Introduction
Subarachnoid hemorrhage (SAH) refers to bleeding
within the subarachnoid space, which is a critical condition with
high morbidity and mortality. The annual incidence of SAH has been
estimated to be approximately 9/100,000 in the general population,
worldwide (1). Cerebral vasospasm
(CVS) is one of the most serious complications of SAH and is an
independent risk factor for poor prognosis (2). CVS most commonly occurs within 3 to
15 days from the onset of SAH, and reaches peak severity between 7
and 10 days. CVS is characterized by upregulated vasoconstrictor
and downregulated vasodilator expression, as well as vascular
smooth muscle cell hypercontractility (3).
Endothelin-1 (ET-1) is a potent vasoconstrictor
peptide of endothelial origin and may be associated with the
pathogenesis of CVS following aneurysmal SAH (4). ET-1-induced vasoconstriction is
mediated by two G-protein-coupled receptors: Endothelin receptor
type A (ETA) and endothelin receptor type B
(ETB) (5). In
experimental animal models of SAH, it has been demonstrated that
cerebral arteries are more sensitive to ET-1 (6,7), and
increased levels of ETA and ETB mRNA have
been reported (8,9). Additionally, ET-1 receptor
antagonists, particularly antagonists of both ETA and
ETB receptors, prevent and relieve SAH-induced CVS
(10,11). Some scholars have speculated that
increased levels of ET-1 in the cerebrospinal fluid (CSF) of
patients with SAH may induce hypercontractility in cerebral
arteries, thereby contributing to the occurrence of CVS (12).
The present study aimed to investigate the
expression levels of ET-1 in the CSF of patients with aneurysmal
SAH. In addition, arterial contractility and ET-1 receptor
expression (ETA and ETB mRNA) was analyzed
in vitro. Based on the present study, the therapeutic
applications of the antagonist for ET-1 receptors should be
assessed in further studies.
Materials and methods
Participants and CSF sample
collection
The present study was approved by the Institutional
Review Board and Ethics Committee of the First Affiliated Hospital
of Xi'an Jiaotong University (Xi'an, China; approval no. 2016-074).
A total of 16 patients were enrolled, who were diagnosed with
aneurysmal SAH based on computed tomography (CT) and/or digital
subtraction angiography (DSA) evidence. The individual clinical
severity of SAH was assessed according to the World Federation of
Neurological Surgeons scale and the Fisher CT grading system
(13,14). Additionally, 12 patients with
hydrocephalus were enrolled as controls.
The inclusion criteria were as follows: i) Between
the ages of 18 and 80 years old; ii) diagnosed with aneurysmal SAH
by CT and/or DSA; iii) admission with-in 48 h from onset of SAH;
iv) Fisher grade III or IV, which is radiologically typical and
prone to CVS (14); and v) consent
to participate was provided.
CSF samples were collected from patients with
aneurysmal SAH and hydrocephalus, which were classified into a SAH
group and NPH group, respectively. All CSF samples were obtained
via lumbar puncture or lumbar subarachnoid space catheter. The CSF
samples were collected within seven-point time frame covering 0–10
days after the onset of SAH. All CSF samples were centrifuged at
(4,130 × g) for 15 min at room temperature, following which the
supernatant was frozen at −80°C. The hemorrhagic CSF samples can be
diluted by fresh-made MOPS buffer into different concentrations,
including 25 and 50%.
Measurement of ET-1 levels
ET-1 concentrations in CSF samples were measured
using an ELISA kit (cat. no. F20030; Shanghai Xitang Biotechnology
Co., Ltd., Shanghai, China) at the National Key Laboratory of China
Pharmaceutical University.
Animal selection and artery
incubation
Animal experiments were approved by the Animal
Experiment Management Committee of Xi'an Jiaotong University
(Xi'an, China), and all measures were taken to minimize animal
discomfort. Specific pathogen-free Sprague-Dawley rats (age,
6-months; weight, 300–400 g; 30 males and 30 females) were provided
by the Experimental Animal Center of Xi'an Jiaotong University
(Xi'an, China). The rats were maintained in a controlled facility
at 25°C and 50% humidity, under a 12 h light/dark cycle with free
access to standard chow and water.
The rats were sacrificed by carbon dioxide overdose
followed by cervical dislocation. The brain was immediately removed
and immersed in fresh-made ice-cold MOPS buffer. Its constituents
are shown in Table I. The cerebral
basilar arteries were isolated using a stereomicroscope and were
subsequently cut into segments of approximately 1 mm in length. The
artery segments were put in a culture plate with 2 ml medium
(hemorrhagic CSF or clear CSF from SAH and hydrocephalus patients,
respectively) containing antibiotics (1×105 U/l
penicillin and 100 mg/l streptomycin). The culture plate was put
into a box with a constant temperature of 37°C, which was
continuously ventilated with 5% CO2 for 6, 12 or 24
h.
 | Table I.Constituents of MOPS buffer and 63.5
mM K+ MOPS buffer (/L). |
Table I.
