Introduction
Acute myeloid leukaemia (AML) is a heterogeneous
group of disorders characterized by the clonal proliferation of
progenitor cells or primitive hematopoietic stem cells (1). Due to the development of allogeneic
hematopoietic stem cell transplantation (allo-HSCT) for patients
with AML, in particular the extensive development of haploidentical
allo-HSCT in China, the therapeutic efficacy of treatments for AML
have significantly improved throughout previous decades (2). However, the treatment of refractory
and relapsed patients remains a significant clinical challenge that
has yet to be overcome (3).
AML is typically diagnosed using morphologic,
immunologic, cytogenetic and molecular biologic (MICM)
classification techniques. However, the accumulation of
somatically-acquired genetic changes in hematopoietic progenitor
cells serves a vital role in the pathogenesis of AML, including
gene mutations, copy number alterations and chromosomal
translocation, which provides clinicians with a novel method to
diagnose AML (4). Due to the
successful application of next generation sequencing (NGS), NGS has
become widely used in the analysis of clinical and biological
heterogeneity of AML in a clinical setting (5). The study conducted by
Corces-Zimmerman et al (6)
demonstrated that preleukemic mutation in AML affected the
regulation of epigenetic systems, and promoted the survival of
hematopoietic stem cells via resistance to chemotherapy. In
addition, cyclin D1 and cyclin D2 mutations have been identified to
be frequently-occurring events in adult patients with AML at
t(8;21)(q22;q22), and may serve as additional therapeutic targets
for AML. Furthermore, the inhibition of mutant isocitrate
dehydrogenase [NADP(+)] 2, mitochondrial via AG-221 or DNA
methyltransferase activity by 5-azacytidine has been demonstrated
to improve the sensitivity of patients with AML to epigenetic
therapy (7,8). These data indicate that mutations in
AML exert important functions in the development, treatment and
prognosis of AML.
Recently, a spectrum of somatic mutations that were
detected by targeted NGS have been identified by Feng et al
(9). This mutation spectrum
contained 112 genes and was based on 121 adult patients with acute
leukaemia, and has subsequently been used for the analysis of gene
mutations and mutation frequency in malignant hematologic disorders
(10). In the present study,
amongst the 112-gene mutation panel, a total of 61 gene mutations
were determined in the 62 patients with AML. Based on these data,
single gene mutations and co-mutations in AML were analysed,
followed by the associations with clinical features and the
prognosis of AML. The aim of the present study was to provide novel
information pertaining to the mechanism of action of AML, with
particular emphasis on the roles of co-occurrence gene mutations,
in order to provide more efficient therapeutics and to guide the
individual course of treatment for patients with AML.
Materials and methods
Patients and specimen collection
Bone marrow samples were collected from 62 patients
with AML (29 males and 33 females, aged between 15–75 years old)
who were diagnosed for the first time at Provincial Hospital
affiliated to Shandong University (Jinan, China) from January 2016
to December 2016. The diagnosis and categories of AML were
performed according to the criteria recommended by the World Health
Organization in 2008 (11), and
was combined with the MICM characteristics (12). Bone marrow mononuclear cells were
isolated by density gradient centrifugation with 2,000 × g for 15
min at 4°C. The present study was approved by the Ethics Committees
of Shandong Provincial Hospital and all participants provided
written informed consent. The clinical and pathological information
of the 62 patients with AML are summarized in Table I.
 | Table I.Clinical and pathological information
of 62 patients with AML. |
Table I.
Clinical and pathological information
of 62 patients with AML.
|
Characteristics | Mean | N |
|---|
| Age at study entry,
years (range) | 43.32 (15–75) | – |
| Sex |
|
|
|
Male | – | 29/62 |
|
Female | – | 33/62 |
| WBC count at
diagnosis (range) |
|
|
| WBC
(109/l) | 31.35
(0.80–280.70) | – |
| Bone marrow blast
count (range) | 63.22
(5.83–99.00) | – |
| AML FAB
subtype |
|
|
| AML
with minimal maturation (M0) | – |
0/62 |
| AML
without maturation (M1) | – |
3/62 |
| AML
with maturation (M2) | – | 14/62 |
| Acute
myelomonocytic leukemia (M4) | – | 12/62 |
| Acute
monoblastic or monocytic leukemia (M5) | – | 11/62 |
| Acute
erythroid leukemia (M6) | – |
3/62 |
| Acute
megakaryoblastic leukemia (M7) | – |
1/62 |
|
Unclassified | – | 18/62 |
|
Immunophenotype |
|
|
|
CD13 | – | 58/62 |
|
CD15 | – | 39/62 |
|
CD33 | – | 60/62 |
|
CD34 | – | 48/62 |
|
CD117 | – | 58/62 |
|
MPO | – | 39/62 |
|
CD64 | – | 40/62 |
|
HLA-DR | – | 58/62 |
|
CD56 | – | 22/62 |
|
CD38 | – | 61/62 |
| Cytogenetics |
|
|
|
Abnormal karyotype | – | 28/62 |
| Normal
karyotype | – | 22/62 |
|
Information missing | – | 12/62 |
| Risk |
|
|
|
High | – | 12/62 |
|
Medium | – | 31/62 |
|
Low | – | 10/62 |
|
Information missing | – |
9/62 |
| Induction
therapy |
|
|
| IA | – | 32/62 |
| DA | – | 10/62 |
|
Others | – |
8/62 |
|
Information missing | – | 12/62 |
| Response
evaluation |
|
|
|
Achieving CR | – | 27/62 |
| NR | – | 19/62 |
|
Unevaluated | – |
8/62 |
|
Information missing | – |
8/62 |
| Consolidation
therapy after CR |
|
|
|
Chemotherapy | – | 30/62 |
|
HSCT | – | 14/62 |
|
Information missing | – | 18/62 |
DNA isolation
For the bone marrow samples, red blood cells were
lysed using Red Blood Cell Lysis buffer (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). The remaining cells
were subsequently counted, and ~1.0×107 karyocytes were
used to isolate genomic DNA using the Column Blood DNAOUT kit
(Tiandz Inc., Beijing, China) according to the manufacturer's
protocols.
Detection of gene mutations
A specific target panel for malignant hematologic
disorders, which covered hotspots or complete coding regions of 112
genes (Table II) known to be
recurrently mutated and/or associated with malignant hematologic
disorders was used in the present study (9). A DNA library was constructed using
Ion Proton™ Ion kits (Ion AmpliSeq™ Library Kit 2.0–96 rxns),
according to the manufacturer's protocol (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Subsequent to preparation of the template,
the Ion Proton sequencing platform was applied to sequence the
exons of these genes using the Ion PI Hi-Q OT2 200 Kit (A26434) and
Ion PI Hi-Q Sequencing 200 Kit (A26433). Then, the results were
mapped to the National Center for Biotechnology Information hg19
RefSeq with a mean of >97% coverage of the targeted regions at
an average depth of 800X. The genetic mutation analysis was
completed by Ion Reporter system and Variant Reporter software v2.0
(Thermo Fisher Scientific, Inc.). All putative mutations were
compared against multiple databases, including dbSNP (13), 1,000 genomes (14), Polyphen-2 (15), and Catalogue of Somatic Mutations
In Cancer (16). The detection
rate of 5% mutation frequency was 97–98%.
 | Table II.Genes closely associated with
diseases of the blood system. |
Table II.
