mao L, Ding J, Zha Y, et al: HOXC9 links
cell-cycle exit and neuronal differentiation and is a prognostic
marker in neuroblastoma. Cancer Res. 71:4314–4324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambros IM, Hata J, Joshi VV, et al:
Morphologic features of neuroblastoma (Schwannian stroma-poor
tumors) in clinically favorable and unfavorable groups. Cancer.
94:1574–1583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimada H, Ambros IM, Dehner LP, et al:
The international neuroblastoma pathology classification (the
Shimada system). Cancer. 86:364–372. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brodeur GM: Neuroblastoma: biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheung NK and Dyer MA: Neuroblastoma:
developmental biology, cancer genomics and immunotherapy. Nat Rev
Cancer. 13:397–411. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sidell N: Retinoic acid-induced growth
inhibition and morphologic differentiation of human neuroblastoma
cells in vitro. J Natl Cancer Inst. 68:589–596. 1982.PubMed/NCBI
|
|
10
|
Hämmerle B, Yañez Y, Palanca S, et al:
Targeting neuroblastoma stem cells with retinoic acid and
proteasome inhibitor. PLoS One. 8:e767612013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reynolds CP, Matthay KK, Villablanca JG
and Maurer BJ: Retinoid therapy of high-risk neuroblastoma. Cancer
Lett. 197:185–192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Volchenboum SL and Cohn SL: Progress in
defining and treating high-risk neuroblastoma: lessons from the
bench and bedside. J Clin Oncol. 27:1003–1004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pevny L, Simon MC, Robertson E, et al:
Erythroid differentiation in chimaeric mice blocked by a targeted
mutation in the gene for transcription factor GATA-1. Nature.
349:257–260. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asnagli H, Afkarian M and Murphy KM:
Cutting edge: Identification of an alternative GATA-3 promoter
directing tissue-specific gene expression in mouse and human. J
Immunol. 168:4268–4271. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Lim KC, Onodera K, et al: Rescue
of the embryonic lethal hematopoietic defect reveals a critical
role for GATA-2 in urogenital development. EMBO J. 17:6689–6700.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Craven SE, Lim KC, Ye W, Engel JD, de
Sauvage F and Rosenthal A: Gata2 specifies serotonergic neurons
downstream of sonic hedgehog. Development. 131:1165–1173. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dasen JS, O’Connell SM, Flynn SE, et al:
Reciprocal interactions of Pit1 and GATA2 mediate signaling
gradient-induced determination of pituitary cell types. Cell.
97:587–598. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto M, Ko LJ, Leonard MW, Beug H,
Orkin SH and Engel JD: Activity and tissue-specific expression of
the transcription factor NF-E1 multigene family. Genes Dev.
4:1650–1662. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Whyatt DJ, deBoer E and Grosveld F: The
two zinc finger-like domains of GATA-1 have different DNA binding
specificities. EMBO J. 12:4993–5005. 1993.PubMed/NCBI
|
|
20
|
Tsarovina K, Pattyn A, Stubbusch J, et al:
Essential role of Gata transcription factors in sympathetic neuron
development. Development. 131:4775–4786. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kornhauser JM, Leonard MW, Yamamoto M,
LaVail JH, Mayo KE and Engel JD: Temporal and spatial changes in
GATA transcription factor expression are coincident with
development of the chicken optic tectum. Brain Res Mol Brain Res.
23:100–110. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pata I, Studer M, van Doorninck JH, et al:
The transcription factor GATA3 is a downstream effector of Hoxb1
specification in rhombomere 4. Development. 126:5523–5531.
1999.PubMed/NCBI
|
|
23
|
Tsarovina K, Reiff T, Stubbusch J, et al:
The Gata3 transcription factor is required for the survival of
embryonic and adult sympathetic neurons. J Neurosci.
30:10833–10843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Esch H and Devriendt K: Transcription
factor GATA3 and the human HDR syndrome. Cell Mol Life Sci.