Constituents of MOPS buffer and 63.5
mM K+ MOPS buffer (/L).
| Constituents | MOPS buffer | 63.5 mM
K+ MOPS buffer |
|---|
|
Na2HPO4·12H2O | 0.4295 g | 0.4295 g |
| MOPS | 0.418 g | 0.418 g |
| EDTA | 0.006 g | 0.006 g |
| NaCl | 8.15 g | 4.945 g |
| KCl | 0.35 g | 4.475 g |
|
CaCl2 | 0.1775 g | 0.1775 g |
| MgSO4
·7H2O | 0.295 g | 0.295 g |
| Glucose | 1 g | 1 g |
In vitro myographic experiments
Artery segments were placed in MOPS fluid and
threaded by two filaments (diameter, 40 µm) prior to mounting in a
small vessel PowerLab myograph (Danish Myo Technology A/S, Aarhus,
Denmark). One filament was connected to a tension transducer linked
to the PowerLab system, and the other was connected to the
fine-tuning device that adjusts the distance between the two
filaments. Then, the installed vascular rings were laid in a bath
chamber containing 5 ml MOPS fluid with continuous ventilation and
a pH of 7.4. All of the vessels were allowed to equilibrate for 30
min at room temperature. When the baseline was stable, the initial
vascular tension was changed to 1.5 mN. Following this, 5 ml
fresh-made potassium rich (63.5 mM K+) MOPS fluid was
added into the bath chamber twice to evaluate contractility. The
constituents of (63.5 mM K+) MOPS buffer are also shown
in Table I. Between the twice
potassium rich MOPS buffer, the vessel segments were washed using
common MOPS buffer three times. Vessels with a contraction
difference of <10% were included for further analyses. Finally,
ET-1 (ALX-155-001-PC05, Enzo Life Sciences, Inc., Farmingdale, NY,
USA) and sarafotoxins 6c (S6c, ET-1 analogue; S6545, Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) were added into the bath chamber at
gradually increasing concentrations to obtain the concentration
response curves. The concentration of S6c ranged from
10−11 to 10−7.5 mol/l, while that of ET-1
ranged from 10−11 to 10−7 mol/l. The
contractile response of the vessels was expressed as a percentage
of the contraction induced by potassium rich MOPS fluid. The
maximal contraction was indicated by Emax, and
pEC50 denoted the negative logarithm of the
concentration that elicits half of the maximal response.
Detection of ETA and
ETB mRNA expression
Total cellular RNA was extracted from rat cerebral
arteries, including anterior, middle and posterior cerebral artery
as well as basilar artery that had been incubated at 37°C for 24 h.
Reverse transcription of total RNA to single-stranded cDNA was
performed using SuperScipt™ IV Reverse Transcriptase (18090010,
Thermo Fisher Scientific, Inc., Waltham, MA USA) in a 12 µl
reaction system. RNA was incubated at 65°C for 5 min for
denaturation, following which it was immediately cooled on ice. The
reverse transcription master mix was added to the tube, and the
reaction was performed at 42°C for 1 h and 70°C for 5 min.
Polymerase chain reaction (PCR) was performed using a SYBR PreMix
Ex Taq™ kit (Takara Bio, Inc., Otsu, Japan) in an iCycler iQ5
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). This system
automatically monitors the binding of fluorescent SYBR Green dye to
double-stranded DNA during each PCR amplification cycle. PCR was
performed in a 10 µl reaction system. The PCR amplification
procedure started at 50°C for 2 min and 95°C for 10 min, followed
by 40 cycles at 95°C for 15 sec, 60°C for 60 sec and 72°C for 15
sec, and ended at 55°C for 30 sec. Dissociation curves were
identified for the PCR products, and the 2−∆∆Cq method
was used to analyze the mRNA levels (15). The primer sequences used for RT-PCR
were listed in Table II.
 | Table II.Primer sequences used for polymerase
chain reaction. |
Table II.
Primer sequences used for polymerase
chain reaction.
| Gene | Forward primer
sequences (5′-3′) | Reverse primer
sequences (3′-5′) |
|---|
| ETA |
GTCGAGAGGTGGCAAAGACC |
ACAGGGCGAAGATGACAACC |
| ETB |
GATACGACAACTTCCGCTCCA |
GTCCACGATGAGGACAATGAG |
|
EF-1α |
CCCCTCCAGGACGTTTACAAA |
CACACGGCCCACAGGTACA |
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
statistical analyses. Continuous data were presented as the mean ±
standard error. The comparison between groups was performed using a
one-way analysis of variance or Kruskal-Wallis test, based on the
homogeneity of variance. Multiple comparisons among groups were
performed using the Student-Neuman-Keuls method. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Alterations in CSF ET-1 expression
levels
The clinical data of enrolled patients were
summarized in Table III. The
ET-1 levels in the CSF samples that were collected within
seven-time frames following the onset of SAH are presented in
Table IV. The ET-1 levels in CSF
appeared to increase in the initial five days, reaching a peak
within 3 to 5 days after onset of SAH, but then gradually subsided.