Genes closely associated with
diseases of the blood system.
| No. | Gene name | No. | Gene name | No. | Gene name | No. | Gene name | No. | Gene name | No. | Gene name | No. | Gene name |
|---|
| 1 | ABL1 | 18 | MYC | 35 | SRSF2 | 52 | NF1 | 69 | CCND1 | 86 | PTPN11 | 103 | CSF3R |
| 2 | BRAF | 19 | ABCB1 | 36 | BIRC3 | 53 | MAPK1 | 70 | CEBPA | 87 | STX11 | 104 | EZH2 |
| 3 | CUX1 | 20 | SF1 | 37 | CBL | 54 | ZRSR2 | 71 | EP300 | 88 | U2AF2 | 105 | IDH2 |
| 4 | FANCA | 21 | MAFB | 38 | DNM2 | 55 | IKZF1 | 72 | GATA2 | 89 | CRLF2 | 106 | MAF |
| 5 | IL7R | 22 | PRPF40B | 39 | FAT1 | 56 | TET2 | 73 | KIT | 90 | TRAF3 | 107 | PAX5 |
| 6 | MPL | 23 | ECT2L | 40 | JAK1 | 57 | TAL1 | 74 | NOTCH2 | 91 | BCL6 | 108 | RB1 |
| 7 | PDGFRB | 24 | WT1 | 41 | MYH11 | 58 | ATM | 75 | PTEN | 92 | CREBBP | 109 | SMC3 |
| 8 | XPO1 | 25 | ALAS2 | 42 | PRF1 | 59 | CDKN1A | 76 | ARID1A | 93 | ETV6 | 110 | SF3B1 |
| 9 | ZMYM3 | 26 | RUNX1 | 43 | KMT2D | 60 | EGFR | 77 | ADAMTS13 | 94 | IDH1 | 111 | ASXL1 |
| 10 | SUZ12 | 27 | DDX3X | 44 | SF3A1 | 61 | FLT3 | 78 | FBXW7 | 95 | SH2D1A | 112 | WAS |
| 11 | UNC13D | 28 | FANCG | 45 | SETBP1 | 62 | JAK3 | 79 | TP53 | 96 | NRAS | – | – |
| 12 | WHSC1 | 29 | ITK | 46 | STXBP2 | 63 | NOTCH1 | 80 | BCL2 | 97 | RAB27A | – | – |
| 13 | AKT1 | 30 | MYD88 | 47 | XIAP | 64 | RELN | 81 | LYST | 98 | EED | – | – |
| 14 | CALR | 31 | PIK3CA | 48 | CCND3 | 65 | SMC1A | 82 | EPHA7 | 99 | DIS3 | – | – |
| 15 | CYLD | 32 | CXCR4 | 49 | DNMT3A | 66 | PRMT5 | 83 | GATA3 | 100 | PHF6 | – | – |
| 16 | FANCC | 33 | SH2B3 | 50 | FGFR3 | 67 | FAM46C | 84 | KRAS | 101 | U2AF1 | – | – |
| 17 | MUM1 | 34 | SAMHD1 | 51 | JAK2 | 68 | TNFAIP3 | 85 | NPM1 | 102 | PRDM1 | – | – |
Statistical analysis of gene
mutations
The distribution of detected mutations in the 62
patients was presented using the ggplot2 (version 2.2.1, http://cran.r-project.org/web/packages/ggplot2/)
(17) in R software. The mutation
frequency of each gene was calculated and the high frequency
mutated genes (mutation frequency >10%) were selected for
subsequent analysis.
Single gene mutation analysis
Associations between high frequency mutated genes
(mutation frequency >10%) and clinical characteristics were
analysed using the Pearson's χ2 test (18) in R 3.4.1 software. In addition,
high frequency gene mutation profiles were extracted from The
Cancer Genome Atlas (TCGA) database (http://cancergenome.nih.gov/). Then,
prognosis-associated gene mutations were analysed using Cox
univariate regression analysis in a survival package (version
2.40.1; http://cran.r-project.org/package=survival) (19), and the survival results of the high
frequency gene mutations were also analysed using Kaplan-Meier
survival curves and log-rank tests (20).
Combined gene mutation analysis
Associations between co-mutations with a high
frequency and clinical characteristics were analysed using the lm
function (https://www.rdocumentation.org/packages/stats/versions/3.4.1/topics/lm)
(21) in R 3.4.1 software. The
multiple regression model was performed by forced entry linear
regression in limma of package R and bilateral P<0.05 was
considered statistically significant. The clinical features that
were significantly associated with combined gene mutations were
subjected to analysis using the Gene Ontology (GO) (22,23)
