Introduction
Human glioma is one of the most common primary
tumors of the central nervous system, with a high proliferative and
invasive capacity (1). Although
chemotherapy, radiotherapy and microsurgery have previously been
used to treat gliomas, the majority of patients succumb to the
disease within two years of the diagnosis (2). Therefore, there is an urgent requirement
to develop novel and effective therapies for patients with
glioma.
Cell growth and cell death are two critical factors
involved in the homeostasis of normal cells; however, in cancer
cells, homeostasis is often disrupted by the dysregulation of the
cell cycle mechanism (3). The
mammalian cell cycle is divided into four distinct phases:
G1, S, G2 and M. Cyclins, cyclin-dependent
kinases (CDKs) and CDK inhibitors are critical regulators of
mammalian cell cycle progression (4).
Each phase of the cell cycle is regulated by various CDKs and their
individual regulatory cyclins. Induction of cell cycle arrest at
different cell cycle checkpoints contributes to various antitumor
effects (5). The induction of
apoptosis, which typically involves the activation of various
caspases, including caspase-3, represents an important underlying
mechanism for the elimination of tumor cells (6).
Arctigenin is a natural lignan compound that is
extracted from the seeds of Arctium lappa and possesses
various pharmacological properties, including antiproliferative,
anti-inflammatory, antioxidant, antiviral, immunomodulation,
neuroprotective and antidiabetic effects (7). Arctigenin has demonstrated anticancer
activities in numerous types of cancer, including gastric cancer
(8), breast cancer (9) and ovarian cancer (10). However, the effects of arctigenin on
the aggressive phenotypes of human glioma cells remain unclear. In
the present study, the effects of arctigenin on the proliferation,
colony formation and invasion of glioma cells were investigated,
and the effects on the cell cycle distribution and cell apoptosis
were also assessed.
Materials and methods
Cell culture and treatment
U87MG and T98G human glioma cells were purchased
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). Normal human astrocytes (NHA) were purchased from ScienCell
Research Laboratories, Inc., (Carlsbad, CA, USA). Cells were
cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; both Invitrogen;
Thermo Fisher Scientific, Inc. Waltham, MA, USA), and were treated
with various concentrations (1, 5, 10, 20 and 40 µM) of arctigenin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 24 or 48 h
at 37°C before being subjected to further analyses. Dimethyl
sulfoxide (DMSO; 0.5%) was used as the vehicle control. In specific
cases, the cells were pre-treated for 1 h with 10 µM Z-DEVD-FMK
(Calbiochem; EMD Millipore, Billerica, MA, USA), a general
inhibitor of caspase-3, prior to their exposure to arctigenin.
Cell viability assay
Cell viability was evaluated using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich; Merck Millipore). The cells were seeded into
96-well plates (2×104 cells/well) and incubated with 1,
5, 10, 20 or 40 µM arctigenin for 48 h. Subsequently, MTT solution
was added to each well and the cells were incubated in the dark at
37°C for 4 h. The formazan crystals were solubilized in 100 µl DMSO
and the plates were evaluated using a multiwell spectrophotometer
(VersaMax Microplate Reader; Molecular Devices, LLC, Sunnyvale, CA,
USA) at a wavelength of 570 nm. All experiments were performed in
triplicate and the relative cell viability (%) was normalized to
the vehicle-treated control cells.
Bromodeoxyuridine (BrdU) incorporation
assay
Cells were seeded into 96-well plates
(5×103 cells/well) and allowed to adhere overnight.
Arctigenin was added to the culture medium and incubated with the
cells for 48 h. BrdU reagent (5 µM; Sigma-Aldrich; Merck Millipore)
was added to each well and the cells were incubated for an
additional 2–4 h. The cells were then incubated with a mouse
anti-BrdU monoclonal primary antibody (1:300; cat. no. B8434) for 2
h at 37°C and a fluorescein isothiocyanate (FITC)-labeled
anti-mouse IgG secondary antibody (1:2,000; cat. no. F9137; both
Sigma-Aldrich; Merck Millipore) for 30 min at room temperature. The
cells were subsequently stained with DAPI (Sigma-Aldrich; Merck
Millipore) prior to imaging using fluorescence microscopy. The
number of BrdU-positive cells was determined amongst 500 randomly
selected tumor cells.
Colony formation assay
Cells (1,000 cells/well) seeded into 6-well plates
and exposed to arctigenin (10 or 20 µM) for 48 h at 37°C, and were
subsequently incubated in fresh medium for an additional 10 days at
37°C. The cells were then fixed in 4% paraformaldehyde and stained
with 0.1% crystal violet (Sigma-Aldrich; Merck Millipore). The
colonies containing >50 cells were identified and counted using
a phase-contrast microscope.