However, comparison between the seven groups showed no significant
difference (P>0.05).
 | Table III.Demographic and clinical data of
enrolled patients. |
Table III.
Demographic and clinical data of
enrolled patients.
|
Characteristics | SAH group | NPH group |
|---|
| Gender
(male/female) | 8/8 | 6/6 |
| Age (years) | 52.38±13.24 | 30.75±3.234 |
| Fisher grade III
(n) | 11 | – |
| Fisher grade IV
(n) | 5 | – |
| Hunt-Hess grade
II–III (n) | 12 | – |
| Hunt-Hess grade
IV–V (n) | 4 | – |
 | Table IV.ET-1 expression levels in the
hemorrhagic CSF samples. |
Table IV.
ET-1 expression levels in the
hemorrhagic CSF samples.
| Sampling time (T;
days) | ET-1 (pg/ml) |
|---|
| T≤1.5 | 93.78±13.10 |
| 1.5<T≤2.5 | 105.01±17.28 |
| 2.5<T≤3 | 102.74±9.58 |
| 3<T≤5 | 107.54±18.98 |
| 5<T≤7 | 102.18±12.58 |
| 7<T≤10 | 104.02±17.42 |
| T>10 | 91.75±4.14 |
Contraction of arteries incubated with
hemorrhagic CSF of different concentrations
In order to determine the best functional
concentration, the hemorrhagic CSF was diluted in MOPS buffer and
classified into three different concentrations (25, 50 or 100%).
Rat basilar arteries were incubated with CSF of different
concentrations for 12 h, and in vitro contraction responses
to ET-1 or S6c were observed. Statistical analysis demonstrated a
positive association between the concentration and contraction
degree, with a significant difference (P<0.05). Based on this
result, 100% hemorrhagic CSF to was used at the culture media (data
not shown).
Contraction of arteries for different
incubation durations
The Emax and pEC50 of unin.
cubated fresh arteries and those of arteries incubated with clear
CSF or hemorrhagic CSF for 6, 12 or 24 h are presented in Table V. The contraction curves are
presented in Figs. 1 and 2. In unincubated fresh arteries, ET-1
induced significant vasoconstriction, while S6c resulted in no
vasoconstrictive effects.
 | Table V.Emax and pEC50
of artery rings. |
Table V.
Emax and pEC50
of artery rings.
|
|
| S6c | ET-1 |
|---|
|
|
|
|
|
|---|
| Incubation
duration | Incubation
medium | n | Emax (%
K+) |
pEC50 | n | Emax (%
K+) |
pEC50 |
|---|
| Control | Not incubated | 7 | 0±0.00 | 0±0.00 | 7 | 156.1±8.71 | 8.9±0.12 |
| 6 h | SAH | 7 | 51.43±19.64 | 9.6±0.24 | 7 | 156.1±15.81 | 8.5±0.05 |
|
| NPH | 7 | 122.0±15.85 | 9.2±0.07 | 7 | 170.0±31.85 | 9.3±0.07 |
| 12 h | SAH | 7 | 99.00±28.89 | 9.7±0.14 | 7 | 153.0±16.53 | 9.4±0.08 |
|
| NPH | 7 | 77.14±17.73 | 9.5±0.01 | 7 | 137.7±8.26 | 9.5±0.06 |
| 24 h | SAH | 7 | 199.3±10.70 | 9.6±6.82 | 7 | 242.1±33.13 | 9.7±7.04 |
|
| NPH | 7 | 132.3±7.00 | 9.7±0.18 | 7 | 132.0±13.78 | 9.7±0.11 |
After a 6 h incubation, all of the artery rings
contracted, and those cultivated in clear CSF exhibited a more
marked contraction. Statistical analysis demonstrated significant
differences in the context of S6c concentrations ranging from
10−9.5 to 10−7.5 mol/l, or ET-1
concentrations ranging from 10−10 to 10−8.5
mol/l (Fig. 1A).
After a 12 h incubation, all of the artery rings
contracted, and those cultivated in hemorrhagic CSF demonstrated a
larger contraction. Statistical analysis revealed significant
differences in the context of S6c concentrations ranging from
10−9.5 to 10−8.5 mol/l, or ET-1
concentrations ranging from 10−9.5 to 10−7.5
mol/l (Fig. 1B).