and the Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway
(24) analyses using Database for
Annotation, Visualisation and Integrated Discovery v.6.8 software
(25,26) with the threshold of P<0.05,
which was considered to indicate a statistically significant
difference. In addition, the prognosis-associated co-mutations were
analysed using the aforementioned method for single gene
mutations.
Results
Mutations in patients with AML
A total of 61 gene mutations were detected based on
the 112 genetic mutations associated with AML Among of the 62
enrolled patients, a total of 60 cases (96.77%) presented with at
least one mutation, and 52 out of 62 (83.87%) patients exhibited ≥2
mutations. Specifically, 9 cases (14.52%) had 2 mutations, 11
patients (17.74%) had 3 mutations, 15 patients (24.19%) had 4
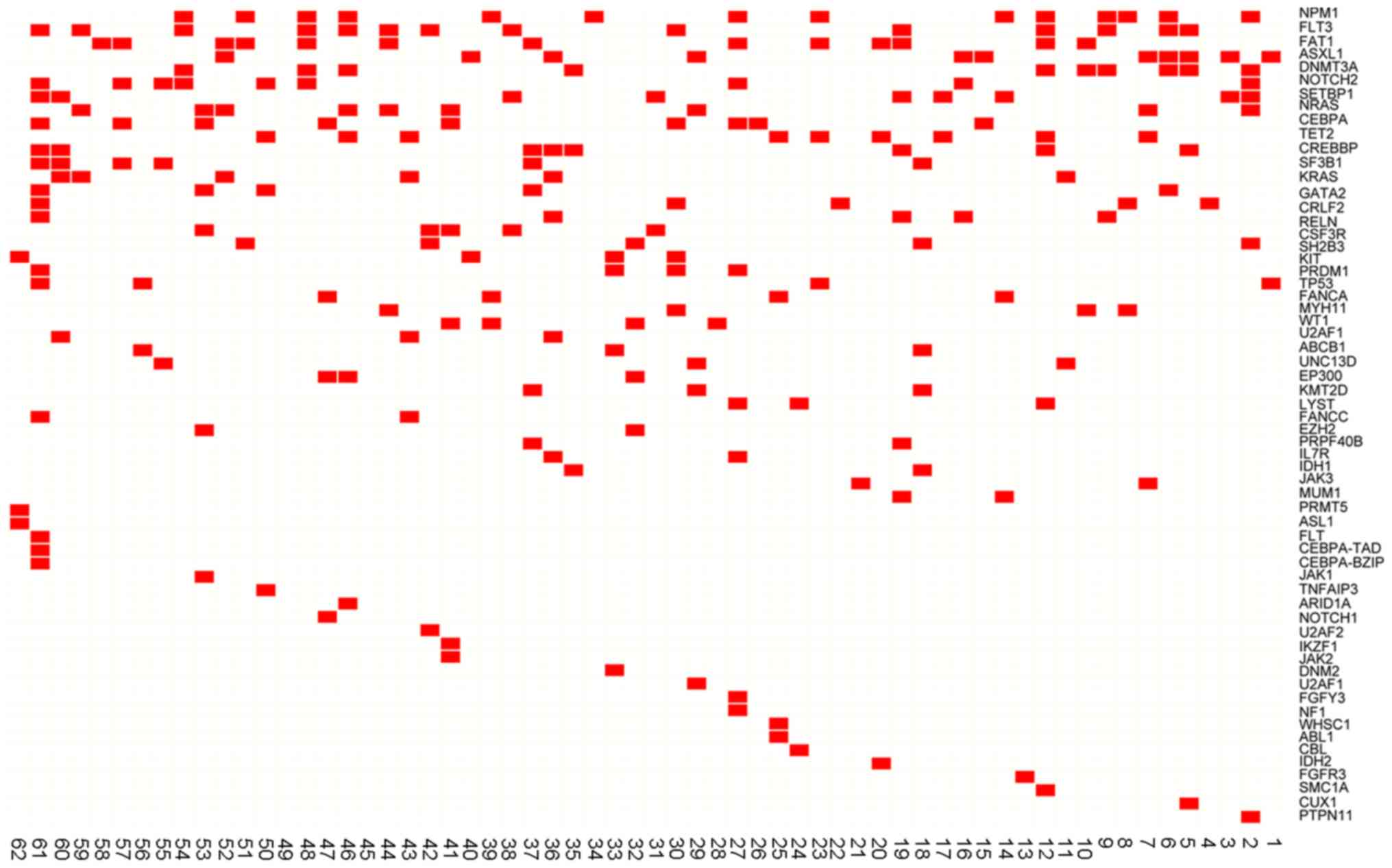
mutations and 17 patients (27.42%) had >5 mutations (Fig. 1). Nucleophosmin 1 (NPM1),
Fms related tyrosine kinase 3 (FLT3), FAT atypical cadherin
1 (FAT1), ASXL transcriptional regulator 1 (ASXL1)
and DNA methytransferase 3α (DNMT3A) were the 5 most
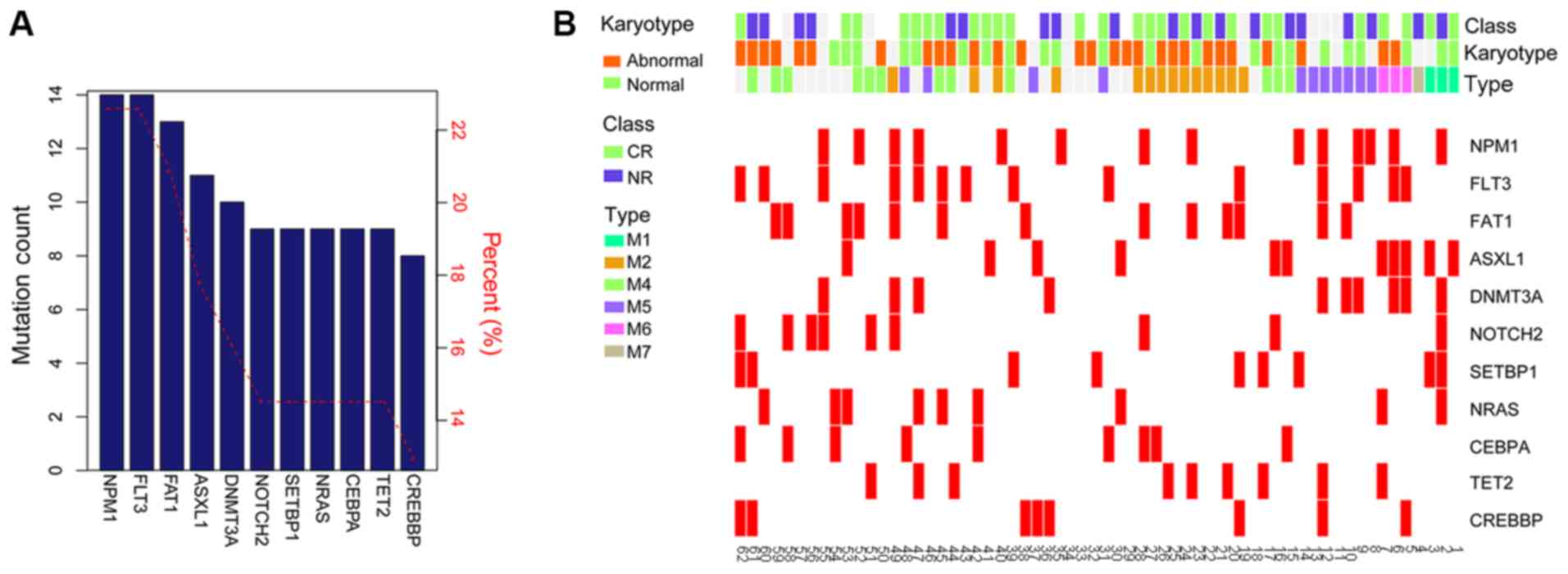
frequently identified mutations in patients with AML. Using a
cut-off frequency of >10%, a total of 11 high frequency
mutations were screened, including NPM1 (22.58%),
FLT3 (22.58%), FAT1 (20.97%), ASXL1 (17.74%),
DNMT3A (16.13%), Notch 2 (NOTCH2; 14.52%), SET
binding protein 1 (SETBP1; 14.52%), NRAS proto-oncogene,
GTPase (NRAS; 14.52%), CCAAT enhancer binding protein α
(CEBPA; 14.52%), Tet methylcytosine dioxygenase 2 (14.52%)
and cyclic adenosine 5′-phosphate response element-binding protein
binding protein (CREBBP; 14.52%) (Fig. 2A). The distribution of high
frequency mutations in clinical characteristics are presented in
Fig. 2B. The frequencies and types
of variants of the 11 high frequency mutations are presented in
Table III.
 | Table III.Frequencies and types of variants of
11 high frequency mutations. |
Table III.
Frequencies and types of variants of
11 high frequency mutations.
| Mutated genes
(sample number) | Type of variant
(Mutant amino acid) | Frequency, % |
|---|
| NPM1 (11) | p.W288fs | >10.00 |
|
| p.W288fs | >10.00 |
|
| p.W288fs | >10.00 |
|
| p.W288fsX12 | >10.00 |
|
| p.W288fs | >10.00 |
|
| p.K193R |
5.00 |
|
| p.E245Q | 45.02 |
|
| p.W288fs | >10.00 |
|
| p.W288fs | >10.00 |
|
| p.K193R |
4.80 |
|
| p.W288Cfs | >10.00 |
| FLT3 (23) | p.D835γ | 15.47 |
|
| p.V491L | 32.25 |
|
| ITD | >10.00 |
|
| p.A680V |
9.73 |
|
| p.D835γ | 41.16 |
|
| p.836_837del | 44.29 |
|
| ITD | + |
| FAT1 (21) | p.V2089I | 54.45 |
|
| p.A4551G | 49.27 |
|
| p.L2822P | 52.81 |
|
| p.V5911 | 50.91 |
|
| p.A4551G | 48.07 |
|
| p.R1257q | 46.51 |
|
| p.Q587K |
9.41 |
|
| p.A4551G | 48.14 |
|
| p.Q587K |
7.75 |
|
| p.Y4232C | 52.24 |
|
| p.V3694I | 58.18 |
| ASXL1 (18) | p.G652S | 50.30 |
|
| p.G652S | 51.03 |
|
| p.W898X | 22.89 |
|
| p.W898X | 42.25 |
|
| p.C687X | 41.15 |
|
| p.G652S | 51.97 |
|
| p.G652S | 57.19 |
|
| p.G1954A | 54.18 |
|
| p.G652S | 54.72 |
|
| p.G652S | 58.47 |
|
| p.G652S | 52.21 |
|
| p.G652S | 57.60 |
| DNMT3A (16) | p.R882H | 42.57 |
|
| p.R882H | 43.58 |
|
| p.R882C | 47.07 |
|
| p.R882H | 44.92 |
|
| p.V716D | 45.72 |
|
| p.R882C | 52.86 |
|
| p.R882P | 31.13 |
|
| p.R882C | 42.50 |
|
| p.R882H | 49.56 |
|
| p.R882C | 47.38 |