Matrigel invasion assay
A total of 2×104 cells were suspended in
serum-free DMEM containing various concentrations of arctigenin (10
or 40 µM) and added to the Matrigel-coated upper chambers of
Transwell inserts. Medium supplemented with 10% FBS was added to
the lower chambers. Following incubation for 48 h at 37°C, the
cells on the upper surface of the filters were removed using cotton
swabs. The cells that had invaded to the lower surface of the
filters were fixed in 70% ethanol, stained with Giemsa
(Sigma-Aldrich; Merck Millipore) and counted.
Cell cycle analysis
Following treatment with arctigenin for 48 h, the
cells were harvested by trypsinization, washed twice with PBS and
fixed in ice-cold 70% ethanol. The cells were then washed again,
subjected to RNase A at a final concentration of 0.05 mg/ml
(Sigma-Aldrich; Merck Millipore), and incubated with 10 µg/ml
propidium iodide (PI; Sigma-Aldrich; Merck Millipore) at 4°C for 15
min in the dark. Cell cycle analysis was conducted using a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Apoptosis analysis
In order to analyze cell apoptosis in
arctigenin-treated cells, FITC-labeled annexin V/PI staining was
performed using the FITC-labeled Annexin V Apoptosis Detection kit,
according to the manufacturer's instructions (Nanjing Keygen
Biotech Co., Ltd., Nanjing, China). Briefly, 1×106
cells/well were washed with PBS and resuspended in 500 µl binding
buffer containing 2.5 µl annexin V-FITC and 5 µl PI. The cells
samples were subjected to flow cytometry using a FACSCalibur flow
cytometer and analyzed using CellQuest™ software (version 5.1; BD
Biosciences). A total of 20,000 events from each cell sample were
obtained.
Western blot analysis
The cells were collected and lysed in lysis buffer
(50 mmol/l Tris-HCl, pH 7.4; 150 mmol/l NaCl; 1% NP-40 and 0.5%
sodium deoxycholate), supplemented with 1 mM
phenylmethanesulphonylfluoride, 10 mg/ml aprotinin and 10 mg/ml
leupeptin (all Roche Diagnostics GmbH, Mannheim, Germany). Protein
concentration was evaluated using a BCA Protein Assay kit (Beyotime
Institute of Biotechnology, Haimen, China), and 40 µg total
protein/lane was separated using 10% SDS-PAGE and transferred onto
nitrocellulose membranes. The membranes were blocked with 5%
non-fat dry milk for 1 h at room temperature, followed by
incubation with various primary antibodies (Table I) at 4°C overnight. Membranes were
subsequently washed three times with Tris buffer (pH 7.4). The
membranes were then incubated for 1 h at room temperature with
horseradish peroxidase (HRP)-conjugated secondary antibodies
(Table I). Subsequently, the
membranes were incubated with Amersham ECL Western Blotting
Detection Reagent (GE Healthcare Life Sciences, Chalfont, England)
according to the manufacturer's instructions, and the blots were
visualized by exposure to X-ray films in the dark. Densitometric
analyses were conducted using Scion Image software (version 4.1;
Scion Corporation, Frederick, MD, USA) using β-actin for
normalization.
 | Table I.Antibodies used in the present
study. |
Table I.
Antibodies used in the present
study.
| Antibody | Source | Catalog no. | Dilution |
|---|
| Anti-p21 | CST | 2947 | 1:300 |
| Anti-RB | CST | 9309 | 1:300 |
| Anti-p53 | CST | 2524 | 1:300 |
| Anti-cyclin D1 | CST | 2978 | 1:300 |
| Anti-CDK4 | CST |
12790 | 1:300 |
| Anti-p27 | CST | 3698 | 1:300 |
| Anti-CDK2 | SCBT | sc-6248 | 1:300 |
| Anti-SKP2 | SCBT | sc-7164 | 1:300 |
| Anti-CDK6 | SCBT | sc-7961 | 1:300 |
| Anti-cleaved
caspase-3 | SCBT | sc-22171 | 1:300 |
| Anti-BAX | SCBT | sc-20067 | 1:300 |
| Anti-Bcl-2 | SCBT | sc-7382 | 1:300 |
| Anti-β-actin | SCBT | sc-47778 | 1:500 |
| HRP-conjugated
anti-mouse IgG | SCBT | sc-2386 |
1:3,000 |
| HRP-conjugated
anti-rabbit IgG | SCBT | sc-2379 |
1:3,000 |
Statistical analysis
Data are presented as the mean ± standard deviation.
All analyses were performed using one-way analysis of variance
followed by Tukey's post hoc test, using SPSS version 13.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant result.