After a 24 h incubation, all of the artery rings
contracted, and those cultivated in hemorrhagic CSF exhibited a
more marked contraction. Statistical analysis demonstrated
significant differences in the context of S6c concentrations
ranging from 10−9 to 10−7.5 mol/l, or ET-1
concentrations ranging from 10−9 to 10−7
mol/l (Fig. 1C).
Contraction response to S6c or
ET-1
In arteries incubated with hemorrhagic CSF, S6c and
ET-1 both induced vasoconstriction. Following the administration of
S6c, the contraction degree was positively associated with the
incubation period. When compared to fresh arteries, statistical
analysis revealed significant differences in the context of S6c
concentrations, ranging from 10−9.5 to 10−7.5
mol/l; there was also a significant difference in contraction
degree between arteries incubated for 12 or 24 h (Fig. 2). Following the administration of
ET-1, the contraction of arteries incubated for 6 h were not
significantly different compared with the fresh arteries, and the
arteries incubated for 24 h demonstrated the most remarkable
contraction; statistical analysis revealed a significant difference
in contraction degree between 24-h incubated arteries and fresh
arteries in the context of a ET-1 concentration ranging from
10−9.5 to 10−7 mol/l.
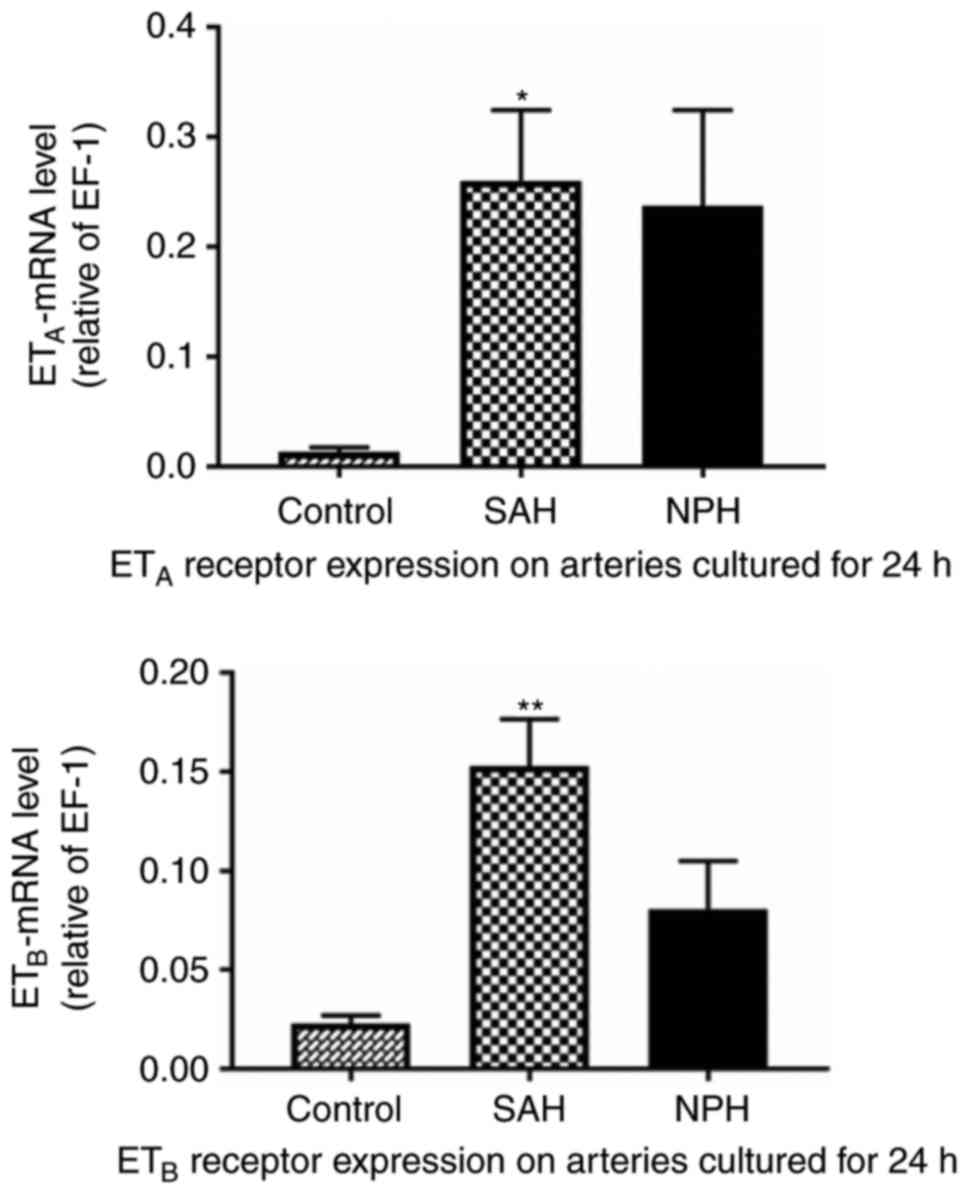
Expression of ETA and
ETB mRNA
The detected expression levels of ETA and
ETB mRNA are summarized in Table VI and Fig. 3. The expression levels of
ETA mRNA in the incubation groups were significantly
higher compared with the control group (P<0.05). The expressions
level of ETB mRNA in the hemorrhagic CSF-incubation
group was significantly higher compared with the control group
(P<0.01), while there was no significant difference in the
expression level of ETB mRNA between the clear
CSF-incubated arteries and the control arteries (P>0.05). There
was no significant difference in the expression levels of
ETA or ETB mRNA between the hemorrhagic
CSF-incubated arteries and the clear CSF-incubated arteries
(P>0.05).