| NOTCH2 (15) | p.I1689F | 47.85 |
|
| p.I1689F | 48.99 |
|
| p.1689F | 50.89 |
|
| p.I1789F | 51.70 |
|
| p.1689F | 48.41 |
|
| p.1689F | 48.94 |
|
| p.I1689F | 50.12 |
|
| p.I1689F | 51.42 |
|
| p.I1689F | 49.20 |
| SETBP1(15) | p.P1563L | 20.00 |
|
| p.D868N |
1.65 |
|
| p.E1466D | 51.09 |
|
| p.A1193T | 65.89 |
|
| p.E1466D | 54.58 |
|
| p.E1466D | 52.11 |
|
| p.E1466D | 47.83 |
|
| p.R627C | 51.31 |
|
| p.R627C | 46.66 |
| NRAS (15) | p.G12D |
1.80 |
|
| p.G12D |
4.74 |
|
| p.G12C |
4.03 |
|
| p.G12D |
6.61 |
|
| p.Q61R |
1.80 |
|
| p.G13D |
4.85 |
|
| p.G12D | 30.81 |
|
| p.G12D | 46.83 |
|
| p.G12D |
1.75 |
|
| p.G13V |
6.36 |
|
| p.Q16H | 22.49 |
| CEBPA (15) | p.G32fs | 25.35 |
|
| p.K313delinsQK | 59.26 |
|
| p.A66fs | >10.00 |
|
| p.A303P | 48.16 |
|
| p.P23fs | 46.12 |
|
| p.A72LfsX35 | + |
|
| p.L317delinsRL | 48.27 |
|
| p.P23fs |
2.70 |
| TET2 (15) | p.F868L | 51.68 |
|
| p.S1039L | 48.33 |
|
| p.Q1523X |
2.20 |
|
| p.I1762V | 47.20 |
|
| p.Q324H |
5.88 |
|
| p.R550X | 10.64 |
|
| p.S1039L | 50.94 |
|
| p.R814C | 49.51 |
|
| p.S1039L | 50.76 |
| CREBBP (13) | p.R1140Q |
9.29 |
|
| p.R1140Q |
4.17 |
|
| p.V1924M | 41.56 |
|
| p.R1140Q |
5.20 |
|
| p.R1140Q |
4.35 |
|
| p.R1140Q | 5.21 |
|
| p.R1140Q | 5.75 |
|
| p.R1140Q | 6.96 |
Single mutation analysis
In order to examine the significance of acquired
genetic mutations in the development of AML, the present study
initially analysed the association between single mutations and
clinical features, including white blood cell (WBC) count at
diagnosis, French-American-British (FAB) subtype (27), and karyotype using Pearson's
χ2 test. As a result, FLT3, NRAS and CEBPA
mutations were significantly associated with WBC count, while
ASXL1 and DNMT3A mutations were significantly
associated with the FAB subtypes. The DNMT3A mutation was
also significantly associated with the variation of the karyotype
(Table IV). The survival
information of 11 high frequency mutations in AML was extracted
from TCGA database, and survival prognosis analysis was performed.
The results revealed that 3 single mutations were identified to be
negatively associated with a poor overall survival (OS) in patients
with AML, including FLT3, NPM1 and DMT3A (Fig. 3).
 | Table IV.Associations between mutations and
clinical features. |
Table IV.
Associations between mutations and
clinical features.
| A, WBC count at
diagnosis |
|---|
|
|---|
|
| WBC (H/L) |
|
|---|
|
|
|
|
|---|
| Mutations | Mutation | Non-mutation | P-value |
|---|
| FLT3 | 7/7 | 10/38 | 0.04402 |
| NRAS | 6/4 | 11/32 | 0.009661 |
| CEBPA | 5/4 | 12/41 | 0.049879 |
|
| B, AML FAB
subtype |
|
|
| FAB subtype,
M1/M2/M4/M5/M6/M7 |
|
|
|
|
|
|
Mutations |
Mutation |
Non-mutation | P-value |
|
| ASXL1 | 2/1/3/0/3/0 | 1/13/9/11/0/1 | 0.000115 |
| DNMT3A | 1/1/0/5/2/0 | 2/13/12/6/1/1 | 0.007636 |
|
| C,
Cytogenetics |
|
|
| Karyotype,
abnormal/normal |
|
|
|
|
Mutations |
Mutation |
Non-mutation | P-value |
|
| DNMT3A | 2/8 | 26/14 | 0.01446 |
Combined mutation analysis
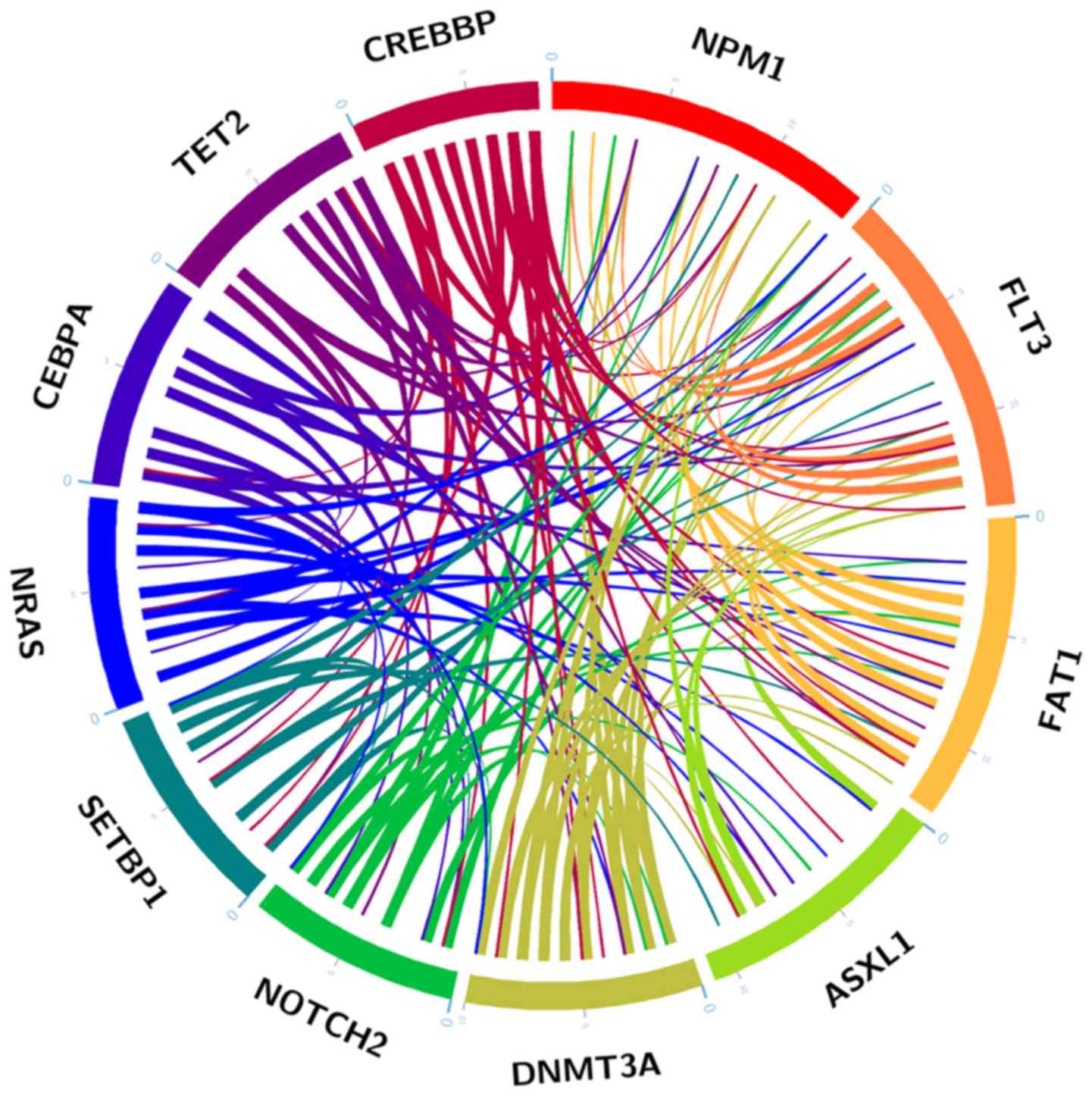
The mutation analysis revealed that 56.45% of
patients (35/62) exhibited >2 high frequency mutations (Fig. 4), indicating that co-occurrence
gene mutations were a common phenomenon in AML. The present study
subsequently analysed the association between co-mutations of 11