Results
Arctigenin inhibits the growth of
glioma cells in vitro
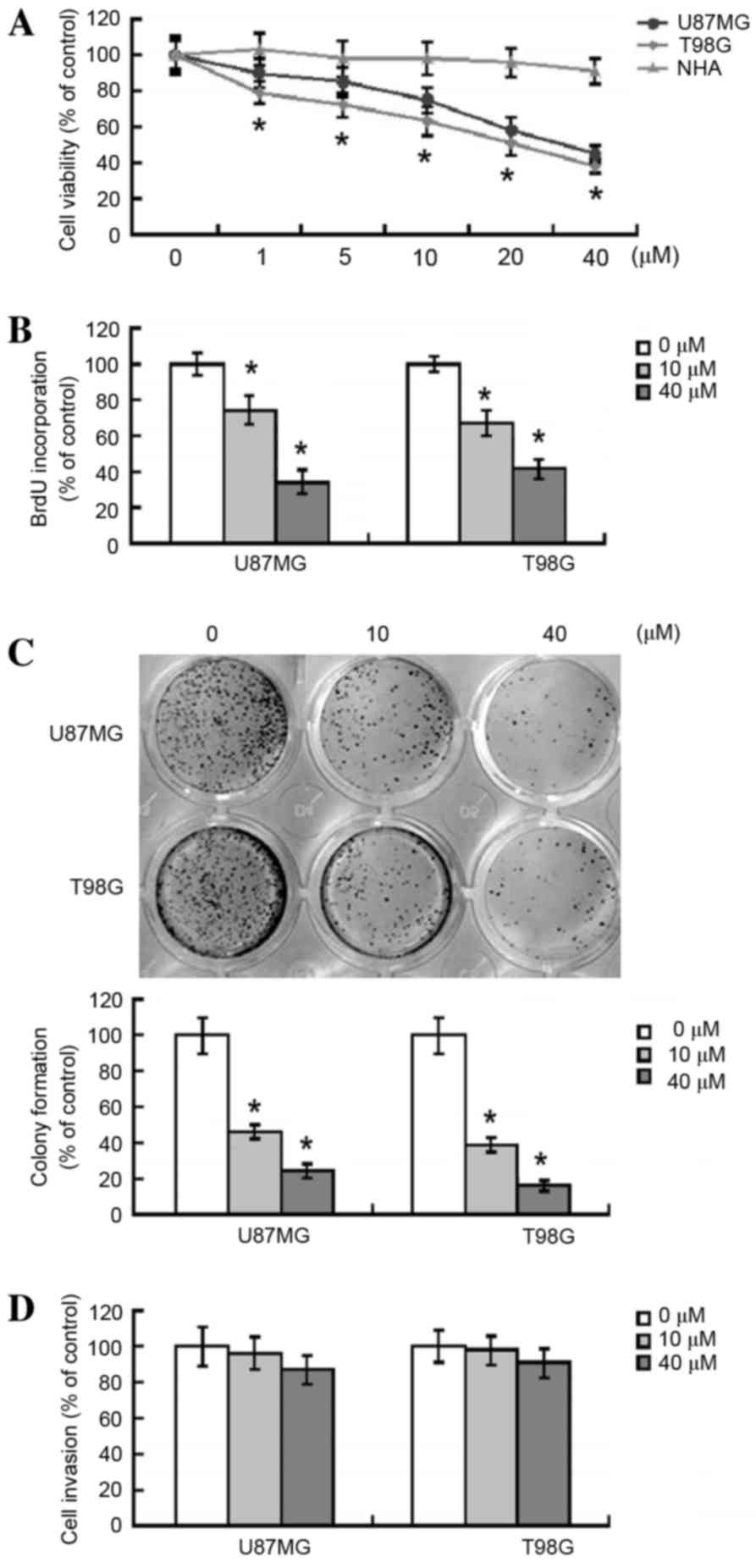
At all the concentrations investigated, arctigenin
treatment was observed to significantly suppress the viability of
U87MG and T98G glioma cells, as compared with vehicle-treated
control cells (Fig. 1A). Furthermore,
this suppression was determined to be concentration-dependent.
Treatment with 40 µM arctigenin decreased the viability of U87MG
and T98G cells by 47% (P=0.0027) and 40% (P=0.0012), respectively.
By contrast, arctigenin (≤40 µM) was not observed to result in
significant cytotoxicity to NHAs (Fig.
1A). Unless otherwise indicated, two concentrations for
arctigenin (10 and 40 µM) were used in the following
experiments.
The antiproliferative activity of arctigenin in
human glioma cells was further investigated using BrdU
incorporation assays (Fig. 1B). The
results demonstrated that treatment with arctigenin (10 and 40 µM)
significantly inhibited the proliferation of U87MG (P=0.0305 and
P=0.0046, respectively) and T98G (P=0.0279 and P=0.0113,
respectively) cells. The effect of arctigenin on the
anchorage-independent growth of glioma cells was also assessed
using clonogenic assays. As presented in Fig. 1C, exposure of U87MG and T98G cells to
arctigenin induced a significant 60–75% reduction in colony
formation, as compared with vehicle-treated cells (P<0.05).
Arctigenin does not affect the
invasive capacity of glioma cells
The effect of arctigenin on the invasive capacity of
glioma cells was also examined. As presented in Fig. 1D, arctigenin was not observed to have
a significant effect on the invasive ability of U87MG and T98G
cells through Matrigel-coated membranes (P=0.324 vs.
vehicle-treated cells).