 | Table VI.Expression levels of ETA
and ETB mRNA. |
Table VI.
Expression levels of ETA
and ETB mRNA.
|
| ETB
mRNA | ETA
mRNA |
|---|
|
|
|
|
|---|
| Groups | n | Relative expression
level | n | Relative expression
level |
|---|
| Control | 5 | 0.0229±0.0041 | 5 | 0.0136±0.0038 |
| NPH | 4 | 0.0808±0.0242 | 5 | 0.2375±0.0875 |
| SAH | 5 | 0.1531±0.0235 | 5 | 0.2606±0.0644 |
Discussion
CVS is a serious complication of SAH, which may lead
to fatal outcomes. The pathogenetic mechanisms remain poorly
understood (16). Previous studies
have demonstrated how vasospasm may develop following SAH, which
involves altered expression levels of various vasoactive substances
and specific receptors, as well as the matrix metalloproteinase
family, inflammatory pathways, altered metal ion concentrations in
CSF and high cholesterol levels (17–19).
Among the mechanisms noted above, an increased level of contractile
vasoactive substances and unregulated expression of their receptors
are the most immediate causes for CVS following SAH. As a
well-established, potent and long-lasting vasoconstrictor, ET-1
serves a crucial role in regulating the vascular tension (20). Previous studies have demonstrated
that ET-1 levels in both plasma and CSF may increase in patients
with SAH (21), and ET-1 causes
dose-dependent vasoconstriction in animal models (7). The present study also drew similar
conclusions, with the results indicating that ET-1 levels in the
CSF of patients with SAH increased, and arteries incubated with
hemorrhagic CSF from patients with SAH constricted in a
dose-dependent manner in response to ET-1 and S6c. The present
study used hemorrhagic CSF from patients with SAH to incubate rat
basilar arteries, as this can better mimic the reaction of cerebral
vessels of patients following SAH onset (22). However, the definitive pathogenic
effects of ET-1 in CVS remain unclear. In the current study, the
dynamic alterations in ET-1 expression in the CSF of SAH patients
were demonstrated, and the contractility and expression levels of
endothelin receptors in rat cerebral arteries incubated in various
conditions were investigated.
Suzuki et al (23) revealed that the ET-1 expression
levels in both the plasma and CSF of patients with SAH classified
as Fisher grade III to IV were significantly higher, compared with
those in patients with SAH classified as Fisher grade I or II. The
present study only included patients with SAH classified as Fisher
grade III or IV, who were more prone to develop CVS; CSF samples
were collected within seven-time frames covering 0–10 days after
SAH onset to observe the dynamic alterations in ET-1 levels. The
findings of the current study indicated that the ET-1 expression
levels in CSF increased in the initial 5 days, reaching a peak
within 3 to 5 days following SAH onset, and then gradually
subsided. It was speculated that these alterations may be due to a
sequential process including blood flowing into the subarachnoid
space, erythrocyte splitting, and their splitting products, such as
oxyhemoglobin and oxygenase-1. These can stimulate cerebral vessels
both physically and chemically (24,25).
Furthermore, previous studies demonstrated that patients with SAH
may be at a high risk of developing CVS within 4–10 days following
aneurysm rupture (3). This
clinical phenomenon is consistent with the results of the present
study, in that the time period of the higher CVS risk was similar
to the higher levels of ET-1 detected in the CSF of SAH patients.
The peak expression of ET-1 in the CSF of SAH patients appeared
within 3–5 days and remained at a high level until 10 days after
SAH onset. Therefore, it was speculated that ET-1 expression in the
CSF may be a potential biomarker to predict CVS following SAH.
However, statistical analysis revealed no significant difference,
which may be due to the limited sample size; therefore, the exact
role of ET-1 expression in patients with SAH requires further
research.
To evaluate the expression of endothelin receptors
and the arterial contractility, an in vitro experiment was
performed using artery samples isolated from rat models. The
arterial rings were incubated with hemorrhagic CSF or clear CSF,
and ET-1 and S6c were administered to induce arterial contraction.