high frequency mutations and clinical features, including age at
the time of diagnosis, sex, bone marrow blast proportion, FAB
subtype, karyotype and first course therapeutic response using a
multiple regression model. Consequently, a total of 3 combined
mutations were identified to be markedly associated with the
clinical features of AML. Specifically, the combined mutations
FLT3-NOTCH2 and DNMT3A-CEBPA were significantly
associated with WBC and cytogenetics, respectively, while the
SETBP1-CREBBP combined mutation was significantly associated
with response evaluation and consolidation therapy following
complete remission (CR) in AML (Table
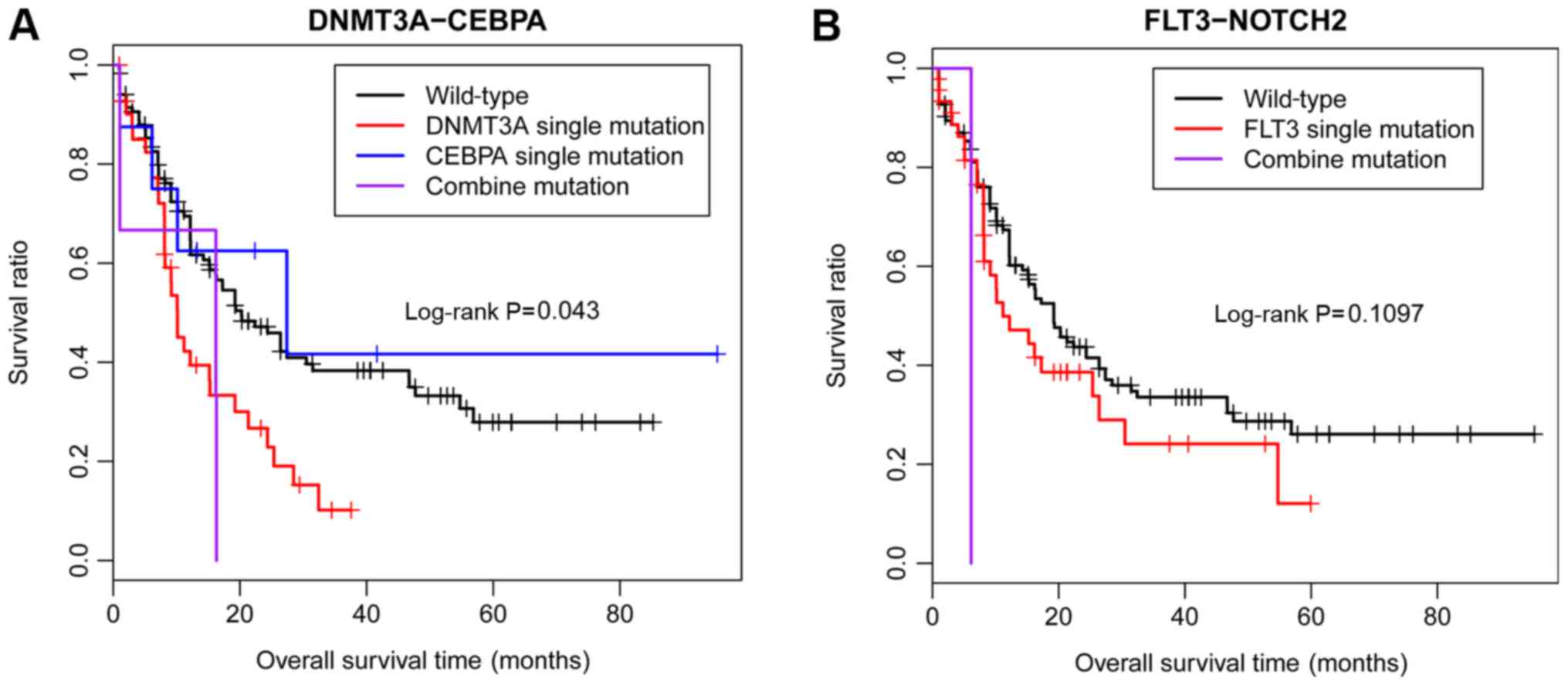
V). According to the TCGA, among these 3 significant
co-mutations, only DNMT3A-CEBPA was significantly associated
with a poor OS in patients with AML, and no significant difference
was identified in the co-mutation of FLT3-NOTCH2 due to the
small sample size (Fig. 5).
However, no information about the co-mutation of
SETBP1-CREBBP was available in TCGA database; therefore, the
present study did not analyse the association between prognosis and
the co-mutation SETBP1-CREBBP in patients with AML.
 | Table V.Associations between clinical
features and 11 high-frequency mutations by multi-factor
analysis. |
Table V.
Associations between clinical
features and 11 high-frequency mutations by multi-factor
analysis.
| Clinic
characteristics | NPM1 | FLT3 | FAT1 | ASXL1 | DNMT3A | NOTCH2 | SETBP1 | NRAS | CEBPA | TET2 | CREBBP |
|---|
| Age at study entry,
years | 0.490 | 0.491 | 0.209 | 0.153 | 0.116 | 0.519 | 0.985 | 0.212 | 0.263 | 0.637 | 0.820 |
| Sex,
male/female | 0.875 | 0.033 | 0.590 | 0.695 | 0.080 | 0.760 | 0.750 | 0.569 | 0.750 | 0.680 | 0.793 |
| WBC, H/L | 0.695 | 0.034 | 0.269 | 0.705 | 0.902 | 0.003 | 0.092 | 0.108 | 0.645 | 0.061 | 0.577 |
| Bone marrow blast
count | 0.647 | 0.114 | 0.717 | 0.801 | 0.777 | 0.907 | 0.151 | 0.698 | 0.464 | 0.955 | 0.412 |
| AML FAB subtype,
M0/M1/M2/M4/M5/M6/M7 | 0.707 | 0.536 | 0.442 | 0.242 | 0.045 | 0.811 | 0.978 | 0.145 | 0.739 | 0.279 | 0.638 |
| Cytogenetics,
abnormal/normal | 0.204 | 0.137 | 0.735 | 0.438 | 0.021 | 0.277 | 0.186 | 0.863 | 0.001 | 0.872 | 0.904 |
| High risk,
high/medium/low | 0.111 | 0.971 | 0.292 | 0.933 | 0.826 | 0.976 | 0.528 | 0.929 | 0.530 | 0.664 | 0.163 |
| Response
evaluation, CR/NR | 0.952 | 0.529 | 0.668 | 0.176 | 0.148 | 0.409 | 0.036 | 0.793 | 0.365 | 0.277 | 0.003 |
| Consolidation
therapy following CR, | 0.804 | 0.532 | 0.184 | 0.427 | 0.535 | 0.801 | 0.024 | 0.916 | 0.905 | 0.664 | 0.046 |
|
chemotherapy/hematopoietic stem cell
transplantation |
Functional analysis of combined
mutations
To additionally investigate the functions of the
combined mutations, the 3 co-mutations were subjected to GO and
KEGG pathway analyses. The GO analysis revealed that these 3
co-mutations were significantly enriched in 15 biological
processes, including ‘hemopoietic or lymphoid organ development’
(P=2.15×10−3), ‘negative regulation of cell
differentiation’ (P=1.49×10−3), ‘haemopoiesis’
(P=1.78×10−3) and ‘immune system development’
(P=2.42×10−3). Concomitantly, these 3 co-mutations were
also significantly enriched in 3 KEGG pathways, including ‘AML’
(P=0.045), ‘pathways in cancer’ (P=0.023) and the ‘Notch signalling
pathway’ (P=0.036; Fig. 6).