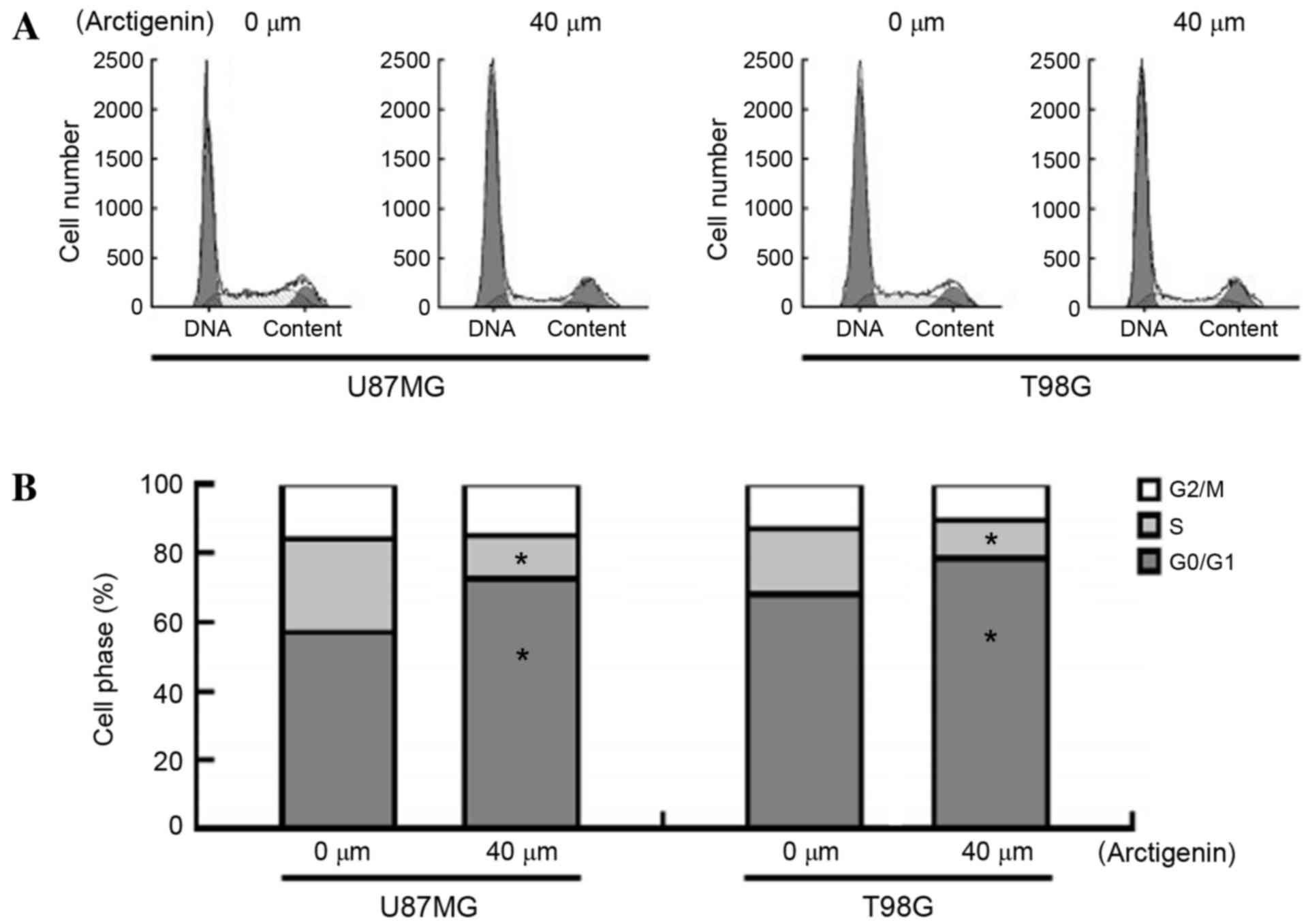
Arctigenin induces
G0/G1 cell cycle arrest in glioma cells
To elucidate the mechanisms underlying the
antiproliferative activity of arctigenin, PI staining was performed
to evaluate the cell cycle progression in response to arctigenin
exposure (Fig. 2A and B). The results
demonstrated that treatment of U87MG cells with arctigenin
significantly increased the proportion of cells in the
G0/G1 phase (71.2±1.9 vs. 57.8±1.4%;
P=0.0143) and reduced the number of cells in the S phase (12.4±0.7
vs. 25.3±1.2%; P=0.005) compared with the vehicle-treated cells.
Similar results were noted in T98G cells following treatment with
arctigenin, indicating that arctigenin is able to induce cell cycle
arrest at the G0/G1 phase.