It was demonstrated that S6c induced a significant contraction in
CSF-incubated arteries but not in fresh arteries, whereas ET-1
induced contraction in both CSF-incubated and fresh arteries. A
potential explanation for this phenomenon is that S6c is a
selective ETB receptor agonist, and ETB
receptors are infrequently expressed in fresh vessels. However,
they may greatly increase following CSF incubation. Nevertheless,
ET-1 induces arterial contraction by both ETA receptors
and ETB receptors, and ETA receptors are
widely expressed in both fresh and CSF-incubated arteries (26). In the present study, as ET-1 or S6c
concentrations increased, the arterial contraction increased. In
arteries incubated in clear CSF, an incubation period of 6 h was
associated with the most obvious arterial contraction, whereas in
arteries incubated in hemorrhagic CSF, an incubation period of 12
or 24 h was associated with the strongest arterial contraction, and
the contraction degree was associated with the incubation duration
and CSF concentration. This discrepancy may be because there were
not only vasoconstrictors but also a small quantity of short-acting
vasodilators in hemorrhagic CSF (27,28).
Furthermore, significantly elevated expression
levels of both ETA and ETB mRNA in arteries
incubated with hemorrhagic CSF were detected, compared with the
expression levels in fresh arteries. However, there was no
significant difference in the expression levels of ETA
or ETB mRNA between the hemorrhagic CSF-incubated
arteries and the clear CSF-incubated arteries. Zuccarello et
al (29) proposed that
ETA receptors induce vasoconstriction, while
ETB receptors mediate vasodilation in physiological
conditions. Certain reports have also speculated that SAH may
result in an altered endothelial-dependent action of ET-1 and may
lead to the upregulation of ETB receptors in vascular
smooth muscle cells, thereby contributing to vasoconstriction
(30,31). It was therefore considered that the
enhanced contractility of hemorrhagic CSF-incubated arteries in the
present study may have been more associated with upregulated
expression of ETB receptors, rather than ETA
receptors. In previous studies, scholars reported that clazosentan,
a nonpeptide selective ETA receptor antagonist,
significantly reduces the occurrence of CVS following SAH, but that
it did not improve clinical outcomes (32–34).
This may be because this selective ETA receptor
antagonist cannot antagonize ETB receptors, which may
serve a more important role in the pathogenesis of CVS following
SAH (32). According to the result
of the present study and the unsatisfactory treatment effect of
clazosentan, the therapeutic efficacy of ET receptor antagonists
acting on both ETA receptor and ETB receptor
requires further study.
The role of ET-1 and its receptors in the
pathogenetic mechanism of CVS remains unclear. Previous studies
have indicated that the mitogen-activated protein kinase (MAPK)
signaling pathway may be a promising research avenue, as the
activation of MAPKs may increase the expression of endothelin
receptors (35). However, the role
of MAPK phosphatases, the endogenous MAPK inhibitors, has not been
investigated in CVS. Additionally, the elevated expression of
ETA receptors was reported to be associated with the
activation of extracellular regulated protein kinase in vascular
endothelial cells, as well as intracellular protein kinase C,
protein kinase A and phosphatidylinositol 3-kinase (36). The causative role of ET-1 and its
receptors in CVS warrants further research.
The sample size in the current study was limited,
and in the future studies the cohort size of patients with SAH
should be expanded to obtain more CSF samples. Additionally,
hemodynamic parameters will be recorded using CT perfusion
techniques, including cerebral blood flow, cerebral blood volume,
and mean transit time, to further investigate the effects of ET-1
on cerebral vessels, particularly microvessels.
In conclusion, ET-1 and its receptors may be
involved in the pathogenic mechanism of CVS following SAH. It was
demonstrated that the dynamic alterations in ET-1 expression levels
in CSF were parallel with the clinical development of CVS in
patients with SAH, indicating that ET-1 may be a helpful biomarker
for the prediction and diagnosis of CVS. The expression levels of
ETA and ETB mRNA were upregulated in CSF
following SAH, which may be associated with the hypercontractility
of cerebral arteries. ET-1 receptors may provide novel therapeutic
targets in CVS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (Grant no. 81271227) and the
National Key Technology R&D Program of China (Grant no.
2014BAI04B05).
Availability of data and materials
All data generated and analyzed during the present
study is included in this published article.
Authors' contributions
GL, YXC and CX conceived and designed the
experiments. YWC, WL and XD performed the experiments. YWC, WL and
RJ analyzed the data. HY and XL helped testing the levels of ET-1
in the CSF samples. JL designed the concentration gradient of the
drugs. YWC wrote the manuscript. All authors have read and approved
this manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board and Ethics Committee of the First Affiliated Hospital
of Xi'an Jiaotong University (Xi'an, China; approval no. 2016-074).