Analysis of the clinical features of
patients with combined mutations
Finally, the present study analysed the common
clinical features of patients with these 3 co-mutations. A total of
3 patients with AML were identified to possess the
FLT3-NOTCH2 mutation. All of these patients presented with
positive aminopeptidase N (CD13), myeloid cell surface antigen CD33
(CD33), myeloperoxidase (MPO), high affinity immunoglobulin γ Fc
receptor I (CD64), human leukocyte antigen-DR isotype (HLA-DR) and
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (CD38) expression
(Table VI). In addition, 3
patients were identified to possess the SETBP-CREBBP
mutation, and presented with positive CD13, CD33, hematopoietic
progenitor cell antigen CD34 (CD34), mast/stem cell growth factor
receptor Kit (CD117), MPO, HLA-DR, neural cell adhesion molecule 1
(CD56) and CD38 expression, and abnormal cytogenetics (Table VII). However, no patients in our
study were identified as having the DNMT3A-CEBPA
co-mutation.
 | Table VI.Clinical features of 3 patients with
concurrent FLT3 and NOTCH2 mutations. |
Table VI.
Clinical features of 3 patients with
concurrent FLT3 and NOTCH2 mutations.
|
| Sample ID |
|---|
|
|
|
|---|
| Clinical
features | Sample 2 | Sample 9 | Sample 15 |
|---|
| Age, years | 46 | 58 | 67 |
| Sex | Female | Male | Male |
| White blood cell
(*109/l) | 61.51 | 184.23 | 21.42 |
| Bone marrow blast
count | 64.5 | 89 | 70 |
| Diagnosis | M4 | Unclassified | M5 |
|
Immunophenotype |
|
|
|
|
CD13 | + | + | + |
|
CD15 | + |
| + |
|
CD33 | + | + | + |
|
CD34 | + | + |
|
|
CD117 | + | + |
|
|
MPO | + | + | + |
|
CD64 | + | + | + |
|
HLA-DR | + | + | + |
|
CD56 | + |
|
|
|
CD38 | + | + | + |
| Cytogenetics | Abnormal | Normal | Normal |
| Risk | Medium | Medium | Medium |
| Response
evaluation | NR | Unevaluated | CR |
 | Table VII.Clinical features of 3 patients with
concurrent SETBP1 and CREBBP mutations. |
Table VII.
Clinical features of 3 patients with
concurrent SETBP1 and CREBBP mutations.
|
| Sample ID |
|---|
|
|
|
|---|
| Clinical
features | Sample 2 | Sample 3 | Sample 44 |
|---|
| Age, years | 46 | 23 | 33 |
| Sex | Female | Male | Female |
| WBC
(109/l) | 61.51 | 33.72 | 6.23 |
| Bone marrow blast
count | 64.5 | 63 | 45 |
| Diagnosis | M4 | Unclassified | M2 |
|
Immunophenotype |
|
|
|
|
CD13 | + | + | + |
|
CD15 | + | + |
|
|
CD33 | + | + | + |
|
CD34 | + | + | + |
|
CD117 | + | + | + |
|
MPO | + | + | + |
|
CD64 | + | – | – |
|
HLA-DR | + | + | + |
|
CD56 | + | + | + |
|
CD38 | + | + | + |
| Cytogenetics | Abnormal | Abnormal | Abnormal |
| Risk | Medium | High | Low |
| Response
evaluation | NR | NR | CR |
Discussion
Molecular abnormalities in multiples genes are
involved in the pathogenesis of AML, and have been demonstrated to
affect the overall prognosis of AML (28). In the present study, a total of 11
high frequently mutations were identified. Among them, the
mutations of FLT3, NRAS, CEBPA, ASXL1 and DNMT3A were
significantly associated with the clinical features of patients
with AML. A total of 3 co-mutations, FLT3-NOTCH2,
DNMT3A-CEBPA and SETBP1-CREBBP, were identified to be
significantly associated with the clinical features and prognosis
of patients with AML. Functional enrichment analysis demonstrated
that mutations in these genes were significantly enriched in the
biological process of immune system development, indicating that
these combined mutations may serve a critical role in the
development of AML.
Genetic mutations are significantly associated with
the prognosis and recurrence of AML (6,29).
In previous studies, multiple gene mutations have been identified
in AML, including FLT3 (30), GATA2 (31), IDH (32) and CPM1 (33). Among these mutations, FLT3,
which is the encoding gene of Fms-like receptor tyrosine kinase 3
receptor, is one of the most frequently-occurring mutations
detected in AML (34,35). In the present study, the
FLT3 mutation was identified in 22.58% patients; however,
this frequency was decreased compared with the previously described
rate (~30%) (36). This
discrepancy may be due to differences in the ethnicity,
geographical region and sample size of the investigated subjects
between these studies. In the present study, the FLT3
mutation was also identified to be negatively associated with the
survival of patients with AML based on data from TCGA database,
which was consistent with the results obtained by a previous study
(35). NOTCH2 receptor
signalling was demonstrated to govern the differentiation of
dendritic cells in the spleen and intestine (37). Additionally, NOTCH2 also
controls the rate of generation of long- and short-term
repopulating stem cells in mice (38). In the present study, a
NOTCH2 mutation was detected in 14.52% of patients with AML,
and the co-mutation of FTL3-NOTCH2 was significantly
associated with the variation of WBCs in patients with AML.
Additional analysis revealed that patients with the
FLT3-NOTCH2 co-mutation also exhibited positive CD13, CD33,
MPO, CD64, HLA-DR and CD38 expression, indicating that
FLT3-NOTCH2 may serve critical roles in the regulation of
the immune response.
SETBP1, which is recurrent in myelodysplastic
syndromes (MDS) and often co-exists with cytogenetic markers in the
progression of AML (39), was also
within the top 10 mutations in the present study, with an
occurrence of 14.52%. A previous study demonstrated that the
SETBP1 mutation was detected in 17% of patients with
secondary AML, which was similar to the results obtained in the
present study (40). Cristóbal
et al suggested that overexpressed SETBP1 predicted
an adverse outcome in patients with AML (41). Taken together, these data
demonstrate that gene mutations frequently occur in the development
of AML and exert crucial functions in regulating the prognosis of
AML. In MDS, the SETBP1 mutation promotes the leukemic
transformation of patients with the ASXL1 mutation (42), indicating that the co-mutation of
SETBP1 and ASXL1 may serve a promotive role in the
development of AML. Notably, ASXL1 was significantly
associated with the FAB subtypes in the present study, suggesting
that the SETBP1-ASXL1 mutation was associated with the
clinical features of patients with AML. In the present study, the
co-mutation of SETBP1 and CREBBP was identified in
patients with AML, and this was significantly associated with the
response evaluation and consolidation therapy following CR,
indicating that this co-mutation served an important role in the
treatment and prognosis of AML. In addition, the co-mutation of
SETBP1 and CREBBP consistently presented with
abnormal cytogenetics, and positive CD13, CD33, CD34, CD117, CD56,
CD38 and MPO expression, indicating that these features may be
utilized as potential biomarkers for the diagnosis of patients with
AML who present with the SETBP1 and CREBBP
co-mutation. However, the OS was not significantly different in AML
patients with or without mutant SETBP1 and CREBBP;
therefore, the underlying mechanism of action requires additional
investigation.
DNMT3A is essential for the differentiation
of hematopoietic stem cells and its mutations have been identified
in 4–22% of AML cases (43,44).