58:1296–1300. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pattyn A, Simplicio N, van Doorninck JH,
Goridis C, Guillemot F and Brunet JF: Ascl1/Mash1 is required for
the development of central serotonergic neurons. Nat Neurosci.
7:589–595. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lim KC, Lakshmanan G, Crawford SE, Gu Y,
Grosveld F and Engel JD: Gata3 loss leads to embryonic lethality
due to noradrenaline deficiency of the sympathetic nervous system.
Nat Genet. 25:209–212. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Milo M, Cacciabue-Rivolta D, Kneebone A,
et al: Genomic analysis of the function of the transcription factor
gata3 during development of the mammalian inner ear. PLoS One.
4:e71442009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ory DS, Neugeboren BA and Mulligan RC: A
stable human-derived packaging cell line for production of high
titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc
Natl Acad Sci USA. 93:11400–11406. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen QR, Song YK, Wei JS, et al: An
integrated cross-platform prognosis study on neuroblastoma
patients. Genomics. 92:195–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Asgharzadeh S, Pique-Regi R, Sposto R, et
al: Prognostic significance of gene expression profiles of
metastatic neuroblastomas lacking MYCN gene amplification. J Natl
Cancer Inst. 98:1193–1203. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui H, Hu B, Li T, et al: Bmi-1 is
essential for the tumorigenicity of neuroblastoma cells. Am J
Pathol. 170:1370–1378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cui H, Ma J, Ding J, Li T, Alam G and Ding
HF: Bmi-1 regulates the differentiation and clonogenic self-renewal
of I-type neuroblastoma cells in a concentration-dependent manner.
J Biol Chem. 281:34696–34704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lambert DG, Ghataorre AS and Nahorski SR:
Muscarinic receptor binding characteristics of a human
neuroblastoma SK-N-SH and its clones SH-SY5Y and SH-EP1. Eur J
Pharmacol. 165:71–77. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sidell N, Altman A, Haussler MR and Seeger
RC: Effects of retinoic acid (RA) on the growth and phenotypic
expression of several human neuroblastoma cell lines. Exp Cell Res.
148:21–30. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pedersen WA, Becker LE and Yeger H:
Expression and distribution of peripherin protein in human
neuroblastoma cell lines. Int J Cancer. 53:463–470. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sommer L, Shah N, Rao M and Anderson DJ:
The cellular function of MASH1 in autonomic neurogenesis. Neuron.
15:1245–1258. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Horton S, Meredith A, Richardson JA and
Johnson JE: Correct coordination of neuronal differentiation events
in ventral forebrain requires the bHLH factor MASH1. Mol Cell
Neurosci. 14:355–369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alam G, Cui H, Shi H, et al: MYCN promotes
the expansion of Phox2B-positive neuronal progenitors to drive
neuroblastoma development. Am J Pathol. 175:856–866. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grandori C, Cowley SM, James LP and
Eisenman RN: The Myc/Max/Mad network and the transcriptional
control of cell behavior. Annu Rev Cell Dev Biol. 16:653–699. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Adhikary S and Eilers M: Transcriptional
regulation and transformation by Myc proteins. Nat Rev Mol Cell
Biol. 6:635–645. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schwab M, Alitalo K, Klempnauer KH, et al:
Amplified DNA with limited homology to myc cellular oncogene is
shared by human neuroblastoma cell lines and a neuroblastoma
tumour. Nature. 305:245–248. 1983. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kohl NE, Kanda N, Schreck RR, et al:
Transposition and amplification of oncogene-related sequences in
human neuroblastomas. Cell. 35:359–367. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akter J, Takatori A, Hossain MS, et al:
Expression of NLRR3 orphan receptor gene is negatively regulated by
MYCN and Miz-1, and its downregulation is associated with
unfavorable outcome in neuroblastoma. Clin Cancer Res.
17:6681–6692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Seeger RC, Siegel SE and Sidell N:
Neuroblastoma: clinical perspectives, monoclonal antibodies, and
retinoic acid. Ann Intern Med. 97:873–884. 1982. View Article : Google Scholar : PubMed/NCBI
|