Animal experiments were approved by the Animal Experiment
Management Committee of Xi'an Jiaotong University (Xi'an, China),
and all measures were taken to minimize animal discomfort.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Rooij NK, Linn FH, van der Plas JA,
Algra A and Rinkel GJ: Incidence of subarachnoid haemorrhage: A
systematic review with emphasis on region, age, gender and time
trends. J Neurol Neurosurg Psychiatry. 78:1365–1372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bederson JB, Connolly ES Jr, Batjer HH,
Dacey RG, Dion JE, Diringer MN, Duldner JE Jr, Harbaugh RE, Patel
AB and Rosenwasser RH: American Heart Association: Guidelines for
the management of aneurysmal subarachnoid hemorrhage: A statement
for healthcare professionals from a special writing group of the
stroke council, American heart association. Stroke. 40:994–1025.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cossu G, Messerer M, Oddo M and Daniel RT:
To look beyond vasospasm in aneurysmal subarachnoid haemorrhage.
Biomed Res Int. 2014:6285972014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mascia L, Fedorko L, Stewart DJ, Mohamed
F, terBrugge K, Ranieri VM and Wallace MC: Temporal relationship
between endothelin-1 concentrations and cerebral vasospasm in
patients with aneurysmal subarachnoid hemorrhage. Stroke.
32:1185–1190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao L, Xu CB, Zhang Y, Cao YX and
Edvinsson L: Secondhand smoke exposure induces
Raf/ERK/MAPK-mediated upregulation of cerebrovascular endothelin
ETA receptors. BMC Neurosci. 12:1092011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie A, Aihara Y, Bouryi VA, Nikitina E,
Jahromi BS, Zhang ZD, Takahashi M and Macdonald RL: Novel mechanism
of endothelin-1-induced vasospasm after subarachnoid hemorrhage. J
Cereb Blood Flow Metab. 27:1692–1701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zubkov AY, Rollins KS, Parent AD, Zhang J
and Bryan RM Jr: Mechanism of endothelin-1-induced contraction in
rabbit basilar artery. Stroke. 31:526–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hino A, Tokuyama Y, Kobayashi M, Yano M,
Weir B, Takeda J, Wang X, Bell GI and Macdonald RL: Increased
expression of endothelin B receptor mRNA following subarachnoid
hemorrhage in monkeys. J Cereb Blood Flow Metab. 16:688–697. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Itoh S, Sasaki T, Asai A and Kuchino Y:
Prevention of delayed vasospasm by an endothelin ETA receptor
antagonist, BQ-123: Change of ETA receptor mRNA expression in a
canine subarachnoid hemorrhage model. J Neurosurg. 81:759–764.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zuccarello M, Boccaletti R, Romano A and
Rapoport RM: Endothelin B receptor antagonists attenuate
subarachnoid hemorrhage-induced cerebral vasospasm. Stroke.
29:1924–1929. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kikkawa Y, Matsuo S, Kameda K, Hirano M,
Nakamizo A, Sasaki T and Hirano K: Mechanisms underlying
potentiation of endothelin-1-induced myofilament Ca(2+)
sensitization after subarachnoid hemorrhage. J Cereb Blood Flow
Metab. 32:341–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Assenzio B, Martin EL, Stankevicius E,
Civiletti F, Fontanella M, Boccaletti R, Berardino M, Mazzeo A,
Ducati A, Simonsen U and Mascia L: Cerebrospinal fluid from
patients with subarachnoid haemorrhage and vasospasm enhances
endothelin contraction in rat cerebral arteries. PLoS One.
10:e01164562015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogungbo B: The world federation of
neurological surgeons scale for subarachnoid hemorrhage. Surg
Neurol. 59:236–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fisher CM, Kistler JP and Davis JM:
Relation of cerebral vasospasm to subarachnoid hemorrhage
visualized by computerized tomographic scanning. Neurosurgery.
6:1–9. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bacigaluppi S, Zona G, Secci F, Spena G,
Mavilio N, Brusa G, Agid R, Krings T, Ottonello G and Fontanella M:
Diagnosis of cerebral vasospasm and risk of delayed cerebral
ischemia related to aneurysmal subarachnoid haemorrhage: An
overview of available tools. Neurosurg Rev. 38:603–618. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mehta V, Russin J, Spirtos A, He S,
Adamczyk P, Amar AP and Mack WJ: Matrix metalloproteinases in
cerebral vasospasm following aneurysmal subarachnoid hemorrhage.
Neurol Res Int. 2013:9437612013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tuttolomondo A, Di Sciacca R, Di Raimondo
D, Pedone C, La Placa S, Pinto A and Licata G: Effects of clinical
and laboratory variables and of pretreatment with cardiovascular
drugs in acute ischaemic stroke: A retrospective chart review from
the GIFA study. Int J Cardiol. 151:318–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tuttolomondo A, Pecoraro R, Casuccio A, Di
Raimondo D, Buttà C, Clemente G, Della Corte V, Guggino G, Arnao V,
Maida C, et al: Peripheral frequency of CD4+ CD28- cells in acute
ischemic stroke: Relationship with stroke subtype and severity
markers. Medicine (Baltimore). 94:e8132015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barton M: The discovery of
endothelium-dependent contraction: The legacy of Paul M. Vanhoutte.