In the present study, the DNMT3A mutation was identified in
16.12% of patients with AML. The present study also demonstrated
that the DNMT3A mutation was significantly associated with
the WBC count; however, it was not associated with other mutations
in the patient cohort. The DNMT3A mutation was also revealed
to be negatively associated with the prognosis of AML, which was
consistent with the results obtained by a previous study (45). Although no DNMT3A-CEBPA
co-mutation was identified in the present study, the data from TCGA
database demonstrated that the co-mutation of DNMT3A-CEBPA
was significantly associated with a poor prognosis in patients with
AML. Therefore, additional investigations examining the association
between the co-mutation of DNMT3A-CEBPA and clinical
features should be performed, with a larger patient cohort.
As a result of previous in-depth investigations,
several signalling pathways have been demonstrated to be involved
in the development and prognosis of AML: The study by
Quintás-Cardama et al (46)
demonstrated that mutations in the tumor protein p53 pathway are
associated with the lowest survival rates in patients with AML.
Ufkin et al (47)
hypothesized that miR-125a regulated cell proliferation and
apoptosis in AML via the ErbB pathway. In the present study, the
mutated genes that were significantly associated with clinical
features were also subjected to functional enrichment analysis. The
data revealed that these genes were significantly enriched in the
biological processes of ‘negative regulation of cell
differentiation’ and ‘immune system development’. Curran et
al suggested that targeting the innate immune system may serve
as an underlying therapy for AML (48). Additionally, the co-mutations were
significantly enriched in the ‘Notch signalling pathway’. Takam
Kamga et al (49)
demonstrated that Notch signalling enhanced bone marrow stromal
cell-mediated chemoresistance in AML, and the activation of Notch
antagonizes DNA-binding protein Ikaros-based tumor suppression in
T-cell ALL (50). These data
indicated that these clinical features and mutations of the
associated genes may promote the development of ALL via
dysregulating the differentiation of hematopoietic cells and the
immune response.
In conclusion, FLT3, NOTCH2, and
DNMT3A were the 3 mutations with the highest frequencies
identified in AML. Specifically, the mutations in FLT3 and
DNMT3A were significantly associated with a poor prognosis
in patients with AML. In addition, co-mutations of
FLT3-NOTCH2 and SETBP1-CREBBP were significantly
associated with the clinical features of patients with AML, and may
serve a critical role in AML, via regulating the differentiation of
hematopoietic cells and the immune response. Genome sequencing is
an important method for the detection of mutations in patients with
AML, which may provide useful information in understanding the
mechanism of AML, which would assist in guiding individual
treatment strategies.
Acknowledgements
Not applicable.
Funding
The present study received funding from the National
Natural Science Foundation (grant nos. 81473486 and 81770210), the
Technology Development Projects of Shandong Province (grant nos.
2014GSF118021 and 2017GSF18189), the Taishan Scholar Foundation of
Shandong Province and The Key Research and Development Project of
Shandong Province, China (grant no. 2015GSF118025).
Availability of data and materials
The software packages and raw data used to support
the results of the present study are available from the
corresponding author upon request.
Authors' contributions
YL (first author), XinW and HX made substantial
contributions to the conception and design of the present study,
and drafted the manuscript. XLiu, CZ and WZ performed the data
acquisition. XG, DY and XLv performed the data analysis and
interpretation. YL (11th author), MD and XiaW contributed to the
design of the study, and performed the bioinformatic analysis. All
authors have read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of Shandong Provincial Hospital. All participants
provided written informed consent.
Patient consent for publication
All participants provided written informed
consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khwaja A, Bjorkholm M, Gale RE, Levine RL,
Jordan CT, Ehninger G, Bloomfield CD, Estey E, Burnett A,
Cornelissen JJ, et al: Acute myeloid leukaemia. Nat Rev Dis
Primers. 2:160102016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan Y, Liu D, Wei Y, Su D, Lu C, Hu Y and
Zhou F: Azelaic acid exerts antileukemic activity in acute myeloid
leukemia. Front Pharmacol. 8:3592017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang H, Zheng QL, Fang P, Zhang J, Zhang
T, Liu W, Guo M, Robinson CL, Chen SB, Chen XP, et al: Targeting
the PI3K/AKT pathway via GLI1 inhibition enhanced the drug
sensitivity of acute myeloid leukemia cells. Sci Rep. 7:403612017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanders MA and Valk PJ: The evolving
molecular genetic landscape in acute myeloid leukaemia. Curr Opin
Hematol. 20:79–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kohlmann A, Grossmann V, Nadarajah N and
Haferlach T: Next-generation sequencing-feasibility and
practicality in haematology. Br J Haematol. 160:736–753. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Corces-Zimmerman MR, Hong WJ, Weissman IL,
Medeiros BC and Majeti R: Preleukemic mutations in human acute
myeloid leukemia affect epigenetic regulators and persist in
remission. Proc Natl Acad Sci USA. 111:2548–2553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shih AH, Meydan C, Shank K,
Garrett-Bakelman FE, Ward PS, Intlekofer AM, Nazir A, Stein EM,
Knapp K, Glass J, et al: Combination targeted therapy to disrupt
aberrant oncogenic signaling and reverse epigenetic dysfunction in
IDH2- and TET2-mutant acute myeloid leukemia. Cancer Discov.
7:494–505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yen K, Travins J, Wang F, David MD, Artin
E, Straley K, Padyana A, Gross S, DeLaBarre B, Tobin E, et al:
AG-221, a first-in-class therapy targeting acute myeloid leukemia
harboring oncogenic IDH2 mutations. Cancer Discov. 7:478–493. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng J, Li Y, Jia Y, Fang Q, Gong X, Dong
X, Ru K, Li Q, Zhao X, Liu K, et al: Spectrum of somatic mutations
detected by targeted next-generation sequencing and their
prognostic significance in adult patients with acute lymphoblastic
leukemia. J Hematol Oncol. 10:612017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng J, Gong XY, Jia YJ, Liu KQ, Li Y,
Dong XB, Fang QY, Ru K, Li QH, Wang HJ, et al: Spectrum of somatic
mutations and their prognostic significance in adult patients with
B cell acute lymphoblastic leukemia. Zhonghua Xue Ye Xue Za Zhi.
39:98–104. 2018.(In Chinese). PubMed/NCBI
|
|
11
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A and Bloomfield CD: The 2008
revision of the World Health Organization (WHO) classification of
myeloid neoplasms and acute leukemia: Rationale and important
changes. Blood. 114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Löffler H: Morphology, immunology,
cytochemistry, and cytogenetics and the classification of subtypes
in AML. Haematol Blood Transfus. 33:239–242. 1990.PubMed/NCBI
|
|
13
|
Sherry ST, Ward MH, Kholodov M, Baker J,
Phan L, Smigielski EM and Sirotkin K: dbSNP: The NCBI database of
genetic variation. Nucleic Acids Res. 29:308–311. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
1000 Genomes Project Consortium, ;
Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker
RE, Kang HM, Marth GT and McVean GA: An integrated map of genetic
variation from 1,092 human genomes. Nature. 491:56–65. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adzhubei I, Jordan DM and Sunyaev SR:
Predicting functional effect of human missense mutations using
PolyPhen-2. Curr Protoc Hum Genet Chapter. 7:Unit7.202013.
|
|
16
|
Forbes SA, Bindal N, Bamford S, Cole C,
Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al:
COSMIC: Mining complete cancer genomes in the catalogue of somatic
mutations in cancer. Nucleic Acids Res. 39:D945–D950. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito K and Murphy D: Application of ggplot2
to pharmacometric graphics. CPT Pharmacometrics Syst Pharmacol.