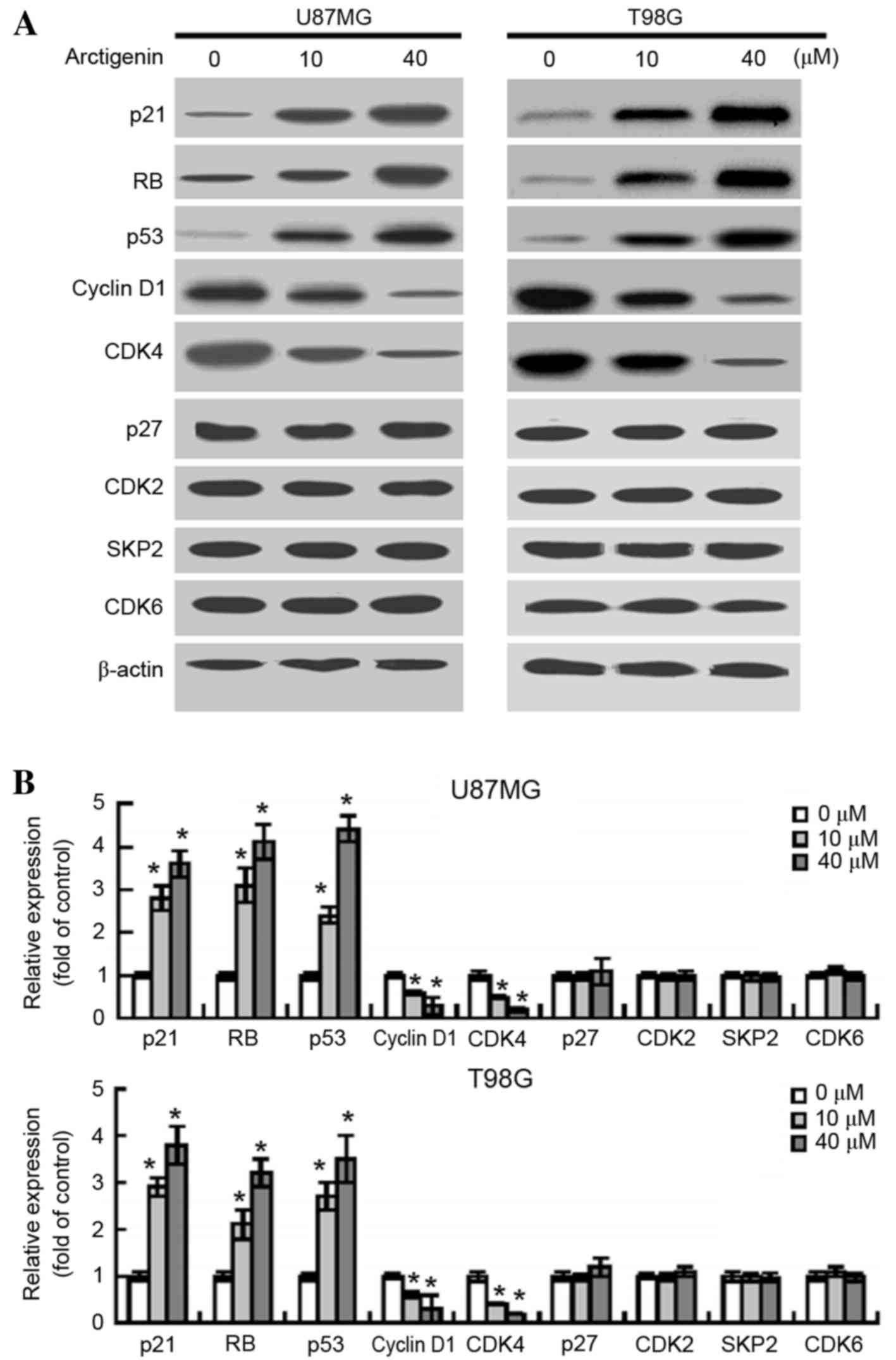
Arctigenin affects the expression of
certain cell cycle-related proteins
Following exposure of the U87MG and T98G cells to
arctigenin, changes in the gene products that regulate cell cycle
progression were examined. Following treatment with 10 and 40 µM
arctigenin, western blot analysis revealed increased protein
expression levels of p21 (P=0.0226 and P=0.0102, respectively),
retinoblastoma protein (RB; P=0.0147 and P=0.0079, respectively)
and p53 (P=0.0368 and P=0.0054, respectively) in U87MG cells
(Fig. 3A and B). By contrast,
following treatment with 10 and 40 µM arctigenin, the protein
expression levels of cyclin D1 (P=0.0412 and P=0.0308,
respectively) and CDK4 (P=0.0375 and P=0.0116, respectively) were
significantly decreased in U87MG cells. However, no significant
variations in the expression levels of p27, CDK2, S-phase
kinase-associated protein 2 (SKP2) and CDK6 were observed between
the vehicle- and arctigenin-treated glioma cells. Similar results
were detected in T98G cells in response to treatment with
arctigenin (Fig. 3A and B).