Pharmacol Res. 63:455–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kessler IM, Pacheco YG, Lozzi SP, de
Araújo AS Jr, Onishi FJ and de Mello PA: Endothelin-1 levels in
plasma and cerebrospinal fluid of patients with cerebral vasospasm
after aneurysmal subarachnoid hemorrhage. Surg Neurol. 64 Suppl
1:S1:2–5; discussion S1:5. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peterson JW, Kwun BD, Teramura A, Hackett
JD, Morgan JA, Nishizawa S, Bun T and Zervas NT: Immunological
reaction against the aging human subarachnoid erythrocyte. A model
for the onset of cerebral vasospasm after subarachnoid hemorrhage.
J Neurosurg. 71:718–726. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki R, Masaoka H, Hirata Y, Marumo F,
Isotani E and Hirakawa K: The role of endothelin-1 in the origin of
cerebral vasospasm in patients with aneurysmal subarachnoid
hemorrhage. J Neurosurg. 77:96–100. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang KC, Tang SC, Lee JE, Lai DM, Huang
SJ, Hsieh ST, Jeng JS and Tu YK: Prognostic value of intrathecal
heme oxygenase-1 concentration in patients with Fisher Grade III
aneurysmal subarachnoid hemorrhage. J Neurosurg. 121:1388–1393.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kolias AG, Sen J and Belli A: Pathogenesis
of cerebral vasospasm following aneurysmal subarachnoid hemorrhage:
Putative mechanisms and novel approaches. J Neurosci Res. 87:1–11.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao L, Ping NN, Cao YX, Li W, Cai Y,
Warfvinge K and Edvinsson L: The effects of MEK1/2 inhibition on
cigarette smoke exposure-induced ET receptor upregulation in rat
cerebral arteries. Toxicol Appl Pharmacol. 304:70–78. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Onoue H, Kaito N, Akiyama M, Tomii M,
Tokudome S and Abe T: Altered reactivity of human cerebral arteries
after subarachnoid hemorrhage. J Neurosurg. 83:510–515. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Tian Y, Wang D, Wei H, Zhao Z,
Jiang R, Yue S and Zhang J: High Angiopoietin-1 levels predict a
good functional outcome within 72 h of an aneurysmal subarachnoid
hemorrhage: A prospective study from a single center. J Neurol Sci.
356:72–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zuccarello M, Lewis AI and Rapoport RM:
Endothelin ETA and ETB receptors in subarachnoid hemorrhage-induced
cerebral vasospasm. Eur J Pharmacol. 259:R1–R2. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clozel M, Gray GA, Breu V, Löffler BM and
Osterwalder R: The endothelin ETB receptor mediates both
vasodilation and vasoconstriction in vivo. Biochem Biophys Res
Commun. 186:867–873. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hansen-Schwartz J, Hoel NL, Zhou M, Xu CB,
Svendgaard NA and Edvinsson L: Subarachnoid hemorrhage enhances
endothelin receptor expression and function in rat cerebral
arteries. Neurosurgery. 52:1188–1194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Macdonald RL, Kassell NF, Mayer S,
Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S and Pasqualin
A: CONSCIOUS-1 Investigators: Clazosentan to overcome neurological
ischemia and infarction occurring after subarachnoid hemorrhage
(CONSCIOUS-1): Randomized, double-blind, placebo-controlled phase 2
dose-finding trial. Stroke. 39:3015–3021. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Macdonald RL, Higashida RT, Keller E,
Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A,
et al: Randomised trial of clazosentan, an endothelin receptor
antagonist, in patients with aneurysmal subarachnoid hemorrhage
undergoing surgical clipping (CONSCIOUS-2). Acta Neurochir Suppl.
115:27–31. 2013.PubMed/NCBI
|
|
34
|
Macdonald RL, Higashida RT, Keller E,
Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A,
et al: Randomized trial of clazosentan in patients with aneurysmal
subarachnoid hemorrhage undergoing endovascular coiling. Stroke.
43:1463–1469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suzuki H, Hasegawa Y, Kanamaru K and Zhang
JH: Mitogen-activated protein kinases in cerebral vasospasm after
subarachnoid hemorrhage: A review. Acta Neurochir Suppl.
110:133–139. 2011.PubMed/NCBI
|
|
36
|
Chen QW, Edvinsson L and Xu CB: Role of
ERK/MAPK in endothelin receptor signaling in human aortic smooth
muscle cells. BMC Cell Biol. 10:522009. View Article : Google Scholar : PubMed/NCBI
|