2:e792013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plackett RL: Karl pearson and the
Chi-squared test. Int Stat Rev. 51:59–72. 1983. View Article : Google Scholar
|
|
19
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goel MK, Khanna P and Kishore J:
Understanding survival analysis: Kaplan-Meier estimate. Int J
Ayurveda Res. 1:274–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang YY, Zhou XB, Wang QZ and Zhu XY:
Quality of reporting of multivariable logistic regression models in
Chinese clinical medical journals. Medicine (Baltimore).
96:e69722017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
The Gene Ontology Consortium: The gene
ontology resource: 20 years and still GOing strong. Nucleic Acids
Res. 47:D330–D338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanehisa M, Sato Y, Furumichi M, Morishima
K and Tanabe M: New approach for understanding genome variations in
KEGG. Nucleic Acids Res. 47:D590–D595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposed revised
criteria for the classification of acute myeloid leukemia: A report
of the French-American-British Cooperative Group. Ann Intern Med.
103:620–625. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukherjee A, Nan X, Ensor J, Randhawa JK,
Pingali SRK, Zieske AW, Olsen RJ, Chung B and Iyer SP: An integer
weighted genomic mutation score (GMS) using next generation
sequencing is predictive of prognosis in intermediate risk AML
patients. Blood. 130:39402017.
|
|
29
|
Klco JM, Miller CA, Griffith M, Petti A,
Spencer DH, Ketkar-Kulkarni S, Wartman LD, Christopher M, Lamprecht
TL, Helton NM, et al: Association between mutation clearance after
induction therapy and outcomes in acute myeloid leukemia. JAMA.
314:811–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wander SA, Levis MJ and Fathi AT: The
evolving role of FLT3 inhibitors in acute myeloid leukemia:
Quizartinib and beyond. Ther Adv Hematol. 5:65–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pasquet M, Bellanné-Chantelot C, Tavitian
S, Prade N, Beaupain B, Larochelle O, Petit A, Rohrlich P, Ferrand
C, Van Den Neste E, et al: High frequency of GATA2 mutations in
patients with mild chronic neutropenia evolving to MonoMac
syndrome, myelodysplasia, and acute myeloid leukemia. Blood.
121:822–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Im AP, Sehgal AR, Carroll MP, Smith BD,
Tefferi A, Johnson DE and Boyiadzis M: DNMT3A and IDH mutations in
acute myeloid leukemia and other myeloid malignancies: Associations
with prognosis and potential treatment strategies. Leukemia.
28:1774–1783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Etchin J, Sanda T, Mansour MR, Kentsis A,
Montero J, Le BT, Christie AL, McCauley D, Rodig SJ, Kauffman M, et
al: KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has
selective anti-leukaemic activity in preclinical models of T-cell
acute lymphoblastic leukaemia and acute myeloid leukaemia. Br J
Haematol. 161:117–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Höckendorf U, Yabal M and Jost PJ:
RIPK3-dependent cell death and inflammasome activation in FLT3-ITD
expressing LICs. Oncotarget. 7:57483–57484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kurtz SE, Wilmot B, McWeeney S, Vellanki
A, Local A, Benbatoul K, Folger P, Sheng S, Zhang H, Howell SB, et
al: CG'806, a first-in-class FLT3/BTK inhibitor, exhibits potent
activity against AML patient samples with mutant or wild type FLT3,
as well as other hematologic malignancy subtypes. Clin Cancer Res.
23:442017.
|
|
36
|
Nishida A, Yuasa M, Kageyama K, Ishiwata
K, Takagi S, Yamamoto H, Asano-Mori Y, Yamamoto G, Uchida N, Izutsu
K, et al: High disease-free and overall survival rate following
allogeneic hematopoietic stem cell transplantation for FLT3-mutated
acute myeloid leukemia even in non-remission status. Blood.
128:22832016.PubMed/NCBI
|
|
37
|
Lewis KL, Caton ML, Bogunovic M, Greter M,
Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, et
al: Notch2 receptor signaling controls functional differentiation
of dendritic cells in the spleen and intestine. Immunity.
35:780–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Varnum-Finney B, Halasz LM, Sun M, Gridley
T, Radtke F and Bernstein ID: Notch2 governs the rate of generation
of mouse long- and short-term repopulating stem cells. J Clin
Invest. 121:1207–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fernandez-Mercado M, Pellagatti A, Di
Genua C, Larrayoz MJ, Winkelmann N, Aranaz P, Burns A, Schuh A,
Calasanz MJ, Cross NC and Boultwood J: Mutations in SETBP1 are
recurrent in myelodysplastic syndromes and often coexist with
cytogenetic markers associated with disease progression. Br J
Haematol. 163:235–239. 2013.PubMed/NCBI
|
|
40
|
Makishima H, Yoshida K, Nguyen N,
Przychodzen B, Sanada M, Okuno Y, Ng KP, Gudmundsson KO,
Vishwakarma BA, Jerez A, et al: Somatic SETBP1 mutations in myeloid
malignancies. Nat Genet. 45:942–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cristóbal I, Blanco FJ, Garcia-Orti L,
Marcotegui N, Vicente C, Rifon J, Novo FJ, Bandres E, Calasanz MJ,
Bernabeu C and Odero MD: SETBP1 overexpression is a novel
leukemogenic mechanism that predicts adverse outcome in elderly
patients with acute myeloid leukemia. Blood. 115:615–625. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Inoue D, Kitaura J, Matsui H, Hou HA, Chou
WC, Nagamachi A, Kawabata KC, Togami K, Nagase R, Horikawa S, et
al: SETBP1 mutations drive leukemic transformation in ASXL1-mutated
MDS. Leukemia. 29:847–857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ley TJ, Ding L, Walter MJ, McLellan MD,
Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, et
al: DNMT3A mutations in acute myeloid leukemia. N Engl J Med.
363:2424–2433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abdel-Wahab O, Pardanani A, Rampal R,
Lasho TL, Levine RL and Tefferi A: DNMT3A mutational analysis in
primary myelofibrosis, chronic myelomonocytic leukemia and advanced
phases of myeloproliferative neoplasms. Leukemia. 25:1219–1220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kao HW, Liang DC, Kuo MC, Wu JH, Dunn P,
Wang PN, Lin TL, Shih YS, Liang ST, Lin TH, et al: High frequency
of additional gene mutations in acute myeloid leukemia with MLL
partial tandem duplication: DNMT3A mutation is associated with poor
prognosis. Oncotarget. 6:33217–33225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Quintás-Cardama A, Hu C, Qutub A, Qiu YH,
Zhang X, Post SM, Zhang N, Coombes K and Kornblau SM: p53 pathway
dysfunction is highly prevalent in acute myeloid leukemia
independent of TP53 mutational status. Leukemia. 31:1296–1305.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ufkin ML, Peterson S, Yang X, Driscoll H,
Duarte C and Sathyanarayana P: miR-125a regulates cell cycle,
proliferation, and apoptosis by targeting the ErbB pathway in acute
myeloid leukemia. Leuk Res. 38:402–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Curran E, Corrales L and Kline J:
Targeting the innate immune system as immunotherapy for acute
myeloid leukemia. Front Oncol. 5:832015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takam Kamga P, Bassi G, Cassaro A, Midolo
M, Di Trapani M, Gatti A, Carusone R, Resci F, Perbellini O,
Gottardi M, et al: Notch signalling drives bone marrow stromal
cell-mediated chemoresistance in acute myeloid leukemia.
Oncotarget. 7:21713–21727. 2016.PubMed/NCBI
|
|
50
|
Witkowski MT, Cimmino L, Hu Y, Trimarchi
T, Tagoh H, McKenzie MD, Best SA, Tuohey L, Willson TA, Nutt SL, et
al: Activated Notch counteracts Ikaros tumor suppression in mouse
and human T-cell acute lymphoblastic leukemia. Leukemia.
29:1301–1311. 2015. View Article : Google Scholar : PubMed/NCBI
|