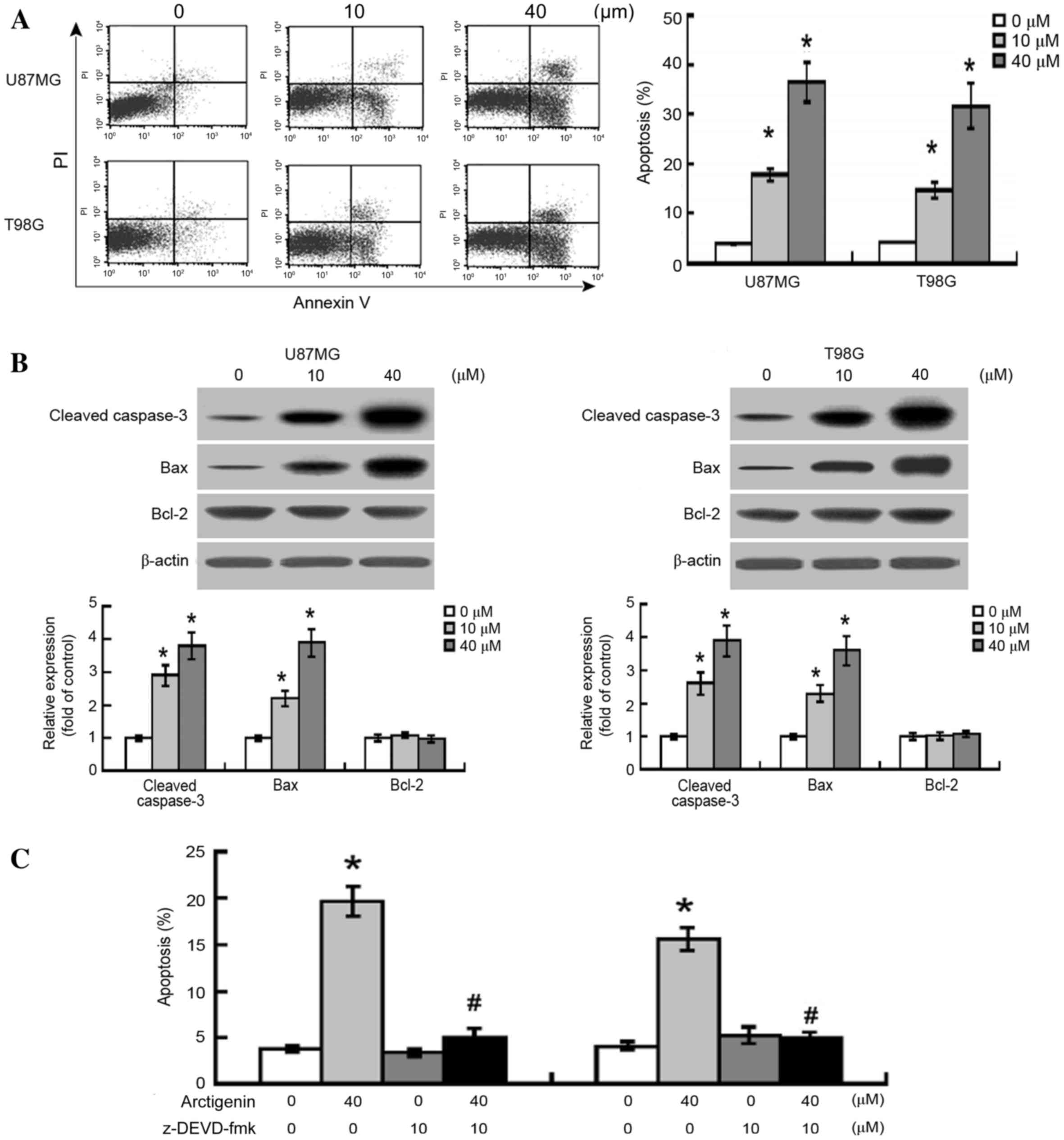
Arctigenin induces caspase-3-dependent
apoptosis in glioma cells
Flow cytometric analysis revealed that treatment
with arctigenin (10 and 20 µM) significantly induced apoptotic
response in U87MG and T98G cells (P<0.0001; Fig. 4A). Western blot analysis also
demonstrated that increased expression levels of the apoptosis
mediator cleaved caspase-3 were present in the arctigenin-treated
cells (Fig. 4B). Furthermore, the
expression levels of the pro-apoptotic protein B-cell lymphoma-2
(Bcl-2)-associated X-protein (BAX) were significantly increased
following treatment with 10 and 40 µM arctigenin in U87MG (P=0.0042
and P<0.0001, respectively) and T98G (P=0.0015 and P<0.0001,
respectively) cells, compared with the vehicle-treated cells.
However, no significant difference was observed in the protein
expression levels of the anti-apoptotic protein Bcl-2 between the
vehicle-treated and the arctigenin-treated cells.
In order to further evaluate the significance of
caspase activation in glioma cells following arctigenin treatment,
the general caspase-3 inhibitor, Z-DEVD-FMK, was used. As presented
in Fig. 4C, arctigenin-induced
apoptosis was significantly suppressed by the pre-treatment of
glioma cells with Z-DEVD-FMK, indicating that the
arctigenin-induced apoptosis of glioma cells may be mediated via
caspase-3 activation.
Discussion
In the present study, it was identified that
arctigenin (≤40 µM) significantly inhibited the growth of U87MG and
T98G cells, induced G0/G1 cell-cycle arrest
and triggered cell apoptosis in glioma cells. However, within the
concentration range used, arctigenin was not observed to affect the
invasive capacity of glioma cells. Western blot analysis
demonstrated that arctigenin is able to enhance the protein
expression levels of p21, RB and p53, and significantly decrease
the expression levels of cyclin D1 and CDK4. However, arctigenin
was not revealed to affect the expression levels of p27, CDK2, SKP2
and CDK6 in glioma cells. Arctigenin was also able to increase the
expression levels of the apoptosis mediator cleaved caspase-3, and
the pro-apoptotic BAX protein, in glioma cells. These results
suggest that arctigenin may be potentially effective in inhibiting
the proliferation of human glioma cells.
Numerous natural herb products have previously been
demonstrated to exert antiproliferative effects in various types of
cancer cells in vitro and in vivo, including in
glioma cells (11–13). For example, mangiferin, a flavonoid
extracted from the leaves of the Anacardiaceae plant, is
able to inhibit proliferation and induce apoptosis in glioma cells
via the induction of microRNA-15b and the inhibition of matrix
metalloproteinase 9 (MMP-9) expression levels (11). Similarly, escin, which is the primary
active compound of Aesculus hippocastanum (the horse
chestnut) seed extract, has been revealed to reduce cell
proliferation and induce apoptosis in certain glioma cell lines
(12). Paeoniflorin is one of the
active ingredients present in Paeonia, and is able to
suppress proliferation and trigger apoptosis in human glioma cells
via the upregulation of microRNA-16 and the downregulation of MMP-9
expression (13). Previous studies
have demonstrated that arctigenin exhibits antitumor effects in
various types of tumor cells (7–9,14). In the present study, it was identified
that arctigenin, within the concentration range evaluated,
significantly suppressed the viability of the U87MG and T98G cells,
as compared with the vehicle-treated cells, in a
concentration-dependent manner. The antiproliferative activity of
arctigenin in glioma cells was further demonstrated using BrdU
incorporation assays and colony formation assays.
Dysregulation of cell cycle progression has been
observed in glioma cells (15,16). In
order to elucidate the underlying mechanisms of the
arctigenin-mediated inhibition of glioma growth, the effects of
arctigenin on the cell cycle distribution were analyzed in U87MG
and T98G human glioma cells in the present study. It was observed
that arctigenin was effective in inducing
G0/G1 cell cycle arrest in glioma cells,
which was consistent with a previous report that demonstrated
gastric cancer cell G0/G1 arrest following
treatment with arctigenin (7). At the
molecular level, arctigenin enhanced the expression levels of p21,
RB, p53, cyclin D1, and CDK4. However, arctigenin did not
significantly alter the expression levels of p27, CDK2, SKP2 and
CDK6. The tumor suppressor gene p53 acts as a transcription factor
to induce the expression of p21WAF1/CIP1/Sdi1, which is an
inhibitor of CDKs (17). Accumulated
p21 inhibits RB phosphorylation and activation, which is required
for cell cycle progression (18).
Therefore, arctigenin has the potential to alter the expression of
numerous genes involved in the regulation of the cell cycle, which
may explain its induction of cell cycle arrest at the
G0/G1 phase.
The effects of arctigenin on cell apoptosis were
also investigated by flow cytometric analysis, demonstrating that
arctigenin induced the apoptosis of U87MG and T98G cells. Caspases,
which are a family of inactive proenzymes, have a crucial role in
the apoptotic signaling pathway (19). Caspase-3 protein interacts with
caspase-8 and caspase-9, and its role in apoptosis is to cleave and
activate caspases 6, 7 and 9 in order to break down apoptotic cells
prior to their removal (19). Western
blot analysis indicated that arctigenin enhanced the expression
levels of cleaved caspase-3, which suggests that caspase-3 is
involved in arctigenin-triggered apoptosis. Concordantly,
arctigenin-mediated apoptosis was significantly inhibited by
pretreatment of the U87MG and T98G cells with Z-DEVD-FMK, a
caspase-3 inhibitor, indicating that the arctigenin-induced
apoptosis of glioma cells may be mediated via caspase-3 activation.
The pro-apoptotic activity of arctigenin has also been demonstrated
in numerous other types of cancer, including breast and ovarian
cancer (8,10,20).
In conclusion, arctigenin has demonstrated
growth-suppressive effects in U87MG and T98G human glioma cells,
and is associated with the induction of
G0/G1-phase cell cycle arrest and
caspase-3-dependent cell apoptosis. Further studies are required to
investigate the in vivo effects of arctigenin on the growth
of gliomas in animal models.
References
|
1
|
Yu X, Zhang W, Ning Q and Luo X:
MicroRNA-34a inhibits human brain glioma cell growth by
down-regulation of Notch1. J Huazhong Univ Sci Technolog Med Sci.
32:370–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grossman SA, Ye X, Piantadosi S, Desideri
S, Nabors LB, Rosenfeld M and Fisher J: NABTT CNS and Consortium:
Survival of patients with newly diagnosed glioblastoma treated with
radiation and temozolomide in research studies in the United
States. Clin Cancer Res. 16:2443–2449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borgne A and Golsteyn RM: The role of
cyclin-dependent kinases in apoptosis. Prog Cell Cycle Res.
5:453–459. 2003.PubMed/NCBI
|
|
5
|
Ruijtenberg S and van den Heuvel S:
Coordinating cell proliferation and differentiation: Antagonism
between cell cycle regulators and cell type-specific gene
expression. Cell Cycle. 15:196–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Indovina P, Pentimalli F, Casini N, Vocca
I and Giordano A: RB1 dual role in proliferation and apoptosis:
Cell fate control and implications for cancer therapy. Oncotarget.
6:17873–17890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kudou N, Taniguchi A, Sugimoto K, Matsuya
Y, Kawasaki M, Toyooka N, Miyoshi C, Awale S, Dibwe DF, Esumi H, et
al: Synthesis and antitumor evaluation of arctigenin derivatives
based on antiausterity strategy. Eur J Med Chem. 60:76–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong JB, Hong SC, Jeong HJ and Koo JS:
Arctigenin induces cell cycle arrest by blocking the
phosphorylation of Rb via the modulation of cell cycle regulatory
proteins in human gastric cancer cells. Int Immunopharmacol.
11:1573–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsieh CJ, Kuo PL, Hsu YC, Huang YF, Tsai
EM and Hsu YL: Arctigenin, a dietary phytoestrogen, induces
apoptosis of estrogen receptor-negative breast cancer cells through
the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic Biol
Med. 67:159–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang K, Li LA, Meng YG, You YQ, Fu XY and
Song L: Arctigenin promotes apoptosis in ovarian cancer cells via
the iNOS/NO/STAT3/survivin signalling. Basic Clin Pharmacol
Toxicol. 115:507–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao J, Liu L, Zhong Z, Xiao C and Zhang
J: Mangiferin regulates proliferation and apoptosis in glioma cells
by induction of microRNA-15b and inhibition of MMP-9 expression.
Oncol Rep. 33:2815–2820. 2015.PubMed/NCBI
|
|
12
|
Çiftçi GA, Işcan A and Kutlu M: Escin
reduces cell proliferation and induces apoptosis on glioma and lung
adenocarcinoma cell lines. Cytotechnology. 67:893–904. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Qi Z, Wei Z, Liu S, Wang P, Chen Y
and Zhao Y: Paeoniflorin inhibits proliferation and induces
apoptosis of human glioma cells via microRNA-16 upregulation and
matrix metalloproteinase-9 downregulation. Mol Med Rep.
12:2735–2740. 2015.PubMed/NCBI
|
|
14
|
Yang S, Ma J, Xiao J, Lv X, Li X, Yang H,
Liu Y, Feng S and Zhang Y: Arctigenin anti-tumor activity in
bladder cancer T24 cell line through induction of cell-cycle arrest
and apoptosis. Anat Rec (Hoboken). 295:1260–1266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouyang Q, Xu L, Cui H, Xu M and Yi L:
MicroRNAs and cell cycle of malignant glioma. Int J Neurosci.
126:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Changa YC, Choua FP, Huanga HP, Hsub JD
and Wang CJ: Inhibition of cell cycle progression by penta-acetyl
geniposide in rat C6 glioma cells. Toxicol Appl Pharmacol.
198:11–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomez-Manzano C, Fueyo J, Kyritsis AP,
McDonnell TJ, Steck PA, Levin VA and Yung WK: Characterization of
p53 and p21 functional interactions in glioma cells en route to
apoptosis. J Natl Cancer Inst. 89:1036–1044. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Igata M, Motoshima H, Tsuruzoe K, Kojima
K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D,
et al: Adenosine monophosphate-activated protein kinase suppresses
vascular smooth muscle cell proliferation through the inhibition of
cell cycle progression. Circ Res. 97:837–844. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao X, Zhu F, Zhao Z, Liu C, Luo L and Yin
Z: Arctigenin enhances chemosensitivity of cancer cells to
cisplatin through inhibition of the STAT3 signaling pathway. J Cell
Biochem. 112:2837–2849. 2011. View Article : Google Scholar : PubMed/NCBI
|